Abstract
The extended-spectrum beta-lactamase (ESBL)-producing phenotype is frequent among Enterobacter isolates at the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. We examined the clonal relatedness and characterized the ESBLs of a collection of these strains. Clonal relatedness was determined by pulsed-field gel electrophoresis. Isoelectric focusing (IEF) and transconjugation experiments were performed. ESBL gene families were screened by colony hybridization and PCR for blaTEM, blaSHV, blaCTX-M, blaIBC, blaPER, blaOXA, blaVEB, and blaSFO; and the PCR products were sequenced. The 17 Enterobacter isolates studied comprised 15 distinct genotypes. All isolates showed at least one IEF band (range, one to five bands) whose appearance was suppressed by addition of clavulanate; pIs ranged from 5.4 to ≥8.2. Colony hybridization identified at least one family of beta-lactamase genes in 11 isolates: 10 harbored blaTEM and 9 harbored blaSHV. PCR screening and sequence analysis of the PCR products for blaTEM, blaSHV, and blaCTX-M identified TEM-1 in 11 isolates, SHV-12 in 7 isolates, SHV-1 in 1 isolate, a CTX-M-2-like gene in 2 isolates, and CTX-M-26 in 1 isolate. In transconjugation experiments with four isolates harboring blaTEM-1 and blaSHV-12, both genes were simultaneously transferred to the recipient strain Escherichia coli HB101. Plasmid mapping, PCR, and Southern analysis with TEM- and SHV-specific probes demonstrated that a single transferred plasmid carried both the TEM-1 and the SHV-12 genes. The widespread presence of ESBLs among Enterobacter isolates in Tel Aviv is likely due not to clonal spread but, rather, to plasmid-mediated transfer, at times simultaneously, of genes encoding several types of enzymes. The dominant ESBL identified was SHV-12.
Enterobacter spp. are leading nosocomial pathogens (26) that commonly cause pneumonia (25) and that are the most frequent gram-negative organisms causing bloodstream infections in intensive care units (14, 33). More than one-third of the Enterobacter sp. isolates in intensive care units reporting to the National Nosocomial Infection Surveillance System (26) are resistant to extended-spectrum cephalosporins. Moreover, treatment with extended-spectrum cephalosporins may lead to the emergence of resistance to these antimicrobial agents among susceptible strains of Enterobacter spp. (8, 19, 35). Emergence of resistance results in increased rates of mortality, longer hospital stays, and higher hospital charges (9, 10).
The resistance of Enterobacter spp. to beta-lactam antibiotics is most frequently mediated by hyperproduction of chromosomal AmpC beta-lactamase, caused either by induction or, more likely, by selection of derepressed mutants (1). In the last decade, the production of plasmid-mediated extended-spectrum beta-lactamases (ESBLs) has been recognized as an additional important emerging mechanism of resistance among members of the family Enterobacteriaceae (3, 17), including clinical isolates of Enterobacter spp. (31). The most common ESBLs found in clinical isolates of Enterobacter spp. belong to the TEM-, SHV-, and CTX-M-derived β-lactamases (2, 5, 36). However, other ESBLs have recently been reported in Enterobacter spp., including IBC-1, which was detected in an Enterobacter cloacae isolate in Greece (15, 18); VEB-1, which was found in clinical isolates of E. cloacae and Enterobacter sakazakii in Bangkok, Thailand (16); and SFO-1, which was detected in E. cloacae isolates in Japan (24). The prevalence of ESBLs among members of the family Enterobacteriaceae may vary significantly between geographical areas (40). For example, Schwaber et al. (34) recently reported that less than 2% of 152 U.S. isolates of Enterobacter spp. were confirmed to produce ESBLs. In sharp contrast, we recently investigated the occurrence of the ESBL-producing phenotype among members of the family Enterobacteriaceae at the Tel Aviv Sourasky Medical Center, Tel Aviv, Israel, and found that 22% of the Enterobacter clinical isolates in our institution had this phenotype (27). To understand the widespread occurrence of the ESBL phenotype in Enterobacter spp. at the Tel Aviv Sourasky Medical Center, we examined the genetic relatedness between these ESBL producers and characterized the ESBL enzyme families produced by these strains.
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Seventeen unique Enterobacter sp. patient isolates identified as ESBL producers (27) were studied. All the strains were isolated over a 6-month period, from June 2000 through December 2000, at the Tel Aviv Sourasky Medical Center, a 1,200-bed, tertiary-care, university-affiliated hospital. Bacterial identification to the species level and the MICs of the broad-spectrum cephalosporins cefotaxime, ceftazidime, and cefepime, the monobactam antibiotic aztreonam, and the ureidopenicillin piperacillin were performed with an automated identification and microdilution system (Microscan, Dade International, Inc., West Sacramento, Calif.) by using an overnight panel; and the results were recorded and interpreted according to NCCLS guidelines (28). Escherichia coli 4107 (blaTEM-26; pI 5.6), Klebsiella oxytoca 4076 (K1; pI 6.5), E. coli 4075 (TEM-1; pI 5.4), E. cloacae 4080 (P99; pI 7.8), and E. coli 4133 (SHV-1; pI 7.6) were used as standards for isoelectric focusing (IEF). These strains, as well as E. coli J53(pMG267) (blaCTX-M-14) and E. coli J53 R55 (blaOXA-3), which were used as positive controls, and E. coli ATCC 25922, which was used as a negative control, were used in the PCR assays. A strain carrying blaPER-1 was used as a positive control in the PCR assay for the presence of this gene. E. coli HB101 (Strr Amps; Promega) was used as the recipient strain in the conjugation experiments to study plasmid-transferable beta-lactamases. E. coli 4107 (blaTEM-26), E. coli J53(pAFF2) (blaSHV-5), and E. coli J53(pMG203) (blaOXA-4) were used as positive controls and Pseudomonas aeruginosa PU21 was used as a negative control for colony hybridization.
