Abstract
Increased drug resistance in Candida glabrata (a model non-albicans Candida) calls for the identification of potential molecular targets for the development of effective drugs. Hypoxia (a state of low oxygen) is an important host factor, which affects the virulence of the pathogen and efficacy of drugs. In the present study, in vitro characterization of 13 null mutants of C. glabrata were done under hypoxic condition (1% O2). These mutants have a major role to play in cellular pathways, viability and pathogenesis (cell wall biosynthesis, ergosterol synthesis, calcium–calcineurin, etc.). The in vitro growth, biofilm formation and susceptibility of biofilm to antifungal drugs of these mutants were compared with the control. Hypoxia reduced the susceptibility of planktonic cells to fluconazole. The mutants ecm33Δ, kre1Δ, rox1Δ, and kre2Δ showed maximum reductions in their biofilm activities (>20%). The selected mutants (upc2BΔ, kre2 Δ, ecm7Δ, rox1 Δ, mid1Δ, ecm33Δ, cch1Δ, kre1Δ) showed reduced biofilm activities (>30%) in the presence of 16 μg ml−1 fluconazole under hypoxia. Functional analysis revealed that Kre1, Ecm33, Upc2B, Kre2, Ecm7, Cch1, Mid1 and Rox1 can be explored as a potential drug target for developing novel antifungal drugs.
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0821-7) contains supplementary material, which is available to authorized users.
Keywords: Hypoxia, Candida glabrata, Deletion mutants, Biofilm, Drug targets
Introduction
Fungal infections often contribute to high morbidity and mortality, which require the immediate attention of researchers to explore the host of factors and molecular determinants that are required for their virulence and pathogenesis. Candidiasis, a condition resulting from the infection of Candida, ranges from superficial to deeply invasive and systemic infections. Oral thrush and vulvovaginitis are the most common superficial forms of candidiasis, while candidemia (bloodstream infection) is the most common invasive infection (Giri and Kindo 2012). In the USA, candidemia infection is the fourth most widely hospital-acquired blood stream infection (BSI), while in Europe it stands between the sixth and tenth position of BSI (Wisplinghoff et al. 2004; Mean et al. 2008). Immune-suppressed state and implantation devices such as catheter, parenteral nutrition and the overuse of antibiotics are common factors that facilitate Candida infection (Rodrigues et al. 2014).
The trend of causatives of candidiasis is now shifting from albicans to non-albicans Candida (NAC) species worldwide (Guinea 2014; Yapar 2014). C. glabrata is one of the most pathogenic non-albicans Candida species after C. albicans, which is inherently resistant to azole drugs (Kaur et al. 2005; Caggiano et al. 2015). Among NAC species, C. glabrata is the major cause of Candida infection in Northern Europe and the USA, while most reported cases in Spain and Brazil are of C. parapsilosis (Guinea 2014). Many studies from different parts of India have also reported the shift in Candida epidemiology from albicans to drug-resistant NAC species, such as C. glabrata, C. parapsilosis and C. tropicalis (Chander et al. 2013; Juyal et al. 2013; Pahwa et al. 2014; Tak et al. 2014).
The pathogenic behavior of microbes is affected by several host factors, such as pH, temperature, hypoxia and nutritional availability. Hypoxia is a low oxygen state, which modulates the transcription of a subset of genes that ultimately affects pathogenicity (Synnott et al. 2010; Gleason et al. 2011). Inside the human body, 2.5–9.0% oxygen level is reported in normal tissues, while wounds and tumors have much lower oxygen levels (≤1.0%) (Dewhrist 1998; Nizet and Johnson 2009). Adaptation to hypoxia is required for the survival and virulence of pathogenic fungi, e.g., C. albicans, Cryptococcus neoformans and Aspergillus fumigatus (Grahl et al. 2012). In general, hypoxic response of fungal pathogens includes upregulation of genes involved in heme biosynthesis, fatty acid synthesis, ergosterol biosynthesis, glycolysis, adhesins and hyphal transition (Setiadi et al. 2006; Askew et al. 2009; Mundy and Cormack 2009; Synnott et al. 2010; Gleason et al. 2011).
The threat of upcoming pathogenic NAC species and their rising resistance to existing antifungals have encouraged us to characterize the deletion mutants of C. glabrata with an aim to identify the molecular drug targets in C. glabrata (a model NAC species). An effective drug target must be crucial for survival and virulence of pathogen and must not share similarity with any of the host proteins (Spitzer et al. 2011; Pierce and Lopez-Ribot 2013). It is well proven that drugs screened under normoxic condition did not show in vivo efficacy because of the hypoxic environment of the tissue (Pellegrini et al. 2015). Therefore, we aimed to characterize the molecular drug targets under hypoxia, a metabolic stress present inside the host tissue. In the present study, an approach of reverse genetics was applied to characterize 13 selected deletion mutants of C. glabrata, which were prepared earlier in a multi-centric study, under hypoxic as well as normoxic conditions, (Schwarzmuller et al. 2014). The deletion mutants were selected from different pathways on the basis of their requirement for viability and virulence of C. glabrata, e.g., cell wall biosynthesis (ecm33∆, kre1∆, kre2∆), DNA checkpoint pathway (dun1∆), ergosterol pathway (erg5∆), calcium–calcineurin pathway (cch1∆, mid1∆, ecm7∆), transporters (atm1∆, cdr1∆) and selected transcription factors (pdr1∆, rox1∆, upc2B∆).
