Abstract
Several proteins and peptides of microbial origin are reported for their elicitor properties, which play a vital role in the development of local and systemic resistances in plants. In this study, the efficacy of total crude proteins (TCP) extracted from six different Trichoderma spp. (T. asperellum, T. harzianum, T. atroviride, T. virens, T. longibrachiatum, and T. brevicompactum) was evaluated for their ability to elicit defense responses in pearl millet against downy mildew disease. Priming of pearl millet seeds (with or without mannitol) with different concentrations of TCP from Trichoderma spp. does not affect the seed germination and seedling vigor significantly. Under greenhouse conditions, a varied level of disease protection was recorded with TCP of different Trichoderma spp., and furthermore, its efficacy was found increased when treated with mannitol. Total crude protein extracts of T. atroviride (75 µg/ml) with mannitol recorded significantly higher disease protection of 53.6% in comparison with respective controls. Furthermore, this observation was supported by elevated levels of peroxidase (7.7 U @ 36 h after inoculation) and lipoxygenase (29.5 U @ 48 h after inoculation) and hypersensitive necrotic spots (56% @ 24 h after inoculation). The present study illustrated the capability of TCP extracted from different Trichoderma spp. to elicit the disease resistance mechanism in pearl millet seedlings against Sclerospora graminicola.
Keywords: Sclerospora graminicola, Trichoderma spp., Total crude protein, Pearl millet, Seed priming
Introduction
Pearl millet [Pennisetum glaucum (L.) R. Br] is one of the most important millet crop grown and consumed in semi-arid regions of the world, including India. In India, pearl millet is grown on 7.95 m ha with a total production of ~8.9 million tonnes and productivity of 1106 kg/ha (ICRISAT 2014). Downy mildew of pearl millet caused by Sclerospora graminicola (Sacc.) J. Schröt. is an epidemic disease resulting in a considerable yield loss. Seed treatment with chemicals such as Metalaxyl (Apron 35WS) and RidomilMZ-72 (Metalaxyl + Mancozeb) is an effective measure of managing of downy mildew pathogen (Aliyu et al. 2011). Use of chemicals for plant disease management is proving to be unsustainable and hazardous to the environment (Myers et al. 2016). Development of downy mildew disease resistant cultivars of pearl millet is still under progress (Upadhyaya et al. 2016). Although the existing pearl millet hybrids give better grain yields than local open-pollinated cultivars, the genetically uniform single-cross hybrid cultivars are more vulnerable to epidemics of downy mildew disease. Hence, use of biocontrol agents for the management of seed-borne diseases is gaining more attention. In this regard, several microbes such as plant growth promoting rhizobacteria (PGPR), actinomycetes, endophytic bacteria, plant growth promoting fungi (PGPF), Trichoderma spp. have been successfully demonstrated to suppress S. graminicola infection in pearl millet (Niranjan Raj et al. 2003a, b, 2011; Chandrashekhara et al. 2007; Jogaiah et al. 2016).
Among the different biocontrol agents, Trichoderma were found to be the most promising agent as reviewed by Waghunde et al. (2016). The advantages of the use of Trichoderma spp. are that they are ubiquitous in nature, well-adaptable, plant symbionts which are reported to be beneficial for plant growth and soil health. Upon root colonization, Trichoderma spp. cause significant changes to the plant proteome and metabolome, resulting in enhancement of the crop productivity and ability to withstand abiotic and biotic stresses (Shoresh et al. 2010; Harman et al. 2004). Different mechanisms through which Trichoderma promote plant growth and suppress plant diseases have been studied, which includes production of plant hormones (Carvajal et al. 2009), mobilizing plant nutrients (Altomare et al. 1999), antibiotics (Vinale et al. 2008), mycoparasitism, cell wall-degrading enzymes (Reithner et al. 2011), and competition for nutrients, which is known as niche exclusion.
Systemic acquired resistance (SAR) and induced systemic resistance (ISR) are two types of induced resistance in plants. Elicitation of defense responses by the application of molecules derived from biocontrol agents that activate plant resistance against pathogens has emerged as an efficient and eco-friendly novel approach for managing crop diseases (Pel and Pieterse 2013). The appliances of such elicitors or resistance inducers at low amounts are not only efficient against a broad range of pathogens in different plants, but also improve crop yield without emphasizing selective pressures on pathogen populations (Katiyar et al. 2015).
In the process of plant disease development, an early interaction between host and pathogen determines the fate of disease. Host plants always try to resist microbial infection and proliferation by strengthening their structural barriers and biochemical defense mechanism. Biocontrol agents or elicitor molecules are well known to modify the host response towards the pathogen infection which further suppresses proliferation of pathogen, thereby restricting the disease establishment. Typical defense response includes reinforcement of cell wall by cross-linking of cell wall structural proteins and callose deposition, cell wall lignification, production and localized/systemic accumulation of pathogenesis-related (PR) proteins and phytoalexins, oxidative burst [reactive/nitrogen oxygen species (R/NOS)], and hypersensitive response (HR) (Agrios 2004). The whole process is underpinned by modification in several biochemical reactions within plant system.
Enhanced enzyme activity of peroxidase (POX) and lipoxygenase (LOX) during incompatible plant–pathogen interaction was reported in several host-pathogen systems. Being a member of PR proteins family, POX involved in strengthening the cell wall through cross-linking the phenolic monomers using hydrogen peroxide (H2O2) as an oxidant, cross-linking of cell wall proteins and subarization, etc. Furthermore, it creates a noxious environment by increasing the concentration of R/NOS leading to the HR, thereby restricting the pathogen establishment. In addition, POX are known for their activity of reactive oxygen species scavenging which generates under abiotic and biotic stresses, capable of damaging the cellular components (Scandalios 2005; Almagro et al. 2009). Lipoxygenase (LOX) are involved in the oxidation of polyunsaturated fatty acids to produce an unsaturated fatty acid hydroperoxide, thereby producing the substrate for further enzymatic reaction to produce oxylipins (Porta and Rocha-Sosa 2002). With respect to the disease protection, LOX involved in synthesis of signaling compounds (jasmonic acid) (Creelman and Mullet 1997) and development of hypersensitive reactions (Rustérucci et al. 1999).
