Abstract
Cytokines play crucial roles in the communication between brain cells including neurons and glia, as well as in the brain-periphery interactions. In the brain, cytokines modulate long-term potentiation (LTP), a cellular correlate of memory. Whether cytokines regulate LTP by direct effects on neurons or by indirect mechanisms mediated by non-neuronal cells is poorly understood. Elucidating neuron-specific effects of cytokines has been challenging because most brain cells express cytokine receptors. Moreover, cytokines commonly increase the expression of multiple cytokines in their target cells, thus increasing the complexity of brain cytokine networks even after single-cytokine challenges. Here, we review evidence on both direct and indirect-mediated modulation of LTP by cytokines. We also describe novel approaches based on neuron- and synaptosome-enriched systems to identify cytokines able to directly modulate LTP, by targeting neurons and synapses. These approaches can test multiple samples in parallel, thus allowing the study of multiple cytokines simultaneously. Hence, a cytokine networks perspective coupled with neuron-specific analysis may contribute to delineation of maps of the modulation of LTP by cytokines.
Keywords: synapse, LTP, hippocampus, FASS-LTP, inflammation
1. Introduction
Brain plasticity underlies our ability to learn and modify our behavior, and can be compromised in neuropsychiatric and neurodegenerative diseases. Brain plasticity relies on synaptic plasticity, which strengthens or weakens synapses. One of the most widely used models for studying molecular mechanisms of synaptic plasticity is long-term potentiation (LTP), a cellular correlate of memory characterized by a rapid and remarkably persistent increase in synaptic transmission elicited by brief patterns of afferent activity (1). Experimental support that LTP is causally linked to synaptic processes underlying memory continues to build (2, 3). Notably, a recent study demonstrated that fear conditioning (a type of associative memory) can be inactivated and reactivated by long-term depression (LTD) and LTP respectively (4), supporting a causal link between these synaptic processes and memory. The critical elements for establishing LTP involve membrane depolarization and NMDA receptors (NMDAR) activation (2), which allows calcium influx (5), activation of intracellular pathways (e.g., calcium/calmodulin kinases, PKA, and the Rac/Pak/LIMK cascade), and morphological adaptations in spines that are essential for stable LTP (6). LTP can be modulated by soluble messengers of the brain, such as cytokines and classic neuromodulators (e.g., norepinephrine, dopamine and acetylcholine). While the role of classic neuromodulators on LTP has been extensively studied, the effects of cytokines on LTP are relatively unexplored. Importantly, a growing body of evidence indicates that cytokine networks modulate LTP under both physiological and pathological conditions (7). In this review, we illustrate examples of direct vs indirect modulation of synaptic transmission and LTP by cytokines. We show that cytokines can directly target synapses, and present a novel approach using isolated synaptosomes which allows the study of LTP directly at the synapse. We conclude with a perspective on strategies for dissecting the identity of cytokines able to modulate LTP directly in neurons. The information provided by these novel approaches may reveal key nodes on the topology of brain cytokine-cell networks.
Cytokines constitute an extremely elaborated network of peptide signaling molecules (~5–20 kDa) that are fundamental in cell signaling. Cytokines act through receptors, and are especially important for immune cells, which synthesize and release cytokines in response to infections or tissue damage. Notably, the pattern of released cytokines depends on the nature of the antigenic stimulus, and the cell source that is being stimulated (8). A number of factors further contribute to the high complexity of cytokine-cell networks. A prominent factor is the cytokine’s pleiotropy nature, by which a given cytokine can induce differential, even opposite cell responses (9, 10). In addition, cytokines can cross-talk with signaling from other soluble factors; a cross-talk that is time, concentration and tissue-specific (10). When acting on the brain, cytokines can induce fever, sleep and sickness behavior; they can also modify the mood, memory consolidation and cognition, as well as regulate neuroendocrine stress responses (8). Indeed, a large number of cytokines can be released under multiple physiological and pathological contexts including learning, arousal, stress and neurodegeneration (7). Brain cytokine levels are generally low at physiological-basal conditions but dramatically increase in response to infection, pathology (e.g., Aβ, α-synuclein) or damage (e.g., damage-associated molecular patterns, PAMP’s). Based on the emerging understanding that inflammation-mediated signaling leads to cognitive deficits (11, 12), it is commonly believed that neuronal functions can be impaired by high concentrations of inflammatory cytokines (e.g., IL-1β, IL-6, IL-18, tumor necrosis factor-α (TNFα), interferon (IFN)-α and IFNγ), whereas anti-inflammatory cytokines (e.g., IL-4, and IL-10) could have a protective role (13).
