Abstract
Background
Rates of weight normalization and obesity remission after Roux-en-Y gastric bypass (GB) are unknown. This study evaluated weight loss, rates of achieving body mass index (BMI) < 25 or 30 kg/m2, recidivism, and predictors of success following GB.
Methods
We retrospectively studied weight and BMI at baseline, 2 and 6 months, and annually at 1–7 years in 219 patients undergoing GB at the University of Michigan from January 2008 to November 2010.
Results
Follow-up was excellent for a population traditionally associated with high attrition rates with data availability of 157/219, 145/219, 144/219, 134/219, 123/219, 82/161, and 29/64 patients at 1–7 years respectively. Mean baseline BMI was 47.0 kg/m2. Weight normalization (BMI < 25 kg/m2) occurred in 2.3–6.8% of patients. More importantly, 47% of patients achieved remission of obesity (BMI < 30 kg/m2) at some time point and 24% (52/219) at the last observed time point. BMI < 30 kg/m2 was associated with a lower initial BMI and follow-up for more than 2 years.
Conclusions
Rates of weight normalization are low after GB, however a large number of patients achieved BMI < 30 kg/m2. While the percent total weight loss and excess weight loss are both quite high in the entire cohort and this is likely associated with significant health benefits, our results still underscore the need to address obesity with intensive clinical attention earlier in its course.
Keywords: Obesity, Bariatric Surgery, Weight loss, Metabolism, Gastric Bypass, Weight normalization
INTRODUCTION/PURPOSE
Obesity afflicts tens of millions of Americans and the number of Americans with morbid obesity and number of global citizens who are overweight or obese are still on the rise [1, 2]. A recent study demonstrated the low likelihood of a patient with morbid obesity achieving normal weight, defined as body mass index (BMI) less than 25 kg/m2, with medical therapy with a reported annual probability of 1 in 1,290 for males and 1 in 677 for females with baseline BMI 40–45 kg/m2 [3]. A 2014 systematic review of primary case based interventions for weight loss showed a mean weight loss of only 1.36 kg at 12 months and 1.23 kg at 24 months [4]. Specialized weight management programs have demonstrated significantly greater weight loss, for example with a very low calorie diet or meal replacement approach [5, 6]. However, head-to-head comparisons of medical weight loss approaches with weight loss surgery by randomized controlled trials have consistently favored a surgical approach for greater weight loss [7–13]. Some of these studies were limited by short term follow up of one year, weight loss being a secondary outcome, or having relatively low weight loss in the medical group compared to results achieved in other studies of medical weight management programs. Nonetheless, all are well-designed studies that showed a significantly greater weight loss with surgical intervention including two studies with 5-year follow up [11, 13]. While there are many published series just demonstrating the excess of weight loss, no known studies in the literature report rates of weight normalization following weight loss surgery.
Given this paucity of data and to provide some degree of comparison to medical weight loss for patients interested in achieving milestone weights, we investigated the likelihood of achieving normal body mass index in a cohort of patients undergoing Roux-en-Y gastric bypass. Other variables assessed included significant weight regain of either 50% or 100% of weight lost at 2 years and percent total body weight loss at 1–7 years. Given the low number of patients achieving BMI < 25 kg/m2, we also investigated the likelihood of obesity remission (achieving BMI <30 kg/m2), rates of relapse to BMI > 30 kg/m2, and characteristics of patients achieving a non-obese state compared with patients who remain with BMI > 30 kg/m2. The relevance of achieving BMI targets of either 25 or 30 kg/m2 is highlighted by a recent systematic review demonstrating an increase in mortality as BMI increases above 20–25 kg/m2 with further increases as BMI rises [14]. Blood pressure was also assessed at each visit.
METHODS
All patients over 17 years of age undergoing laparoscopic Roux-en-Y gastric bypass (GB) for obesity at the University of Michigan between January 2008 and November 2010 were included in our retrospective study. We have previously described our cohort MI-BaSiC [15]. All patients had either BMI > 40 kg/m2 or BMI > 35 kg/m2 with an obesity-related comorbidity and had previously been unsuccessful with medical treatment for obesity, consistent with Medicare and Medicaid requirements for insurance coverage of bariatric surgery. Exclusion criteria included previous bariatric surgery or the inability to complete the surgery related to unexpected operative finding. We identified 219 patients (183 female) who met these criteria and were included for analysis.
Data Collection
The University of Michigan Adult Bariatric Surgery Program is a multidisciplinary approach to weight loss [16]. Patients presenting for evaluation for bariatric surgery first undergo rigorous medical, dietary, and psychological evaluation. After surgery, patients follow up with the surgeon and the dieticians at two weeks and at two months postoperatively. Subsequent follow-up is with the endocrinologist and the dietician at the Post-Bariatric Endocrine Clinic housed in the Metabolism, Endocrinology and Diabetes Division of the Department of Medicine, where patients are asked to present at 6 and 12 months postoperatively and annually thereafter to be evaluated and treated for long-term bariatric-related complications (e.g. vitamin deficiencies and post-bariatric hypoglycemia), weight regain, and obesity-related comorbidities.
