Abstract
Studies using traditional treatment strategies for mild traumatic brain injury (TBI) have produced limited clinical success. Interest in treatment for mild TBI is at an all time high due to its association with the development of chronic traumatic encephalopathy and other neurodegenerative diseases, yet therapeutic options remain limited. Traditional pharmaceutical interventions have failed to transition to the clinic for the treatment of mild TBI. As such, many pre-clinical studies are now implementing non-pharmaceutical therapies for TBI. These studies have demonstrated promise, particularly those that modulate secondary injury cascades activated after injury. Because no TBI therapy has been discovered for mild injury, researchers now look to pharmaceutical supplementation in an attempt to foster success in human clinical trials. Non-traditional therapies, such as acupuncture and even music therapy are being considered to combat the neuropsychiatric symptoms of TBI. In this review, we highlight alternative approaches that have been studied in clinical and pre-clinical studies of TBI, and other related forms of neural injury. The purpose of this review is to stimulate further investigation into novel and innovative approaches that can be used to treat the mechanisms and symptoms of mild TBI.
Keywords: Mild traumatic brain injury, Supplementation, Alternative therapies, Chronic symptoms, Secondary injury cascades
Introduction
Traumatic brain injury (TBI) continues to be a major cause of morbidity and mortality worldwide. Recently, mild repetitive TBI was found to be a possible contributor to the development of neurodegenerative diseases.1 In this review, we focus on the secondary injury effects of mild TBI, and the various effective supplement and alternative strategies being investigated in both pre-clinical and clinical studies. Preventing primary injury, such as the acceleration/deceleration from collision or skull impact, can only be targeted through patient education or improved protective equipment. As such, TBI research has focused on the treatment of secondary injury effects, which we will focus on in this review. Secondary injury cascades following mild TBI have been linked to the development of progressive degeneration and disease burden.2 Important cascades include oxidative stress, endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and neuroinflammation. These cascades are thought to contribute to the development of seizures, sleep disruption, depression, impulsivity, and cognitive decline, which can manifest months to years after initial injury.3 To date, no effective treatments are available to target these secondary injury cascades. A recent interest has focused on the benefits of supplements, nutrition, and alternative therapies for the treatment of mild TBI and subsequent symptoms. Supplements have shown beneficial outcomes when administered in pre-clinical models of TBI,4 and clinical case series have also alluded to the importance of supplements and nutrition for recovery following mild TBI in patients.5 Furthermore, alternative therapies such as music therapy, acupressure, and acupuncture have been used to treat symptoms associated with mild TBI. In this review, we provide an overview of both acute and chronic mild TBI-related symptoms, discuss the secondary mechanisms associated with mild TBI, and highlight supplements, nutritional interventions, and alternative therapies that warrant further investigation.
Acute changes
Neurological manifestations of mild TBI
TBI is classified along a continuum with mild, moderate, and severe categories.6 The pathophysiology of mild TBI remains poorly understood due to the absence of diagnosis using standard clinical imaging, or other symptomatic measurements.7 Symptoms are on a spectrum, and range from momentary disorientation to slight amnesia.8 The limited range of symptom presentation makes it difficult to localize focal brain lesions.
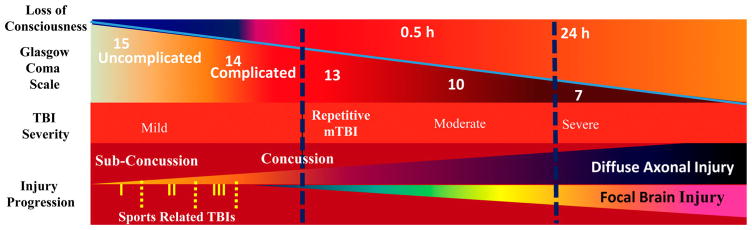
The American Congress of Rehabilitation Medicine (ACRM, 1993) defines mild TBI as an alteration of brain function caused by external forces with one or more of the following: loss of consciousness (LOC) lasting 0–30 minutes, amnesia <24 hours, focal neurologic deficits that may or may not be transient, and mental state alteration at the time of the accident.9 In 2004, the World Health Organization (WHO) redefined the criteria for mild TBI. They specified that the GCS score should be 13–15 at time of presentation, instead of limiting it to a score of >13 within 30 minutes.10 In all likelihood though, these guidelines do not reflect the modern viewpoints of TBI, in which the spectrum of injury has now increased to recognize not only concussive injury, but also subconcussive injury as well as frequency of exposure.11 A diagram of clinically defined injury severity in regards to LOC and the GCS has been portrayed in Fig. 1.
Figure 1.
Schematic showing the progression of injury severity in correlation to loss of consciousness, diffuse axonal injury, and the Glasgow Coma Scale score.
Acute and subacute symptoms
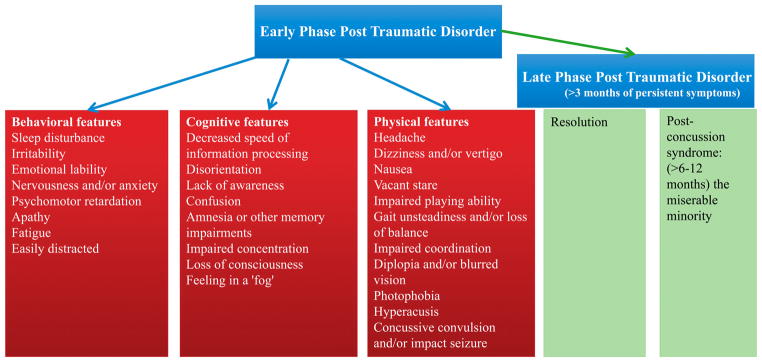
At least 80% of patients report one or more symptoms in the acute period after mild TBI. Up to 20% of athletes suspected of having a concussion are asymptomatic, indicating possible subconcussive injuries.12,13 Patients typically present with cognitive, physical, and behavioral signs and symptoms. The most frequent symptoms include headache, dizziness, and memory impairment. LOC is not a requirement for the diagnosis of concussion; therefore, the primary diagnostic symptoms of mild TBI evaluate neurosensory performance.14 Dizziness has been reported as the most common symptom of mild TBI, closely followed by headache.15 Cognitive disorders, sleep disorders, and hearing disorders such as, tinnitus, hearing loss, and central auditory processing disorder are also common.16 In a study of over 500 mild TBI patients, the authors concluded that neurosensory symptoms can persist for several months after injury, and can include blurry vision and personality changes.17 An overview of common symptoms that are evaluated after mild TBI have been highlighted in Fig. 2.
Figure 2.
Symptoms associated with acute phase and late phase post-traumatic disorder.
Concussion
Concussion has multiple definitions without a consensus. Concussion remains a clinical diagnosis that is subject to variability. Concussion is often referred to as the mildest end of the mild TBI spectrum. The American Academy of Neurology (AAN) divided concussion into three different grades to establish management guidelines for sports injuries. These guidelines include: Grade 1-no LOC, momentary confusion, mental status abnormalities <15 minutes, Grade 2-no LOC, transient confusion, mental status abnormalities lasting 15 minutes to 1 hour, and Grade 3-LOC, confusion, mental status abnormalities that may last more than 1 hour.18 The thresholds between grades are arbitrary, and in light of these findings, the AAN released updated guidelines that focus more on the management of concussion.19 Management is dependent upon salient features from a historical and physical exam. No single test score can be the basis of a concussion diagnosis. The success of concussion care is dictated by acute symptom treatment and timely recovery.20
Chronic changes
Common symptoms
Patients exposed to repetitive mild TBI can develop chronic symptoms that are both functional and behavioral in nature. Franke and colleagues have shown that mild TBI induces slowing of EEG oscillations.21 This slowing may account for the earlier onset of mild cognitive impairment in this population.22 Furthermore, patients frequently present with deficits in executive function.23 While the above two symptoms are the most debilitating, other common symptoms that are chronic and frequent following mild TBI include headache, fatigue, and irritability.24 Several of these symptoms have been linked to sleep disturbance that is frequently reported after repetitive mild TBIs.25 In rare situations, repetitive mild TBI can also lead to the onset of post-traumatic epilepsy. The etiology however is not fully understood.26 The persistence of symptoms can negatively affect the patient and prevent a return to work.27 We address how supplements and alternative therapies target some of these symptoms later in this review.