Elicitors obtained from the cell wall of fungi are reported to elicit host plant resistance against a broad range of pathogens (Wiesel et al. 2014). A number of such inducers have been examined against downy mildew disease of pearl millet, including oligosaccharides, N-acetylchito oligosaccharides, β-aminobutyric acid, and 3,5-dichloroanthranilic acid (Sharathchandra et al. 2004; Nandini et al. 2013; Shailasree and Melvin 2015; Lavanya and Amruthesh 2016). Trichoderma, being one of the most studied biocontrol agents, suppress a wide range of phytopathogens including S. graminicola incitant of downy mildew disease in pearl millet. Trichoderma follow a wide range of mechanisms, while imparting resistance in host plant among which involvement of protein/peptides is recently reported (Vinale et al. 2008). The present study was aimed to evaluate the efficacy of the total crude protein (TCP) extracted from Trichoderma spp. as elicitor against downy mildew disease of pearl millet. Furthermore, we studied nature of resistance offered by the crude protein elicitors.
Materials and methods
Seed source and inoculum
Seeds of pearl millet cv. 7042S, highly susceptible to downy mildew disease, were obtained from the International Crop Research Institute in Semi-arid Tropics (ICRISAT), Patencheru, India and used throughout the experiment. The collected susceptible seed samples were surface sterilized with 0.2% sodium hypochlorite for 1 min and rinsed in sterile distilled water (SDW) for 2–3 times.
A sick plot of downy mildew pathogen, Sclerospora graminicola, is maintained at the Department of Studies in Biotechnology, University of Mysore, Mysuru (N 24º18′, E 79º 26′, 903 m altitude) since the last 30 years under the ICAR—All India Co-ordinated Pearl Millet Improvement Project (AICPMIP) program. The inoculum for the experiment was collected from field-grown-infected plants showing typical symptoms. The leaves showing profuse sporulation on the abaxial surface were selected and collected during late evenings. The leaves from the infected plants were washed with running water to remove debris and existing sporangia, blot-dried and incubated overnight in a moist chamber at 20 °C under 80% relative humidity (RH) in dark conditions. Fresh sporangia formed on the leaves were harvested using a sterile brush in distilled water and spore concentration was adjusted to 4 × 104 per ml using hemocytometer and used as inoculum for further studies (Safeeulla 1976).
Extraction of total crude protein (TCP) from Trichoderma
Six Trichoderma spp., viz T. asperellum, T. harzianum, T. atroviride, T. virens, T. longibrachiatum, and T. brevicompactum, which were already tested and found significant in suppressing downy mildew disease in pearl millet (data not shown) were obtained from department stock cultures. The selected Trichoderma spp. were mass-cultivated on potato dextrose broth for 12–14 days at 28 ± 2 °C. Towards the end of the incubation period, mycelia were harvested, washed in SDW and blot-dried. The mycelial mat was crushed in sterilized, pre-chilled pestle and mortar into a fine powder using liquid nitrogen. Extraction of TCP was done by following the phenolic extraction method as described by Hurkman and Tanaka (1986) with slight modifications (Anup et al. 2015). Quantification of protein content was done following Bradford method (Bradford 1976) using BSA as standard and stored at −80 °C.
Effect of seed priming with TCP extracted from Trichoderma spp. on pearl millet seed quality parameters
Sterilized seeds were coated uniformly with extracted crude proteins in different concentrations 25, 50, 75, and 100 μg/ml alone and also with the same concentration of crude protein with 1% mannitol as a priming agent for 12 h at room temperature on a shaker at 150 rpm separately. The seeds treated with sterile distilled water (SDW) and 1% mannitol served as controls. Germination test was done by the paper towel method according to ISTA (2005). Seedling vigor was analyzed as per the method of Abdul Baki and Anderson (1973). The vigor index (VI) was calculated using the formula:
Evaluation of TCP extracted from Trichoderma spp. against S. graminicola infection in pearl millet
Crude protein-treated and control seeds were germinated in petriplates lined with wet blotter sheets (25 seeds/plate) for 3 days. Three-day-old seedlings were root-dip inoculated with a zoospore suspension of 4 × 104 ml−1 and incubated in the dark at 25 ± 1 °C for 24 h (Safeeulla 1976). Pearl millet seedlings were observed under a stereobinocular microscope for HR response which was visualized in the form of light–dark brown and black necrotic spots or streaks on coleoptile and root regions up to 24 h of incubation at regular intervals of 2 h. A number of seedlings showing HR reaction during the experimental time were recorded and the percentage was calculated. The experiment was repeated three times with 100 seedlings of four replications for each experiment:
Lignification in the cell wall of control and treated seedlings were visualized as explained by Sherwood and Vance (1976). The epidermal peelings of seedlings were collected and placed in 2% phloroglucinol in 95% ethanol for 2 h. The peelings were then placed on a slide with a drop of 35% HCl and heated over a low flame until the veins turned reddish purple. The slides were then observed under a microscope for the intensity of coloration and the percentage of lignified cells was counted in ten randomly selected microscopic fields and the average was tabulated. The experiment was repeated thrice consisting of three replicates of ten seedlings in each treatment.
Hydrogen peroxide (H2O2) deposition was studied by following the method of Thordal-Christensen et al. (1997). Epidermal peelings of treated and control seedlings were placed in 3,3-diamino benzidine (DAB) solution at 1 mg/ml, pH 3.8 for 8 h under white light at 25 °C. The samples were placed in 96% ethanol and boiled for 10 min. After cooling, the samples were kept in fresh ethanol at room temperature for 4 h before being photographed. The peelings were then observed under a microscope for H2O2 staining. The cells with brown color staining deposition were counted in randomly selected ten microscopic fields and the percentage was tabulated. The experiment was repeated thrice consisting of three replicates of ten seedlings.