Determination of ESBL-producing phenotype.
The ESBL-producing phenotypes of the 17 isolates were determined by Kirby-Bauer disk diffusion test methodology. The zones of inhibition of each isolate were tested on Mueller-Hinton agar plates (Hy-Labs, Rehovot, Israel) with disks containing 30 μg of cefpodoxime, cefotaxime, and ceftazidime, alone and in combination with 10 μg of clavulanic acid (CA; Oxoid, Basingstoke, England). An organism was classified as having an ESBL-producing phenotype if the zone of inhibition produced by at least one combination disk was ≥5 mm larger than that produced by the corresponding lone antibiotic-impregnated disk (28). E. coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603 were used as negative and positive controls for ESBL production, respectively.
PFGE analysis.
Pulsed-field gel electrophoresis (PFGE) was performed with all of the 17 ESBL-positive Enterobacter strains. Bacterial DNA was prepared and cleaved with 20 U of SpeI endonuclease (New England Biolabs, Boston, Mass.), as described previously (29). Plugs were loaded onto a 1% agarose gel (BMA Products, Rockland, Maine) prepared and run in 0.5× Tris-borate-EDTA buffer on a CHEF-DR III apparatus (Bio-Rad Laboratories, Inc., Hercules, Calif.). Electrophoresis was performed at 6 V/cm and 14°C. The run time was 23 h, with pulse times ranging from 5 to 40 s. Gels were stained with ethidium bromide, destained in distilled water, and photographed under UV light with a GelDoc camera (Bio-Rad). PFGE DNA macrorestriction patterns were visually compared and interpreted according to the criteria established by Tenover et al. (38).
Beta-lactamase characterization.
Enterobacter sp. clinical isolates were cultured on tryptic soy broth (Biolife Italiana, Milan, Italy) and harvested, and cell lysates were prepared by sonication. The protein concentrations in the sonic extracts of the cells were determined with the Bradford reagent (Bio-Rad). Detection of beta-lactamases and determination of pIs were performed by IEF electrophoresis with Ampholine PAGplate pH gradient 3 to 9.5 gels in a Multiphor II electrophoresis system apparatus (Amersham Biosciences, Uppsala, Sweden). Beta-lactamases with known pIs (pIs 5.4, 5.6, 6.5, 7.6, and 7.8) were electrophoresed in parallel as controls. Beta-lactamase activity was revealed with nitrocefin (0.5 mg/ml; Calbiochem-Novabiochem Corp., San Diego, Calif.) (29) and with CA prior to testing with nitrocefin for determination of the CA inhibition effect.
Screening for ESBL gene families by colony hybridization.
Clinical isolates of Enterobacter spp. were grown overnight on Mueller-Hinton agar plates containing 100 μg of ampicillin per ml. Single colonies were transferred to Hybond-N+ membranes (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) that were placed on Whatman papers sequentially saturated with a denaturation solution (0.5 M NaOH, 1.5 M NaCl) and a neutralization solution (0.5 M Tris-HCl, 1.5 M NaCl). The DNA was allowed to cross-link to the membrane for 2 min at 302 nm. The probes were prepared by labeling the DNA fragments with [α-32P]dCTP with a random primer DNA labeling mixture (Biological Industries, Beit Haemek, Israel). The TEM-specific probe was prepared from the 467-bp PCR fragment of blaTEM-26, the SHV-specific probe was prepared from the 480-bp PCR fragment of blaSHV-5 (30), and the OXA-4-specific probe was prepared from the 813-bp PCR fragment of blaOXA-4.
Detection of ESBL genes by PCR.
Bacterial cell lysates were used as templates in the specific PCR amplifications for detection of the blaTEM, blaSHV, blaCTX-M, blaIBC, blaPER, blaOXA, blaVEB, and blaSFO genes. Cell lysates were prepared from overnight cultures grown on solid medium (Luria-Bertani medium [LB] plus 12 μg of ceftazidime per ml) by inoculating a single colony into 100 μl of double-distilled water, boiling at 95°C for 10 min, and discarding the cellular debris by centrifugation (12,000 × g, 2 min, 4°C). PCRs were performed with Hot-StarTaq DNA polymerase (Qiagen, Hilden, Germany), according to the instructions of the manufacturer, in the presence of 1 μl of the template DNA preparation in a total volume of 50 μl. The PCR conditions were as follows: 15 min at 95°C and 35 cycles of 1 min at 94°C, 1 min at an annealing temperature designed for each primer set (Table 1), and 1 min at 72°C, followed by 10 min at 72°C. The primer pairs used (Metabion GmbH, Martinsried, Germany) and their respective annealing temperatures and expected product lengths are listed in Table 1. The resulting PCR products were analyzed and visualized in a 1.5% agarose gel with ethidium bromide staining and UV light. PCR analysis was performed with primers specific for the 16S rRNA gene (32). These primers were used as controls to determine whether the cell lysate could be amplified.
TABLE 1.