Dementia pugilistica and chronic symptoms
Athletes exposed to repetitive concussions during contact sports are at an increased risk for the development of cognitive deficits.28 This has been historically characterized in boxers’ who have histories of multiple bouts of head injury. Dementia Pugilistica was the first neurotrauma-related neurodegenerative disease described. It was first found in boxers’ who received repetitive punches to the head. Symptoms developed months to years after repetitive injury and were correlated to distinct pathological changes within the brain. Parkinsonism was also linked with dementia pugilistica in select cases. When tremor and rigidity dominate the clinical picture, the term ‘pugilistic parkinsonism’ is applied.29 Chronic onset dementia pugilistica has been proposed in the past decade. This phenotype has been associated with a clinical history of aggressive and violent behavior, but warrants further investigation.30
In other sports, some of the deficits following repetitive neurotrauma include mood and behavioral changes. Retired National Football League (NFL) players, for example, have a threefold increased risk of developing depression after retirement if they had received multiple concussions throughout their career.31 A nine year clinical follow-up study showed a link between concussions and depression.32 Further neuropsychological examination of former NFL players validated this relationship between a self-reported concussion history and depression.33 In addition to depression, retired NFL players who sustained three or more concussions reported increased cognitive symptoms, such as memory consolidation deficits.34 Additional studies also found cognitive deficits in retired NFL athletes after neuropsychological examination.35 Cognitive impairments have also been correlated with the incidence and frequency of head impacts in young football players equipped with helmet-mounted accelerometers.36 Moreover, after pathological examination of brains from the Mayo clinic, of the brains exhibiting signs of chronic disease, 33% of patients had participated in contact sports.37 Although these studies supply important information, the studies are retrospective in nature, and require further prospective investigation to eliminate recall bias.
CTE: Clinical manifestations
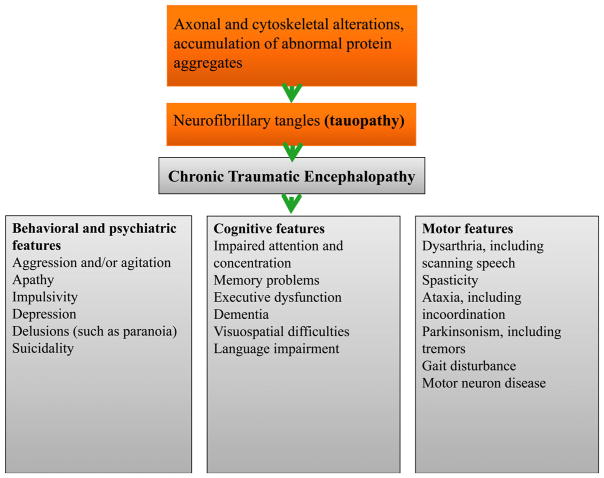
The symptoms of CTE are insidious Fig. 3. At first, patients have disturbances associated with poor attention or concentration, which can trigger impulsivity. The initial symptoms usually appear around ages 35–45 years.38 They are followed by headaches, short-term memory difficulty and aggressive tendencies. Typically a long latency (8 years) exists between head injury and the development of CTE symptoms.39 Recent evidence suggests that some athletes develop symptoms of CTE while actively playing contact sports.40 At this point, it is unclear whether symptoms denote prolonged PCS or the early manifestations of CTE.29 A case series reported that headache is a persistent and early symptom in nearly 50% of patients who develop a comprehensive description of clinical CTE.41 They also listed a broad range of clinical manifestations, in the domains of cognition, behavioral changes, and mood disturbances that warrant further investigation with prospective studies.
Figure 3.
Symptoms associated with chronic traumatic encephalopathy.
Because CTE is still a post-mortem diagnosis characterized by neurofibrillary tangles, these symptom classifications are still being debated. Due to the subtle clinical features common among the general population, it is challenging to differentiate CTE from other diseases without pathologic confirmation.20 Mez and colleagues evaluated available case series and grouped CTE symptoms into stages: Stage 1, attention loss and headache, Stage 2 depression and short-term memory loss, Stage 3 cognitive impairment, and Stage 4 poor verbal skill and aggression.42 Stage 1 symptoms are likely ‘preclinical’ because the pathology is unlikely to cause long-term clinical manifestations at this point in the disease process.43
Secondary effects of TBI
The primary effects of TBI are currently untreatable; however, secondary injury effects, which include cellular stress, immunotoxicity, oxidative stress, and inflammation can be treated.44 At the cellular level, TBI can deplete intracellular energy stores and cause subsequent failure of ATP-dependent ion pumps.45 Pump failure can trigger neuronal membrane depolarization and increase glutamate release.44 Augmented extracellular glutamate levels can activate glutamate receptors, which are known to allow positively charged ions into the cell.45 An excessive influx of positive ions can trigger immunoexcitotoxicity and neuronal cell death.46 Neuronal cells activate intrinsic mechanisms that produce and conserve energy to avoid imminent cell death. Mechanisms include catabolism, autophagy, phospholipase activation, and the organelle stress response.44,47
Reactive oxygen species
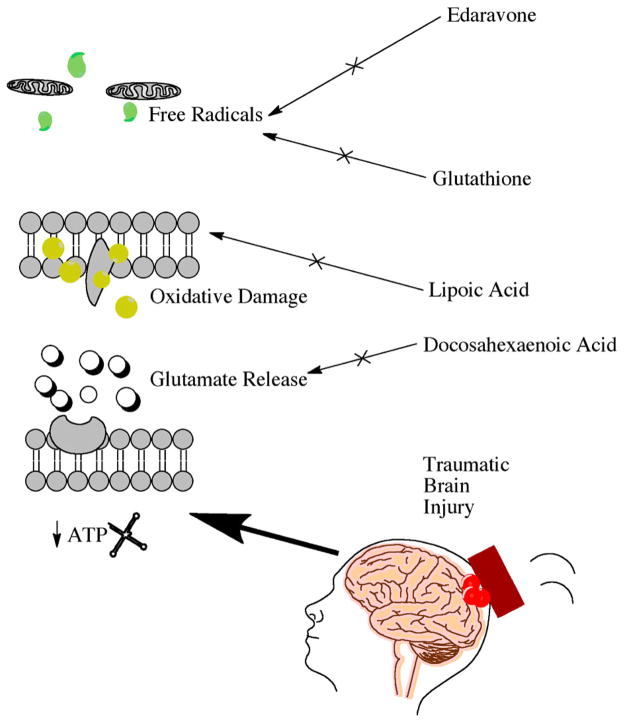
As mentioned before, augmented glutamate levels can activate catabolic processes that are known to increase intracellular reactive oxygen species (ROS). ROS production can lead to membrane lipid oxidation, an additional secondary injury mechanism observed in TBI.44 Therefore, compounds that can decrease the amount of ROS have a potential role in TBI therapy. ROS damage can also be reduced through implementation of free radical scavengers.48 Many antioxidants can donate electrons to free radicals in order to neutralize toxic effects. Glutathione and edaravone are free radical scavengers that are also potent antioxidants that provide beneficial peripheral effects. Roth and colleagues delivered glutathione, directly into the brain through a thinned skull and observed reduced ROS levels and an amelioration of brain injury markers.49 Edaravone reduced oxidative stress and increased neuronal survival in a weight-drop model of TBI.50 The use of free radical scavengers is an intriguing strategy for reducing TBI-induced oxidative stress Fig. 4. This strategy was also effective in other neural injury models such as ischemic stroke; however, the short half-lives of these compounds limits the brain bioavailability.
Figure 4.
Key supplements under investigation and the proposed mechanisms of action.
Lipid peroxidation
Due to a longer half-life, lipid peroxidase inhibitors have been shown to be more neuroprotective than direct free radical scavengers.48 Found endogenously in the brain, lipoic acid (LPA) is a potent antioxidant that can reduce lipid peroxidation. LPA has been shown to decrease neuronal cell death when administered after TBI.51 LPA can also increase the activity of antioxidants such as superoxide dismutase and glutathione. However, as mentioned before these antioxidants have a very short half-life and are only effective after mild bouts of oxidative stress.48 Another way to inhibit lipid peroxidation is to chelate free iron. The iron chelator, deferoxamine, has shown improved outcome in animal models of TBI.48 Iron chelation can prevent the catalyzation of lipid breakdown and lipid peroxide formation in the brain.48
Nutrition and supplements
Nutrition and supplementation may be a viable treatment option for the secondary effects of TBI. While evidence-based guidelines for nutrition are suggested for thoracic trauma, few studies have provided specific recommendations for TBI. In 2011 a consensus was established that advanced nutritional planning should be started 24–48 hours after TBI.52 A multi-center cohort study published in 2012 found that patients receiving enteral nutrition within 48 hours of injury had better survival rates and improved GCS scores.53 Other studies recommend an increased caloric intake, with the addition of 1–1.5 g/kg of protein, for two weeks post-injury.54
Specific nutrients can be chosen for supplementation based on their putative roles in the mechanisms of brain injury. The primary effects of TBI cause neuronal membrane dysfunction and vasculature damage which can lead to ischemia.55 Ischemia can alter cellular metabolism and result in ROS production, which can cause further damage to neuronal membranes.56 When neuronal membranes are damaged, intracellular Ca2+ homeostasis is perturbed, which can cause ER stress and potentially apoptosis. Nutritional targets may reduce the secondary effects of TBI, such as inflammation, ROS production, and neuronal cell death. Commonly used dietary supplements for TBI are discussed below.