Modulation in defense enzyme activities after priming pearl millet seeds with TCP extracts of Trichoderma spp
Crude protein-treated and control seedlings were raised and challenge-inoculated with pathogen as explained above. Seedlings were harvested at different time intervals after pathogen inoculation, ground to a fine powder in liquid nitrogen, and used for enzyme extraction. The protein content of the extract was estimated by the standard method (Bradford 1976) with bovine serum albumin (Sigma, USA) as standard.
Peroxidase (POX) (EC 1.11.1.7) assay
For POX assay, 1 g of seedlings ground in liquid nitrogen was extracted with 10 mM potassium phosphate buffer (pH 6.9) at 4 °C and supernatant was used as the enzyme source. Enzyme assay was done by following the procedure of Hammerschmidt et al. (1982). The reaction mixture (3 ml) consisted of 0.25% (v/v) guaiacol in 10 mM potassium phosphate buffer (pH 6.9) containing 10 mM hydrogen peroxide. The reaction was initiated by the addition of 5 µl of crude enzyme extract. Formation of tetraguaiacol was measured as increase in absorbance at 470 nm spectrophotometrically (Hitachi U-3900, Japan). One unit of enzyme activity was represented as change in OD at 470 nm min−1 mg−1 protein. The experiment was performed thrice and enzyme activity was tabulated.
Lipoxygenase (LOX) (EC 1.13.11.12) assay
Lipoxygenase (LOX) activity was measured by the procedure of Borthakur et al. (1987). The supernatant of 0.5 g seedling extracts with 5 ml of 0.2 M sodium phosphate buffer (pH 6.5) was used as the enzyme source. The substrate, linoleic acid (10 mM) was prepared according to the method of Axelrod et al. (1981). The activity was determined spectrophotometrically by monitoring the appearance of the conjugated diene hydroperoxide at 234 nm.
The reaction mixture contained 2.7 ml of sodium phosphate buffer (0.2 M, pH 6.5) and 0.3 ml of substrate. The reaction was initiated by adding the enzyme extract (100 µl) and change in absorbance at 234 nm was recorded for 3 min using Hitachi U-3900 spectrophotometer. One unit of enzyme activity was expressed as change in OD at 234 nm min−1 mg−1 protein. The experiment was performed thrice and enzyme activity was tabulated.
Greenhouse studies
Seeds were primed with TCP of Trichoderma spp. as explained earlier. Treated and control seeds were sown in earthen pots containing sterile potting mixture (2:1:1, soil:sand:farm yard manure) and maintained under greenhouse condition (90–95% RH, 20–25 °C temperature). Two-day-old seedlings were challenge-inoculated with zoospore suspension of S. graminicola at a concentration of 4 × 104 zoospores ml−1 (Singh and Gopinath 1985). All the pots were arranged in randomized block design and maintained under greenhouse conditions. Seedlings were regularly observed for the typical disease symptoms such as chlorosis, sporulation on the abaxial leaf surface, and stunted growth. Disease incidence was recorded at 15, 30, and 45 days after challenge inoculation.
Statistical analysis
The data obtained from laboratory and greenhouse experiments were analyzed separately, and percentage data were ARCSINE transformed and subjected to analysis of variance (ANOVA) using SPSS Inc. version 17.0. The significant differences between the treatment means were compared using the highest significant difference (HSD) as obtained by Tukey test at P ≤ 0.05 levels.
Results
Effect of seed priming with Trichoderma protein extracts on pearl millet seed quality parameters
Seed priming with crude protein extracts of Trichoderma spp. at four concentrations was found to be not significant (P ≤ 0.05) in increasing or decreasing the growth parameters such as root length, shoot length, seed germination, and seedling vigor when compared to their respective controls (Table 1). However, when TCP was applied with 1% mannitol, a significant (P ≤ 0.05) increase in % seed germination was recorded in comparison with the control, 1% mannitol and metalaxyl treatment. However, variation observed in seedling vigor was not significant (P ≤ 0.05) among the different treatments (Table 1).
Table 1.
Effect of seed priming with TCP of different Trichoderma spp. on seed germination and seedling vigor of pearl millet
| Treatment | Conc. µg/ml | T. asperellum | T. harzianum | T. virens | T. longibrachiatum | T. atroviride | T. brevicompactum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % G | SV | % G | SV | % G | SV | % G | SV | % G | SV | % G | SV | ||