Materials and methods
Cultures, culture conditions and chemicals
Strain and deletion mutants used in this study (Table 1) were prepared by Schwarzmuller et al. (2014) and thankfully provided by Dr. Rupinder Kaur, Staff Scientist and Group Leader, Laboratory of Fungal Pathogenesis, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India. Strains were routinely maintained in YPD media at 37 °C (1% yeast extract, 2% Bacto-Peptone and 2% dextrose; Difco). Biofilm assays were performed in RPMI-1640 medium supplemented with 50 mM HEPES (HiMedia) and l-Glutamine, pH 7.0 (referred as to RPMI henceforth) and SD medium (0.67% yeast nitrogen base w/o amino acids with ammonium sulfate supplemented with 2% dextrose; Difco) in sterile 96-well polystyrene cell culture plates with flat bottom (HiMedia). All other routine chemicals of molecular grade were procured from Merck and plastic wares from Tarson. Fluconazole (FLU), menadione and 2,3-bis(2-methoxy-4-nitro-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) were purchased from HiMedia. To monitor cell growth under hypoxia, a mixture of O2: 1%; CO2: 5% and N2: 94% was maintained inside a hypoxia incubator (Make: New Brunswick, Model: Galaxy 170R).
Table 1.
C. glabrata strains used in this study
| Sr. no. | Strain/mutants (systematic name of ORF from Candida Genome Database, CGD) | Genotype |
|---|---|---|
| 1 | ATCC 2001 | C. glabrata wild-type strain |
| 2 | pdr1Δ (CAGLOA00451 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT pdr1Δ::NAT1 |
| 3 | cch1Δ (CAGLOB02211 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT cch1Δ::NAT1 |
| 4 | upc2BΔ (CAGLOF07865 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT upc2BΔ::NAT1 |
| 5 | cdr1Δ (CAGLOM01760 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT cdr1Δ::NAT1 |
| 6 | ecm33Δ (CAGLOM01826 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT ecm33Δ::NAT1 |
| 7 | mid1Δ (CAGLOM03597 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT mid1Δ::NAT1 |
| 8 | kre1Δ (CAGLOM04169 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT kre1Δ::NAT1 |
| 9 | atm1Δ (CAGLOM13739 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT atm1Δ::NAT1 |
| 10 | rox1 Δ (CAGLOD05434 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT rox1Δ::NAT1 |
| 11 | kre2 Δ (CAGLOH07403 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT kre2Δ::NAT1 |
| 12 | ecm7Δ (CAGLOM00748 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT ecm7Δ::NAT1 |
| 13 | dun1Δ (CAGLOL07326 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT dun1Δ::NAT1 |
| 14 | erg5Δ (CAGLOM07656 g) | C. glabrata his3Δ::FRT leu2Δ::FRT trp1Δ::FRT erg5Δ::NAT1 |
Antifungal susceptibility assay
Antifungal susceptibility was studied in 96-well round-bottom plate following M27-A2 guidelines of broth microdilution (NCCLS 2002). Overnight-grown culture of C. glabrata in YPD was re-inoculated in fresh YPD broth and incubated for 2 h at 37 °C. Log-phase cells were diluted to 2.5 × 103 cells ml−1 in RPMI and 100 μl of this suspension was added to wells of microtiter plate, containing 100 μl of fluconazole (FLU) at log2 concentration range of 0.5–16 μg ml−1, with a drug-free control in RPMI. Plates were incubated at 35 °C for 48 h under normoxic or hypoxic conditions. After incubation, the absorbance of culture in wells was measured at 600 nm in an ELISA reader (Multiskan Go, Thermo Scientific). The concentrations of FLU required for 50% inhibition of growth of different strains (IC50) were determined for comparison.
Biofilm formation assay
A biofilm was developed in 96-well flat-bottom microtiter plates (Riera et al. 2012). The cell suspension was prepared in PBS at a concentration of 107 cells ml−1 and 100 μl of suspension was added to each well. The plates were incubated for 90 min at 37 °C on normoxic condition for the adhesion phase. Adhesion was visualized under inverted light microscope. Wells were washed twice with 200 μl of PBS to remove non-adhered cells. In each well, 200 μl of culture media were added and plates were incubated at 37 °C under hypoxic or normoxic conditions for 48 h with shaking. Afterward, wells were washed twice with 200 μl of PBS to remove non-adhered cells and the biofilm was quantified by the XTT reduction assay by reading the absorbance at 492 nm through an ELISA Reader (Multiskan Go, Thermo Scientific).
XTT reduction assay
The XTT reduction assay was performed as described previously by Tsang et al. (2012) for the quantification of the biofilm. The XTT solution was prepared by mixing 1 mg of XTT salt in 1 ml of PBS; the solution was syringe filtered (pore size 0.22 μm) and finally stored at −20 °C. Menadione solution (0.4 mM) was prepared freshly in acetone. After washing wells with PBS, 158 μl of PBS, 40 μl XTT and 2 μl menadione was added to each well and the plates were incubated for 2 h in the dark at 37 °C. After incubation, 100 μl of solution was transferred to a new plate and the absorbance was measured at 492 nm using an ELISA Reader (Multiskan Go, Thermo Scientific). The biofilm formed by each deletion mutant was compared with the control under hypoxic and normoxic conditions. The percentage decrease in the mean biofilm activity of the mutant with respect to its control (at the respective condition) was calculated and shown as percent relative reduction in metabolic activity (RRMA).
Antifungal susceptibility of the biofilm
In vitro biofilm was developed, in the presence of FLU (at a log2 concentration range of 0.5–16 μg ml−1), with a drug-free control under hypoxic or normoxic conditions for 48 h at 37 °C (Riera et al. 2012; Tsang et al. 2012). The biofilm development was quantified by an XTT reduction assay and represented in terms of relative reduction in metabolic activity (RRMA).
Statistical analysis
Experiments were performed in triplicate and the averages of the values are shown in the tables and figures along with standard deviations. Student’s t test was applied for analyzing the significant differences between the values. p value <0.05 was considered as significant and represented by ‘*’ in the figures.