Inflammatory cytokines impair neuronal function in the adult brain by their direct effect on neurons or by indirect mechanisms mediated by non-neuronal cells (e.g., microglia and astrocytes). The effects of inflammatory cytokines on brain mechanisms have been studied in vitro using brain slices (14, 15), as well as in vivo by systemic treatment (16), direct infusion in the brain (17, 18), and by transgenic cytokine overexpression (19, 20). In these experimental systems, elucidating neuron-specific effects of cytokines has been challenging because both neurons and non-neuronal brain cells commonly express cytokine receptors (21). Moreover, cytokines can induce the expression and release of multiple cytokines in their target cells (20–24), thus increasing the complexity of the stimuli sensed by neurons after a challenge with a single cytokine (Fig. 1). Clarification of the brain cytokine networks and how their final effectors impact neuronal activity directly during both physiological and pathological contexts may help to identify specific therapeutic targets for inflammation-related cognitive decline.
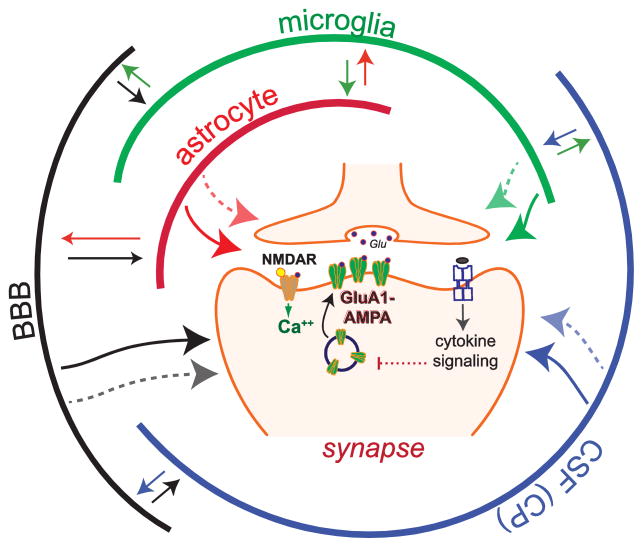
Fig. 1.
Cytokines and cytokine networks modulate LTP by targeting synapses. This simplified model illustrates LTP, which relies on the NMDAR-dependent insertion of GluA1-containing AMPA receptors at the postsynaptic surface. In this model communication via cytokines is depicted by arrows, which can bi-directionally connect multiple cell populations. Cytokine networks enable local interactions between neuronal and non-neuronal cells (e.g., astrocytes, microglia, vascular endothelial cells) in the brain, as well as brain-periphery communication via the brain-blood barrier (BBB) and the choroid plexus (CP). The BBB releases cytokines and regulates the flux of cytokines from the blood; the CP produces cerebrospinal fluid (CSF) and cytokines, and regulates the transport of cytokines and immune cells from blood vessels. LTP modulation by cytokines has been widely studied, however, for most cytokines, is unclear if they modulate LTP by directly targeting synapses (one-direction arrows) or by indirect mechanisms relying on cytokine networks maintained by non-neuronal cells interactions. Cytokines can induce the expression and release of multiple cytokines in their target cells, thus activating cytokine networks, which could modulate synaptic transmission by targeting synapses via both cytokine-dependent and -independent mechanisms (dotted arrows).
2. Modulation of synaptic transmission by cytokine-cell networks
Cytokine networks are composed of the cytokine themselves, their receptors and their regulators. In the brain, cytokine networks are fundamental for the dynamic interaction between neurons, glia, endothelial cells, and immune cells including monocytes and lymphocytes (Fig. 1). Immune cells reach the CNS via blood (10) and, potentially, via the recently discovered meningeal lymphatic vessels (25). At the synapse, the central element of neural connectivity, pre- and post-synaptic elements interact with processes of neighboring astrocyte and microglia via hormones, neurotransmitters and cytokines; these interactions have led to the concept of tri, tetra, and multipartite synapses (26). Importantly, cytokines networks can locally modulate synaptic transmission via glia-neuron signaling. For instance, a recent report has demonstrated that TNFα (600 pM but not 60 pM) increases presynaptic activity as measured by the frequency of miniature postsynaptic excitatory currents (mEPSCs), in mouse hippocampal slices (27). Using elegant genetic models, the authors demonstrated that TNFα activates TNFR1 at astrocytes, which then signal to the neurons via glutamate/NMDAR to increase presynaptic activity. Relevant for neuroinflammation, this TNFα-mediated modulation of astrocyte-neuron communication contributes to memory impairments in a model of multiple sclerosis (experimental autoimmune encephalomyelitis, EAE) (27). Similar to the TNFα/TNFR1-astrocyte-glutamate/NMDAR-synapse network, neurons and glia also interact via fractalkine (CX3CL1), a chemokine that is expressed by neurons and acts through a receptor (CX3CR1) that is present on microglia. Microglial CX3CR1 activation by fractalkine induces the release of adenosine, which activates A2AR receptors on microglia (and possibly astrocytes) causing the release of D-serine which acts as a co-agonist at the NMDA receptor, thus potentiating NMDA-mediated excitatory postsynaptic potentials (fEPSPs) (28). Overall, these data illustrate that cytokines/chemokines impact synaptic transmission via glia-released NMDAR-activating factors (e.g., glutamate and D-serine). Still incompletely defined, however, is which cytokines, if any, modulate synaptic transmission and plasticity as final effectors, acting directly on neuronal elements (soma, dendrites and synapses) without the glia as intermediary.