Vitamins and minerals
Vitamin and mineral supplementation have been shown to improve outcome in pre-clinical models of mild TBI.46 Vitamin D deficient rodents displayed worsened outcome after TBI.57 The combination therapy of Vitamin D (1μg/kg) and progesterone (16 mg/kg) was shown to reduce markers of inflammation and neuronal cell death in a model of TBI.58 Progesterone has even shown neuroprotective effects in patients exposed to TBI.59 Another vitamin to consider for TBI clinical trial therapy is Vitamin E. Interestingly, Vitamin E supplementation (2 IU/g) improved cognition following repetitive concussive brain injury in mice.60 Vitamin E also reduced oxidative stress and improved learning and memory in a fluid percussion model of TBI.61
The water-soluble vitamin nicotinamide improved recovery in rodents following mild TBI when given with progesterone.62 Continuous infusion of nicotinamide (150 mg/kg per day) provided the most robust neuroprotection following mild TBI in rodents.63 This sustained administration paradigm has been linked to improved functional recovery.64 In contrast, folic acid administration for TBI therapy has been controversial. Naim and colleagues showed that folic acid can increase functional recovery following mild TBI in piglets.65 Vonder Haar and colleagues however showed no treatment effect with folic acid administration in rodents following mild TBI.66 This may indicate that vitamin supplementation may have species-specific effects. Vitamins have not yet been used in clinical trials for TBI. They may be best used as adjuvant agents to improve the effectiveness of promising therapeutics.46
Zinc, a cofactor of superoxide dismutase, has shown promise as a supplement to TBI therapy. In addition to oxidative stress reduction, zinc was also shown to reduce inflammation, apoptosis, and autophagy in pre-clinical models of neural injury.54 Zinc supplementation (180 ppm) for four weeks was also shown to decrease depression and anxiety in rats following TBI.67 Hypozincemia has been linked to depression and could be correlated to the incidence of depression after TBI.68 Zinc serum levels are reduced in mild TBI patients, possibly due to the hypoalbuminemia that can develop after injury, which can disrupt serum zinc availability.69 This finding however has not yet been verified for mild TBI and warrants a double blind clinical trial. Introducing high levels of zinc into the diets of TBI patients may help to preserve brain tissue and reduce neuropsychiatric symptoms.
Magnesium is another mineral that has been shown to improve recovery following TBI in pre-clinical models.70 Furthermore, Wahls and colleagues found that magnesium was one of the four key nutrients that correlated with improved somatic scores when supplemented following mild TBI in humans.71 Magnesium is commonly depleted following TBI likely through interaction with transient receptor potential melastatin, which leads to neuronal cell death.72 Magnesium readily enters the brain following mild TBI in rodents, but has not been shown to significantly increase in the CNS when supplemented in humans. Sen and Gulati found that co-administration of magnesium with mannitol may increase brain bioavailability in humans.73 Further work is needed to determine if combined therapy might improve the efficacy of magnesium supplementation in humans following mild TBI.
Pharmaceutical supplements
The detrimental effects of neural injury can also be reduced with cyclooxygenase (COX) inhibition. TBI is proposed to activate COX2, which is known to induce chronic inflammation after neural injury. Indomethacin, a non-selective COX inhibitor, has been shown to improve neurological outcomes following sports-related concussion.74 The anti-inflammatory drug, carprofen, also improved TBI outcome by reducing TBI lesion size and increasing neurogenesis in rodents.75 Used only for investigational study, Rolipram, an antidepressant drug, is a selective phosphodiesterase-4 inhibitor that was shown to be neuroprotective in a mouse model of TBI.76 Rolipram was shown to improve synaptic plasticity after TBI, and even improve cognitive performance.77 These compounds have been shown to be neuroprotective in a variety of pre-clinical TBI models; however, no compounds have survived the rigor of clinical trials to become viable treatments. Proposed reasons are due to the heterogeneity of TBI. Ongoing trials have grouped patients into more distinct categories, and may found better results for select subgroups of TBI.
Docosahexanoic acid
Docosahexaenoic acid (DHA) is an omega-3 fatty acid primarily stored in neuronal cell phospholipids.46 Phospholipase activation can release free fatty acids into the cell to promote cellular homeostasis. DHA release has been shown to counteract excessive glutamate activity following mild TBI in rodents Fig. 4.78 DHA has also been shown to promote synapse formation and upregulate glutamate receptor expression; thereby reducing excitotoxicity.79 A beneficial prophylaxis for patients suffering from TBI may be DHA. Studies show that DHA supplementation improved cognition and reduced markers of neuronal cell death in an impact-acceleration TBI model.80 DHA treatment reduced aberrant protein aggregation and improved neurobehavioral outcome in a controlled cortical impact model of TBI.81 Moreover, endogenous DHA depletion prior to TBI caused worsened outcomes in cognitive and motor function.79 A parent compound of DHA, α-linolenic acid, has also been shown to have neuroprotective effects preclinically.82 The results of DHA supplementation in an animal model of TBI, suggested both pre-injury neuroprotection and post-injury reduction of beta-amyloid precursor protein.83 DHA may protect neurons by decreasing nitric oxide levels and subsequent ROS.84 DHA may also indirectly decrease ROS by increasing activity of glutathione peroxidase and glutathione reductase.84 DHA supplementation post-TBI can reduce protein oxidation and restore neuronal cell homeostasis in order to manage the enduring effects of the injury.85 DHA is a safe and easily accessible compound that could benefit people exposed to a neurotraumatic event. To successfully implement DHA in a clinical trial, it will be necessary to perform human pilot studies in order to determine the most effective dosing strategy.
Dietary supplements in pre-clinical models of TBI
Dietary supplements have been shown to improve outcome in models of experimental TBI; however, clinical trials using supplements have continued to fail. New supplements that show promise in experimental TBI models include: curcumin, sulforaphane, and resveratrol.
Curcumin normalized brain-derived neurotrophic factor levels, and improved motor and learning performance in animals exposed to TBI.86 Sulforaphane was shown to improve blood–brain barrier integrity,87 reduce cerebral edema and improve cognition in a rodent model of TBI.88 Resveratrol has been shown to reduce ROS, suppress excitotoxicity, and reduce inflammation in a controlled cortical impact model of TBI.89 Resveratrol also reduced lipid peroxidation, decreased TBI lesion size, and specifically protected astrocytes after experimental TBI.90 Lipoic acid stabilized neuronal plasma membranes, prevented NADPH oxidative stress, and improved behavior following mild TBI in rats.91 Ansari and colleagues showed that different types of antioxidants are depleted at various time points following mild TBI.92 Therefore, targeting mild TBI with different types of antioxidants may be a viable approach to treatment. These supplements have not been used in clinical trials for TBI. A combination of supplementation and pharmaceutical intervention should be the focus for clinical trial paradigms moving forward.
Supplements in clinical trials for TBI
New efforts are being made to improve clinical trials for the treatment of TBI. TBI patients have shown decreased melatonin concentrations, likely contributing to their sleep disturbances.93 Melatonin is currently in Phase I clinical trials for youth TBI to assess GABAergic effects.94 Melatonin is promising in that it is endogenous and has been shown to be decreased even after mild TBI. Choline administration, enhanced cerebral blood flow in TBI patients;95 however, in a follow-up randomized, double-blinded, multi-center COBRIT trial, choline was shown to have no difference from placebo.96 Another clinical trial compound for TBI is an extract, known as enzogenol. Enzogenol (1000 mg/kg) was shown to improve cognition when administered to TBI patients in a randomized, controlled study.97 It had no adverse effects and the promising pilot data warrants verification with a larger more definitive clinical trial. Due to the complex pathophysiology of TBI, a combination of nutrients and supplements may be essential for optimal treatment. The overall goal is to implement new supplements such as, Melatonin, Choline, and Enzogenol into pharmaceutical-based clinical trials for TBI. A combined approach of supplement and pharmaceutical treatment will likely be the most beneficial.