Results
Deletion mutants of C. glabrata, used in this study, were prepared in an earlier study by Schwarzmuller et al. (2014). The use of URA3 marker was avoided, since it is known to alter the virulence properties of Candida (Lay et al. 1998; Brand et al. 2004). The HTL strain (his3∆::FRT leu2∆::FRT trp1∆::FRT; isogenic to ATCC2001) has displayed similar growth properties and rates as the parental wild-type strain (ATCC2001), on both minimal and rich media, under hypoxic as well as normoxic conditions (data not shown). The auxotrophic markers of the mutant (HIS3, LEU2 and TRP1) did not influence the in vitro growth and survival of the mutants in immunocompetent mice when compared to the parental wild type strain (ATCC2001) (Jacobsen et al. 2010).
Antifungal susceptibility assay
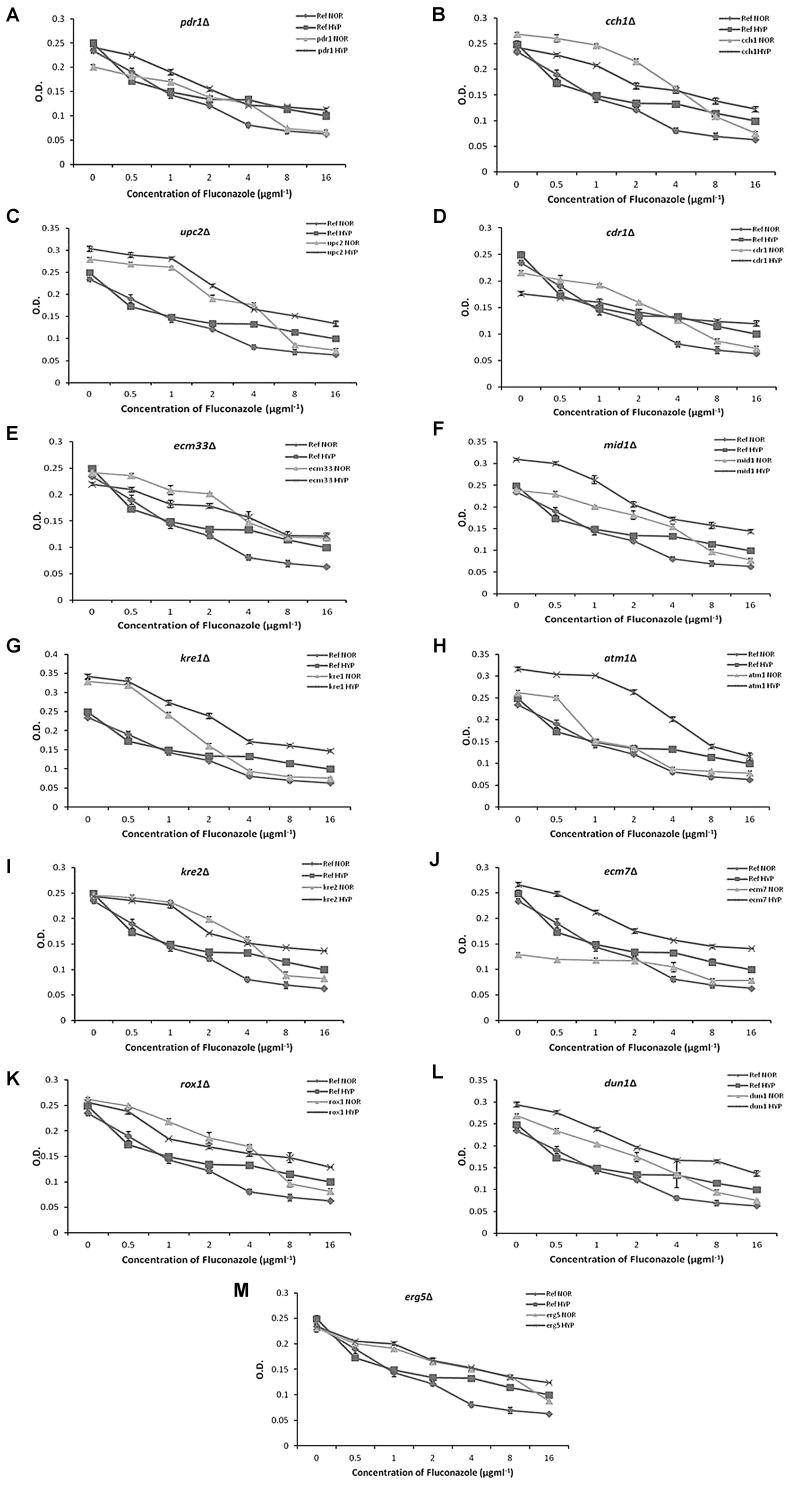
The IC50 values of FLU for the strains under hypoxia and normoxia are shown in Table 2. No deletion was found to increase the susceptibility to FLU above the control strain under hypoxic condition. Some deletions such as pdr1Δ, kre1Δ and atm1Δ showed IC50 values similar to the control under hypoxia. For each mutant, a line graph was plotted between the mean absorbance (O.D.600nm) of cultures and the presence of different concentrations of FLU (Fig. 1a–m).
Table 2.
IC50 of fluconazole for the strains under normoxia and hypoxia
| Sr. no. | Strain/mutant | IC50 value (µg ml−1) under normoxia | IC50 value (µg ml−1) under hypoxia |
|---|---|---|---|
| 1 | ATCC2001 | 2–4 | 4–8 |
| 2 | pdr1Δ | 2–4 | 4–8 |
| 3 | cch1Δ | 4–8 | >16 |
| 4 | upc2BΔ | 4–8 | 8–16 |
| 5 | cdr1Δ | 2–4 | >16 |
| 6 | ecm33Δ | 4–8 | >16 |
| 7 | mid1Δ | 4–8 | 8–16 |
| 8 | kre1Δ | 2–4 | 4–8 |
| 9 | atm1Δ | 2–4 | 4–8 |
| 10 | rox1 Δ | 4–8 | >16 |
| 11 | kre2 Δ | 4–8 | >16 |
| 12 | ecm7Δ | >16 | >16 |
| 13 | dun1Δ | 4–8 | 8–16 |
| 14 | erg5Δ | 8–16 | >16 |
Fig. 1.