Oligonucleotides used for PCR amplification of ESBL genes
| Primer type and gene family | Sequencea | Annealing temp (°C) | Reference or source | PCR product size (kb) |
|---|---|---|---|---|
| Screening primers | ||||
| 16S | For: 5′-CCGCACAAGCGGTGGAGCA-3′ | 42 | 32 | 0.4 |
| Rev: 5′-AGGCCCGGGAACGTATTCAC-3′ | ||||
| TEM | For: 5′-TCAACATTTCCGTGTCG-3′ | 42 | This study | 0.86 |
| Rev: 5′-CTGACAGTTACCAATGCTTA-3′ | ||||
| SHV | For: 5′-ATGCGTTATATTCGCCTGTG-3′ | 47 | This study | 0.78 |
| Rev: 5′-AGATAAATCACCACAATGCGC-3′ | ||||
| CTX-M | For: 5′-RGMAGYGYRMCGCTKYATGCSC-3′ | 55 | This study | 0.73 |
| Rev: 5′-ARTARGTSACCAGAAYVAGCGG-3′ | ||||
| IBC | For: 5′-GGGCGTACAAAGATAATTTCC-3′ | 47 | This study | 0.94 |
| Rev: 5′-GAAGCAACGTCGGCTTGAACG-3′ | ||||
| VEB | For: 5′-ACGGTAATTTAACCAGATAGG-3′ | 46 | This study | 0.97 |
| Rev: 5′-ACCCGCCATTGCCTATGAGCC-3′ | ||||
| SFO | For: 5′-GTTAATCCATTTTATGTGAGG-3′ | 44 | This study | 0.94 |
| Rev: 5′-CAGATACGCGGTGCATATCCC-3′ | ||||
| PER | For: 5′-ATGAATGTCATTATAAAAGC-3′ | 42 | 39 | 0.93 |
| Rev: 5′-AATTTGGGCTTAGGGCAGAA-3′ | ||||
| OXA-4 | For: 5′-ACACAATACATATCAACTTCGC-3′ | 42 | 34 | 0.81 |
| Rev: 5′-AGTGTGTTTAGAATGGTGATC-3′ | ||||
| CTX-M | For: 5′-CGYTTTSCIATGTGCAG-3′ | 54 | This study | 0.55 |
| Rev: 5′-ACCGCRATATCRTTGGT-3′ | ||||
| OXA-3 | For: 5′-TTCAAGCCAAAGGCACGATAG-3′ | 42 | 34 | 0.7 |
| Rev: 5′-TTCGAGTTGACTGCCGGGTTG-3′ | ||||
| Full gene amplification and sequencing primers | ||||
| TEM | For: 5′-KACAATAACCCTGRTAAATGC-3′ | 42 | This study | 0.94 |
| Rev: 5′-AGTATATATGAGTAAACTTGG-3′ | ||||
| SHV | For: 5′-TTTATCGGCCYTCACTCAAGG-3′ | 50 | This study | 0.93 |
| Rev: 5′-GCTGCGGGCCGGATAACG-3′ |
For, forward primer; Rev, reverse primer. R, A or G; M, A or C; Y, C or T; K, G or T; S, G or C; I, Inosin.
Sequence analyses.
Sequences were analyzed with an ABI PRISM 3100 Genetic Analyzer (PE Biosystems) with DNA Sequencing Analysis Software and 3100 Data Collection Software (version 1.1).
The nucleotide and deduced protein sequences were analyzed and compared by use of the software available online at http://www.mbio.ncsu.edu/BioEdit/bioedit.html.
Transconjugation experiments.
The Enterobacter sp. and E. coli HB101 isolates studied were grown overnight on LB plates containing either 12 μg of ceftazidime per ml or 200 μg of streptomycin per ml, cross-spread on Mueller-Hinton agar plates, and incubated overnight at 37°C. Colonies appearing in the cross area were pooled and spread on LB plates containing 12 μg of ceftazidime per ml and 200 μg of streptomycin per ml. Isolated colonies, obtained after overnight incubation at 37°C and referred to as transconjugant colonies, were further analyzed.
Plasmid isolation and Southern analysis.
Plasmid DNA was isolated from two selected clinical Enterobacter strains and their E. coli transconjugants by using a Plasmid Midi kit (Qiagen). Plasmid DNA was digested with SmaI, XhoI, and BamHI endonucleases (MBI Fermentas); and the resulting restriction pattern was visualized in a 1% agarose gel by ethidium bromide staining. DNA was transferred from the agarose gel to a positively charged Hybond N+ membrane (Amersham Biosciences, Little Chalfont, United Kingdom) and cross-linked with UV light. The TEM- and SHV-specific probes used in the Southern analysis were the same as those used in the colony hybridization described above.
RESULTS
Bacterial strains and antibiotic susceptibilities.
Seventeen unique patient Enterobacter sp. isolates with an ESBL-producing phenotype were studied. All were identified to the species level: 14 as E. cloacae and 3 as E. aerogenes. The MICs for these isolates show that the MICs of at least one of the cephalosporins tested were within the susceptible range for 8 (47%) isolates and within the intermediately susceptible range for 2 (12%) isolates. Seven strains (41%) were resistant to all cephalosporins and aztreonam (Table 2).
TABLE 2.