3. Suppression of LTP by cytokines
Recent reports have shown that the Nlrp3 inflammasome controls systemic inflammation in both brain and periphery (29, 30). Following Nlrp3 inflammasome activation, caspase-1 can cleave the precursors of IL-1β and IL-18, thus converting these cytokines into mature forms that can be secreted from the cell. In rodents, in vivo (31, 32) and in vitro (14, 15) electrophysiological recordings have shown that IL-1β suppresses LTP in the hippocampus, a brain region containing key neuronal circuitries for memory formation. While IL-18 also impairs LTP in the hippocampus, the naturally occurring IL-1 receptor antagonist (IL-1RA) blocks the suppression of LTP by IL-18 (33), thus indicating that IL-18 impairs LTP indirectly, via an IL-1-dependent mechanism. Similarly, it has been shown that the suppression of hippocampal LTP by IFNγ is associated with an increased IL-1β signaling following IFNγ-dependent microglia activation (34, 35). Overall, these data indicate that, in the hippocampus, IL-18 and IFNγ activate cellular-molecular cascades that increase the levels of IL-1β, which may impair LTP directly in neurons. It is noteworthy that hippocampal neurons express high levels of IL-1 receptor type-1 (IL-1R1, the ligand binding subunit) (23, 36) and its accessory receptor subunits (37). Thus, the hippocampus is well positioned to be modulated by IL-1.
A principle concept that has evolved in the field is that IL-1β is the final common effector for many cytokine networks modulating LTP and memory. Although IL-1β may also act as an intermediate factor further amplifying inflammation by stimulating the synthesis of TNFα, IL-6 and GM-CSF (Granulocyte-macrophage colony-stimulating factor) in glia (38), several reports strongly suggest that IL-1β is a main final effector for the inflammation-induced deficits in both LTP and memory. For instance, age-related cognitive impairments by low-grade inflammation are attenuated in the IL-1 receptor deficient mouse (29), while brain infusion of IL-1RA blocks the suppression of LTP and memory following peripheral inflammatory challenges in aged rats (39–41). Similarly, pre-incubation of hippocampal slices with IL-1RA prevented the sepsis-induced impairments of LTP in mice (42). Consistent with these data, two experimental strategies to reduce brain IL-1 signaling in vivo (infusion of either an IL-1 receptor blocking antibody or IL-1RA) block the impairment on LTP and memory following amyloid-beta (Aβ)-induced inflammation (43, 44). Further supporting the idea that IL-1β is one of the final effectors for the impairment of LTP, some reports suggest that the anti-inflammatory cytokines IL-4 and IL-10 rescue LTP by reducing IL-1β signaling in models of age- and LPS-driven inflammation (45, 46). However, all the evidence described above does not conclusively establish that IL-1β impairs LTP and memory by acting directly on neurons as a final effector during the activation of cascades of inflammatory cytokines (e.g., IL-18 and IFNγ). Thus, a timely question is how to dissect neuron-targeting effectors directly impairing LTP?
4. Facilitation of LTP by cytokines
In apparent contradiction with clinical studies showing that cytokine-driven neuroinflammation contributes to Alzheimer’s disease and other neurodegenerative diseases (11, 12, 47), there is evidence that inflammatory cytokines may play physiological roles in the healthy brain (i.e., in the absence of immune challenges or age-related inflammation). For instance, TNFα has been involved in AMPA receptor scaling, a homeostatic (non-Hebbian) form of plasticity that regulates neuronal firing rate by altering the quantity of postsynaptic AMPA receptors (48). Also, knockout mice for IL-1R1 (49), IL-6 (50) and TNF receptor-2 (TNFR2) (51) exhibit memory impairments, thus indicating that endogenous signaling by these cytokines contributes to synaptic plasticity and memory. The critical role of physiological levels of IL-1β on memory and LTP has been further demonstrated by genetic (e.g., IL-1RA transgenic overexpression (18, 52)) and pharmacological manipulations (e.g., the maintenance of LTP in the hippocampus is blocked by the IL-1 receptor antagonist, IL-1RA (53)). IFNγ, a molecule crucial for the immune response against viruses, is another inflammatory cytokine that can facilitate LTP and memory (54, 55). According to a recent report using IFNγ-deficient mice, endogenous IFNγ signaling can facilitate LTP by blocking GABA-mediated inhibition in the hippocampus (55). IFNγ could reduce GABA release/production directly on interneurons or indirectly via glia-dependent mechanisms. Alternatively, IFNγ could increase the sensitivity to GABA on excitatory neurons. At present however, none of these possible mechanisms has been tested.