Nutrition
While the hope is that optimal nutrition can improve TBI outcome, it is intuitive to think that malnutrition can adversely affect outcome. In a retrospective study, body mass index less than 18.5 was associated with higher mortality in intensive care patients and longer hospital stays.98 In a mild TBI clinic study, patients were surveyed about their nutritional intake. None of the patients met the recommended daily allowance for all 14 specified nutrients, and worsened outcome scores were linked with the lowest nutrient intake compared to those who had the highest nutrient intake.71 TBI patients who were malnourished at the time of admission exhibited lower albumin and prealbumin levels and were correlated to have poor neurological scores.99 The above evidence suggests that implementing nutritional supplementation can improve outcomes following mild TBI.
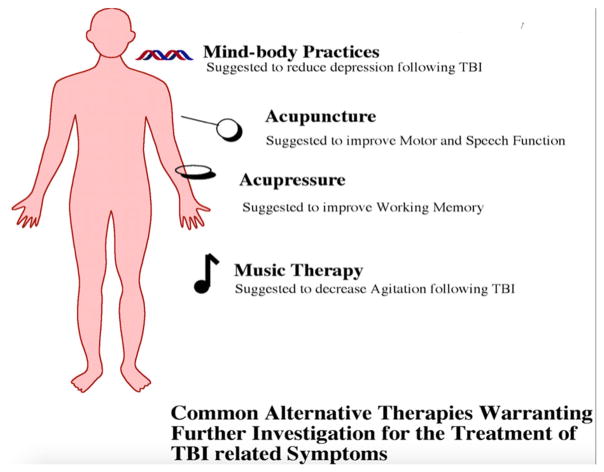
Alternative therapy
In addition to ‘natural products’ (e.g., herbal remedies, vitamins), alternative therapies can be used for TBI, which include: acupuncture, acupressure, mind-body practices, and music therapy Fig. 5.
Figure 5.
Alternative therapy approaches for the treatment of TBI.
Acupuncture
Acupuncture involves the stimulation of specific ‘acu-points’, or meridian points, on the skin to normalize aberrant flow of Qi by the insertion of fine needles either by hand or through electrical stimulation.100
A Cochrane systematic review on the safety and efficacy of acupuncture in the acute management and/or rehabilitation of individuals with TBI had identified four randomized controlled trials studies with a total of 294 participants.100 All trials had two groups to compare needle acupuncture to electrical acupuncture. After acute TBI, acupuncture improved overall functional outcome on the GCS,101 the Glasgow Outcome Score (GOS),101,102 and motor and speech functions.101 In the rehabilitation phase after TBI, acupuncture improved overall functional outcome on the Barthel Index,103 Modified Barthel Index,104 motor function on the Fugl-Meyer Assessment,104 and muscle strength grading.103 However, though all four studies were described as randomized, they were deemed by the review authors to carry a high risk of bias. Randomization procedures were not described, blinding information was not available, no placebo or sham control group was used, and variation in the acupuncture treatments existed among the different participants. Consequently, the authors noted that the current evidence in this review were greatly insufficient to support recommendations for acupuncture as a standard procedure for TBI in clinical practice. Moreover, no adverse effects of acupuncture were reported in any of the clinical trials reviewed. Although serious adverse effects are rare, the incidence of minor adverse events may be considerable.105 In the few studies that have reported adverse effects, 38% of all patients experienced some bleeding,106 28% reported at least one adverse event,107 and 45% reported an aggravation of pain.108 The potential side effects of acupuncture in the context of TBI treatment and rehabilitation remain unclear and understudied.
In a recent population-based investigation from Taiwan, TBI patients who underwent acupuncture treatment were found to require less medical care within the first year.109 In a follow-up study, TBI patients with acupuncture treatment were found to have a lower incidence of stroke compared to patients not receiving acupuncture treatment.110 Despite having a large sample size, the database lacked detailed patient information on lifestyle, physical, psychiatric, and laboratory examinations.110 Additionally, the mode of acupuncture treatment for patients with TBI varied with Traditional Chinese Medical physicians.110 In another study, involving 24 adult TBI survivors randomized to acupuncture of control arms, the authors demonstrated that acupuncture had beneficial effects on perception of sleep, or sleep quality.111 The acupuncture group also showed improvements on measures of cognition and depression not observed in the control group.111 Unfortunately, this study had a risk of bias comparable to the studies identified in the Cochrane review, including failure to report randomization sequence and failure to blind observers. Clinical trials with lower risk of bias and investigations into the specific acupuncture points are necessary to better determine the efficacy and safety of acupuncture in individuals with TBI.
Acupressure
Acupressure is another form of Traditional Chinese Medicine that involves the application of pressure to specific ‘acupoints’ to affect the flow of Qi. Distinct from acupuncture, it is non-invasive, using hands, fingers, or thumbs instead of needles.
In a randomized, placebo-controlled, single-blind study, the authors demonstrate enhanced working memory among TBI patients after acupressure treatments.112 Forty-two adults with mild TBI were randomized to receive active acupressure treatment (eight treatments over four weeks), or sham acupressure treatment.112 Cognitive function (working memory, attention, motor speed, and spatial problem solving) was assessed using a battery of neuropsychological tests. Neurophysiological measures were also added to delineate mechanisms underlying potential cognitive change associated with the treatment. To do so, event-related potentials were recorded during performance on specific cognitive tasks,112 as previous studies have shown that even mild TBI affects the amplitude and latency of these recordings.113 Compared with those in the placebo group, those in the active acupressure group demonstrated a reduction in P300 latency (increased speed with which memory operations occur) and amplitude (the degree of memory engagement).112 The active-treatment group also showed improvements in attention as compared with the placebo group on the Stroop task and Digit Span task.112 There were no significant between-group differences on other measures of neuropsychological function, or measures of anxiety, depression, or loneliness.112 Strengths of this study included a strong effect size (Cohen’s d = 0.68 for digit span involvement) and the use of multiple behavioral and physiological measures of treatment-associated change.113 Limitations include the lack of long-term follow-up to assess for the enduring nature of acupuncture treatment effects. Adverse effects were also not reported. In addition, individuals with mild TBI were identified via the Brain Injury Screening Questionnaire, which has good reliability and validity, but is still a self-report measure to detect TBI.114
Mind-body practices
Mind-body practice uses the power of thoughts and emotions to influence physical health, and vice-versa. Some examples of mind-body medicine practices are meditation, hypnosis, tai chi, qi gong, and yoga. Preliminary studies show that these interventions can enhance parasympathetic tone, heart rate variability, neuroplasticity, EEG synchrony, long-term potentiation, and oxygenation in mental health care.115
Yoga, in particular, is a mind-body practice with origins in ancient Indian philosophy that typically combines physical postures, breathing techniques, and meditation or relaxation. However, there were only ten individuals who attended weekly yoga classes and four individuals in the no-treatment group. The small sample sizes precluded the analysis of between-group analyses. In another yoga study, Schmid et al. demonstrated that yoga may be feasibly delivered in a one-to-one setting and improve multiple aspects of physical function in individuals with chronic TBI.116 Only three patients were involved in the study, and assessments were taken before and after the 8-week yoga intervention.116 Further investigation of yoga for the treatment of TBI is warranted.
Tai Chi and qi gong also involves certain postures and gentle movements with mental focus, breathing and relaxation. They are rooted in the Chinese philosophical tradition of Taoism. In contrast to qi gong, ta chi movements, if practiced quickly, can be a form of combat or self-defense. In a study involving 18 participants, with nine each assigned to a control (waiting list) or Tai Chi group, Gemmell and Leathem found Tai Chi to reduce confusion, tension and fear among TBI patients.117 There was however no between-group differences in mood, self-esteem, or mental well-being after three weeks of completing the Tai Chi course.117 Qi gong, however, improved mood and self-esteem among individuals with TBI.118 In this study, 20 individuals were randomly assigned to receive the qi gong intervention (n = 10), or to a non-exercise leisure activity control group (n = 10).118 After eight weeks, self-esteem and mood were improved in the qi gong group compared to controls.118 Future large-scale randomized control trials are needed to assess the therapeutic potential of Tai Chi and qi gong for the management of TBI.