MICs of beta-lactams for Enterobacter clinical isolates
| Strain | MIC (μg/ml)a
|
||||||
|---|---|---|---|---|---|---|---|
| AMP | A/S | ATM | CTX | CAZ | CEP | IMP | |
| E. aerogenes 1063 | >32 | >32 | >64 | >64 | >64 | >64 | 2 |
| E. aerogenes 1220 | >32 | >32 | 2 | 8 | >64 | <1 | <0.5 |
| E. aerogenes 1620 | >16 | >32 | >64 | >64 | >64 | >64 | <0.5 |
| E. cloacae 1061 | >32 | >32 | >64 | 16 | >64 | 2 | <0.5 |
| E. cloacae 1434 | >32 | >32 | >64 | >64 | >64 | >64 | <0.5 |
| E. cloacae 1330 | >32 | >32 | >64 | >64 | >64 | 2 | <0.5 |
| E. cloacae 1598 | >32 | >32 | >64 | >64 | >64 | 4 | <0.5 |
| E. cloacae 1005 | >32 | >32 | >64 | >64 | >64 | <1 | <0.5 |
| E. cloacae 1018 | >32 | >32 | >64 | 32 | >64 | 8 | <0.5 |
| E. cloacae 1143 | >32 | >32 | >64 | >64 | >64 | 2 | <0.5 |
| E. cloacae 1250 | >32 | >32 | >64 | 8 | >64 | <1 | <0.5 |
| E. cloacae 1262 | >32 | 16 | 16 | 2 | 16 | <1 | <0.5 |
| E. cloacae 1274 | >32 | >32 | 2 | 8 | 16 | 2 | <0.5 |
| E. cloacae 1408 | >32 | >32 | >64 | >64 | >64 | 16 | <1 |
| E. cloacae 1522 | >32 | >32 | >64 | 8 | >64 | <1 | <0.5 |
| E. cloacae 1527 | >32 | >32 | >64 | 8 | >64 | <1 | <0.5 |
| E. cloacae 1538 | >32 | >32 | >64 | >64 | >64 | 2 | <0.5 |
AMP, ampicillin; A/S, ampicillin sulbactam; ATM, aztreonam; CTX, cefotaxime; CAZ, ceftazidime; CEP, cefepime; IMP, imipenem.
PFGE analysis.
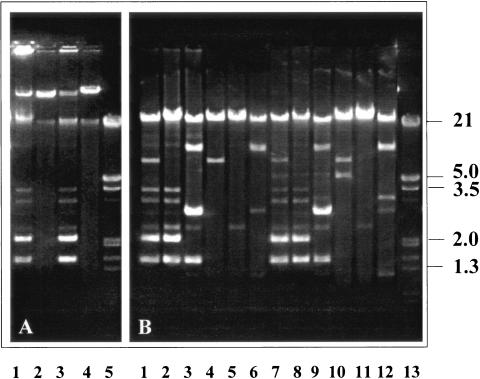
The 17 clinical isolates possessed 15 distinct PFGE patterns; 13 clones included a single isolate each. Two clones, clone C and clone E (including subtype E′), included two isolates each (Fig. 1 and Table 3).
FIG. 1.
PFGE of the Enterobacter sp. clinical isolates. Lane λ, bacteriophage lambda concatemers as molecular size markers; the remaining lanes contain different clinical isolates, as indicated above each lane.
TABLE 3.
IEF analysis and ESBL genes of ESBL-producing Enterobacter sp. isolates
| Strain | PFGE type | pI(s) | Detection and characterization of ESBL genes
|
||||
|---|---|---|---|---|---|---|---|
|
blaTEM
|
blaSHV
|
blaCTX-M by PCR | |||||
| PCR | Colony hybridization | PCR | Colony hybridization | ||||
| E. aerogenes 1063 | B | 5.4, 5.8, 6.6, 7.6, 7.8 | TEM-1 | − | SHV-1 | + | CTX-M-2 like |
| E. aerogenes 1220 | K | 8.2 | −a | − | − | − | − |
| E. aerogenes 1620 | I | 5.4, 8.2 | − | + | − | + | − |
| E. cloacae 1061 | C | 5.4, 6.8, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1434 | C | 5.4, 5.6, 6.6, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1330 | E | 5.4, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1598 | E′ | 5.4 | TEM-1 | − | − | − | − |
| E. cloacae 1005 | J | 5.4, 5.8, 6.8, 7.2, 7.6, 7.8 | − | − | − | − | − |
| E. cloacae 1018 | A | 5.4, 5.8, 6.8 | TEM-1 | + | − | − | CTX-M-26 like |
| E. cloacae 1143 | D | 6.8, 7.8, 8.2, >8.2 | − | − | − | − | − |
| E. cloacae 1250 | N | 5.4, 7.8, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1262 | L | 5.4, 5.6, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1274 | M | 5.4, 7.8, >8.2 | TEM-1 | + | − | − | CTX-M-2 |
| E. cloacae 1408 | O | 6.8, 7, 7.6, 7.8 | − | − | − | − | − |
| E. cloacae 1522 | F | 5.4, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1527 | G | 5.4, 8.2 | TEM-1 | + | SHV-12 | + | − |
| E. cloacae 1538 | H | 5.6, 6.5, 7.6, 7.8, 8.2 | − | − | − | − | − |
−, tested, but yielded negative results.
Screening for ESBL gene families.
IEF followed by colony hybridization was carried out with all Enterobacter isolates to screen for the presence of ESBL gene families. All isolates showed at least one IEF band indicating hydrolysis of nitrocefin. The pIs of these beta-lactamases inhibited by CA ranged from 5.4 to ≥8.2 (Table 3). Thirteen isolates had a beta-lactamase with a pI of 5.4, and 11 strains had a CA-inhibited beta-lactamase with a pI of 8.2. All isolates were screened for the presence of the blaTEM and blaSHV genes by colony hybridization. Ten of them hybridized with the probe specific for the blaTEM gene, and nine hybridized with the blaSHV gene-specific probe (Table 3). Only the positive control strain hybridized with the probe specific for blaOXA-4, and therefore, PCR screening for this gene was not performed.