Retrospective electronic medical record review was performed, and data were abstracted from visits that occurred preoperatively (within 60 days prior to surgery) and postoperatively at 1 year ± 2 months, 2 years ± 3 months, 3 years ± 6 months, 4 years ± 6 months, 5 years ± 6 months, 6 years ± 6 months, and 7 years ± 6 months. The last eligible visit was determined by the absolute difference between the date of data abstraction and date of surgery; for example, if a patient was 5 years and 364 days out from surgery, 5 years was the last eligible data point. Baseline demographic characteristics included sex and age. Data collected at baseline and follow-up, when available, included weight, height, and body mass index (BMI).
Statistical analysis
Percent excess weight loss (%EWL) was calculated by dividing total weight loss by the difference between actual body weight and ideal body weight, which is the weight at a BMI of 25 kg/m2 for each patient. Percent total weight loss (%TWL) was calculated by dividing the change in weight by the initial weight. Rates of weight normalization and obesity remission were calculated by dividing the number of patients achieving the goal (BMI < 25 or < 30 kg/m2 respectively) by the initial number of patients (219); these are conservative estimates that assume that subjects who dropped out did not achieve the goal.
Summary statistics are presented as means and standard deviations. A two-sample t-statistic and a 2×2 chi-square statistic were used to compare continuous and discrete outcomes between groups. Analyses were performed using SAS 9.4.
RESULTS
Demographic data
The total study population included 219 patients (183 females, 84%). Baseline characteristics of the study population are provided in Table 1. Males were older and had a higher baseline weight but similar baseline BMI compared with females.
Table 1.
Baseline characteristics reported as mean (standard deviation).
| Females | Males | P-value | |
|---|---|---|---|
| Number of patients | 183 | 36 | |
| Age (years) | 43.1 (10.8) | 47.6 (9.2) | 0.01 |
| Weight (kg) | 127.8 (23.9) | 150.8 (31.0) | <0.0001 |
| BMI (kg/m2) | 47.1 (7.9) | 46.9 (7.6) | 0.88 |
Data availability
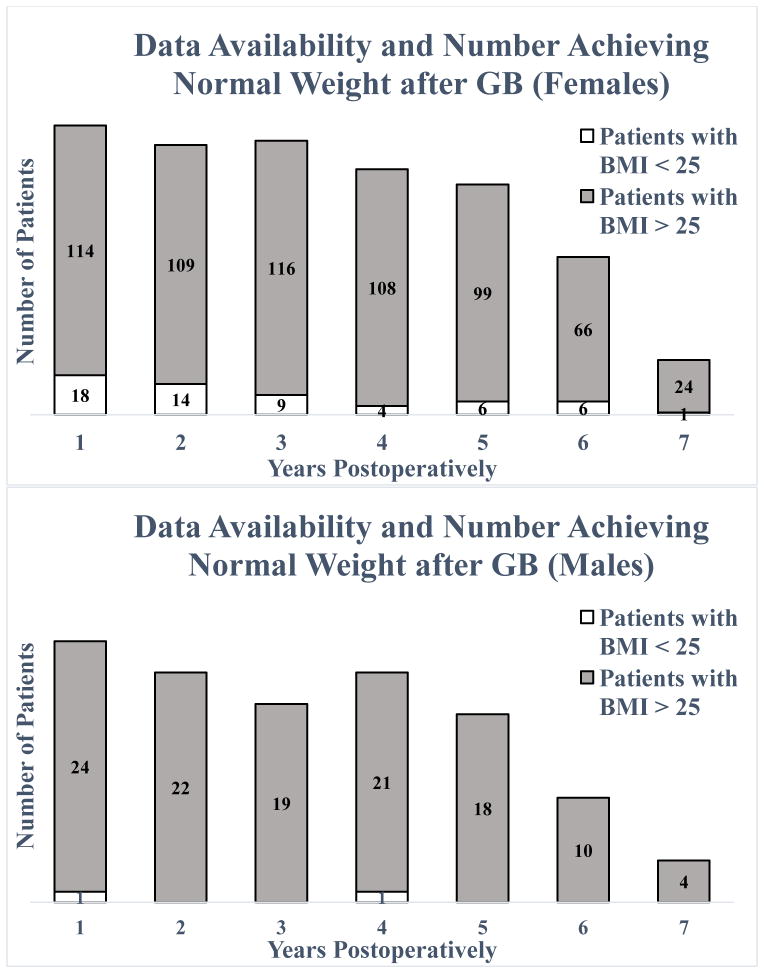
Data were available for 123 and 105 female patients at 2 and 5 years respectively (67% and 57% follow-up rate) and for 22 and 18 male patients at 2 and 5 years (61% and 50% follow-up rate). For all patients, follow-up rate at 2 years was 66% (145/219) and at 5 years was 56% (123/219). Data availability is displayed by number of patients having available data at various time points in Figure 1 and by the last available visit data point as compared to eligibility for data at various time points in Table 2. Based on surgery date and timing of data collection, the number of patients eligible for having six and seven year data availability was 161 and 64 patients respectively since 58 patients only completed five years at the time of chart review. Patients having available data at the last eligible time point were 119/219 (54.3%), which is shown in bold in Table 2.
Figure 1.
Data availability for females (top) and males (bottom) denoting patients who achieved BMI < 25 kg/m2 at a given time point (dotted) and those who did not (solid). The total number of patients at each time is the sum of these two data points. For females, this is 132, 123, 125, 112, 105, 72, and 25 patients at 1–7 years, and for males, this is 25, 22, 19, 22, 18, 10, and 4 patients at 1–7 years. A patient who had normal BMI at multiple visits will be counted at each of those time points. GB, gastric bypass.