Mindfulness-based cognitive therapy (MBCT) incorporates the elements of cognitive-behavioral therapy. Mindfulness-based stress reduction (MBST) identifies our thoughts without passing judgment to those thoughts.119 Initially designed to aid in the prevention of relapse of depression, specifically in individuals with major depressive disorder, MBCT has since been applied to various neurological and psychiatric conditions. To demonstrate the efficacy of MBCT in the treatment of clinically diagnosed depression in TBI patients, Bedard et al. recruited 23 participants for an 8-week study, with a 90-min MBCT session once a week.120 They found that MBCT significantly reduced depression symptoms on all scales compared to baseline.120 Participants also indicated reduced pain intensity and increased energy levels.120 No significant effects were found on anxiety symptoms, pain frequency, and level of functioning.120 In a pilot study, Berdard et al. showed MBST delivered to TBI patients produced a better quality of life and reduced depression symptoms.121 Interestingly, in a one-year follow-up study, Bedard et al. found MBST to offer sustained effects on several key outcome measures, including depression symptoms.122 However, this finding has to be interpreted with caution as there was no control group, and three of the ten original MBST group participants were lost to follow-up. Moreover, whether or not the participants continued to use the skills they acquired was not assessed.
Music therapy
Music therapy is a therapeutic intervention to address the emotional, physical, and spiritual needs of an individual. Music therapy can be broadly categorized as ‘active’, in which people re-create, improvise, or compose music, and ‘receptive’, in which they listen to music.122 Music therapy treatment involves selecting from a range of music-based interventions, using both music and the therapist–patient relationship as agents of change.123
A recent Cochrane systematic review of music therapy as a rehabilitation intervention for people with acquired brain injury (e.g., stroke and TBI) included seven studies with a total of 184 participants.123 Interventions included rhythmic auditory stimulation (RAS)124–126 electronic music making,127 rhythmic-melodic voice training128, and listening to pre-recorded songs 129,130 or live music.129 In stroke patients, RAS may be beneficial for improving gait velocity, cadence, stride length, and stride symmetry.123 While one study observed a significant improvement of upper extremity function during RAS,124 music therapy was found to reduce agitation, and improve orientation levels compared to no music.129 As most of these studies focused on stroke patients, further studies implementing music therapy for TBI patients are needed.
Overall, the evidence presented thus far for the beneficial effects of alternative therapies for TBI patients is insufficient. Most of the studies reviewed have a high risk of bias (e.g., failure to blind, failure to report randomization procedures, and failure to use a placebo or sham control group) and small sample sizes. Moreover, safety data on many of the interventions were not reported at all. Albeit encouraging, additional rigorous and methodically sound randomized control trials are needed before recommendations can be made for clinical practice.
Conclusion
The chronic neurological deficits of TBI are complex and clinical treatments are limited. In this review, we highlighted TBI diagnosis, pharmaceutical targets of TBI, supplementation to traditional treatment for TBI, and the approach of alternative medicine. Ongoing failure of clinical trials for TBI treatment has questioned the direction of therapy. Nutritional Supplementation, including vitamins, has emerged as a promising therapeutic option for TBI. No class I evidence is available for the effectiveness of supplementation; however, several class III studies have shown the minerals magnesium and zinc to be most effective at improving both pre-clinical and clinical TBI outcome. The literature is mixed on supplements such as coline and melatonin. There is class IIC evidence that enzogenol is effective. Alternative therapies may also help to treat neuropsychiatric symptoms associated with TBI, however, definitive clinical trial evidence is lacking. Going forward it will be important to learn how supplementation and alternative therapies can be incorporated with clinical practice to improve outcomes for TBI patients. Combination therapies of supplementation and pharmaceutical agents are likely to yield the best results. Furthermore, the treatment regimens need to be tailored more specifically to the type of TBI based on primary injury and severity. Several clinical trials have failed because heterogeneous TBI patients with a wide range of injury severities were grouped together in the same studies. Specifically targeting key biochemical mechanisms with different agents at the appropriate time points will likely lead to more successful clinical trials going forward.
Acknowledgments
Funding Brandon Lucke-Wold and Aric Logsdon received funding from an American Foundation of Pharmaceutical Education Pre-doctoral Grant. Brandon Lucke-Wold also received funding from an American Medical Association Foundation Seed Grant, Neurosurgery Research and Education Foundation Medical Student Fellowship, and Sigma Xi Grants-in-Aid-of-Research.
Footnotes
Conflicts of interest No conflicts-of-interest to report.
Ethics approval Confirm that all the research meets the ethical guidelines, including adherence to the legal requirements of the study country.
References
- 1.Koliatsos VE, Xu L. The problem of neurodegeneration in cumulative sports concussions: emphasis on neurofibrillary tangle formation. In: Kobeissy FHP, editor. Brain Neurotrauma: molecular, neuropsychological, and rehabilitation aspects. Frontiers in neuroengineering. Boca Raton, FL: CRC Press; 2015. [PubMed] [Google Scholar]
- 2.Lucke-Wold BP, Turner RC, Logsdon AF, Bailes JE, Huber JD, Rosen CL. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J Neurotrauma. 2014;31(13):1129–38. doi: 10.1089/neu.2013.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR. Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imag Behav. 2015;9(3):358–66. doi: 10.1007/s11682-015-9399-z. [DOI] [PubMed] [Google Scholar]
- 4.Wang T, Van KC, Gavitt BJ, Grayson JK, Lu YC, Lyeth BG, et al. Effect of fish oil supplementation in a rat model of multiple mild traumatic brain injuries. Restorat Neurol Neurosci. 2013;31(5):647–59. doi: 10.3233/RNN-130316. [DOI] [PubMed] [Google Scholar]
- 5.Hasadsri L, Wang BH, Lee JV, Erdman JW, Llano DA, Barbey AK, et al. Omega-3 fatty acids as a putative treatment for traumatic brain injury. J Neurotrauma. 2013;30(11):897–906. doi: 10.1089/neu.2012.2672. [DOI] [PubMed] [Google Scholar]
- 6.Gerber DJ, Weintraub AH, Cusick CP, Ricci PE, Whiteneck GG. Magnetic resonance imaging of traumatic brain injury: relationship of T2*SE and T2GE to clinical severity and outcome. Brain Inj. 2004;18(11):1083–97. doi: 10.1080/02699050410001672341. [DOI] [PubMed] [Google Scholar]
- 7.Carson JD, Rendely A, Garel A, Meaney C, Stoller J, Kaicker J, et al. Are Canadian clinicians providing consistent sport-related concussion management advice? Can Fam Phys. 2016;62(6):494–500. [PMC free article] [PubMed] [Google Scholar]
- 8.Lau KM, Madden E, Neylan TC, Seal KH, Maguen S. Assessing for mild TBI among Iraq and Afghanistan veterans: outcomes of injury severity and neurological factors. Brain Inj. 2016;30(3):287–94. doi: 10.3109/02699052.2015.1089601. [DOI] [PubMed] [Google Scholar]
- 9.Ruff RM, Iverson GL, Barth JT, Bush SS, Broshek DK, Policy NAN, et al. Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol: Off J Natl Acad Neuropsychol. 2009;24(1):3–10. doi: 10.1093/arclin/acp006. [DOI] [PubMed] [Google Scholar]
- 10.Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, Holm L, et al. Prognosis for mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;(43 Suppl):113–25. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 11.Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T. Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg. 2013;119(5):1235–45. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- 12.Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc JINS. 2010;16(3):401–11. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- 13.Levin HS, Amparo E, Eisenberg HM, Williams DH, High WM, Jr, McArdle CB, et al. Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg. 1987;66(5):706–13. doi: 10.3171/jns.1987.66.5.0706. [DOI] [PubMed] [Google Scholar]