Antifungal susceptibility assay of C. glabrata mutants by broth microdilution assay. Different dilutions of FLU were tested in RPMI against the mutants. The growth was measured by taking O. D. at 600 nm after 48 h incubation at 35 °C
It is obvious that the mutants behaved differently in broth microdilution assay under hypoxic and normoxic conditions (Table 2). The susceptibility of mutants to FLU was reduced under hypoxia, resulting in increased growth. On the basis of IC50 values, kre1∆, atm1∆ and pdr1∆ did not show further drop in susceptibility over the respective control strain under hypoxic conditions. It indicated that Kre1, Atm1 and Pdr1 may be good drug targets for the planktonic cells of C. glabrata under hypoxic conditions.
Biofilm development
C. glabrata mutants, which reduced the biofilm formation under hypoxic and normoxic conditions in RPMI medium, were selected in this study. Data showed that the control strain did not exhibit any significant difference in biofilm development under hypoxic and normoxic conditions (p value– 0.373118), while the mutants exhibited significant differences in their biofilm activities (Supplementary figure, Fig. S1; Table 3). Most of the mutants showed that hypoxic exposure increased the biofilm formation, when compared with the respective biofilm at normoxia. The percent relative reduction in metabolic activities (RRMA) of the mutants under normoxic and hypoxic conditions ranged between 10.39–50.65% and 0–35.62%, respectively (Table 3).
Table 3.
Mean value of O.D.492nm for biofilm of deletion mutants
| Strain | Normoxic condition | Hypoxic condition | ||
|---|---|---|---|---|
| Mean of O.D.492nm ± SD | Percent RRMA | Mean of O.D.492nm ± SD | Percent RRMA* | |
| ATCC 2001 | 0.077 ± 0.0050 | – | 0.073 ± 0.0020 | – |
| pdr1Δ | 0.050 ± 0.0036 | 35.06 | 0.068 ± 0.0035 | 06.85 |
| cch1Δ | 0.054 ± 0.0020 | 29.87 | 0.080 ± 0.0020 | Nil |
| upc2BΔ | 0.052 ± 0.0026 | 32.47 | 0.072 ± 0.0005 | 01.37 |
| cdr1Δ | 0.068 ± 0.0020 | 11.69 | 0.063 ± 0.0015 | 13.70 |
| ecm33Δ | 0.053 ± 0.0050 | 31.17 | 0.054 ± 0.0041 | 26.03 |
| mid1Δ | 0.053 ± 0.0049 | 31.17 | 0.072 ± 0.0050 | 01.37 |
| kre1Δ | 0.038 ± 0.0055 | 50.65 | 0.047 ± 0.003 | 35.62 |
| atm1Δ | 0.048 ± 0.0047 | 37.66 | 0.060 ± 0.0049 | 17.81 |
| rox1Δ | 0.057 ± 0.0026 | 25.97 | 0.057 ± 0.0011 | 21.92 |
| kre2Δ | 0.057 ± 0.0051 | 25.97 | 0.058 ± 0.0005 | 20.55 |
| ecm7Δ | 0.058 ± 0.0045 | 24.68 | 0.066 ± 0.0035 | 09.59 |
| dun1Δ | 0.069 ± 0.0065 | 10.39 | 0.064 ± 0.0047 | 12.33 |
| erg5Δ | 0.062 ± 0.0035 | 19.48 | 0.060 ± 0.0056 | 17.81 |
* Percent RRMA Relative reduction in the mean metabolic activity of biofilm in percent, with reference to the mean metabolic activity of wild-type strain, ATCC 2001
As shown in Table 3, mutants (pdr1Δ, cch1Δ, upc2BΔ, ecm33Δ, mid1Δ, kre1Δ, atm1Δ, rox1Δ, kre2Δ, and ecm7Δ) showed high reductions in the biofilm activities (>20%) under normoxic condition, whereas under hypoxic condition only few mutants (ecm33Δ, kre1Δ, rox1Δ, and kre2Δ) showed high reduction in their biofilm activities (>20%). C. glabrata mutants, kre1∆ and ecm33∆ showed maximum decrease in biofilm activities (35.62 and 26.03%, respectively) upon hypoxic exposure, indicating them to be the most promising drug targets under hypoxia.
Antifungal susceptibility of biofilm
Antifungal resistance of Candida biofilm is about thousand times more than planktonic cells (LaFleur et al. 2006). Antifungal susceptibility of the biofilm assay showed that all mutants exhibited reduced biofilm in the presence of FLU under normoxia and hypoxia. The percent relative metabolic activities of biofilm of deletion mutants in the presence of FLU is shown as bar graphs (Supplementary figure, Fig. S2). Interestingly, no change in antifungal susceptibility of biofilm was observed for cch1∆, atm1∆ and upc2B∆ (Table 4).
Table 4.