Table 2.
Final available data point vs. eligibility for follow-up visits.
| Last follow-up visit | Last eligible visit (years) | |||
|---|---|---|---|---|
| 5 | 6 | 7 | Total | |
| 2 months | 1 | 6 | 1 | 8 |
| 6 months | 4 | 3 | 1 | 8 |
| 1 year | 3 | 6 | 9 | 18 |
| 18 months | 0 | 3 | 0 | 3 |
| 2 years | 2 | 3 | 1 | 6 |
| 3 years | 5 | 2 | 6 | 13 |
| 4 years | 2 | 11 | 4 | 17 |
| 5 years | 41 | 14 | 4 | 59 |
| 6 years | 0 | 49 | 9 | 58 |
| 7 years | 0 | 0 | 29 | 29 |
| Total | 58 | 97 | 64 | 219 |
Achievement of normal weight
The number of female patients achieving a BMI of 25 kg/m2 or lower during the 7 years of observation was 31 (16.9%) with 5 (2.7%) of these patients reaching normal weight by year 2 and sustaining it at 5 years or longer. The latest available measurement of BMI was below 25 kg/m2 for 15 (8.2%) female patients with the majority of these data points at 5 years or beyond. There were only 2 males who had a BMI of less than 25 kg/m2 during follow-up with 1 subject having normal weight at 1 year and no subsequent weight measurements available and the other achieving normal weight at 4 years with no further data points. For all patients, a conservative estimate for the probability of achieving and sustaining normal weight with Roux-en-Y gastric bypass in this population, determined by the number of patients who are normal weight at two years and sustained at least 5 years postoperatively, is 2.3% (5/219). A more liberal estimate would be 6.8% (15/219), determined by the number of patients who are normal weight at the last available data point. Overall, 33 out of 219 patients (15.1%) of patients achieved BMI of less than 25 kg/m2 at any point postoperatively.
Achievement of BMI < 30 kg/m2
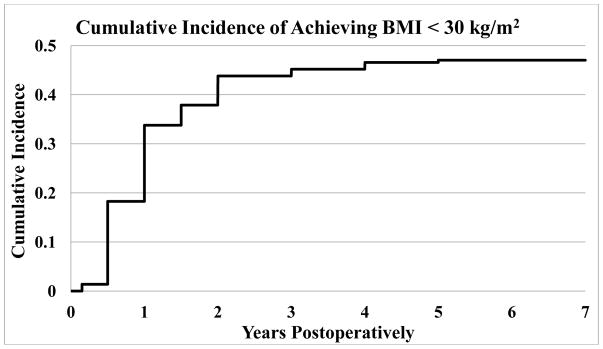
The number of patients reaching BMI < 30 kg/m2 at any time during 7 years of follow-up was 103 (47.0%). The majority of patients who achieve this goal do so within the first year. Figure 2 displays the cumulative incidence of a patient achieving BMI < 30 kg/m2 at that time point or earlier, as a percentage of the total number of patients who underwent surgery. Supplemental Table 1 shows when patients first and last reached BMI < 30 kg/m2. Nineteen patients had BMI < 30 kg/m2 at only one visit. Of the patients who reached BMI < 30 kg/m2, 52 out of 103 patients (50.5%) remained below this threshold at the last recorded visit. Supplemental Table 2 shows when these 52 patients first and last reached BMI < 30 kg/m2.
Figure 2.
Cumulative incidence of achieving BMI < 30 kg/m2 by time. Incidence is displayed as patients who had achieved BMI < 30 kg/m2 at or before the given time point divided by the total number of patients who underwent surgery. Data availability at 1–7 years was 157, 145, 144, 134, 123, 82, and 29 patients respectively out of 219 total patients.
Association of follow-up and weight loss maintenance
Patients who followed up for less than two years were less likely to obtain a BMI < 30 kg/m2 than patients who followed up (a) for more than two years but not including the final eligible visit or (b) until the final eligible visit (24.3% vs. 49.2% and 52.9% respectively, p = 0.014 for comparison between the first two groups). Additionally, 61% of patients who achieved BMI < 30 kg/m2 followed up through the final eligible visit vs. 48% of patients who did not achieve this goal (p = 0.056).
