Abstract
Background:
Persistent psychological stress often leads to anxiety disorders and depression. Benzodiazepines and selective serotonin reuptake inhibitors are popular treatment options but have limited efficacy, supporting the need for alternative treatment. Based on our recent preclinical work suggesting a causal link between neurobehavioral deficits and elevated oxidative stress, we hypothesized that interventions that mitigate oxidative stress can attenuate/overcome neurobehavioral deficits.
Methods:
Here, we employed the rat social defeat model of psychological stress to determine whether increasing antioxidant levels using grape powder would prevent and/or reverse social defeat-induced behavioral and cognitive deficits. Furthermore, a hippocampal-derived HT22 cell culture model of oxidative stress was employed to identify the individual beneficial constituent(s) of grape powder and the underlying mechanism(s) of action.
Results:
Grape powder treatment prevented and reversed social defeat-induced behavioral and cognitive deficits and also decreased social defeat-induced increase in plasma corticosterone and 8-isoprostane (systemic and oxidative stress markers, respectively). And grape powder treatment replenished social defeat-induced depleted pool of key antioxidant enzymes glyoxalase-1, glutathione reducatse-1, and superoxide dismutase. Grape powder constituents, quercetin and resveratrol, were most effective in preventing oxidative stress-induced decreased cellular antioxidant capacity. Grape powder protected oxidative stress-induced cell death by preventing calcium influx, mitochondrial dysfunction, and release of cytochrome c.
Conclusions:
Grape powder treatment by increasing antioxidant pool and preventing cell damage and death prevented and reversed social defeat-induced behavioral and cognitive deficits in rats. Quercetin and resveratrol are the major contributors towards beneficial effects of grape powder.
Keywords: Social defeat, grape powder, oxidative stress, resveratrol, quercetin
Significance Statement
Current pharmacological therapies used for treatment of stress-associated mental comorbidities such as anxiety, depression, and cognitive impairment are less efficacious and associated with severe side effects. Therefore, alternative treatments are needed. Since stress is a daily unavoidable phenomenon, use of naturally available substances as a potential therapy for stress-associated diseases would be beneficial. In this study, we propose the potential protective role of grape powder in preventing/reversing psychological stress-induced behavioral and cognitive deficits.
Introduction
Stress in life is unavoidable; yet, persistent psychological stress often leads to development of anxiety, depression, and cognitive impairment (Somers et al., 2006; Cohen et al., 2007). The current pharmacotherapy includes use of benzodiazepines and selective serotonin reuptake inhibitors. Though these pharmacological interventions are regarded as the “gold standard” of treatment, these are associated with severe side effects including, but not limited to, tolerance, withdrawal effects, memory dysfunction, and significant weight gain (Gudex, 1991; Ashton, 1994; Trindade et al., 1998; Ferguson, 2001; Barker et al., 2004). Therefore, improvement in therapeutic interventions with higher efficacy and fewer side effects is needed. Animal studies have provided novel insights and the opportunity for identification of novel molecular targets and alternative treatment strategies for anxiety and depression. Several groups have suggested potential involvement of oxidative stress mechanisms in anxiety and depression (Oliveira-Dos-Santos et al., 2000; Masood et al., 2008; Salim et al., 2011a), revealing a potentially new therapeutically relevant avenue. Our own work has suggested that psychological stress induced via social defeat causes behavioral and cognitive deficits in rats while increasing oxidative stress systemically as well as in the brain (Patki et al., 2013a). Increase in oxidative stress was associated with reduced systemic and cerebral antioxidant status. Our hypothesis is that if a rise in oxidative stress causes behavioral and cognitive deficits, interventions that mitigate oxidative stress should attenuate/overcome neurobehavioral deficits. The resent study addresses this hypothesis. Using a rat model of social defeat, we explored the beneficial effects of a standardized, freeze-dried grape powder (GP; California Table Grape Commission), rich in polyphenols, on psychological stress-induced behavioral and cognitive impairment in rats.