Reduction in metabolic activities of biofilm of deletion mutants when compared with the control in the presence of 16 μg ml−1 fluconazole
| Sr. no. | Strain | Percent RRMA* under normoxia | Percent RRMA* under hypoxia |
|---|---|---|---|
| 1 | pdr1Δ | 11.00 | 23.48 |
| 2 | cch1Δ | 39.00 | 38.19 |
| 3 | upc2BΔ | 45.15 | 49.66 |
| 4 | cdr1Δ | 27.64 | 20.18 |
| 5 | ecm33Δ | 26.96 | 32.20 |
| 6 | mid1Δ | Nil | 32.92 |
| 7 | kre1Δ | 04.52 | 31.32 |
| 8 | atm1Δ | 15.58 | 14.02 |
| 9 | rox1Δ | 11.01 | 34.59 |
| 10 | kre2Δ | 20.36 | 46.14 |
| 11 | ecm7Δ | 18.14 | 46.35 |
| 12 | dun1Δ | 12.62 | 21.02 |
| 13 | erg5Δ | 03.14 | 23.11 |
* Percent RRMA relative reduction in the mean metabolic activity of biofilm in percent, with reference to the mean metabolic activity of wild-type strain, ATCC 2001
When compared with the normoxic results, hypoxia highly increased the biofilm susceptibility to FLU for pdr1∆, mid1∆, kre1∆, rox1∆, kre2∆, ecm7∆, dun1∆ and erg5∆ deletion mutants. Some mutants (cch1∆, upc2B∆ and ecm33∆) did not show much differences in percent RRMA at hypoxia and normoxia, but showed large reductions in biofilm activities and so can be considered as potential drug targets. The deletion mutants, dun1∆, erg5∆, pdr1∆ and cdr1∆, showed percent RRMA >20% under hypoxic condition, whereas all mutants except atm1∆ showed percent RRMA> 30%, under hypoxic condition. Under hypoxic condition, Upc2B, Kre2, Ecm7, Rox1, Mid1, Ecm33, Cch1 and Kre1, appeared to be the most potential drug targets (percent RRMA of deletion mutants >30%) to sensitize the biofilm in the presence of FLU (Table 4).
Discussion
Adaptations to hypoxia are required for the survival and enhanced virulence of pathogenic fungi such as C. albicans, C. glabrata, Cryptococcus neoformans and Aspergillus fumigatus (Grahl et al. 2012; Gupta et al. 2014, 2015). The function of most of the genes analyzed in this study is still unknown in C. glabrata. This study has also revealed the functions of the selected genes in virulence.
Yeast Atm1 is an ATP binding cassette (ABC) transporter found in mitochondrial inner membrane with ABC domain-facing mitochondrial metrics (Leighton and Schatz 1995). It has a role in the generation of cytosolic Fe/S proteins, which catalyzes several metabolic reactions like isomerization, dehydration and electron transport in the redox reaction (Cammack 1992; Johnson 1998). Earlier, it has been shown that the absence of ATM1 in yeast results in defective mitochondrial respiration and increases intracellular oxidative stress (Kispal et al. 1999). In S. cerevisiae, the deletion mutant of ATM1 is inviable in aerobic condition, but exhibits slow growth in anaerobic condition (Leighton and Schatz 1995). Similarly, we observed that the biofilm of atm1∆ was severely compromised under normoxic condition, whereas the decrease was relatively lesser under hypoxic conditions (Table 3). This effect may be due to less dependency on mitochondrial respiration under hypoxic conditions.
In biofilm formation and antifungal susceptibility assays, Kre1 and Ecm33 came out as the most promising drug target under hypoxic conditions (Tables 3, 4). Yeast Kre1 and Ecm33 are key components of cell wall organization and synthesis. Their deletion weakens the cell wall and increases sensitivity (Terashima et al. 2003; Breining et al. 2004). It plays an important role in β-glucan synthesis, a component of fungal cell wall (Boone et al. 1990; Roemer and Bussey 1995). Kre1 is required for the synthesis of β-1,3 glucan, which is a crucial element of the extracellular matrix and has a role in the sequestration of the antifungal molecules, causing drug resistance of the Candida biofilm (Nett et al. 2007, 2010). Ecm33 is a GPI protein and has an important role in establishing and maintaining the cell wall integrity (Groot et al. 2013). In addition to the defective cell wall matrix in kre1∆ and ecm33∆, hypoxic exposure may lead to the reduced ergosterol and fatty acid synthesis, resulting in increased sensitivity. Therefore, the biofilm of C. glabrata kre1∆ and ecm33∆ strain is reduced and the mutant appeared more susceptible in the presence of azole.
C. glabrata Kre2 is uncharacterized and its ortholog in C. albicans is alpha-1,2-mannosyl transferase, which is predicted as type II Golgi membrane protein that adds the second mannose during cell wall mannoprotein biosynthesis. It is required for wild-type virulence and adherence to epithelial cells (Singh et al. 2011). Therefore, in the present study, the kre2∆ mutant of C. glabrata has shown reduction in the biofilm and biofilm susceptibility to FLU (probably due to reduction in ergosterol level at hypoxia) (Tables 3, 4).
Hypoxic response in S. cerevisiae is mostly mediated through Rox1, a transcription factor which represses hypoxic genes under normoxic conditions (Zitomer et al. 1997). Rfg1 of C. albicans is the closest homolog of yeast Rox1, which represses filamentous growth (Kadosh and Johnson 2001). CgROX1 is uncharacterized and this report has revealed for the first time the role of CgRox1 in biofilm formation and in biofilm resistance to azole under hypoxic condition (Tables 2, 3). As shown in supplementary figure S1, the rox1∆ mutant did not show any difference in biofilm activities under normoxic and hypoxic conditions; it suggested that like S. cerevisiae, Cg Rox1 is required for survival in hypoxia.
The highest biofilm susceptibility to FLU (16 μg ml−1) was shown by upc2B∆ (percent RRMA 45.15% in normoxia and 49.66% in hypoxia; Table 4). Therefore, CgUpc2B appeared to be a very potent drug target to sensitize the biofilm in the presence of azole. CgUpc2B was also required for the biofilm synthesis under normoxic condition. Fungal Upc2B is a functional homolog of S. cerevisiae Ecm22 and a study has shown that it is not the main transcriptional regulator of the genes of sterol homeostasis and sterol susceptibility (Nagi et al. 2011). CgUpc2B is not characterized, but this study has indicated that Upc2B regulated biofilm formation and biofilm resistance to FLU.
The results of the present study indicated that Cch1, Mid1 and Ecm7 have a role in biofilm formation under normoxic condition (Table 3). Cch1 is required in biofilm resistance to FLU under normoxic as well as hypoxic conditions, whereas Mid1 and Ecm7 were required for biofilm resistance to FLU under hypoxic condition only (Table 4).