Association between initial BMI and weight loss
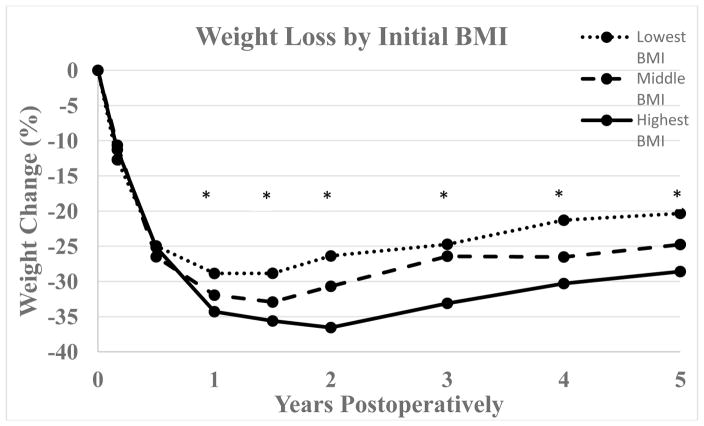
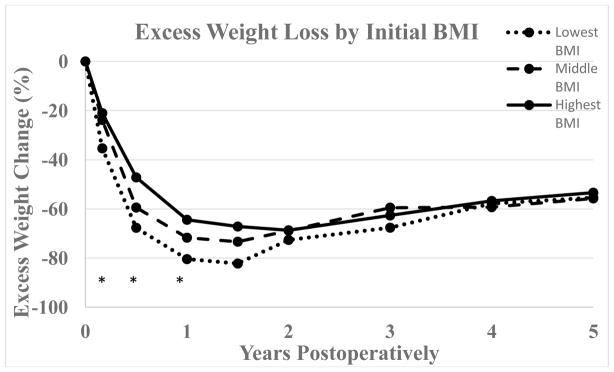
Initial mean BMI of females achieving BMI < 30 kg/m2 was 43.5 versus 50.4 kg/m2 in females who did not reach this cutoff (p < 0.0001). For males the corresponding mean initial BMIs were 44.6 and 48.1 kg/m2 respectively (p = 0.18). Patients who reached BMI < 30 kg/m2 had a greater difference between maximum and minimum BMI than their counterparts who did not (17.6 vs. 14.3 kg/m2, p < 0.0001). Figure 3 shows percent weight loss and figure 4 shows excess weight loss over time for patients divided into three strata based on initial BMI (lowest BMI group < 43 kg/m2, middle BMI group 43–48 kg/m2, highest BMI group > 48 kg/m2). The inter-group differences for %TWL were significant at all time points starting at one year postoperatively with greater percent total weight loss in the group with the highest baseline BMI. Conversely, greater percent excess weight loss was seen in patients with lower initial baseline BMI through one year postoperatively with p-values at 2, 6, and 12 months of < 0.0001, < 0.0001, and 0.004 respectively.
Figure 3.
Percent total weight change following GB for patients divided into three groups based on initial BMI (lowest BMI group < 43 kg/m2, middle BMI group 43–48 kg/m2, highest BMI group > 48 kg/m2) with respective n = 72, 66, and 79. Data availability at 1, 1.5, 2, 3, 4, and 5 years respectively was 51, 36, 48, 47, 49, and 46 patients for the lowest BMI group, 50, 26, 41, 39, 43, and 36 patients for the middle BMI group, and 53, 35, 56, 57, 42, and 41 patients for the highest BMI group. *, p < 0.05.
Figure 4.
Percent excess weight change following GB for patients divided into three groups based on initial BMI (lowest BMI group < 43 kg/m2, middle BMI group 43–48 kg/m2, highest BMI group > 48 kg/m2) with respective n = 72, 66, and 79. Excess weight change was calculated by dividing weight change by the difference between actual body weight and body weight corresponding to BMI = 25 kg/m2. Data availability at 1, 1.5, 2, 3, 4, and 5 years respectively was 51, 36, 48, 47, 49, and 46 patients for the lowest BMI group, 50, 26, 41, 39, 43, and 36 patients for the middle BMI group, and 53, 35, 56, 57, 42, and 41 patients for the highest BMI group. *, p < 0.05.
Percent total weight loss stratified by sex and age
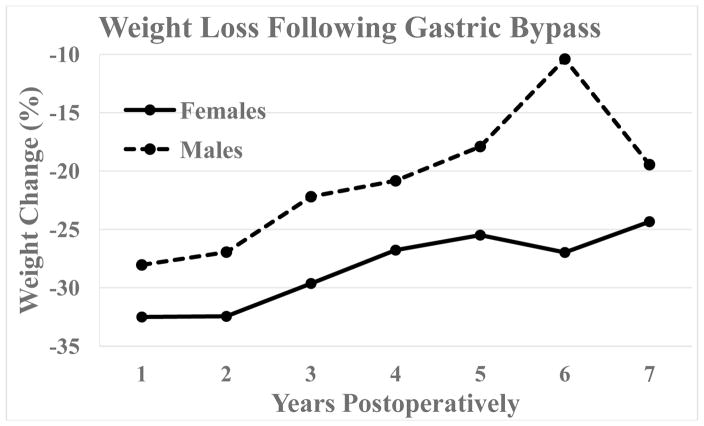
Percent total weight loss for females and males is displayed in Figure 5. While there were no significant differences in %TWL between males and females, there was greater variability in weight loss for males. For both sexes combined, mean %TWL peaked at 1 year with 31.8% and at 5, 6, and 7 years respectively was 24.4%, 25.0%, and 23.6%. Data availability is notably low at 7 years (24 females and 4 males) but more robust prior.
Figure 5.
Percent total weight change over time following Roux-en-Y gastric bypass for females vs. males. Data availability for females is 132, 123, 125, 112, 105, 72, and 25 patients at 1–7 years, and for males, this is 25, 22, 19, 22, 18, 10, and 4 patients at 1–7 years. There were no significant differences between males and females.
The percent of female patients reaching BMI < 30 kg/m2 versus those who did not reach this threshold was similar (87.5% versus 80.0% respectively). Interestingly, there was a significant effect of age on the ability to reach a BMI < 30 kg/m2; the mean age of the subgroup who achieved BMI < 30 kg/m2 was three years younger at baseline at 42±11 versus 45±11 years (p=0.0294).