Analysis of ESBL genes by PCR.
The PCR results showed that one isolate (Enterobacter sp. strain 1063) contained three groups of ESBLs: blaTEM, blaSHV, and blaCTX-M. Seven isolates harbored both blaTEM and blaSHV, two isolates harbored both blaTEM and blaCTX-M, and one isolate harbored only blaTEM. These results were similar to the colony hybridization screening results, with a few exceptions: one isolate positive for blaTEM and blaSHV by colony hybridization did not yield either gene by PCR. On the other hand, two isolates whose colonies did not hybridize with a labeled blaTEM-specific probe yielded a blaTEM product by PCR (Table 3).
PCR analysis for the blaCTX-M family identified three isolates with a blaCTX-M product. All isolates were screened with primers specific for blaOXA-7 and blaPER-1, and all were negative. The results of the screening for blaIBC, blaVEB, and blaSFO were negative as well, although these results must be interpreted with caution due to the absence of suitable positive control strains for these genes.
Sequence analysis of the entire open reading frame of blaTEM revealed that all blaTEM PCR products were TEM-1 (11 isolates) and that all except one of the blaSHV products were SHV-12 (7 isolates); one product was identified as blaSHV-1. PCR detected blaCTX-M in three of our Enterobacter isolates. Sequencing identified two of the three blaCTX-M genes as being similar to blaCTX-M-2 and the second as being similar to blaCTX-M-26 (Table 3). The results of IEF and PCR suggested that the beta-lactamases with a pI of 5.4 were TEM-1 and that in several of the isolates the beta-lactamases with a pI of 8.2 were SHV-12. The band of pI 7.8 could be ascribed to isolates possessing blaCTX-M-2.
SHV-12 was found to coexist with TEM-1 in both isolates belonging to clone C, whereas the other two genetically related Enterobacter strains belonging to clone E and E′ did not carry the same ESBL gene, SHV-12, by IEF and PCR.
Transconjugation experiments.
Successful transconjugation was obtained with four of the isolates, Enterobacter sp. strains 1250, 1061, 1434, and 1262, all of which harbored TEM-1 and SHV-12. The other three Enterobacter strains carrying both TEM-1 and SHV-12 failed to conjugate. Both genes were transferred simultaneously to E. coli HB101 (as detected by PCR and colony hybridization). These transconjugant strains exhibited an ESBL phenotypic profile, based on the disk combination test, and their IEF profiles showed two distinct β-lactamases with pIs of 5.4 and 8.2, as did their parental Enterobacter strains. The transconjugant PCR analysis and sequencing data confirmed that the four transconjugants carried the same beta-lactamases as their parental strains, TEM-1 and SHV-12, proving a transferable ESBL phenotype.
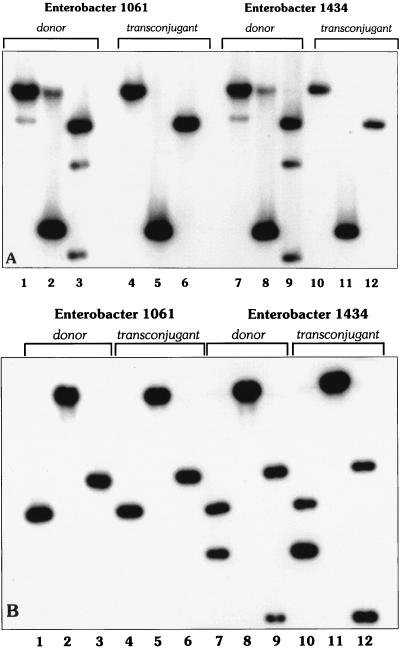
Plasmids were isolated from two representative Enterobacter donor strains (strains 1061 and 1434) and from their respective transconjugants (Fig. 2A). The two donor strains contained various plasmids, one of which was transferred to the transconjugants, suggesting that it carried the ESBL genes.
FIG. 2.
Plasmid DNA (A) and restriction enzyme (B) profiles of two representative Enterobacter isolates and their respective transconjugants carrying both the TEM-1 and the SHV-12 genes. (A) Lane 1, Enterobacter sp. strain 1061; lane 2, transconjugant strain 1061; lane 3, Enterobacter sp. strain 1434; lane 4, transconjugant strain 1434; lane 5, EcoRI-HindIII-digested bacteriophage lambda DNA marker (in kilobases). Only one plasmid was transferred from both Enterobacter strains to the recipient strains. (B) The plasmids from both recipients had identical restriction profiles, suggesting that they are the same plasmid. The restriction patterns of plasmid DNAs digested with the SmaI, XhoI, and BamHI endonucleases are shown in each of the three sets of the following lanes, respectively: lanes 1 to 3, Enterobacter sp. strain 1061; lanes 4 to 6, transconjugant strain 1061; lanes 7 to 9, Enterobacter sp. strain 1434; and lanes 10 to 12, transconjugant strain 1434. Lane 13, DNA size marker.