Many factors may contribute to the dual (physiological-pathological) effects of inflammatory cytokines in the brain, such as the sensitivity of different cell types to a given cytokine concentration, and the selective activation of receptor isoforms. For instance, recent reports indicate that IL-1β activates inflammatory signaling via the IL-1 receptor accessory protein (AcP), whereas the IL-1β-mediated neuronal survival depends on AcPb, an AcP splice variant (37, 56). Similarly, the two TNFα receptors, TNFR-1 and TNFR2, have been proposed to differentially impact the CNS, with TNFR-1 contributing to neuronal damage, whereas TNFR-2 is neuroprotective and facilitates memory (51, 57). In addition to these receptor-dependent mechanisms, a main factor underlying the dual effects of cytokines in the CNS might be the cytokine’s concentration itself. Low IL-1β concentrations (1–3 pM (58)), for instance, enhance LTP in hippocampal slices and facilitate hippocampal-dependent memory (18); whereas pathological-high concentrations suppress both LTP (1–3 nM (15, 58)) and memory (18). Interestingly, there is evidence suggesting that at physiological-low levels IL-1β may specifically target neurons for many reasons: first, neurons but not glia expresses IL-1R1 under basal conditions (23) and, second, neurons but not astrocytes are responsive to low IL-1β concentrations (59). However, whether low IL-1β concentrations support LTP by acting directly on neurons is still unknown.
5. Experimental systems to study LTP modulation by neuron-targeting cytokines
LTP has been studied for decades both in vivo and in vitro, primarily in the hippocampus (60). A number of induction protocols can be used to generate hippocampal LTP; most commonly, a train of electrical stimulation bursts separated by the period of the theta wave is used to initiate LTP in vivo or in brain slices. Although electrophysiological recordings in vivo and in brain slices might better reflect LTP responses found in intact brain circuitries, these approaches are not appropriate for analyzing neurons in the absence of glia, and thus cannot be used to study neuron-specific mechanisms. In contrast, the in vitro culture of primary neurons, an experimental system devoid of glia, opens up the opportunity to test the direct modulation of LTP by neuron-targeting cytokines.
Primary neuronal cultures have shown that neurons are responsive to cytokines and, even more, that inflammatory cytokines such as IL-1β (61, 62) and TNFα (63) can impair activity-dependent signaling and survival in neurons. However, the effect of cytokines on LTP in neuronal cultures has not been reported. Multi-electrode array (MEA) systems, which allow delivery of various patterns of stimulated activity in visually identified neurons, might be an option to study LTP in neuronal cultures (64). Alternatively, electrically-stimulated LTP can be modeled in neuronal cultures by chemical stimulation (chemical-LTP, cLTP), a major technical advance based on the activation of the NMDAR with the NMDAR co-agonist glycine (65, 66). Several studies have demonstrated that cLTP induces a potentiated state that parallels the essential features of electrically-induced LTP. Like electrically-stimulated LTP, cLTP induces insertion of AMPA receptors into the postsynaptic surface (the critical process associated with LTP at excitatory hippocampal synapses (67)) in an NMDAR-, calcium- and calcium/calmodulin kinase (CaMK)-dependent manner (65, 68–70, 71, 72). In addition, paralleling electrically-stimulated LTP, cLTP drives morphological adaptations in spines that are essential for stable LTP, including protein synthesis, and the formation of filamentous actin (F-actin) in spines via Rac/Pak/LIMK signaling (69, 70, 73, 74). Importantly, cLTP is occluded in hippocampal slice cultures by prior induction of LTP by electrical (theta-burts) stimulation, indicating that cLTP and electrophysiological-LTP approaches share underlying cellular processes (66). Thus, cLTP in neuronal cultures offers a simple, robust and neuron-enriched system to test the modulation of LTP by cytokines.
6. Synaptosomes provide an approach to study LTP modulation directly at the synapse
In spite of the advantages of primary neuronal cultures, this experimental system has some limitations. The main disadvantage is that cultured neurons develop in an artificial environment and may not embody all the properties of mature neurons including activity-dependent responses (75). An alternative to neuronal cultures may be to use synaptosomal preparations (presynaptic terminals attached to postsynaptic structures), which can be isolated from adult and even aged animals, thus offering the possibility of modeling mature synapses. Indeed, synaptosomes have been widely used to study synaptic mechanisms by biochemical, structural and functional analysis (76–79). To focus on synapse-specific mechanisms, we have recently developed a novel flow-cytometry-based approach to study LTP in isolated synaptosomes, termed Fluorescence Analysis of Single-Synapse Long-Term Potentiation (FASS-LTP) (56, 80). FASS-LTP focuses on the insertion of AMPA receptors into the post-synaptic surface after cLTP. Specifically, following cLTP, the activity-dependent increase in surface GluA1-containing AMPA receptors is tracked by flow cytometry in isolated synaptosomes. Overall, the technique consists of cLTP stimulation directly in synaptosomal fractions, immunofluorescence labeling for surface GluA1 and flow cytometry analysis (Fig. 2). Alternatively, surface levels of AMPA receptors after cLTP can be quantified by [3H]-AMPA binding (81). Importantly, cLTP response in synaptosomes mechanistically parallels the facilitation of synaptic transmission following electrically-induced LTP in brain slices (e.g., dependence on CaMKII and BDNF signaling) (80). Moreover, cLTP increases GluA1-PSD95 physical interaction in size-sorted synaptosomes (80). Importantly, FASS-LTP can test multiple samples in parallel (~40) using a minimal amount of tissue (milligrams) for each assay, thus opening the opportunity of studying multiple cytokines in parallel in brain samples from rats, mice and humans (postmortem) (80). The use of synaptosomes from cryopreserved postmortem tissue provides a unique opportunity to study LTP modulation in the human brain.