Weight regain
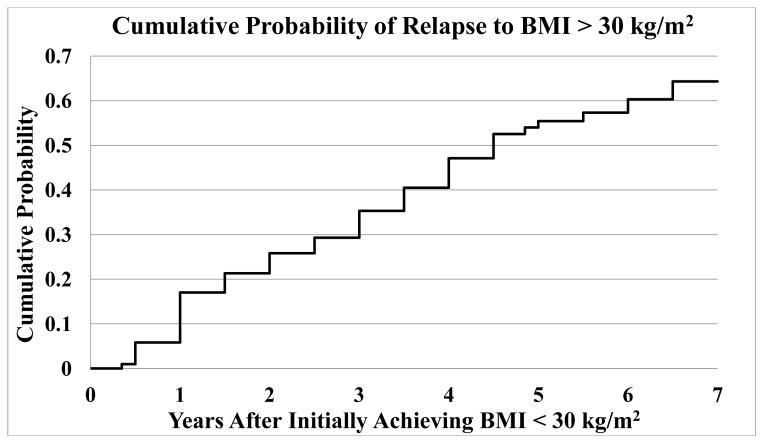
No female patients had a higher postoperative weight than their preoperative weight, while 2 male patients gained weight immediately postoperatively and never subsequently fell below their preoperative weight. Thirteen (7.1%) female patients regained half of the weight they lost after 2 years, all but 1 at 5 years or later. In addition to the 2 male patients who never lost weight, 2 male patients regained half of the weight they lost after 2 years (a rate of 11.2% if all 4 patients are counted). A general trend of weight regain can be seen after 1 year (Figure 5). Figure 6 shows the cumulative risk of a patient who achieved BMI < 30 kg/m2 of relapsing based on the interval between first BMI < 30 kg/m2 and first subsequent BMI > 30 kg/m2.
Figure 6.
Cumulative incidence of relapsing to BMI > 30 kg/m2 by time among patients who had previously achieved BMI < 30 kg/m2. Incidence is displayed as the time difference between these two milestones. 35.7% of patients who reached BMI < 30 kg/m2 maintained this for the duration of the study. Patients who remitted back to BMI < 30 kg/m2 are not taken into account in this figure. Data availability at 1–7 years was 157, 145, 144, 134, 123, 82, and 29 patients respectively out of 219 total patients.
Change in Blood pressure
A significant decrease was seen for both systolic and diastolic blood pressure at all follow-up points. Maximal effect was achieved at 1 year with a mean decrease of 14±7 mmHg (p < .0001). Blood pressure at five years remained improved with a mean decrease of 11±3 mmHg (p < 0.0001) with similar changes at 6 and 7 years. There were no significant differences between sexes in regards to blood pressure. There was a slight difference in systolic blood pressure between patients who achieved BMI < 30 kg/m2 and those who did not at initial visit (135 vs. 140 mmHg respectively, p = 0.048) as well as at the final visit (122 vs. 129 mmHg respectively, p = 0.005). These differences may be attributable to age differences between the two groups, as a general linear model adjusted for age resulted in no residual difference in the blood pressure at baseline or during follow up (data not shown). On the contrary, the diastolic blood pressures at baseline were comparable between those who had remission of obesity with BMI < 30 kg/m2 versus those who did not fully remit (75±9 versus 75±10 mmHg respectively at baseline and 72±11 versus 72±11 mmHg at the last visit). The change in diastolic blood pressure from initial to last visit was −4±12 mmHg versus −4 ±14 mmHg, which was significant within each group (p<0.01) but was not significantly different between the two groups.
DISCUSSION
In this study, we investigated the cumulative likelihood of achieving two important milestones after long-term follow-up in our cohort undergoing gastric bypass surgery. Achieving remission of obesity appears to be a more attainable outcome occurring in nearly half of the patients and being sustainable in a substantial percentage. Having a lower BMI at baseline and following up for a longer period are both associated with a higher likelihood of reaching remission of obesity. Our observational retrospective cohort study is the first to our knowledge to directly examine rates of achieving normal weight following weight loss surgery such as laparoscopic Roux-en-Y gastric bypass. While we do not want to send the message that achieving enough weight loss to reach a BMI of 25 or 30 kg/m2 is the ultimate goal of weight loss surgery, we investigated milestone weight loss to help guide more realistic expectations from surgical intervention. The data add to the growing body of evidence that gastric bypass provides sustainable weight loss in patients with morbid obesity. It is difficult to compare our results for weight normalization to rates reported for primary care based medical weight loss strategies due to differences in study design and statistical methods [3]. Regardless, despite excellent weight loss in the majority of patients undergoing gastric bypass, the rate of achieving BMI < 25 kg/m2 remains low (2.3–6.8%), underscoring the challenge of weight normalization in patients with a very high initial body mass index (the mean preoperative BMI was 47.0 kg/m2 in our study population). There has been interesting investigation done related to the metabolic adaptation that occurs following weight loss that has demonstrated patients experiencing greater than expected reductions in resting metabolic rate, suggesting one mechanism through which the body defends against weight loss and favors returning to a desired weight set point [17, 18]. However, one recent small study showed that patients undergoing GB experience no significant metabolic adaptation (only the expected decline in resting metabolic rate) whereas participants with the television show “The Biggest Loser” who employed a medical weight loss strategy had much lower resting metabolic rates despite similar weight loss, highlighting a potential benefit for weight loss maintenance following weight loss surgery versus medical weight loss [19].