The proteins, Cch1, Mid1 and Ecm7, are involved in Ca2+ transport across the membrane (Teng et al. 2013). It has been previously reported that a drop in intracellular Ca2+ level results in increased susceptibility to FLU (Kaur et al. 2004). Earlier, it has been shown that amlodipine besylate, an inhibitor of mammalian Ca2+ channel, reduced the virulence of C. albicans and C. glabrata clinical isolates, in vitro (Gupta et al. 2016).
Though the other mutants (pdr1∆, dun1∆, erg5∆) slightly regulated the virulence properties, they are required to be addressed for functional characterization. CgPdr1 belongs to the family of zinc finger transcription factors. It is functionally similar to Pdr1 and Pdr3 of S. cerevisiae. CgPdr1 is the transcriptional regulator of multi-drug transporters belonging to the ABC family, such as CgCdr1 and CgCdr2. Deletion of CgPDR1 results in loss of major drug transporters and decreases resistance to azoles (Tsai et al. 2006; Vermitcky et al. 2006). This study has also shown that loss of PDR1 decreased biofilm resistance to FLU (Table 4). The biofilm activity of pdr1∆ was reduced by 35.06% in normoxia; whereas hypoxia partially rescued the loss of biofilm (Table 3).
CgDun1 is uncharacterized and its role in biofilm formation and increase in biofilm resistance to FLU under hypoxia has been reported in this study. In yeast, Dun1 is downstream to Rad53 in the DNA checkpoint pathway and gets activated by phosphorylation at its FHA domain through activated Rad53. Dun1 on activation promotes the synthesis of ribonucleotide reductase through multiple mechanisms (Sanvisen et al. 2013).
CgErg5 is also uncharacterized and appears to play an important role in biofilm formation under normoxic as well as hypoxic conditions, whereas it is required for biofilm resistance to FLU under hypoxic condition. Erg5 is a C-22 sterol desaturase enzyme required for sterol synthesis in yeast. ERG5 deletion is viable in S. cerevisiae and is reported to upregulate the ERG3 expression (Arthington-Skaggs et al. 1996; Skagg et al. 1996).
Conclusion
The present study was undertaken to identify the molecular drug targets under hypoxic condition to develop an effective drug against C. glabrata. The results indicated that Kre1, Ecm33, Upc2B, Kre2, Ecm7, Cch1, Mid1 and Rox1 are potential drug alone or in combination with azoles. None of these proteins has human homologs (except Cch1), hence the risk of cross-reactivity of the drug with host proteins will be the least, resulting in minimal side effects. Kre1 and Ecm33 have emerged as the most potential candidates as drug targets against growth, biofilm and biofilm resistance in the pathogen. These targets may be further characterized by site-directed mutagenesis for the identification of the target domain for the synthesis of inhibitors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Effect of hypoxia on mutants’ biofilm formation. The biofilm was formed in RPMI media for 42 h at 37 °C under hypoxia and normoxia and the metabolic activity of the biofilm formed was analyzed by XTT reduction assay. Optical density (O.D.) was measured at 492 nm. (EPS 17606 kb)
Fig. S2 Antifungal susceptibility of C. glabrata mutants’ biofilm. Different concentration of fluconazole was added during biofilm formation of C. glabrata mutants. The biofilm formed after 48 h at 37 °C under hypoxic and normoxic conditions. The metabolic activity of biofilm formed was quantified by XTT reduction assay and the per cent reduction in the biofilm activity of each mutant, with reference to the biofilm activity of control strain, is shown as % Relative Metabolic Activity. (EPS 11093 kb)
Acknowledgements
We thank Prof. Brendan P. Cormack, Professor of Molecular Biology and Genetics, School of Medicine, John Hopkins University, Maryland, Baltimaore, and Dr. Rupinder Kaur, Laboratory of Fungal Pathogenesis, Centre for DNA Fingerprinting and Diagnostics, Hyderabad, India, for providing the C. glabrata deletion mutants. PG is supported by the INSPIRE fellowship from the Department of Science and Technology, Government of India. We acknowledge the support of the Director, Defence Institute of Physiology and Allied Sciences (DIPAS), DRDO, Delhi-54, along with Dr. Amitabha Chakrabarti, Sc. ‘F’ and Dr. Anju Bansal, Sc. ‘F’, DIPAS, for allowing us to use the facilities for hypoxia exposure and microbial culture in their laboratory. We are also thankful to Dr. Ashish Thapliyal, HOD, Biotechnology, Graphic Era University, for his constant support during the course of this study. This work was financially supported by Graphic Era University, Dehradun.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest regarding any issue related to this manuscript and the data presented.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13205-017-0821-7) contains supplementary material, which is available to authorized users.