Restriction analysis of plasmid DNA from donors and transconjugants revealed that each DNA fragment in the transconjugants corresponded to a similar band in the respective donor strain (Fig. 2B). DNA fragments of the same sizes in both Enterobacter donors and the respective transconjugants were hybridized with the TEM- and SHV-specific probes, proving the presence of both genes on the same plasmid (Fig. 3A and B). Enterobacter sp. strain 1434 and its transconjugant showed an additional DNA fragment that hybridized with the SHV-specific probe, suggesting the presence of an additional copy of the blaSHV gene on the plasmid. Interestingly, Southern analysis showed an additional DNA fragment that hybridized with the TEM-specific probe in both the Enterobacter donor strains and the transconjugants (Fig. 3A).
FIG. 3.
Southern blot analysis of plasmid DNA from Enterobacter sp. strains 1061 and 1434 and their transconjugants. Plasmid DNA was digested with the SmaI, XhoI, and BamHI endonucleases in lanes 1 to 3, 4 to 6, 7 to 9, and 10 to 12, respectively, and hybridized with TEM-specific (A) and SHV-specific (B) probes. Hybridization with the TEM-specific and SHV-specific probes proves the existence of these genes on the same plasmid. Enterobacter sp. strain 1064 carried two copies of TEM, one of which was transferred together with SHV to the transconjugant. The plasmid of Enterobacter sp. strain 1434 had two copies of SHV, and both were transferred to the transconjugant, together with TEM.
DISCUSSION
In a recent study (27) we showed that the ESBL phenotype occurred in 22% of Enterobacter isolates at Tel Aviv Sourasky Medical Center. In the present study, we attempted to determine the resistance determinants that conferred this phenotype and to understand the molecular epidemiology of these organisms.
We characterized 17 unique patient isolates of Enterobacter spp. identified phenotypically as ESBL producers. We were able to prove by IEF and genetic sequencing the presence of at least one ESBL in 9 of the 17 isolates. We believe, however, on the basis of the IEF and colony hybridization results, that at least 13 isolates and, more likely, all 17 isolates carried an ESBL gene. For those isolates from which we failed to amplify and sequence an ESBL gene, we suspect the presence of other classes of ESBLs or technical problems with the PCR primers. There are two main mechanisms for the spread of ESBLs: the spread of an ESBL-producing clone or dissemination of an ESBL-encoding plasmid among various clones. In several studies, clonal spread was found to account for the dispersion of ESBL-producing Enterobacter spp. (12, 18, 23). In other cases, multiple clones were found, although usually only one was shown to be dominant (4, 11). In one of these cases, one specific enzyme was found among polyclonal isolates of Enterobacter (4). In one report from South Korea, SHV-12 was detected in several genetically unrelated Enterobacter isolates. This finding indicates that the ESBL phenotype had spread due not to an outbreak of a resistant strain but rather to the dissemination of the SHV-12 beta-lactamase (21). In France, where one E. aerogenes clone dominated, two populations of this clone were found: one carried the gene for TEM-24 and the other carried the gene for SHV-4 (23). Later, the TEM-24-carrying group spread further, causing a wide outbreak, while the SHV-carrying strains disappeared. In our study, PFGE typing revealed that Enterobacter isolates belonged to distinct clones, with only 2 of 17 cases explained by clonal spread. IEF, colony hybridization, and PCR results were consistent with the presence of various enzymes belonging to different gene families of ESBLs, SHV and CTX-M. Interestingly, TEM-1 was detected in all 11 isolates from which the TEM gene was sequenced. This finding does not explain the ESBL phenotype; moreover, one would not expect TEM-1 to confer any evolutionary advantage to AmpC-carrying organisms such as Enterobacter spp. The major ESBL gene, blaSHV-12, was found to coexist with blaTEM-1 in seven isolates. Moreover, conjugation experiments with four strains identified TEM-1 and SHV-12 as beta-lactamases transferred together. Indeed, plasmid isolation followed by Southern analysis proved the coexistence of these two genes on the same plasmid. We hypothesize that TEM-1-encoding plasmids, which have the ability to spread rapidly between Enterobacter isolates, acquired SHV-12. A similar linkage between a blaTEM gene (TEM-1B) and a blaSHV gene (SHV-12) was found in an Enterobacter amnigenus strain isolated in Thailand (6) and an E. cloacae strain isolated in Guangzhou, China (7) and in E. cloacae and E. aerogenes isolates in South Korea (20, 21). In all these cases, both genes cotransferred to a recipient E. coli strain in transconjugation experiments, as in our case. The coexistence of blaTEM and blaSHV in clinical isolates of E. cloacae was also reported in Italy, where the rate of coexistence was 50% (37), and in Philadelphia, Pa. (22). In France, on the other hand, one E. cloacae isolate was found to carry a TEM-1 gene together with CTX-M-1 on the same 55-kb plasmid, and another isolate was found to carry both TEM-1 and CTX-M-3 on the same 180-kb plasmid. Both plasmids were transferred to E. coli by transconjugation and, as revealed by IEF, expressed two beta-lactamases (one with a pI of 5.4 corresponding to TEM-1 and one with a pI of 8.4 corresponding to both CTX-M enzymes) (13).
In several isolates we found discrepancies between the results of IEF, colony hybridization, and PCR. Such discrepancies are not unusual and may be related to various degrees of expression of the ESBL genes, the existence of ESBL genes that belong to other families or that do not react with the specific primers used, and other factors. For example, the CTX-M family of enzymes is fairly diverse, with various blaCTX-M genes showing as little as 67% homology with one another.
Taken together, the high prevalence of ESBLs in Enterobacter spp. at the Tel Aviv Sourasky Medical Center is attributable not to clonal spread but, rather, to the dissemination of plasmids carrying different class A enzymes among genetically diverse isolates.