| Crude protein | 25 | 89 ± 1.46a | 1566 ± 13.94a | 89 ± 1.46a | 1566 ± 43.94a | 89 ± 1.27ab | 1569 ± 75.17a | 89 ± 1.89a | 1570 ± 40.59a | 89 ± 1.27ab | 1584 ± 13.73a | 89 ± 1.27ab | 1584 ± 70.35a |
| 50 | 89 ± 1.46a | 1577 ± 93.84a | 89 ± 1.46a | 1577 ± 93.84a | 89 ± 2.25ab | 1614 ± 24.42a | 89 ± 1.27a | 1569 ± 47.21a | 89 ± 2.25ab | 1634 ± 41.29a | 89 ± 2.54ab | 1569 ± 60.92a | |
| 75 | 89 ± 2.15a | 1603 ± 59.80a | 89 ± 2.15a | 1603 ± 59.80a | 90 ± 1.33ab | 1622 ± 58.15a | 89 ± 1.46a | 1590 ± 19.85a | 90 ± 1.33ab | 1653 ± 29.13a | 90 ± 0.75ab | 1590 ± 53.71a | |
| 100 | 90 ± 3.27a | 1597 ± 58.23a | 90 ± 2.76a | 1605 ± 34.72a | 91 ± 0.83ab | 1614 ± 77.66a | 90 ± 1.98a | 1590 ± 13.16a | 91 ± 0.83ab | 1644 ± 8.30a | 91 ± 1.61ab | 1584 ± 37.84a | |
| Crude protein +1% M | 25 | 89 ± 1.89a | 1616 ± 25.31a | 89 ± 1.89a | 1616 ± 25.31a | 91 ± 2.07ab | 1684 ± 59.83a | 90 ± 2.36a | 1651 ± 43.06a | 91 ± 2.07ab | 1642 ± 77.63a | 90 ± 1.33ab | 1648 ± 106.24a |
| 50 | 90 ± 1.33a | 1637 ± 67.30a | 90 ± 1.33a | 1637 ± 67.30a | 91 ± 1.61ab | 1645 ± 37.02a | 91 ± 1.39a | 1686 ± 63.27a | 90 ± 0.75ab | 1696 ± 42.78a | 90 ± 0.78ab | 1655 ± 127.11a | |
| 75 | 91 ± 0.83a | 1684 ± 58.56a | 91 ± 0.83a | 1684 ± 60.18a | 92 ± 0.83ab | 1648 ± 46.85a | 91 ± 1.39a | 1681 ± 67.17a | 92 ± 0.83a | 1716 ± 54.92a | 92 ± 0.40a | 1665 ± 54.89a | |
| 100 | 91 ± 0.83a | 1668 ± 62.59a | 91 ± 1.39a | 1668 ± 56.12a | 92 ± 1.48a | 1627 ± 91.99a | 90 ± 1.98a | 1678 ± 96.61a | 92 ± 1.48a | 1709 ± 109.05a | 91 ± 1.39ab | 1636 ± 127.33a | |
| Control | – | 89 ± 0.72a | 1560 ± 24.57a | 89 ± 0.72a | 1560 ± 24.57a | 89 ± 0.72c | 1560 ± 24.57a | 89 ± 0.72a | 1560 ± 24.57a | 89 ± 0.72c | 1560 ± 24.57a | 89 ± 0.72c | 1560 ± 24.57a |
| 1% M | – | 91 ± 1.39a | 1644 ± 62.74a | 91 ± 1.39a | 1644 ± 62.74a | 91 ± 1.39ab | 1644 ± 62.74a | 91 ± 1.39a | 1644 ± 62.74a | 91 ± 1.39ab | 1644 ± 62.74a | 91 ± 1.39ab | 1644 ± 62.74a |
| Metalaxyl* | – | 89 ± 1.89a | 1547 ± 54.09a | 89 ± 1.89a | 1547 ± 54.09a | 89 ± 1.89c | 1547 ± 54.09a | 89 ± 1.89a | 1547 ± 54.09a | 89 ± 1.89c | 1547 ± 54.09a | 89 ± 1.89c | 1547 ± 54.09a |
% Values were arcsine transformed before subjecting to the statistical analysis. Values are the mean within column sharing the same letters that are not significantly different according to Tukey’s HSD at P ≤ 0.05
% G percent germination, SV seedling vigor, M Mannitol
* Metalaxyl was used as seed dressing at the rate of 6 g/kg seeds
Evaluation of TCP of Trichoderma spp. against S. graminicola infection in pearl millet
Effect of TCP extracted from Trichoderma spp. was evaluated for the expression of HR response, i.e., brown necrotic spots/streaks in pearl millet-treated seedlings upon challenge inoculation with S. graminicola. Seed treatment with mannitol alone did not record significant (P ≤ 0.05) increase in number of HR in comparison with respective controls (Fig. 1). Crude proteins of all Trichoderma with or without mannitol recorded significantly (P ≤ 0.05) increased HR response to S. graminicola infection in comparison with control. In all the treatments, the efficiency of crude protein was found enhanced when they were treated with mannitol. Among the six crude protein extracts tested with/without mannitol, T. atroviride + mannitol recorded highest of 56% of HR followed by T. virens + mannitol (39%) and T. harzianum + mannitol (35%) at 24 h after pathogen inoculation (h.a.i) (Fig. 1).
Fig. 1.
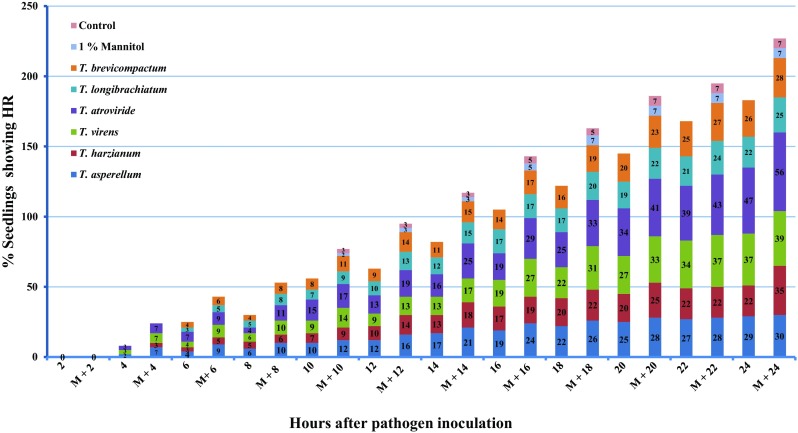
Hypersensitive reaction studies of TCP treatments of Trichoderma spp. at a concentration of 75 µg/ml in different time intervals. Values inside the bars indicate the percent seedlings showing hypersensitive reaction. Mean value of three replicates was represented in the graph. On the X axis, values indicate time interval in hours after pathogen inoculation, two modes of treatments were done, i.e., crude protein treatments without mannitol (2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h of time intervals) and crude protein treatments with 1% mannitol (M + 2, M + 4, M + 6, M + 8, M + 10, M + 12, M + 14, M + 16, M + 18, M + 20, M + 22 and M + 24 hours of time intervals)
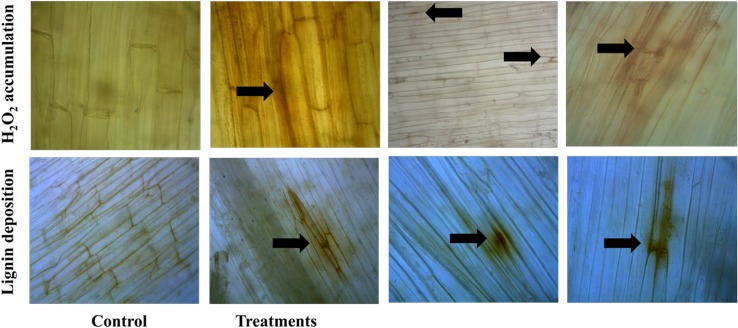
A constitutive level of lignification was observed in both inoculated and uninoculated seedlings in the coleoptiles and root regions. However, TCP of T. atroviride + mannitol-treated seedlings had shown lignification with higher intensity (21%) and H2O2 accumulation of 17% as early as 8 h postinoculation. Whereas, in the case of control and mannitol treatment only, 2% H2O2 and 4% lignin deposition were recorded at 24 h postinoculation (Fig. 2).