Given the low number of patients achieving BMI < 25 kg/m2, we also wanted to focus on another important clinical goal of obesity remission (BMI < 30 kg/m2). Taking into account that the mean BMI prior to surgery in our cohort was 47.1 kg/m2, this target is associated with significant estimated health benefits and likely brings a mortality benefit for patients undergoing GB [14, 16]. It is both noteworthy and encouraging that nearly half of the patients in the study achieved BMI < 30 kg/m2 with just over half of those patients (about one quarter of the overall sample) having BMI < 30 kg/m2 at their final weigh-in. There was no difference between sexes, although this may reflect the low number of males in the study. Initial BMI was inversely correlated to probability of achieving BMI < 30 kg/m2.
Division of patients into three strata based on BMI cutoffs showed greater percent weight loss in patients with the highest BMI beyond one year but greater excess weight loss in patients with lower initial baseline BMI through one year. There is significant heterogeneity in previous studies as to whether baseline BMI is associated with better or worse outcomes depending on whether %EWL or %TWL is used [20], but in general %TWL is preferred as the outcome measure in long-term studies of bariatric surgery if one wishes to reduce variability based on initial BMI [21, 22]. Our data support a conclusion that higher BMI is associated with improved long-term %TWL following GB but a lower rate of achieving BMI < 30 kg/m2.
Rates of significant weight regain (>50% of the weight lost at 2 years) at 5–7 years were encouragingly low, and fewer than 1% of patients had a weight higher than their preoperative weight at any point. Recidivism is apparent in regards to both general weight regain and in patients relapsing back to BMI > 30 kg/m2. This has been recently evaluated systemically with potential contributing etiologies identified as psychiatric, physical inactivity, endocrinopathies/metabolic, and dietary non-compliance [23].
Improvement in blood pressure
Overall, there was significant improvement in primarily systolic blood pressure whether the subjects reached a BMI of 30 kg/m2 or not, with a clinically small, but statistically significant, difference between the final blood pressures attained in the patients who did or did not reach this threshold. Whether such a difference will ultimately affect overall cardiovascular risk remains to be seen in our cohort. Based on the recently published SPRINT trial [24], there may be a benefit in both cardiovascular and all-cause mortality with blood pressure lowering in the average range seen in our patients. Diastolic blood pressures attained as a result of gastric bypass are significantly lower compared to baseline blood pressures regardless of the whether they reached BMI < 30 kg/m2 or not.
Attrition and importance of long-term follow-up
Studies of bariatric surgery are known to have poor follow-up rates with a recent large systematic review showing a mean of 40% attrition at one year and even higher rates at study completion [25], highlighting the challenges of this patient population. Our follow-up was notably better than this with 71.7% at 1 year, 54.3% of patients completing the study and data availability of over 60% at 2 years and over 50% at 5 years. Follow-up of 2 years or greater was associated with higher likelihood of achieving BMI < 30 kg/m2, suggesting early dropout predicts poor success, although this conclusion is limited by lack of follow-up data for these patients and it may be that more successful patients return to clinic at a higher rate.
Study limitations and strengths
Surgical complications are often brought up as a significant downside of weight loss surgery when comparisons to medical weight loss are entertained; previous studies report short-term complications rates of 9.5% (2.7% major) with predictive factors available to help patients assess risks and benefits of surgery [26–28]. We have previously reported the rate of surgical complications as 10.2% within 30 days of surgery with reoperation rates of 3.0% in a patient population that includes the study sample of this investigation [15]. A potential critique of this study is the question of whether patients with obesity achieving BMI < 25 kg/m2 reflect malnutrition rather than success. Malnutrition following gastric bypass is uncommon in clinical practice, although rates are difficult to estimate definitively due to lack of studies with very long term follow-up and no clear definition [29]. While two patients in our study had BMI < 18.5 kg/m2 at any point, this does not necessarily correlate with malnutrition which can occur at high or low body mass index and requires clinical evaluation. While albumin is a poor marker of nutritional status [30], we did evaluate this in patients with BMI < 25 kg/m2, and only one patient had a sustained albumin level < 3.5 g/dL. This patient was also felt clinically to have malnutrition and underwent reversal of GB due to gastroesophageal ulcer and stenosis felt to be attributable to smoking. Other study limitations include the retrospective study design which may introduce selection or other bias, incomplete data availability which may favor follow-up of patients who are doing well or poorly, and patient selection from a single tertiary care referral center which may limit generalizability. Strengths of the study include large sample size, sequential patient inclusion which makes the results more generalizable to all patients in comparison to the more stringent requirements for RCTs, and long-term follow up of 5–7 years.