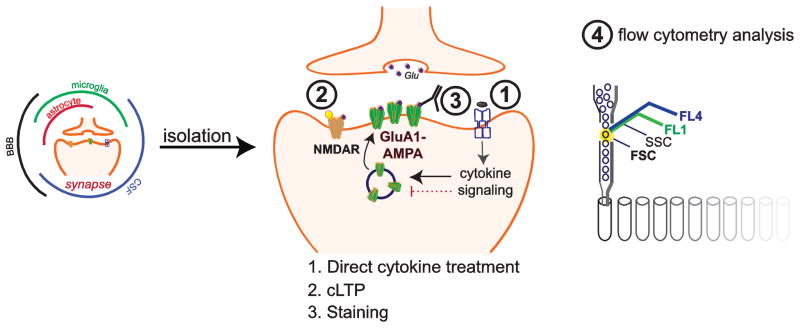
Fig. 2.
Multiplex analysis of cytokine-mediated modulation of LTP directly at the synapse using FASS-LTP. The synaptosome-enriched fraction (P2 fraction) can be rapidly isolated to study synapse-specific effects of cytokines. After isolation, synaptosomes can be treated with cytokines (1) before cLTP (2), which is induced by NMDAR activation using glycine (white triangle) and KCl depolarization. Cytokine treatment and cLTP is followed by immunolabeling for surface (no permeabilization) GluA1 (3) to identify potentiated synapses. Finally, flow cytometry identifies potentiated synapses by size and GluA1 labeling (4) (see details in (56)). A key advantage of this approach is the possibility of testing multiple agents in parallel (~ 40, (80)).
FASS-LTP-derived data have demonstrated that IL-1β suppresses cLTP directly at the synapse in mice, thus providing unequivocal proof that IL-1β can impair synaptic plasticity via neuron-specific mechanisms (56). Interestingly, data also showed that a low IL-1β concentration (3 pM) suppresses cLTP in synaptosomes from middle-aged but not from young mice, thus indicating that the neuron-specific IL-1β signaling is potentiated in aging (56). This age-related potentiation of IL-1β signaling is associated with increased levels of the IL-1R1-AcP receptor in synaptosomes from the aged hippocampus (56). Whether other cytokines can impair LTP by directly acting on synapses remains an open question. However, the possibility that the signaling from multiple cytokines converges on neurons and contributes to LTP modulation might reflect a more realistic scenario as, for example, brain levels of IL-1β as well as other cytokines (e.g., IL-6 and TNFα) and cytokine receptors increase in parallel during immune responses (8, 82). In addition to inflammatory cytokines (IL-1β, IL-6 and TNFα), anti-inflammatory cytokines such as IL-4 and IL-10 are also dynamically expressed across multiple brain regions as part of positive and negative immune feedback loops (82, 83). Further adding complexity to the brain cytokine’s profile, recent reports show that LTP itself is accompanied by synthesis and release of cytokines in a NMDA-dependent manner (84), suggesting that the interaction between cytokines and LTP is bidirectional. Thus, in vivo, the final effect on LTP and memory may likely reflect the cumulative effects of all neuron-specific cytokines locally released during either physiological or inflammatory processes. Cytokines’ effects on neurons may be additive, synergistic or even opposite. Given the complexity of cytokine signaling in the brain, understanding their overall actions including neuron-specific effects requires a shift in focus from single cytokines to a network of cytokine interactions (83).
Overall, a main advantage of in vitro neuron- and synapse-enriched systems is their simplicity. For the study of cytokines, the possibility of testing multiple samples at once could accelerate the construction of concentration-response (C-R) curves; while the information provided by C-R curves may provide significant information for understanding concentration-dependent dual effects of inflammatory cytokines on LTP. In addition, C-R analysis may guide future studies on LTP modulation to identify cytokine interactions (e.g., additive, synergy, crosstalk or redundancy), by using suboptimal concentrations of multiple cytokines at once. A better understanding of cytokine interactions at the cellular (neuronal) level is fundamental to study the influence of cytokine networks on synaptic plasticity.