Fig. 2.
Localized accumulation of H2O2 and lignin deposition (indicated as arrow marks) as observed in TCP treatments of T. atroviride at a concentration of 75 µg/ml under compound microscope after staining in control and treated seedlings
Modulation in defense enzyme activities after priming pearl millet seeds with TCP extract of Trichoderma atroviride
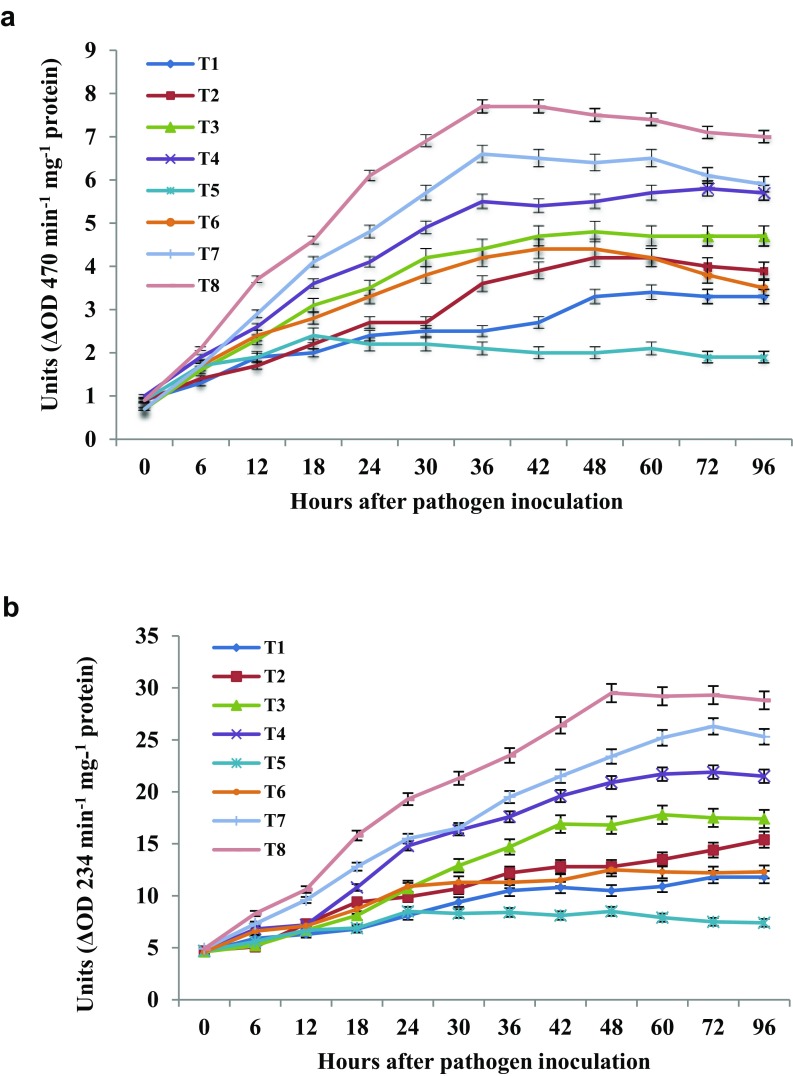
The temporal modulations of upregulation/downregulation of the enzyme activities of the TCP extract of T. atroviride-treated and untreated seedlings with or without pathogen inoculation were examined. Varying patterns of modulation in enzyme activity was observed in seedlings receiving different treatments. Control treatments such as SDW and 1% mannitol treatments showed least enzyme activities at all time intervals tested (Fig. 3a, b). Further upon pathogen inoculation control and only mannitol-treated seedlings not showed any significant (P ≤ 0.05) difference in both POX and LOX activities.
Fig. 3.
Temporal pattern of accumulation of peroxidase (a) and lipoxygenase (b) enzyme in pearl millet seedlings upon seed priming with T. atroviride TCP with/without mannitol. Values are means of three experiments. Lines on the bars indicate the standard error. T1 control, T2 1% mannitol treatment, T3 T. atroviride crude protein treatment, T4 T. atroviride crude protein treatment with 1% mannitol, T5 control + pathogen, T6 1% mannitol treatment + pathogen, T7 T. atroviride crude protein treatment + pathogen, and T8 T. atroviride crude protein treatment with 1% mannitol + pathogen
In the POX assay, significant (P ≤ 0.05) higher activity of 7.7 units was observed in TCP of T. atroviride + mannitol-treated seedlings at 36 h.a.i. Even though the decline in POX activity was observed in treated seedlings at 96 h.a.i, but still, the enzyme activity was significantly (P ≤ 0.05) higher than uninoculated seedlings, which recorded maximum activity at 96 h.a.i (3.3 units in control and 3.9 units in mannitol treatment). Upregulation of POX activity was evident in all treated seedlings, on an average three-fold increase over control seedlings (Fig. 3a).
A similar trend was observed in the case of LOX activity. The time course study of LOX indicated that the initial activity did not differ between the different treatments at 0 h.a.i. However, with the increase incubation time, variation in enzyme activity was observed in TCP + mannitol-treated inoculated seedlings, in which maximum activity was recorded at 48 h.a.i with 29.5 U. Whereas in the case of control uninoculated seedlings shows only marginal changes at all time intervals (Fig. 3b). However, in control-inoculated seedlings, the highest activity was noticed at 24 h.a.i (8.5 U) and it is not significantly (P ≤ 0.05) different from control uninoculated seedlings.