CONCLUSION
A modest proportion of patients undergoing gastric bypass achieve normal body weight, however almost half of patients achieve reversal of obesity with BMI < 30 kg/m2 with 5–7 years of follow-up in a population with mean preoperative BMI of 47.0 kg/m2 likely affording significant metabolic and possibly mortality benefit. Preoperative BMI was correlated with greater total weight loss but negatively associated with achievement of BMI < 30 kg/m2. Longer follow-up time was positively associated with reaching BMI < 30 kg/m2. Realistic goals should be set for patients undergoing gastric bypass, in light of the baseline BMI. Recidivism is seen in this and other studies highlighting the body’s natural defense of current weight [19, 23]. Given the inherent difficulty of achieving normal weight, interventions targeting weight loss prior to reaching the greater stages of obesity are needed. It should be a public health urgency for the United States and other developed countries to make this a priority especially for young adults with BMI levels > 30 kg/m2. We should also carefully examine the option of pursuing surgery at lower BMI cutoffs at which point patients have a greater likelihood of obesity remission. Further studies with larger numbers are needed to more comprehensively delineate whether weight normalization or obesity remission are more feasible goals for specific subgroups of patients, to explore an approach to patients who are lost to follow-up, and to examine the benefit of bariatric surgery at lower preoperative BMI to optimize weight loss success.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the surgeons of the University of Michigan Health System (UMHS) bariatric surgery program: Jonathan Finks MD, Justin Dimick MD, and John Birkmeyer MD as well as the physician assistants Colleen Buda PA-C (Program Director) and Kendra Rogers PA-C. We are also grateful for the dedication and care provided by the entire clinical staff of the UMHS Bariatric Surgery Program and the Division of Metabolism, Endocrinology, and Diabetes. Finally, we would like to thank all our patients who have participated in the UMHS Bariatric Surgery Program and followed in our Post-Bariatric Surgery Clinic.
Funding sources: Supported by the University of Michigan Health System as discretionary funding to EAO. Parker Family Foundation provided support for data acquisition.
Footnotes
Consent Statement: This type of retrospective data analysis is exempt from informed consent. Our study was approved by the IRB prior to collection of any data.
Conflict of interest statement: ATK is an investigator on a clinical study sponsored by Nestle. EAO receives grant support from GI Dynamics and has received grant support and served as an advisor to Amylin Pharmaceuticals LLC, Bristol-Myers-Squibb and AstraZeneca for her work on Lipodystrophy Syndromes. EAO is currently serving as an advisor to AstraZeneca, Aegerion Pharmaceuticals, Ionis Pharmaceuticals and receives grant support from the latter two companies. EAO holds IP in the weight loss space not applicable to this manuscript. All other authors have no conflicts of interest to disclose.
Conflicts of Interest: Authors 1–3 and 5–9 have no conflicts of interest. Author 4 is an investigator on a clinical study sponsored by Nestle. Author 10 receives grant support from GI Dynamics that may be considered relevant for the content of this paper. In addition, author 10 has received grant support and served as an advisor to Amylin Pharmaceuticals LLC, Bristol-Myers-Squibb and AstraZeneca for her work on Lipodystrophy Syndromes. Author 10 is currently serving as an advisor to AstraZeneca Aegerion Pharmaceuticals, Ionis Pharmaceuticals and receiving grant support from the latter two companies. Author 10 holds IP in the weight loss space not applicable to this manuscript.
References
- 1.An R. Prevalence and Trends of Adult Obesity in the US, 1999–2012. ISRN obesity. 2014;2014:185132. doi: 10.1155/2014/185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an Obese Person Attaining Normal Body Weight: Cohort Study Using Electronic Health Records. Am J Public Health. 2015 Sep;105(9):e54–9. doi: 10.2105/AJPH.2015.302773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth HP, Prevost TA, Wright AJ, Gulliford MC. Effectiveness of behavioural weight loss interventions delivered in a primary care setting: a systematic review and meta-analysis. Fam Pract. 2014 Dec;31(6):643–53. doi: 10.1093/fampra/cmu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications. 2014 Jul-Aug;28(4):506–10. doi: 10.1016/j.jdiacomp.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.König D, Zdzieblik D, Deibert P, Berg A, Gollhofer A, Büchert M. Internal Fat and Cardiometabolic Risk Factors Following a Meal-Replacement Regimen vs. Comprehensive Lifestyle Changes in Obese Subjects. Nutrients. 2015 Dec 01;7(12):9825–33. doi: 10.3390/nu7125500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang W, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014 Jul;149(7):707–15. doi: 10.1001/jamasurg.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008 Jan 23;299(3):316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 9.Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014 Jul;149(7):716–26. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikramuddin S, Korner J, Lee W-J, Connett JE, Inabnet WB, Billington CB, et al. Roux-en-Y Gastric Bypass versus Intensive Medical Management for the Control of Type 2 Diabetes, Hypertension and Hyperlipidemia: An International, Multicenter, Randomized Trial. JAMA : the journal of the American Medical Association. 2013;309(21):2240–9. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015 Sep 5;386(9997):964–73. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014 May 22;370(21):2002–13. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schauer PRBD, Kirwan J, editors. Bariatric surgery vs. intensive medical therapy for long-term glycemic control and complications of diabetes: Final 5-year STAMPEDE trial results. American College of Cardiology 2016 Scientific Sessions; 2016; Chicago, IL, USA. [Google Scholar]