References
- Arthington-Skaggs BA, Crowell DN, Yang H, Sturley SL, Bard M. Positive and negative regulation of a sterol biosynthetic gene (ERG3) in the post-squalene portion of the yeast ergosterol pathway. FEBS Lett. 1996;392(2):161–165. doi: 10.1016/0014-5793(96)00807-1. [DOI] [PubMed] [Google Scholar]
- Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, Whiteway M. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog. 2009;5(10):1–10. doi: 10.1371/journal.ppat.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C, Sommer SS, Hensel A, Bussey H. Yeast KRE genes provide evidence for a pathway of cell wall beta-glucan assembly. J Cell Biol. 1990;110:1833–1843. doi: 10.1083/jcb.110.5.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell. 2004;3:900–909. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breining F, Schleinkofer K, Schmitt MJ. Yeast Kre1p is GPI-anchored and involved in both cell wall assemble and architecture. Microbiology. 2004;150:3209–3218. doi: 10.1099/mic.0.27175-0. [DOI] [PubMed] [Google Scholar]
- Caggiano G, Coretti C, Bartolomeo N, Lovero G, De Giglio O, Montagna MT. Candida bloodstream infections in Italy: changing epidemiology during 16 years of surveillance. Biomed Res Int. 2015;2015:1–9. doi: 10.1155/2015/256580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack R. Iron-sulfur clustera in enzymes: themes and variations. In: Cammack R, editor. Iron-sulfur proteins. San Diego: Academic Press; 1992. pp. 281–322. [Google Scholar]
- Chander J, Singla N, Sidhu SK, Gombar S. Epidemiology of Candida blood stream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013;7(9):670–675. doi: 10.3855/jidc.2623. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW. Concepts of oxygen transport at the microcirculatory level. Semin Radiat Oncol. 1998;8:143–150. doi: 10.1016/S1053-4296(98)80040-4. [DOI] [PubMed] [Google Scholar]
- Giri S, Kindo AJ. A review of Candida species causing blood stream infection. Indian J Med Microbiol. 2012;30(3):270–278. doi: 10.4103/0255-0857.99484. [DOI] [PubMed] [Google Scholar]
- Gleason JE, Corrigan DJ, Cox JE, Reddi AR, McGinnis LA, Culotta VC. Analysis of hypoxia and hypoxia-like states through metabolite profiling. PLoS One. 2011;6(9):1–13. doi: 10.1371/journal.pone.0024741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl N, Shepardson KM, Chung D, Cramer RA. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell. 2012;11(5):560–570. doi: 10.1128/EC.00031-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot PWJ, Bader O, Boer AD, Weig M, Chauhan N. Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot Cell. 2013;12(4):470–481. doi: 10.1128/EC.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea J. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect. 2014;20:5–10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- Gupta P, Nath S, Meena RC, Kumar N. Comparative effects of hypoxia and hypoxia mimetic cobalt chloride on in vitro adhesion, biofilm formation and susceptibility to amphotericin B of Candida glabrata. J Mycol Med. 2014;24:169–177. doi: 10.1016/j.mycmed.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Gupta P, Meena RC, Rai N, Kumar N. Effect of hypoxia on in vitro adhesion, biofilm formation and antifungal susceptibility of Candida albicans. Int J Pharm Sci Rev Res. 2015;32:279–283. [Google Scholar]
- Gupta P, Chanda R, Rai N, Kataria VK, Kumar N. Antihypertensive, amlodipine besylate inhibits growth and biofilm of human fungal pathogen Candida. Assay Drug Dev Technol. 2016;14:291–297. doi: 10.1089/adt.2016.714. [DOI] [PubMed] [Google Scholar]
- Jacobsen ID, Brunke S, Seider K, Schwarzmuller T, Firon A, d’Enfért C, Kuchler K, Hube B. Candida glabrata persistence in mice does not depend on host immunosuppression and is unaffected by fungal amino acid auxotrophy. Infect Immun. 2010;78:1066–1077. doi: 10.1128/IAI.01244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK. Iron-sulfur proteins: new role for old clusters. Curr Opin Chem Biol. 1998;2:173–181. doi: 10.1016/S1367-5931(98)80058-6. [DOI] [PubMed] [Google Scholar]
- Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N. Emergence of non albicans Candida species in neonatal candidemia. N Am J Med Sci. 2013;5:541–545. doi: 10.4103/1947-2714.118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh D, Johnson AD. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol Cell Biol. 2001;21(7):2496–2505. doi: 10.1128/MCB.21.7.2496-2505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Castano I, Cormack BP. Functional genomic analysis of fluconazole susceptibility in the pathogenic yeast Candida glabrata: roles of calcium signaling and mitochondria. Antimicrob Agents Chemother. 2004;48(5):1600–1613. doi: 10.1128/AAC.48.5.1600-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Domergue R, Zupancic ML, Cormack BP. A yeast by any other name: Candida glabrata and its interaction with its host. Curr Opin Microbiol. 2005;8:378–384. doi: 10.1016/j.mib.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Prohl C, Lill R. The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J. 1999;18(14):3981–3989. doi: 10.1093/emboj/18.14.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50(11):3839–3846. doi: 10.1128/AAC.00684-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay J, Henry LK, Clifford J, Koltin Y, Bulawa CE, Becker JM. Altered expression of selectable marker URA3 in gene-disrupted Candida albicans strains complicates interpretation of virulence studies. Infect Immun. 1998;66:5301–5306. doi: 10.1128/iai.66.11.5301-5306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J, Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J. 1995;14(1):188–195. doi: 10.1002/j.1460-2075.1995.tb06989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mean M, Marchetti O, Calandra T. Bench-to-bedside review: Candida infections in the intensive care unit. Crit Care. 2008;12:1–9. doi: 10.1186/cc6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy RD, Cormack B. Expression of Candida glabrata adhesions after exposure to chemical preservatives. J Infect Dis. 2009;199:1891–1899. doi: 10.1086/599120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi M, Nakayama H, Tanabe K, Bard M, Aoyama T, Okano M, Higashi S, Ueno K, Chibana H, Niimi M, Yamagoe S, Umeyama T, Kajiwara S, Ohno H, Miyazaki Y. Transcription factors CgUPC2A and CgUPC2B regulate ergosterol biosynthetic genes in Candida glabrata. Genes Cells. 2011;16(1):80–89. doi: 10.1111/j.1365-2443.2010.01470.x. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards (2002) Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa
- Nett J, Lincoln L, Marchillo K, Massey R, Holoyda K, Hoff B, VanHandel M, Andes D. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob Agents Chemother. 2007;51:510–520. doi: 10.1128/AAC.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Sanchez H, Cain MT, Andes DR. Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis. 2010;202:171–175. doi: 10.1086/651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Johnson RS. Interdependence of hypoxia and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa N, Kumar R, Nirkhiwale S, Bandi A. Species distribution and drug susceptibility of Candida in clinical isolates from a tertiary care centre at Indore. Indian J Med Microbiol. 2014;32(1):44–48. doi: 10.4103/0255-0857.124300. [DOI] [PubMed] [Google Scholar]
- Pellegrini P, Haraldsson M, Jensen SJ, Lundback T, De Milito A (2015) A drug-screening model to identify compounds active in cells under metabolic stress. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18–22; Philadelphia, PA. Philadelphia (PA): AACR; Cancer Res., 2015; 75 (15 Suppl): Abstract no. 5509. doi:10.1158/1538-7445.AM2015-5509
- Pierce CG, Lopez-Ribot JL. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Expert Opin Drug Discov. 2013;8:1117–1126. doi: 10.1517/17460441.2013.807245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera M, Mogensen E, d’Enfert C, Janbon G. New regulators of biofilm development in Candida glabrata. Res Microbiol. 2012;163:297–307. doi: 10.1016/j.resmic.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33(5):673–688. doi: 10.1007/s10096-013-2009-3. [DOI] [PubMed] [Google Scholar]
- Roemer T, Bussey H. Yeast Kre1p is a cell surface O-glycoprotein. Mol Gen Genet. 1995;249:209–216. doi: 10.1007/BF00290368. [DOI] [PubMed] [Google Scholar]
- Sanvisens N, Llanos R, Puig S. Function and regulation of yeast ribonucleotide reductase: cell cycle, genotoxic stress, and iron bioavailability. Biomed J. 2013;36(2):51–58. doi: 10.4103/2319-4170.110398. [DOI] [PubMed] [Google Scholar]
- Schwarzmuller T, Ma B, Hiller E, Istel F, Tscherner M, Brunke S, Ames L, Firon A, Green B, Cabral V, Marcet-Houben M, Jacobsen ID, Quintin J, Seider K, Frohner I, Glaser W, Jungwirth H, Bachellier-Bassi S, Chauvel M, Zeidler U, Ferrandon D, Gabaldón T, Hube B, d’Enfert C, Rupp S, Cormack B, Haynes K, Kuchler K. Systematic phenotyping of a large-scale Candida glabrata deletion collection reveals novel antifungal tolerance genes. PLoS Pathog. 2014;10(6):1–19. doi: 10.1371/journal.ppat.1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiadi ER, Doedt T, Cottier F, Noffz C, Ernst JF. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J Mol Biol. 2006;361:399–411. doi: 10.1016/j.jmb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Singh RP, Prasad HK, Sinha I, Agarwal N, Natarajan K. Cap2-HAP complex is a critical transcriptional regulator that has dual but contrasting roles in regulation of iron homeostasis in Candida albicans. J Biol Chem. 2011;286:25154–25170. doi: 10.1074/jbc.M111.233569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs BA, Alexander JF, Pierson CA, Schweitzer KS, Chun KT, Koegel C, Barbuch R, Bard M. Cloning and characterization of the Saccharomyces cerevisiae C-22 sterol desaturase gene, encoding a second cytochrome P-450 involved in ergosterol biosynthesis. Gene. 1996;169(1):105–109. doi: 10.1016/0378-1119(95)00770-9. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Griffiths E, Blakley KM, Wildenhain J, Ejim L, Rossi L, De Pascale G, Curak J, Brown E, Tyers M, Wright GD. Cross-species discovery of syncretic drug combination that potentiates the antifungal fluconazole. Mol Syst Biol. 2011;7:1–14. doi: 10.1038/msb.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG, Butler G. Regulation of the hypoxic response in Candida albicans. Eukaryot Cell. 2010;9:1734–1746. doi: 10.1128/EC.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak V, Mathur P, Varghese P, Gunjiyal J, Xess I, Misra MC. The epidemiological profile of candidemia at an Indian Trauma Care Center. J Lab Physicians. 2014;6(2):96–101. doi: 10.4103/0974-2727.141506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Iida K, Imai A, Nakano M, Tada T, Iida H. Hyperactive and hypoactive mutations in Cch1, a yeast homologue of the voltage-gated calcium-channel pore-forming subunit. Microbiology. 2013;159:970–979. doi: 10.1099/mic.0.064030-0. [DOI] [PubMed] [Google Scholar]
- Terashima H, Hamada K, Kitada K. The localization change of Ybr078w/Ecm33, a yeast GPI-associated protein, from the plasma membrane to the cell wall, affecting the cellular function. FEMS Microbiol Lett. 2003;218:175–180. doi: 10.1111/j.1574-6968.2003.tb11515.x. [DOI] [PubMed] [Google Scholar]
- Tsai HF, Krol AA, Sarti KE, Bennett JE. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob Agents Chemother. 2006;50:1384–1392. doi: 10.1128/AAC.50.4.1384-1392.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang PW, Bandara HMHN, Fong WP. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PLoS One. 2012;7(11):1–11. doi: 10.1371/journal.pone.0050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermitsky JP, Earhart KD, Smith WL, Homayouni R, Edlind TD, Rogers PD. Pdr1 regulates multidrug resistance in Candida glabrata: gene disruption and genome-wide expression studies. Mol Microbiol. 2006;61:704–722. doi: 10.1111/j.1365-2958.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. doi: 10.2147/TCRM.S40160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer RS, Carrico P, Deckert J. Regulation of hypoxic gene expression in yeast. Kidney Int. 1997;51:507–513. doi: 10.1038/ki.1997.71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effect of hypoxia on mutants’ biofilm formation. The biofilm was formed in RPMI media for 42 h at 37 °C under hypoxia and normoxia and the metabolic activity of the biofilm formed was analyzed by XTT reduction assay. Optical density (O.D.) was measured at 492 nm. (EPS 17606 kb)
Fig. S2 Antifungal susceptibility of C. glabrata mutants’ biofilm. Different concentration of fluconazole was added during biofilm formation of C. glabrata mutants. The biofilm formed after 48 h at 37 °C under hypoxic and normoxic conditions. The metabolic activity of biofilm formed was quantified by XTT reduction assay and the per cent reduction in the biofilm activity of each mutant, with reference to the biofilm activity of control strain, is shown as % Relative Metabolic Activity. (EPS 11093 kb)