Disease protection studies
Among the six different Trichoderma spp. TCP treatments, significant (P ≤ 0.05) protection of 53.6% was observed in the seedlings treated with T. atroviride TCP (75 μg/ml) with 1% mannitol followed by TCP of T. virens + mannitol (47.6%). Crude protein treatments T. longibrachiatum had shown least disease protection, which is not significantly (P ≤ 0.05) different from the control treatments. However, metalaxyl positive control treatment had recorded significantly (P ≤ 0.05) highest disease protection of 90.4% with least disease incidence compared to all other treatments (Table 2).
Table 2.
TCP extracts of Trichoderma spp.-mediated downy mildew disease protection studies under greenhouse conditions
| Treatment | Conc. µg/ml | DM disease protection (%) | |||||
|---|---|---|---|---|---|---|---|
| T. asperellum | T. harzianum | T. virens | T. longibrachiatum | T. atroviride | T. brevicompactum | ||
| Crude protein | 25 | 22.53 ± 1.78lm | 19.60 ± 2.96lm | 21.23 ± 1.37lm | 17.30 ± 2.88m | 26.10 ± 1.36klm | 22.90 ± 1.03klm |
| 50 | 28.10 ± 2.65klm | 24.77 ± 1.78klm | 34.93 ± 2.75ijk | 28.10 ± 2.65klm | 30.43 ± 1.36jkl | 26.47 ± 2.30klm | |
| 75 | 40.30 ± 3.22efg | 41.57 ± 3.64def | 43.13 ± 2.24bcd | 30.07 ± 1.55jkl | 42.87 ± 2.48bcd | 30.07 ± 1.55jkl | |
| 100 | 39.40 ± 1.84fgh | 39.20 ± 2.04fgh | 38.90 ± 1.81fgh | 25.93 ± 2.51klm | 40.00 ± 2.74fgh | 28.83 ± 1.22klm | |
| Crude protein + mannitol (1%) | 25 | 26.47 ± 2.30klm | 21.10 ± 1.05lm | 28.10 ± 2.65klm | 25.93 ± 2.51klm | 23.27 ± 2.11klm | 20.50 ± 2.03lm |
| 50 | 39.90 ± 2.20fgh | 26.47 ± 2.30klm | 38.90 ± 1.81fgh | 34.93 ± 2.75ijk | 42.33 ± 1.06cde | 22.13 ± 0.38lm | |
| 75 | 43.07 ± 1.06bcd | 44.77 ± 5.76bcd | 47.60 ± 1.15bc | 37.30 ± 1.38ghi | 53.63 ± 1.76b | 38.90 ± 1.81fgh | |
| 100 | 40.30 ± 3.22efg | 40.30 ± 3.22efg | 38.90 ± 1.81fgh | 28.83 ± 1.22klm | 46.50 ± 0.49bcd | 36.03 ± 1.85hij | |
| Control | – | –n | |||||
| Mannitol (1%) | – | 20.60 ± 1.45lm | |||||
| Metalaxyl* | – | 90.47 ± 1.66a | |||||
% Values were arcsine transformed before subjecting to the statistical analysis
Values are the mean within column sharing the same letters that are not significantly different according to Tukey’s HSD at P ≤ 0.05
* Metalaxyl was used as seed dressing at the rate of 6 g/kg seeds
Discussion
Species of Trichoderma are known to induce systemic resistance against various types of plant disease as the whole organism. Several attempts are made to isolate specific elicitor molecule which is involved in eliciting host resistance against pathogen infection (Djonovic et al. 2006; Mukherjee et al. 2012). Electrolyte leakage, oxidative burst, production of phytoalexins and PR proteins and increased biosynthesis of ethylene have been reported in plant tissues treated with non-specific elicitors (Peever and Higgins 1989) and specific elicitors (Hammond-Kosack et al. 1996). Protein elicitors of various classes have been reported from several species of Phytophthora. Glycoproteins of molecular mass 42 and 32 kDa secreted by Phytophthora sojae and Phytophthora megasperma have been described to induce defense reactions in the non-host plants parsley and tobacco, respectively (Baillieul et al. 1995). In the present study, we studied the possible elicitor properties of TCP of six Trichoderma spp. against downy mildew pathogen of pearl millet. Upon seed treatment, these TCP were not affected the seed quality variables. Treatment with TCP with mannitol was found significant in improving seed germination but not seedling vigor. The experiments indicated that the TCP was not containing any molecule which directly stimulates the plant growth or the concentration of such molecule in crude extract was not sufficient to improve the early plant growth. Further use of mannitol as osmopriming agent may enhance the root length, shoot length, and seedling vigor. Similar observation was reported by Afzal et al. (2011), where they observed that the treatment of mannitol (2%) to marigold (Tagetes spp.) seeds significantly enhanced the seedling vigor with biochemical changes.
Salas-Marina et al. (2015) reported that secretary protein Epl1 and Sm1 from T. atroviride and T. virens were capable of inducing SAR and ISR in tomato against various fungal and bacterial pathogens. Furthermore, overexpression of Ep11 and Sm1 in these strains enhanced their capability to induce disease resistance in tomato. A small cysteine-rich protein fraction from the biocontrol Fusarium oxysporum strain CS-20 control fusarium wilt symptoms by stimulating the defense responses in tomato (Shcherbakova et al. 2016). In this study, seed treatment with TCP of Trichoderma demonstrates the localized deposition of lignin and accumulation of H2O2 during the initial period of infection in pearl millet seedlings. H2O2 is closely associated with lignification of plant cell wall and also accumulates at the site of infection which is related to the lignification process leading to disease resistance in host plant (Olson and Varner 1993; Taiz and Zeiger 2006). Sm1 elicitor from T. virens stimulates hydrogen peroxide production and induces defense genes expression in cotton cotyledons to enhance the resistance against foliar pathogen Colletotrichum spp. (Djonovic et al. 2006). Furthermore, Hückelhoven et al. (1999) correlated the inability of powdery mildew pathogen to cause infection in barley seedlings with strengthened papillae due to the action of H2O2-mediated cross-linking reactions. Similarly, in this study, the highest lignin deposition and H2O2 accumulation were observed in seedlings raised from seeds treated with TCP of T. atroviride and it was in corroboration with increased disease protection. Enhanced activity of POX and LOX is considered as one of the biochemical markers to determine the degree of resistance imparted by biocontrol agent against phytopathogens. Similarly, in our experiment, we found that seedling treated with TCP of T. atroviride with mannitol recorded the significantly (P ≤ 0.05) higher activity of both the enzymes and was well correlated with its ability to suppress S. graminicola infection.