- 14.Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ (Clinical research ed) 2016;353:i2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lager CJ, Esfandiari NH, Subauste AR, Kraftson AT, Brown MB, Cassidy RB, et al. Roux-En-Y Gastric Bypass Vs. Sleeve Gastrectomy: Balancing the Risks of Surgery with the Benefits of Weight Loss. Obesity Surgery. 2016 Jun 24; doi: 10.1007/s11695-016-2265-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christou NV. Impact of obesity and bariatric surgery on survival. World journal of surgery. 2009 Oct;33(10):2022–7. doi: 10.1007/s00268-009-0050-2. [DOI] [PubMed] [Google Scholar]
- 17.Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. The Journal of clinical endocrinology and metabolism. 2012 Jul;97(7):2489–96. doi: 10.1210/jc.2012-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar 9;332(10):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 19.Knuth ND, Johannsen DL, Tamboli RA, Marks-Shulman PA, Huizenga R, Chen KY, et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity (Silver Spring, Md) 2014 Dec;22(12):2563–9. doi: 10.1002/oby.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012 Jan;22(1):70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 21.van de Laar A, de Caluwe L, Dillemans B. Relative outcome measures for bariatric surgery. Evidence against excess weight loss and excess body mass index loss from a series of laparoscopic Roux-en-Y gastric bypass patients. Obes Surg. 2011 Jun;21(6):763–7. doi: 10.1007/s11695-010-0347-0. [DOI] [PubMed] [Google Scholar]
- 22.Sczepaniak JP, Owens ML, Shukla H, Perlegos J, Garner W. Comparability of weight loss reporting after gastric bypass and sleeve gastrectomy using BOLD data 2008–2011. Obes Surg. 2015 May;25(5):788–95. doi: 10.1007/s11695-014-1496-3. [DOI] [PubMed] [Google Scholar]
- 23.Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013 Nov;23(11):1922–33. doi: 10.1007/s11695-013-1070-4. [DOI] [PubMed] [Google Scholar]
- 24.Group TSR. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373(22):2103–16. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Switzer NJ, Merani S, Skubleny D, Pelletier JS, Kanji R, Shi X, et al. Quality of Follow-up: Systematic Review of the Research in Bariatric Surgery. Ann Surg. 2016 May;263(5):875–80. doi: 10.1097/SLA.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 26.Dayer-Jankechova A, Fournier P, Allemann P, Suter M. Complications After Laparoscopic Roux-en-Y Gastric Bypass in 1573 Consecutive Patients: Are There Predictors? Obes Surg. 2016 Jan;26(1):12–20. doi: 10.1007/s11695-015-1752-1. [DOI] [PubMed] [Google Scholar]
- 27.Finks JF, Kole KL, Yenumula PR, English WJ, Krause KR, Carlin AM, et al. Predicting risk for serious complications with bariatric surgery: results from the Michigan Bariatric Surgery Collaborative. Ann Surg. 2011 Oct;254(4):633–40. doi: 10.1097/SLA.0b013e318230058c. [DOI] [PubMed] [Google Scholar]
- 28.Ramanan B, Gupta PK, Gupta H, Fang X, Forse RA. Development and validation of a bariatric surgery mortality risk calculator. Journal of the American College of Surgeons. 2012 Jun;214(6):892–900. doi: 10.1016/j.jamcollsurg.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Faintuch J, Matsuda M, Cruz ME, Silva MM, Teivelis MP, Garrido AB, Jr, et al. Severe protein-calorie malnutrition after bariatric procedures. Obes Surg. 2004 Feb;14(2):175–81. doi: 10.1381/096089204322857528. [DOI] [PubMed] [Google Scholar]
- 30.Banh L. Serum Proteins as Markers of Nutrition: What Are We Treating? Practical Gastroenterology. 2006;30(10):46–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data were available for 123 and 105 female patients at 2 and 5 years respectively (67% and 57% follow-up rate) and for 22 and 18 male patients at 2 and 5 years (61% and 50% follow-up rate). For all patients, follow-up rate at 2 years was 66% (145/219) and at 5 years was 56% (123/219). Data availability is displayed by number of patients having available data at various time points in Figure 1 and by the last available visit data point as compared to eligibility for data at various time points in Table 2. Based on surgery date and timing of data collection, the number of patients eligible for having six and seven year data availability was 161 and 64 patients respectively since 58 patients only completed five years at the time of chart review. Patients having available data at the last eligible time point were 119/219 (54.3%), which is shown in bold in Table 2.
Figure 1.
Data availability for females (top) and males (bottom) denoting patients who achieved BMI < 25 kg/m2 at a given time point (dotted) and those who did not (solid). The total number of patients at each time is the sum of these two data points. For females, this is 132, 123, 125, 112, 105, 72, and 25 patients at 1–7 years, and for males, this is 25, 22, 19, 22, 18, 10, and 4 patients at 1–7 years. A patient who had normal BMI at multiple visits will be counted at each of those time points. GB, gastric bypass.
Table 2.
Final available data point vs. eligibility for follow-up visits.
| Last follow-up visit | Last eligible visit (years) | |||
|---|---|---|---|---|
| 5 | 6 | 7 | Total | |
| 2 months | 1 | 6 | 1 | 8 |
| 6 months | 4 | 3 | 1 | 8 |
| 1 year | 3 | 6 | 9 | 18 |
| 18 months | 0 | 3 | 0 | 3 |
| 2 years | 2 | 3 | 1 | 6 |
| 3 years | 5 | 2 | 6 | 13 |
| 4 years | 2 | 11 | 4 | 17 |
| 5 years | 41 | 14 | 4 | 59 |
| 6 years | 0 | 49 | 9 | 58 |
| 7 years | 0 | 0 | 29 | 29 |
| Total | 58 | 97 | 64 | 219 |