- 14.Stemper BD, Shah AS, Pintar FA, McCrea M, Kurpad SN, Glavaski-Joksimovic A, et al. Head rotational acceleration characteristics influence behavioral and diffusion tensor imaging outcomes following concussion. Annal Biomed Eng. 2015;43(5):1071–88. doi: 10.1007/s10439-014-1171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edmed SL, Sullivan KA, Allan AC, Smith SS. Assessment method influences the severity and type of symptoms reported after self-reported mild traumatic brain injury. J Clin Exp Neuropsychol. 2015;37(6):641–52. doi: 10.1080/13803395.2015.1038984. [DOI] [PubMed] [Google Scholar]
- 16.Skobska OE, Kadzhaya NV, Andreyev OA, Potapov EV. Characterization of vestibular disorders in the injured persons with the brain concussion in acute period. Klin Khir. 2015;7(4):49–51. [PubMed] [Google Scholar]
- 17.Laborey M, Masson F, Ribereau-Gayon R, Zongo D, Salmi LR, Lagarde E. Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: results from a comparative cohort study. J Head Trauma Rehabil. 2014;29(1):E28–36. doi: 10.1097/HTR.0b013e318280f896. [DOI] [PubMed] [Google Scholar]
- 18.McCrory P. Traumatic brain injury: revisiting the AAN guidelines on sport-related concussion. Nat Rev Neurol. 2013;9(7):361–2. doi: 10.1038/nrneurol.2013.88. [DOI] [PubMed] [Google Scholar]
- 19.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development subcommittee of the American academy of neurology. Neurology. 2013;80(24):2250–7. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iverson GL, Silverberg ND, Mannix R, Maxwell BA, Atkins JE, Zafonte R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA Pediatr. 2015;106:21–9. doi: 10.1001/jamapediatrics.2015.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke LM, Walker WC, Hoke KW, Wares JR. Distinction in EEG slow oscillations between chronic mild traumatic brain injury and PTSD. Int J Psychophysiol. 2016;106:21–9. doi: 10.1016/j.ijpsycho.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 22.LoBue C, Denney D, Hynan LS, Rossetti HC, Lacritz LH, Hart J, et al. Self-reported traumatic brain injury and mild cognitive impairment: increased risk and earlier age of diagnosis. J Alzheimers Dis. 2016;51(3):727–36. doi: 10.3233/JAD-150895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libin AV, Scholten J, Schladen MM, Danford E, Shara N, Penk W, et al. Executive functioning in TBI from rehabilitation to social reintegration: COMPASS (goal,) a randomized controlled trial (grant: 1I01RX000637-01A3 by the VA ORD RR&D, 2013-2016) Mil Med Res. 2015;2:32. doi: 10.1186/s40779-015-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivak S, Nosal V, Bittsansky M, Dluha J, Dobrota D, Kurca E. Type and occurrence of serious complications in patients after mild traumatic brain injury. Bratisl Lek Listy. 2016;117(1):22–5. doi: 10.4149/bll_2016_005. [DOI] [PubMed] [Google Scholar]
- 25.Lucke-Wold BP, Smith KE, Nguyen L, Turner RC, Logsdon AF, Jackson GJ, et al. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci Biobehav Rev. 2015;55:68–77. doi: 10.1016/j.neubiorev.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen YW, Smith KE, et al. Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure. 2015;33:13–23. doi: 10.1016/j.seizure.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Xiong C, Martin T, Sravanapudi A, Colantonio A, Mollayeva T. Factors associated with return to work in men and women with work-related traumatic brain injury. Disabil Health J. 2016;9(3):439–48. doi: 10.1016/j.dhjo.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 28.De Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, Twijnstra A. Prediction of post-traumatic complaints after mild traumatic brain injury: early symptoms and biochemical markers. J Neurol Neurosurg Psychiat. 2002;73(6):727–32. doi: 10.1136/jnnp.73.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Experiment Neurol. 2009;68(7):709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and bio-markers. Nat Rev Neurol. 2013;9(4):192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP, Jr, Matthews A, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39(6):903–9. doi: 10.1249/mss.0b013e3180383da5. [DOI] [PubMed] [Google Scholar]
- 32.Kerr ZY, Marshall SW, Harding HP, Jr, Guskiewicz KM. Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med. 2012;40(10):2206–12. doi: 10.1177/0363546512456193. [DOI] [PubMed] [Google Scholar]
- 33.Didehbani N, Munro CC, Mansinghani S, Conover H, Hart J., Jr Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol: Off J Natl Acad Neuropsychol. 2013;28(5):418–24. doi: 10.1093/arclin/act028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CJ, Xiong G, Elkind JA, Putnam B, Cohen AS. Brain injury impairs working memory and prefrontal circuit function. Front Neurol. 2015;6:240. doi: 10.3389/fneur.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford JH, Giovanello KS, Guskiewicz KM. Episodic memory in former professional football players with a history of concussion: an event-related functional neuroimaging study. J Neurotrauma. 2013;30(20):1683–701. doi: 10.1089/neu.2012.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broglio SP, Moore RD, Hillman CH. A history of sport-related concussion on event-related brain potential correlates of cognition. Int J Psychophysiol. 2011;82(1):16–23. doi: 10.1016/j.ijpsycho.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130(6):877–89. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain J Neurol. 2013;136(Pt 1):43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daneshvar DH, Goldstein LE, Kiernan PT, Stein TD, McKee AC. Post-traumatic neurodegeneration and chronic traumatic encephalopathy. Mol Cell Neurosci. 2015;66(Pt B):81–90. doi: 10.1016/j.mcn.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Stein CJ, MacDougall R, Quatman-Yates CC, Myer GD, Sugimoto D, Dennison RJ, et al. Young athletes’ concerns about sport-related concussion: the patient’s perspective. Clin J Sport Med Off J Can Acad Sport Med. 2015;26(5):386–90. doi: 10.1097/JSM.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 41.Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122–9. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mez J, Stern RA, McKee AC. Chronic traumatic encephalopathy: where are we and where are we going? Curr Neurol Neurosci Rep. 2013;13(12):407. doi: 10.1007/s11910-013-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127(1):29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 45.Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int. 2011;2:107. doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maroon JC, Lepere DB, Blaylock RL, Bost JW. Postconcussion syndrome: a review of pathophysiology and potential nonpharmacological approaches to treatment. Phys Sportsmed. 2012;40(4):73–87. doi: 10.3810/psm.2012.11.1990. [DOI] [PubMed] [Google Scholar]
- 47.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 48.Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics: J Am Soc Exp Neurotherapeut. 2010;7(1):51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223–8. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang GH, Jiang ZL, Li YC, Li X, Shi H, Gao YQ, et al. Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma. 2011;28(10):2123–34. doi: 10.1089/neu.2011.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozbal S, Cankurt U, Tugyan K, Pekcetin C, Sisman AR, Gunduz K, et al. The effects of α-lipoic acid on immature rats with traumatic brain injury. Biotech Histochem. 2015;90(3):206–15. doi: 10.3109/10520295.2014.977950. [DOI] [PubMed] [Google Scholar]
- 52.Cook AM, Peppard A, Magnuson B. Nutrition considerations in traumatic brain injury. Nutr Clin Pract. 2008;23(6):608–20. doi: 10.1177/0884533608326060. [DOI] [PubMed] [Google Scholar]
- 53.Chiang YH, Chao DP, Chu SF, Lin HW, Huang SY, Yeh YS, et al. Early enteral nutrition and clinical outcomes of severe traumatic brain injury patients in acute stage: a multi-center cohort study. J Neurotrauma. 2012;29(1):75–80. doi: 10.1089/neu.2011.1801. [DOI] [PubMed] [Google Scholar]
- 54.Scrimgeour AG, Condlin ML. Nutritional treatment for traumatic brain injury. J Neurotrauma. 2014;31(11):989–99. doi: 10.1089/neu.2013.3234. [DOI] [PubMed] [Google Scholar]