Conclusion
The present study demonstrates the ability of the Trichoderma protein elicitors in reducing the downy mildew disease incidence in pearl millet. It is also evident by the biochemical defense activation in defending against the pathogen. It seems that TCP mediated disease protection follows both localized and systemic resistance inductions. In this regard, identification and characterization of specific protein elicitor are required to improve the disease protection efficacy.
Compliance with ethical standards
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdul Baki AA, Anderson JD. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. doi: 10.2135/cropsci1973.0011183X001300060013x. [DOI] [Google Scholar]
- Afzal I, Ashraf S, Qasim M, Basra SMA, Shahid M, Hussain B. Mannitol priming induces biochemical changes and enhances germination capacity and seedling vigor in marigold (Tagetes spp.) Acta Hortic. 2011;898:25–29. doi: 10.17660/ActaHortic.2011.898.1. [DOI] [Google Scholar]
- Agrios GN. Plant pathology. 5. San Diego: Academic Press; 2004. [Google Scholar]
- Aliyu B, Hati SS, Dimari GA, Donli PO. Comparative assessment of metalaxyl enhanced protection of pearl millet varieties in the control of downy mildew. J Cereals Oilseeds. 2011;2:26–31. [Google Scholar]
- Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. J Exp Bot. 2009;60(2):377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- Altomare C, Norvell WA, Bjorkman T, Harman GE. Solubilization of phosphates and micronutrients by the plant growth promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol. 1999;65:2926–2933. doi: 10.1128/aem.65.7.2926-2933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anup CP, Melvin M, Shilpa N, Gandhi MN, Jadhav M, Ali H, Kini KR. Proteomic analysis of elicitation of downy mildew disease resistance in pearl millet by seed priming with β-aminobutyric acid and Pseudomonas fluorescens. J Proteomics. 2015;120:58–74. doi: 10.1016/j.jprot.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Axelrod B, Cheesbrough TM, Laakso S. Lipoxygenase from soybeans. Methods Enzymol. 1981;71:441–451. doi: 10.1016/0076-6879(81)71055-3. [DOI] [Google Scholar]
- Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S. A new elicitor of the hypersensitive response in tobacco: a fungal glycoprotein elicits cell death, expression of defense genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995;8:551–560. doi: 10.1046/j.1365-313X.1995.8040551.x. [DOI] [PubMed] [Google Scholar]
- Borthakur AB, Bhat G, Ramadoss CS. The positional specifications of the oxygenation of linolenic acid catalyzed by two forms of lipoxygenase isolated from Bengal gram (Cicer arietinum) J Biosci. 1987;11:257–263. doi: 10.1007/BF02704676. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carvajal LH, Orduz S, Bissett J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol Control. 2009;51(3):409–416. doi: 10.1016/j.biocontrol.2009.07.018. [DOI] [Google Scholar]
- Chandrashekhara Niranjanraj S, Deepak SA, Amruthesh KN, Shetty NP, Shetty HS. Endophytic bacteria from different plant origin enhance growth and induce downy mildew resistance in pearl millet. Asian J Plant Pathol. 2007;1:1–11. doi: 10.3923/ajppaj.2007.1.11. [DOI] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant Microb Interact. 2006;19:838–853. doi: 10.1094/MPMI-19-0838. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt R, Nuckles EM, Kuc J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Mol Plant Pathol. 1982;20:73–82. doi: 10.1016/0048-4059(82)90025-X. [DOI] [Google Scholar]
- Hammond-Kosack KE, Silverman P, Raskin I, Jones JDG. Race-specific elicitors of Cladosporium fulvum induce changes in cell morphology and the synthesis of ethylene and salicylic acid in tomato plants carrying the corresponding Cf disease resistance gene. Plant Physiol. 1996;110:1381–1394. doi: 10.1104/pp.110.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species-opportunistic a virulent plant symbionts. Nat Rev Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- Hückelhoven R, Fodor J, Preis C, Kogel KH. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol. 1999;119:1251–1260. doi: 10.1104/pp.119.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman WJ, Tanaka CK. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986;8:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) (2014) Proceedings of the consultants’ group meetings on downy mildew and ergot of pearl millet. In: Williams RJ (ed) Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics
- ISTA (2005) International Seed Testing Association. Proceedings of the International Seed Testing Association. International Rules of Seed Testing. Seed Sci Technol 15:1–9
- Jogaiah S, Kurjogi M, Govind SR, Shetty HS, Basappa VA, Tran LSP. Isolation and evaluation of proteolytic actinomycete isolates as novel inducers of pearl millet downy mildew disease protection. Sci Rep. 2016;6:1–13. doi: 10.1038/srep30789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar D, Hemantaranjan A, Singh B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: a review. Ind J Plant Physiol. 2015;20:1–9. doi: 10.1007/s40502-015-0139-6. [DOI] [Google Scholar]
- Lavanya SN, Amruthesh KN. 3, 5-Dichloroanthranilic acid (DCA)—an elicitor induces systemic resistance against downy mildew in pearl millet. Int J Life Sci. 2016;4:97–106. [Google Scholar]
- Mukherjee PK, Buensanteai N, Moran-Diez ME, Druzhinina IS, Kenerley CM. Functional analysis of non-ribosomal peptide synthetases (NRPSs) in Trichoderma virens reveals a polyketide synthase (PKS)/NRPS hybrid enzyme involved in induced systemic resistance response in maize. Microbiology. 2012;158:155–165. doi: 10.1099/mic.0.052159-0. [DOI] [PubMed] [Google Scholar]
- Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, Saal FSV, Welshons WV, Benbrook CM. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health. 2016;15:19. doi: 10.1186/s12940-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandini B, Hariprasad P, Niranjana SR, Shetty HS, Geetha NP. Elicitaion of resistance in pearl millet by oligosaccharides of Trichoderma spp. against downy mildew disease. J Plant Inter. 2013;8:45–55. [Google Scholar]
- Niranjan Raj S, Chaluvaraju G, Amruthesh KN, Shetty HS, Reddy MS, Kloepper JW. Induction of growth promotion and resistance against downy mildew on pearl millet (Pennisetum glaucum) by rhizobacteria. Plant Dis. 2003;87:340–345. doi: 10.1094/PDIS.2003.87.4.380. [DOI] [PubMed] [Google Scholar]
- Niranjan Raj S, Chaluvaraju G, Amruthesh KN, Shetty HS. Induction of growth promotion and resistance against downy mildew on pearl millet (Pennisetum glaucum) by rhizobacteria. Plant Dis. 2003;87:380–384. doi: 10.1094/PDIS.2003.87.4.380. [DOI] [PubMed] [Google Scholar]
- Niranjan Raj S, Lavanya SN, Amruthesh KN, Shetty HS. Comparative evaluation of Pseudomonas fluorescens and their lipopolysaccharides as implicated in induction of resistance against pearl millet downy mildew. Arch Phytopath Plant Protect. 2011;44(13):1285–1299. doi: 10.1080/03235408.2010.493750. [DOI] [Google Scholar]
- Olson P, Varner J. Hydrogen peroxide and lignification. Plant J. 1993;4:887–892. doi: 10.1046/j.1365-313X.1993.04050887.x. [DOI] [Google Scholar]
- Peever TL, Higgins VJ. Electrolyte leakage, lipoxygenase and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989;90:867–875. doi: 10.1104/pp.90.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pel MJC, Pieterse CMJ. Microbial recognition and evasion of host immunity. J Expt Bot. 2013;64:1237–1248. doi: 10.1093/jxb/ers262. [DOI] [PubMed] [Google Scholar]
- Porta H, Rocha-Sosa M. Plant lipoxygenases: physiological and molecular features. Plant Physiol. 2002;130:15–21. doi: 10.1104/pp.010787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithner B, Ibarra-Laclette E, Mach RL, Herrera-Estrella A. Identification of mycoparasitism-related genes in Trichoderma atroviride. Appl Environ Microbiol. 2011;77:4361–4370. doi: 10.1128/AEM.00129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustérucci C, Montillet JL, Agnel JP, Battesti C, Alonso B, Knoll A, Bessoule JJ, Etienne P, Suty L, Blein JP, Triantaphylidès C. Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein of tobacco leaves. J Biol Chem. 1999;274:36446–36455. doi: 10.1074/jbc.274.51.36446. [DOI] [PubMed] [Google Scholar]
- Safeeulla KM. Biology and control of the downy mildew of pearl millet, sorghum and finger millet. Mysore: Wesley Press; 1976. [Google Scholar]
- Salas-Marina MA, Isordia-Jasso M, Islas-Osuna MA, Delgado-Sánchez P, Jiménez-Bremont JF, Rodríguez-Kessler M, Rosales-Saavedra MT, Herrera-Estrella A, Casas-Flores S. The Epl1and Sm1proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front Plant Sci. 2015;6:77. doi: 10.3389/fpls.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- Shailasree S, Melvin P. β-amino butyric acid–resistance inducing agent in pearl millet. J Plant BiochemPhysiol. 2015;2:144. [Google Scholar]
- Sharathchandra RG, Niranjan Raj S, Shetty NP, Amruthesh KN, Shetty HS. A chitosan formulation Elexa induces downy mildew disease resistance and growth promotion in pearl millet. Crop Prot. 2004;23:881–888. doi: 10.1016/j.cropro.2003.12.008. [DOI] [Google Scholar]
- Shcherbakova LA, Odintsova TI, Stakheev AA, Fravel DR, Zavriev SK. Identification of a novel small cysteine-rich protein in the fraction from the biocontrol Fusarium oxysporum strain CS-20 that mitigates Fusarium wilt symptoms and triggers defense responses in tomato. Front Plant Sci. 2016;6:1–15. doi: 10.3389/fpls.2015.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RT, Vance CP. Histochemsitry of papillae formed in reed canary grass leaves in response to infecting pathogenic fungi. Phytopathol. 1976;66:503–510. doi: 10.1094/Phyto-66-503. [DOI] [Google Scholar]
- Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plantresponses to fungal biocontrol agents. Annu Rev Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- Singh SD, Gopinath R. A seedling inoculation technique for detecting downy mildew resistance in pearl millet. Plant Dis. 1985;72:425–428. doi: 10.1094/PD-72-0425. [DOI] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. Massachusetts: Sinauer Associates Inc. Publishers; 2006. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Upadhyaya HD, Reddy KN, Irshad Ahmed M, Ramachandran S, Kumar Vinod, Singh S. Characterization and genetic potential of African pearl millet named landraces conserved at the ICRISAT genebank. Plant Genet Res. 2016;1:1–15. [Google Scholar]
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Barbetti MJ, Li H, Woo SL, Lorito M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol. 2008;72:80–86. doi: 10.1016/j.pmpp.2008.05.005. [DOI] [Google Scholar]
- Waghunde RR, Shelake RM, Sabalpara AN. Trichoderma: a significant fungus for agriculture and environment. Afr J Agric Res. 2016;11:1952–1965. [Google Scholar]
- Wiesel L, Newton AC, Elliott I, Booty D, Gilroy EM, Birch PRJ, Hein I. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front Plant Sci. 2014;5:1–13. doi: 10.3389/fpls.2014.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]