Acknowledgments
We thank Karen Bush and Anne Marie Queenan (R. W. Johnson Pharmaceutical Research Institution, Raritan, N.J.) and George Jacoby (Lahey Clinic, Burlington, Mass.) for providing us with the ESBL-producing bacterial strains used as positive controls in the IEF and PCR experiments and for technical advice.
This work was supported by a grant from the Israel-United States Binational Science Foundation.
REFERENCES
- 1.Barnaud, G., R. Labia, L. Raskine, M. J. Sanson-Le Pors, A. Philippon, and G. Arlet. 2001. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol. Lett. 195:185-190. [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio, G., M. Perilli, S. Stefani, G. Amicosante, and G. Nicoletti. 2002. Prevalence of extended spectrum beta-lactamases among Enterobacteriaceae: an Italian survey. Int. J. Antimicrob. Agents 19:213-217. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, P. A. 2001. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canton, R., A. Oliver, T. M. Coque, C. Varela Mdel, J. C. Perez-Diaz, and F. Baquero. 2002. Epidemiology of extended-spectrum beta-lactamase-producing Enterobacter isolates in a Spanish hospital during a 12-year period. J. Clin. Microbiol. 40:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanal, C., D. Sirot, J. P. Romaszko, L. Bret, and J. Sirot. 1996. Survey of prevalence of extended spectrum beta-lactamases among Enterobacteriaceae. J. Antimicrob. Chemother. 38:127-132. [DOI] [PubMed] [Google Scholar]
- 6.Chanawong, A., F. H. M'Zali, J. Heritage, A. Lulitanond, and P. M. Hawkey. 2001. SHV-12, SHV-5, SHV-2a and VEB-1 extended-spectrum beta-lactamases in gram-negative bacteria isolated in a university hospital in Thailand. J. Antimicrob. Chemother. 48:839-852. [DOI] [PubMed] [Google Scholar]
- 7.Chanawong, A., F. H. M'Zali, J. Heritage, J. H. Xiong, and P. M. Hawkey. 2002. Three cefotaximases, CTX-M-9, CTX-M-13, and CTX-M-14, among Enterobacteriaceae in the People's Republic of China. Antimicrob. Agents Chemother. 46:630-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow, J. W., M. J. Fine, D. M. Shlaes, J. P. Quinn, D. C. Hooper, M. P. Johnson, R. Ramphal, M. M. Wagener, D. K. Miyashiro, and V. L. Yu. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585-590. [DOI] [PubMed] [Google Scholar]
- 9.Chow, J. W., V. L. Yu, and D. M. Shlaes. 1994. Epidemiologic perspectives on Enterobacter for the infection control professional. Am. J. Infect. Control 22:195-201. [DOI] [PubMed] [Google Scholar]
- 10.Cosgrove, S. E., K. S. Kaye, G. M. Eliopoulous, and Y. Carmeli. 2002. Health and economic outcomes of the emergence of third-generation cephalosporin resistance in Enterobacter species. Arch. Intern. Med. 162:185-190. [DOI] [PubMed] [Google Scholar]
- 11.De Gheldre, Y., M. J. Struelens, Y. Glupczynski, P. De Mol, N. Maes, C. Nonhoff, H. Chetoui, C. Sion, O. Ronveaux, and M. Vaneechoutte. 2001. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J. Clin. Microbiol. 39:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumarche, P., C. De Champs, D. Sirot, C. Chanal, R. Bonnet, and J. Sirot. 2002. TEM derivative-producing Enterobacter aerogenes strains: dissemination of a prevalent clone. Antimicrob. Agents Chemother. 46:1128-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutour, C., R. Bonnet, H. Marchandin, M. Boyer, C. Chanal, D. Sirot, and J. Sirot. 2002. CTX-M-1, CTX-M-3, and CTX-M-14 beta-lactamases from Enterobacteriaceae isolated in France. Antimicrob. Agents Chemother. 46:534-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridkin, S. K., and R. P. Gaynes. 1999. Antimicrobial resistance in intensive care units. Clin. Chest Med. 20:303-316, viii. [DOI] [PubMed] [Google Scholar]
- 15.Giakkoupi, P., L. S. Tzouvelekis, A. Tsakris, V. Loukova, D. Sofianou, and E. Tzelepi. 2000. IBC-1, a novel integron-associated class A beta-lactamase with extended-spectrum properties produced by an Enterobacter cloacae clinical strain. Antimicrob. Agents Chemother. 44:2247-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girlich, D., L. Poirel, A. Leelaporn, A. Karim, C. Tribuddharat, M. Fennewald, and P. Nordmann. 2001. Molecular epidemiology of the integron-located VEB-1 extended-spectrum beta-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J. Clin. Microbiol. 39:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacoby, G. A., and A. A. Medeiros. 1991. More extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 35:1697-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kartali, G., E. Tzelepi, S. Pournaras, C. Kontopoulou, F. Kontos, D. Sofianou, A. N. Maniatis, and A. Tsakris. 2002. Outbreak of infections caused by Enterobacter cloacae producing the integron-associated beta-lactamase IBC-1 in a neonatal intensive care unit of a Greek hospital. Antimicrob. Agents Chemother. 46:1577-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye, K. S., S. Cosgrove, A. Harris, G. M. Eliopoulos, and Y. Carmeli. 2001. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob. Agents Chemother. 45:2628-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. H., and S. H. Jeong. 2002. Antibiotic susceptibility of bacterial strains isolated from patients with various infections. Lett. Appl. Microbiol. 34:215-221. [DOI] [PubMed] [Google Scholar]