7. Concluding remarks
Along with classical neuromodulators, cytokines modulate synaptic plasticity via complex cytokine-cell networks. The complexity of brain cytokine networks reflects cytokines’ feedback loops, pleiotropy and cross-talk; and that most, if not all, brain cells are responsive to cytokines.
It is commonly believed that inflammatory cytokines facilitate and impair neuronal functions at physiological-low and pathological-high concentrations, respectively. However, the timing and sequence of cytokine actions are poorly understood. Final effectors directly facilitating or impairing neuronal functions are also mostly unidentified. We propose that dissecting the identity of cytokines/factors able to modulate LTP directly at the synapse may add a point of reference on brain cytokine-cell networks, in both physiological and pathological conditions. Beyond establishing crucial endpoints in the map of cytokine networks (e.g., IL-1β via IL-1R1-AcP at the synapse), dissecting neuron-specific cytokines may significantly contribute to the discovery of therapeutics to prevent LTP suppression. Notably, these therapeutics would selectively target the side-effects of inflammation on synaptic plasticity, while preserving the benefits of brain immune responses (e.g., Aβ clearance by microglia (85)).
In summary, recent advances in the field provide state-of-the-art tools for high-throughput dissection of neuron-specific effectors. Specifically, FASS-LTP may allow scaling up LTP analysis, thus facilitating the characterization (e.g., accurate concentrations and timing) of cytokines able to modulate LTP directly at the synapse. The analysis of multiple cytokine at once and the evaluation of cytokine interactions may further help to reveal key nodes on the topology of cytokine networks in the brain. Hence, a perspective based on cytokine networks coupled with neuron-specific analysis may significantly contribute to elucidate the impact of immune-nervous systems communication on synaptic plasticity.
Highlights.
Cytokines can affect learning and memory by modulating LTP
Cytokines modulate LTP directly in neurons, and indirectly via cell-cytokine networks
Direct cytokine’s effect on LTP can be tested in cultured neurons and in synaptosomes
Synaptosomes allow testing LTP modulation by cytokines directly at the synapse
Acknowledgments
Work in the authors’ lab is supported by National Institutes of Health Grants R21-AG048506, P01-AG000538 and RO1-AG34667 (to C.W.C.), UC MEXUS-CONACYT Grant CN-16-170 (to G.A.P. and C.W.C.). The authors declare no competing financial interests.
Biographies
 Dr. G. Aleph Prieto is an Early-Stage Investigator focused on mechanisms of synaptic plasticity. Dr. Prieto studied biochemistry (B.Sc.), immunology (Master) and neuroscience (PhD) at the National Autonomous University of Mexico (UNAM). Currently, his work aims to dissect molecular pathways involved in synaptic dysfunction in aging and Alzheimer’s disease with the ultimate goal of finding therapeutic strategies to prevent brain impairment. Dr. Prieto has recently developed flow synaptometry approaches to study synapse phenotype and functionality. These highly innovative methods can evaluate synaptic plasticity in the human brain, a previously unattainable goal.
Dr. G. Aleph Prieto is an Early-Stage Investigator focused on mechanisms of synaptic plasticity. Dr. Prieto studied biochemistry (B.Sc.), immunology (Master) and neuroscience (PhD) at the National Autonomous University of Mexico (UNAM). Currently, his work aims to dissect molecular pathways involved in synaptic dysfunction in aging and Alzheimer’s disease with the ultimate goal of finding therapeutic strategies to prevent brain impairment. Dr. Prieto has recently developed flow synaptometry approaches to study synapse phenotype and functionality. These highly innovative methods can evaluate synaptic plasticity in the human brain, a previously unattainable goal.
 Dr. Carl W. Cotman is the Founding Director of the UC Irvine Institute for Memory Impairments and Neurological Disorders (formerly the Institute for Brain Aging and Dementia). His research is focused on identifying and testing interventions to reduce the rate of cognitive decline and promote successful brain aging. He was the first to discover that exercise increases brain derived neurotrophic factor (BDNF). His work has also provided crucial information about the mechanisms causing neuronal degeneration in Alzheimer’s disease (AD) and has been pivotal for developing interventions to promote successful aging such as exercise. Notably, Dr. Cotman has authored more than 700 peer-review scientific articles. H-index: 159, Citations: 100921 (Google Scholar, March 2017).