- 55.Begum G, Harvey L, Dixon CE, Sun D. ER stress and effects of DHA as an ER stress inhibitor. Transl Stroke Res. 2013;4(6):635–42. doi: 10.1007/s12975-013-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ten VS, Starkov A. Hypoxic-ischemic injury in the developing brain: the role of reactive oxygen species originating in mitochondria. Neurol Res Int. 2012;2012:542976. doi: 10.1155/2012/542976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864–74. doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang H, Hua F, Wang J, Yousuf S, Atif F, Sayeed I, et al. Progesterone and vitamin D combination therapy modulates inflammatory response after traumatic brain injury. Brain Inj. 2015:1–10. doi: 10.3109/02699052.2015.1035330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12(2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conte V, Uryu K, Fujimoto S, Yao Y, Rokach J, Longhi L, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90(3):758–64. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 61.Aiguo W, Zhe Y, Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24(3):290–8. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peterson TC, Hoane MR, McConomy KS, Farin FM, Bammler TK, MacDonald JW, et al. A combination therapy of nicotinamide and progesterone improves functional recovery following traumatic brain injury. J Neurotrauma. 2015;32(11):765–79. doi: 10.1089/neu.2014.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vonder HC, Anderson GD, Hoane MR. Continuous nicotinamide administration improves behavioral recovery and reduces lesion size following bilateral frontal controlled cortical impact injury. Behav Brain Res. 2011;224(2):311–7. doi: 10.1016/j.bbr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goffus AM, Anderson GD, Hoane M. Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid Med Cell Longev. 2010;3(2):145–52. doi: 10.4161/oxim.3.2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naim MY, Friess S, Smith C, Ralston J, Ryall K, Helfaer MA, et al. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev Neurosci. 2010;32(5–6):466–79. doi: 10.1159/000322448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vonder HC, Emery MA, Hoane MR. Chronic folic acid administration confers no treatment effects in either a high or low dose following unilateral controlled cortical impact injury in the rat. Restor Neurol Neurosci. 2012;30(4):291–302. doi: 10.3233/RNN-2012-110196. [DOI] [PubMed] [Google Scholar]
- 67.Cope EC, Morris DR, Scrimgeour AG, VanLandingham JW, Levenson CW. Zinc supplementation provides behavioral resiliency in a rat model of traumatic brain injury. Physiol Behav. 2011;104(5):942–7. doi: 10.1016/j.physbeh.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maes M, D’Haese PC, Scharpe S, D’Hondt P, Cosyns P, De Broe ME. Hypozincemia in depression. J Affect Disord. 1994;31(2):135–40. doi: 10.1016/0165-0327(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 69.McClain CJ, Twyman DL, Ott LG, Rapp RP, Tibbs PA, Norton JA, et al. Serum and urine zinc response in head-injured patients. J Neurosurg. 1986;64(2):224–30. doi: 10.3171/jns.1986.64.2.0224. [DOI] [PubMed] [Google Scholar]
- 70.Vonder HC, Peterson TC, Martens KM, Hoane MR. Vitamins and nutrients as primary treatments in experimental brain injury: clinical implications for nutraceutical therapies. Brain Res. 2016;1640(Pt A):114–29. doi: 10.1016/j.brainres.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wahls T, Rubenstein L, Hall M, Snetselaar L. Assessment of dietary adequacy for important brain micronutrients in patients presenting to a traumatic brain injury clinic for evaluation. Nutr Neurosci. 2014;17(6):252–9. doi: 10.1179/1476830513Y.0000000088. [DOI] [PubMed] [Google Scholar]
- 72.Cook NL, Van Den Heuvel C, Vink R. Are the transient receptor potential melastatin (TRPM) channels important in magnesium homeostasis following traumatic brain injury? Magnes Res. 2009;22(4):225–34. doi: 10.1684/mrh.2009.0189. [DOI] [PubMed] [Google Scholar]
- 73.Sen AP, Gulati A. Use of magnesium in traumatic brain injury. Neurotherapeutics. 2010;7(1):91–9. doi: 10.1016/j.nurt.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Girgis H, Palmier B, Croci N, Soustrat M, Plotkine M, Marchand-Leroux C. Effects of selective and non-selective cyclooxygenase inhibition against neurological deficit and brain oedema following closed head injury in mice. Brain Res. 2013;1491:78–87. doi: 10.1016/j.brainres.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 75.Thau-Zuchman O, Shohami E, Alexandrovich AG, Trembovler V, Leker RR. The anti-inflammatory drug carprofen improves long-term outcome and induces gliogenesis after traumatic brain injury. J Neurotrauma. 2012;29(2):375–84. doi: 10.1089/neu.2010.1673. [DOI] [PubMed] [Google Scholar]
- 76.Atkins CM, Cepero ML, Kang Y, Liebl DJ, Dietrich WD. Effects of early rolipram treatment on histopathological outcome after controlled cortical impact injury in mice. Neuroscience lett. 2013;532:1–6. doi: 10.1016/j.neulet.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Titus DJ, Sakurai A, Kang Y, Furones C, Jergova S, Santos R, et al. Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury. J Neurosci: Off J Soc Neurosci. 2013;33(12):5216–26. doi: 10.1523/JNEUROSCI.5133-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menard C, Patenaude C, Gagne AM, Massicotte G. AMPA receptor-mediated cell death is reduced by docosahexaenoic acid but not by eicosapentaenoic acid in area CA1 of hippocampal slice cultures. J Neurosci Res. 2009;87(4):876–86. doi: 10.1002/jnr.21916. [DOI] [PubMed] [Google Scholar]
- 79.Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PloS One. 2014;9(1):e86472. doi: 10.1371/journal.pone.0086472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mills JD, Hadley K, Bailes JE. Dietary supplementation with the omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery. 2011;68(2):474–81. doi: 10.1227/NEU.0b013e3181ff692b. discussion 81. [DOI] [PubMed] [Google Scholar]
- 81.Begum G, Yan HQ, Li L, Singh A, Dixon CE, Sun D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J Neurosci Off J Soc Neurosci. 2014;34(10):3743–55. doi: 10.1523/JNEUROSCI.2872-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Michael-Titus AT. Omega-3 fatty acids and neurological injury. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–6):295–300. doi: 10.1016/j.plefa.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 83.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21(10):1457–67. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Zhao X, Mao ZY, Wang XM, Liu ZL. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14(18):2457–61. doi: 10.1097/00001756-200312190-00033. [DOI] [PubMed] [Google Scholar]
- 85.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeo-stasis in traumatic brain injury. J Neurotrauma. 2007;24(10):1587–95. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 86.Wu A, Ying Z, Schubert D, Gomez-Pinilla F. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabil Neural Repair. 2011;25(4):332–42. doi: 10.1177/1545968310397706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, et al. Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood–brain and blood–spinal cord barriers. J Neurosci: Off J Soc Neurosci. 2014;34(25):8585–93. doi: 10.1523/JNEUROSCI.2935-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett. 2009;460(2):103–7. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gatson JW, Liu MM, Abdelfattah K, Wigginton JG, Smith S, Wolf S, et al. Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg. 2013;74(2):470–4. doi: 10.1097/TA.0b013e31827e1f51. Discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 90.Lin CJ, Chen TH, Yang LY, Shih CM. Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis. 2014;5:e1147. doi: 10.1038/cddis.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucke-Wold BP, Naser ZJ, Logsdon AF, Turner RC, Smith KE, Robson MJ, et al. Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl Res. 2015;166(6):509–28. e1. doi: 10.1016/j.trsl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 92.Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25(5):513–26. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- 93.Seifman MA, Gomes K, Nguyen PN, Bailey M, Rosenfeld JV, Cooper DJ, et al. Measurement of serum melatonin in intensive care unit patients: changes in traumatic brain injury, trauma, and medical conditions. Front Neurol. 2014;5:237. doi: 10.3389/fneur.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barlow KM, Brooks BL, MacMaster FP, Kirton A, Seeger T, Esser M, et al. A double-blind, placebo-controlled intervention trial of 3 and 10 mg sublingual melatonin for post-concussion syndrome in youths (PLAYGAME): study protocol for a randomized controlled trial. Trials. 2014;15:271. doi: 10.1186/1745-6215-15-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leon-Carrion J, Dominguez-Roldan JM, Murillo-Cabezas F, del Rosario Dominguez-Morales M, Munoz-Sanchez MA. The role of citicholine in neuropsychological training after traumatic brain injury. Neuro Rehabil. 2000;14(1):33–40. [PubMed] [Google Scholar]