- 21.Lee, S. H., J. Y. Kim, S. H. Shin, Y. J. An, Y. W. Choi, Y. C. Jung, H. I. Jung, E. S. Sohn, S. H. Jeong, and K. J. Lee. 2003. Dissemination of SHV-12 and characterization of new AmpC-type beta-lactamase genes among clinical isolates of Enterobacter species in Korea. J. Clin. Microbiol. 41:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levison, M. E., Y. V. Mailapur, S. K. Pradhan, G. A. Jacoby, P. Adams, C. L. Emery, P. L. May, and P. G. Pitsakis. 2002. Regional occurrence of plasmid-mediated SHV-7, an extended-spectrum beta-lactamase, in Enterobacter cloacae in Philadelphia teaching hospitals. Clin. Infect. Dis. 35:1551-1554. [DOI] [PubMed] [Google Scholar]
- 23.Mammeri, H., G. Laurans, M. Eveillard, S. Castelain, and F. Eb. 2001. Coexistence of SHV-4- and TEM-24-producing Enterobacter aerogenes strains before a large outbreak of TEM-24-producing strains in a French hospital. J. Clin. Microbiol. 39:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, Y., and M. Inoue. 1999. Characterization of SFO-1, a plasmid-mediated inducible class A beta-lactamase from Enterobacter cloacae. Antimicrob. Agents Chemother. 43:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Nosocomial Infection Surveillance System. 1999. A report from the National Nosocomial Infections Surveillane (NNIS) System. Data summary from January 1990-May 1999. Am. J. Infect. Control 27:520-532. [DOI] [PubMed] [Google Scholar]
- 26.National Nosocomial Infection Surveillance System. 1996. A report from the National Nosocomial Infections Surveillane (NNIS) System. Data summary from October 1986-April 1996. Am. J. Infect. Control 24:380-388. [PubMed] [Google Scholar]
- 27.Navon-Venezia, S., O. Hammer-Münz, D. Schwartz, D. Turner, B. Kuzmenko, and Y. Carmeli. 2003. Occurrence and phenotypic characteristics of extended-spectrum beta-lactamases among members of the family Enterobacteriaceae at the Tel-Aviv Medical Center (Israel) and evaluation of diagnostic tests. J. Clin. Microbiol. 41:155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. Approved standard M100-S12. NCCLS, Wayne, Pa.
- 29.Neuwirth, C., E. Siebor, J. Lopez, A. Pechinot, and A. Kazmierczak. 1996. Outbreak of TEM-24-producing Enterobacter aerogenes in an intensive care unit and dissemination of the extended-spectrum beta-lactamase to other members of the family Enterobacteriaceae. J. Clin. Microbiol. 34:76-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palucha, A., B. Mikiewicz, W. Hryniewicz, and M. Gniadkowski. 1999. Concurrent outbreaks of extended-spectrum beta-lactamase-producing organisms of the family Enterobacteriaceae in a Warsaw hospital. J. Antimicrob. Chemother. 44:489-499. [DOI] [PubMed] [Google Scholar]
- 31.Pitout, J. D., K. S. Thomson, N. D. Hanson, A. F. Ehrhardt, P. Coudron, and C. C. Sanders. 1998. Plasmid-mediated resistance to expanded-spectrum cephalosporins among Enterobacter aerogenes strains. Antimicrob. Agents Chemother. 42:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relman, D. A., J. S. Loutit, T. M. Schmidt, S. Falkow, and L. S. Tompkins. 1990. The agent of bacillary angiomatosis: an approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573-1580. [DOI] [PubMed] [Google Scholar]
- 33.Sanders, W. E., Jr., and C. C. Sanders. 1997. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin. Microbiol. Rev. 10:220-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwaber, M. J., P. M. Raney, J. K. Rasheed, J. W. Biddle, P. Williams, J. E. McGowan, Jr., and F. C. Tenover. 2004. Utility of NCCLS guidelines for identifying extended-spectrum beta-lactamases in non-Escherichia coli and non-Klebsiella spp. of Enterobacteriaceae. J. Clin. Microbiol. 42:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwaber, M. J., C. S. Graham, B. E. Sands, H. S. Gold, and Y. Carmeli. 2003. Treatment with a broad-spectrum cephalosporin versus piperacillin-tazobactam and the risk for isolation of broad-spectrum cephalosporin-resistant Enterobacter species. Antimicrob. Agents Chemother. 47:1882-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.She, D., and Y. Liu. 2002. The expression of AmpC and extended-spectrum beta-lactamases among clinical isolates of Enterobacter cloacae and its impact on antibiotics susceptibility. Zhonghua Yi Xue Za Zhi 82:1355-1358. [PubMed] [Google Scholar]
- 37.Spanu, T., F. Luzzaro, M. Perilli, G. Amicosante, A. Toniolo, and G. Fadda. 2002. Occurrence of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae in Italy: implications for resistance to beta-lactams and other antimicrobial drugs. Antimicrob. Agents Chemother. 46:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weldhagen, G. F., L. Poirel, and P. Nordmann. 2003. Amber class A extended-spectrum β-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob. Agents Chemother. 47:2385-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winokur, P. L., R. Canton, J. M. Casellas, and N. Legakis. 2001. Variations in the prevalence of strains expressing an extended-spectrum beta-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin. Infect. Dis. 32:S94-S103. [DOI] [PubMed] [Google Scholar]