Dr. Carl W. Cotman is the Founding Director of the UC Irvine Institute for Memory Impairments and Neurological Disorders (formerly the Institute for Brain Aging and Dementia). His research is focused on identifying and testing interventions to reduce the rate of cognitive decline and promote successful brain aging. He was the first to discover that exercise increases brain derived neurotrophic factor (BDNF). His work has also provided crucial information about the mechanisms causing neuronal degeneration in Alzheimer’s disease (AD) and has been pivotal for developing interventions to promote successful aging such as exercise. Notably, Dr. Cotman has authored more than 700 peer-review scientific articles. H-index: 159, Citations: 100921 (Google Scholar, March 2017).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 3.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 4.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 6.Baudry M, Zhu G, Liu Y, Wang Y, Briz V, Bi X. Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res. 2015;1621:73–81. doi: 10.1016/j.brainres.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Prieto-Moreno GA, Rosenstein Y. The links between the neuroendocrine and the immune systems: Views of an immunologist. In: Joseph-Bravo P, editor. Molecular Endocrinology. Kerala, India: Research Signpost; 2006. pp. 171–192. [Google Scholar]
- 9.Nathan C, Sporn M. Cytokines in context. J Cell Biol. 1991;113:981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17:49–59. doi: 10.1038/nri.2016.123. [DOI] [PubMed] [Google Scholar]
- 11.Griffin WS. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N Engl J Med. 2013;368:770–771. doi: 10.1056/NEJMcibr1214546. [DOI] [PubMed] [Google Scholar]
- 12.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 13.Lynch MA. Neuroinflammatory changes negatively impact on LTP: A focus on IL-1beta. Brain Res. 2015;1621:197–204. doi: 10.1016/j.brainres.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger FP, Madamba S, Siggins GR. Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 15.Tong L, Prieto GA, Kramar EA, et al. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1beta via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartfai T, Behrens MM, Gaidarova S, Pemberton J, Shivanyuk A, Rebek J., Jr A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci U S A. 2003;100:7971–7976. doi: 10.1073/pnas.0932746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrientos RM, Higgins EA, Sprunger DB, Watkins LR, Rudy JW, Maier SF. Memory for context is impaired by a post context exposure injection of interleukin-1 beta into dorsal hippocampus. Behav Brain Res. 2002;134:291–298. doi: 10.1016/s0166-4328(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 18.Goshen I, Kreisel T, Ounallah-Saad H, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1beta in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164:1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hein AM, Stasko MR, Matousek SB, et al. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 22.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3 doi: 10.1126/scisignal.3105cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 23.Friedman WJ. Cytokines regulate expression of the type 1 interleukin-1 receptor in rat hippocampal neurons and glia. Exp Neurol. 2001;168:23–31. doi: 10.1006/exnr.2000.7595. [DOI] [PubMed] [Google Scholar]
- 24.Docagne F, Campbell SJ, Bristow AF, et al. Differential regulation of type I and type II interleukin-1 receptors in focal brain inflammation. Eur J Neurosci. 2005;21:1205–1214. doi: 10.1111/j.1460-9568.2005.03965.x. [DOI] [PubMed] [Google Scholar]
- 25.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 27.Habbas S, Santello M, Becker D, et al. Neuroinflammatory TNFalpha Impairs Memory via Astrocyte Signaling. Cell. 2015;163:1730–1741. doi: 10.1016/j.cell.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curran B, O’Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108:83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- 34.Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- 35.Kelly RJ, Minogue AM, Lyons A, et al. Glial Activation in AbetaPP/PS1 Mice is Associated with Infiltration of IFNgamma-Producing Cells. J Alzheimers Dis. 2013 doi: 10.3233/JAD-130539. [DOI] [PubMed] [Google Scholar]
- 36.Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- 37.Smith DE, Lipsky BP, Russell C, et al. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30:817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vitkovic L, Chatham JJ, da Cunha A. Distinct expressions of three cytokines by IL-1-stimulated astrocytes in vitro and in AIDS brain. Brain Behav Immun. 1995;9:378–388. doi: 10.1006/brbi.1995.1035. [DOI] [PubMed] [Google Scholar]
- 39.Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24:254–262. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012;32:14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30:7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imamura Y, Wang H, Matsumoto N, et al. Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience. 2011;187:63–69. doi: 10.1016/j.neuroscience.2011.04.063. [DOI] [PubMed] [Google Scholar]
- 43.Schmid AW, Lynch MA, Herron CE. The effects of IL-1 receptor antagonist on beta amyloid mediated depression of LTP in the rat CA1 in vivo. Hippocampus. 2009;19:670–676. doi: 10.1002/hipo.20542. [DOI] [PubMed] [Google Scholar]