- 96.Zafonte R, Friedewald WT, Lee SM, Levin B, Diaz-Arrastia R, Ansel B, et al. The citicoline brain injury treatment (COBRIT) trial: design and methods. J Neurotrauma. 2009;26(12):2207–16. doi: 10.1089/neu.2009.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Theadom A, Mahon S, Barker-Collo S, McPherson K, Rush E, Vandal AC, et al. Enzogenol for cognitive functioning in traumatic brain injury: a pilot placebo-controlled RCT. Eur J Neurol. 2013;20(8):1135–44. doi: 10.1111/ene.12099. [DOI] [PubMed] [Google Scholar]
- 98.Finkielman JD, Gajic O, Afessa B. Underweight is independently associated with mortality in post-operative and non-operative patients admitted to the intensive care unit: a retrospective study. BMC Emergency Med. 2004;4(1):3. doi: 10.1186/1471-227X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baltazar GA, Pate AJ, Panigrahi B, LaBoy S, Prosniak R, Mody A, et al. Malnutrition as measured by albumin and pre-albumin on admission is associated with poor outcomes after severe traumatic brain injury. Am surg. 2015;81(2):E61–3. [PubMed] [Google Scholar]
- 100.Wong V, Cheuk DK, Lee S, Chu V. Acupuncture for acute management and rehabilitation of traumatic brain injury. The Cochrane Library. 2013;11(5):CD007700. doi: 10.1002/14651858.CD007700.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jing D, Guofeng D, Yingxuan S. Control observation on therapeutic effects of acupuncture treatment on acute severe cranio-cerebral injury. Chin Acupuncture Moxibustion. 2002;7:006. [Google Scholar]
- 102.Song Y, Zhang L, Zhang L. Observations on the efficacy of electroacupuncture-assisted treatment for resuscitating coma patients with serious brain trauma. Shanghai J Acupunct Moxibustion. 2007;26(4):11–2. [Google Scholar]
- 103.Cao W, Qin Y, Hou Q. Acupuncture combined with hyperbaric oxygen treatment for post-traumatic brain injury syndrome (Translation) Mod Rehabil J Clin Rehabilitative Tissue Eng. 2001;5(8):110. [Google Scholar]
- 104.Chang Z, Liu P. Rehabilitation and acupuncture treatment for patients with traumatic brain injury (Translation) Chin J Med Dev. 2005;18(5):38–9. [Google Scholar]
- 105.Ernst E, White AR. Prospective studies of the safety of acupuncture: a systematic review. Am J Med. 2001;110(6):481–5. doi: 10.1016/s0002-9343(01)00651-9. [DOI] [PubMed] [Google Scholar]
- 106.Yamashita H, Tsukayama H, Hori N, Kimura T, Tanno Y. Incidence of adverse reactions associated with acupuncture. J Alternat Complement Med. 2000;6(4):345–50. doi: 10.1089/10755530050120718. [DOI] [PubMed] [Google Scholar]
- 107.Melchart D, Hager S, Weidenhammer W, Liao J, Liu Y, Linde K. Adverse effects and concomitant symptoms associated with acupuncture treatment – A pilot study. Akupunktur. 1998;26:87–92. [Google Scholar]
- 108.List T, Helkimo M. Adverse events of acupuncture and occlusal splint therapy in the treatment of craniomandibular disorders. Cranio: J Craniomandib Pract. 1992;10(4):318–24. doi: 10.1080/08869634.1992.11677929. Discussion 24–6. [DOI] [PubMed] [Google Scholar]
- 109.Shih C-C, Lee H-H, Chen T-L, Tsai C-C, Lane H-L, Chiu W-T, et al. Reduced use of emergency care and hospitalization in patients with traumatic brain injury receiving acupuncture treatment. Evid-Based Compl Alt Med. 2013;2013:7. doi: 10.1155/2013/262039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shih C-C, Hsu Y-T, Wang H-H, Chen T-L, Tsai C-C, Lane H-L, et al. Decreased risk of stroke in patients with traumatic brain injury receiving acupuncture treatment: a population-based retrospective cohort study. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0089208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zollman FS, Larson EB, Wasek-Throm LK, Cyborski CM, Bode RK. Acupuncture for treatment of insomnia in patients with traumatic brain injury: a pilot intervention study. J Head Trauma Rehabil. 2012;27(2):135–42. doi: 10.1097/HTR.0b013e3182051397. [DOI] [PubMed] [Google Scholar]
- 112.McFadden KL, Healy KM, Dettmann ML, Kaye JT, Ito TA, Hernández TD. Acupressure as a non-pharmacological intervention for traumatic brain injury (TBI) J Neurotrauma. 2011;28(1):21–34. doi: 10.1089/neu.2010.1515. [DOI] [PubMed] [Google Scholar]
- 113.Hernández T, Palafox C, McFadden K, Ramsberger G, Rings J. Acupressure as a model for complementary and alternative medicine (CAM) treatment following acquired brain injury: translating lessons from the laboratory. Int J Phys Med Rehabil. 2015;3(269):2–00. [Google Scholar]
- 114.Gordon WA, Haddad L, Brown M, Hibbard MR, Sliwinski M. The sensitivity and specificity of self-reported symptoms in individuals with traumatic brain injury. Brain Inj. 2000;14(1):21–33. [PubMed] [Google Scholar]
- 115.Brown RP, Gerbarg PL, Muskin PR. How to use herbs, nutrients and yoga in mental health care. WW Norton & Company; 2009. [Google Scholar]
- 116.Schmid AA, Miller KK, Van Puymbroeck M, Schalk N. Feasibility and results of a case study of yoga to improve physical functioning in people with chronic traumatic brain injury. Disabil Rehabil. 2015;38(9):914–20. doi: 10.3109/09638288.2015.1062927. [DOI] [PubMed] [Google Scholar]
- 117.Gemmell C, Leathem JM. A study investigating the effects of Tai Chi Chuan: individuals with traumatic brain injury compared to controls. Brain Inj. 2006;20(2):151–6. doi: 10.1080/02699050500442998. [DOI] [PubMed] [Google Scholar]
- 118.Blake H, Batson M. Exercise intervention in brain injury: a pilot randomized study of Tai Chi Qigong. Clin Rehabil. 2009;23(7):589–98. doi: 10.1177/0269215508101736. [DOI] [PubMed] [Google Scholar]
- 119.Sipe WE, Eisendrath SJ. Mindfulness-based cognitive therapy: theory and practice. Can J Psychiat. 2012;57(2):63. doi: 10.1177/070674371205700202. [DOI] [PubMed] [Google Scholar]
- 120.Bedard M, Felteau M, Marshall S, Dubois S, Gibbons C, Klein R, et al. Mindfulness-based cognitive therapy: benefits in reducing depression following a traumatic brain injury. Adv Mind-body Med. 2011;26(1):14–20. [PubMed] [Google Scholar]
- 121.Bedard M, Felteau M, Mazmanian D, Fedyk K, Klein R, Richardson J, et al. Pilot evaluation of a mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries. Disabil Rehabil. 2003;25(13):722–31. doi: 10.1080/0963828031000090489. [DOI] [PubMed] [Google Scholar]
- 122.Bedard M, Felteau M, Gibbons C, Klein R, Mazmanian D, Fedyk K, et al. A mindfulness-based intervention to improve quality of life among individuals who sustained traumatic brain injuries: one-year follow-up. J Cognit Rehabil. 2005;23(1):8–13. doi: 10.1080/0963828031000090489. [DOI] [PubMed] [Google Scholar]
- 123.Bradt J, Magee WL, Dileo C, Wheeler BL, McGilloway E. Music therapy for acquired brain injury. The Cochrane Library. 2010;7(7):CD006787. doi: 10.1002/14651858.CD006787.pub2. [DOI] [PubMed] [Google Scholar]
- 124.Thaut M, Kenyon G, Hurt C, McIntosh G, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia. 2002;40(7):1073–81. doi: 10.1016/s0028-3932(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 125.Thaut M, Leins A, Rice R, Argstatter H, Kenyon G, McIntosh G, et al. Rhythmic auditory stimulation improves gait more than NDT/Bobath training in near-ambulatory patients early poststroke: a single-blind, randomized trial. Neurorehabil Neural Repair. 2007;21(5):455–9. doi: 10.1177/1545968307300523. [DOI] [PubMed] [Google Scholar]
- 126.Thaut MH, McIntosh G, Rice R. Rhythmic facilitation of gait training in hemiparetic stroke rehabilitation. J Neurol Sci. 1997;151(2):207–12. doi: 10.1016/s0022-510x(97)00146-9. [DOI] [PubMed] [Google Scholar]
- 127.Paul S, Ramsey D. The effects of electronic music-making as a therapeutic activity for improving upper extremity active range of motion. Occup Ther Int. 1998;5(3):223–37. [Google Scholar]
- 128.Jungblut M, Aldridge D. The music therapy intervention SIPARI with chronic aphasics – research findings (Translation) Neurol Rehabil. 2004;10(2):69–78. [Google Scholar]
- 129.Baker F. The effects of live, taped, and no music on people experiencing posttraumatic amnesia. J Music Ther. 2001;38(3):170–92. doi: 10.1093/jmt/38.3.170. [DOI] [PubMed] [Google Scholar]
- 130.Kim SJ, Koh I. The effects of music on pain perception of stroke patients during upper extremity joint exercises. J Music Ther. 2005;42(1):81–92. doi: 10.1093/jmt/42.1.81. [DOI] [PubMed] [Google Scholar]