- 44.Kitazawa M, Cheng D, Tsukamoto MR, et al. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187:6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loane DJ, Deighan BF, Clarke RM, Griffin RJ, Lynch AM, Lynch MA. Interleukin-4 mediates the neuroprotective effects of rosiglitazone in the aged brain. Neurobiol Aging. 2009;30:920–931. doi: 10.1016/j.neurobiolaging.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10--a role for IL-1 beta? J Neurochem. 2004;88:635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- 47.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 49.Avital A, Goshen I, Kamsler A, et al. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- 50.Hryniewicz A, Bialuk I, Kaminski KA, Winnicka MM. Impairment of recognition memory in interleukin-6 knock-out mice. Eur J Pharmacol. 2007;577:219–220. doi: 10.1016/j.ejphar.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 51.Naude PJ, Dobos N, van der Meer D, et al. Analysis of cognition, motor performance and anxiety in young and aged tumor necrosis factor alpha receptor 1 and 2 deficient mice. Behav Brain Res. 2014;258:43–51. doi: 10.1016/j.bbr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Spulber S, Mateos L, Oprica M, et al. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. J Neuroimmunol. 2009;208:46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Litteljohn D, Nelson E, Hayley S. IFN-gamma differentially modulates memory-related processes under basal and chronic stressor conditions. Front Cell Neurosci. 2014;8:391. doi: 10.3389/fncel.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu PJ, Huang W, Kalikulov D, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147:1384–1396. doi: 10.1016/j.cell.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prieto GA, Snighda S, Baglietto-Vargas D, et al. Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1b in the aged hippocampus. Proc Natl Acad Sci U S A. 2015;112:E5078–5087. doi: 10.1073/pnas.1514486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iosif RE, Ekdahl CT, Ahlenius H, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. J Neuroimmunol. 2003;144:61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 59.Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJ. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith ED, Prieto GA, Tong L, et al. Rapamycin and Interleukin-1beta Impair Brain-derived Neurotrophic Factor-dependent Neuron Survival by Modulating Autophagy. J Biol Chem. 2014;289:20615–20629. doi: 10.1074/jbc.M114.568659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carlos AJ, Tong L, Prieto GA, Cotman CW. IL-1beta impairs retrograde flow of BDNF signaling by attenuating endosome trafficking. J Neuroinflammation. 2017;14:29. doi: 10.1186/s12974-017-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao X, Bausano B, Pike BR, et al. TNF-alpha stimulates caspase-3 activation and apoptotic cell death in primary septo-hippocampal cultures. J Neurosci Res. 2001;64:121–131. doi: 10.1002/jnr.1059. [DOI] [PubMed] [Google Scholar]
- 64.Erickson J, Tooker A, Tai YC, Pine J. Caged neuron MEA: a system for long-term investigation of cultured neural network connectivity. J Neurosci Methods. 2008;175:1–16. doi: 10.1016/j.jneumeth.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 66.Musleh W, Bi X, Tocco G, Yaghoubi S, Baudry M. Glycine-induced long-term potentiation is associated with structural and functional modifications of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci U S A. 1997;94:9451–9456. doi: 10.1073/pnas.94.17.9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992;355:50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- 68.Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 69.Fortin DA, Davare MA, Srivastava T, et al. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30:11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jurado S, Goswami D, Zhang Y, Molina AJ, Sudhof TC, Malenka RC. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77:542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- 72.Man HY, Wang Q, Lu WY, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 73.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 74.Swanger SA, He YA, Richter JD, Bassell GJ. Dendritic GluN2A synthesis mediates activity-induced NMDA receptor insertion. J Neurosci. 2013;33:8898–8908. doi: 10.1523/JNEUROSCI.0289-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 76.Bilousova T, Miller CA, Poon WW, et al. Synaptic Amyloid-beta Oligomers Precede p-Tau and Differentiate High Pathology Control Cases. Am J Pathol. 2016;186:185–198. doi: 10.1016/j.ajpath.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelm BG, Mandad S, Truckenbrodt S, et al. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science. 2014;344:1023–1028. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- 78.Sandoval ME, Horch P, Cotman CW. Evaluation of glutamate as a hippocampal neurotransmitter: glutamate uptake and release from synaptosomes. Brain Res. 1978;142:285–299. doi: 10.1016/0006-8993(78)90636-4. [DOI] [PubMed] [Google Scholar]
- 79.Daniel JA, Malladi CS, Kettle E, McCluskey A, Robinson PJ. Analysis of synaptic vesicle endocytosis in synaptosomes by high-content screening. Nat Protoc. 2012;7:1439–1455. doi: 10.1038/nprot.2012.070. [DOI] [PubMed] [Google Scholar]
- 80.Prieto GA, Trieu BH, Dang CT, et al. Pharmacological Rescue of Long-Term Potentiation in Alzheimer Diseased Synapses. J Neurosci. 2017;37:1197–1212. doi: 10.1523/JNEUROSCI.2774-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corera AT, Doucet G, Fon EA. Long-term potentiation in isolated dendritic spines. PLoS One. 2009;4:e6021. doi: 10.1371/journal.pone.0006021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ansari MA. Temporal profile of M1 and M2 responses in the hippocampus following early 24h of neurotrauma. J Neurol Sci. 2015;357:41–49. doi: 10.1016/j.jns.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 83.Donzis EJ, Tronson NC. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol Learn Mem. 2014;115:68–77. doi: 10.1016/j.nlm.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 85.Guillot-Sestier MV, Town T. Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol Disord Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]