The social defeat (SD) model resembles societal stress in humans and represents an ethologically valid stressor, as it induces long-lasting physiologic and behavioral changes (Hollis and Kabbaj, 2014). Rodents exhibit increased anxiety-like and depression-like behavior, impaired memory, social avoidance, and decreased locomotor and exploratory activity following four consecutive social defeat exposures (Koolhaas et al., 1997; Meerlo et al., 1997; Tidey and Miczek, 1997). Beneficial effects of grapes have been suggested in combating various diseases, including cancer, neurodegenerative, neuropsychiatric, and metabolic disorders (Morre and Morre, 2006; Wang et al., 2009; Chuang and McIntosh, 2011; Solanki et al., 2015). Grape polyphenols, namely resveratrol, quercetin, and kaempferol, are known for their potent antioxidant, antiproliferative, antiinflammatory, cardio-protective, and neuro-protective properties (Shi et al., 2003; Yilmaz and Toledo, 2004; Joseph et al., 2009). However, which grape components are responsible for beneficial effects is not clearly understood. To investigate the potential bioactive component, we used a hippocampus-derived immortalized cell line (HT22), and simulated oxidative stress using buthionine sulfoximine (BSO). Furthermore, the mechanism by which GP modulates oxidative stress pathway and regulates biochemical changes within the hippocampus is unclear. For example, oxidative stress-induced hippocampal neuronal death has been reported (Behl et al., 1997; Liu et al., 2010), but the mechanism is uncertain. Therefore, using the in vitro model of oxidative stress in HT22 cells, we focused our attention on the oxidative stress pathway and investigated the mechanism by which GP modulates oxidative stress pathway and protects the hippocampal neurons from cell death.
Methods
Freeze-Dried GP
Freeze-dried GP was supplied in small, sealed sachets by California Table Grape Commission, CA. The GP is a composite of fresh seeded and seedless red, green, and black grapes that were freeze-dried, ground, and processed to retain the integrity of the bioactive compounds. The GP was stored at -80°C upon receipt. GP was dissolved in tap water at a concentration of 15 g/L. The GP solution was prepared fresh daily and fed orally ad libitum to the rats. This dose was attentively chosen based upon our pilot dose-response studies. Previously, this dose was reported to produce the most pronounced effects on rat behavior (Allam et al., 2013; Patki et al., 2013b; Solanki et al., 2015). Because sugar makes up the majority of the GP, we prepared a placebo control consisting of a 1:1 ratio of glucose and fructose and administered to the rats in a similar fashion as the GP.
Animals and Housing Conditions
Adult male Sprague-Dawley rats and male Long-Evans retired breeders rats were purchased from Charles River Laboratories, Wilmington, MA. Sprague-Dawley rats weighing 275 to 300 g served as control or intruders and retired breeders. Long-Evans rats weighing 400 to 500 g were used as resident for social defeat model. Rats were singly housed in a climate-controlled room on a 12-hour-light/12-hour-dark cycle and provided regular chow diet and/or water/GP/placebo ad libitum. All experiments were conducted following NIH guidelines and approved protocols from the University of Houston Animal Care Committee.
Social Defeat (SD) Rat Model of Psychological Stress
We used the modified version of the resident-intruder paradigm (supplementary Methods). Two experimental designs were followed. In one, protective effect of GP was examined and in another reversal effect of GP was investigated. Rats were randomly assigned to the following groups in both experimental designs (supplementary Figure 1A).
Protective Experimental Design
NC: Naïve control rats, GPNC: Naïve control rats pretreated with GP for 3 weeks, CE: Control exposure where intruders subjected to the resident’s cage in the absence of the resident, SD: Socially defeated rats, GPSD: Socially defeated rats pretreated with grape powder for 3 weeks.
Reversal Experimental Design
NC: Naïve control rats, NCGP: GP treated naïve control rats (3 weeks of GP treatment), CE: Control exposure where intruders subjected to the residents cage when the residents were not present, SD: Socially defeated rats, SDGP: Socially defeated rats treated with grape powder for 3 weeks, SDPL: Socially defeated rats treated with placebo for 3 weeks.
Behavioral Assessments
Measurement of Anxiety-Like Behavior
Anxiety-like behavior was measured using light-dark (LD) test, elevated plus maze (EPM), open field test (OFT), and marble burying (MB) test as previously published by us (Salim et al., 2010; Vollert et al., 2011) (supplementary Methods).
Measurement of Depression-Like Behavior
Depression-like behavior was measured using forced swim test as previously described by us (Solanki et al., 2015) (supplementary Methods).
Measurement of Cognitive Function
Learning and memory function were assessed using the radial arm water maze test as previously published by us (Allam et al., 2013) (supplementary Methods).
One behavioral test was performed on one given day, enabling a rest period of 20 to 24 hours between 2 different behavior tests. The first behavioral test for anxiety-like behavior (OFT) was performed on the day following last day of social defeat (protective effect protocol)/GP treatment (reversal effect protocol). This was then followed by light dark test, EPM test, marble burying test, radial arm water maze test, and forced swim test on separate consecutive days in that order.
Brain Dissections and Collection of Plasma
Rats were anesthetized (isoflurane, #57319-479-06, Phoenix Pharmaceuticals) 24 hours after conclusion of all behavioral tests. The brains were quickly removed and rapidly frozen and stored at -80°C until analysis. Blood collection and plasma separation was performed immediately and samples stored at -80°C. Hippocampus, amygdala, and prefrontal cortex (PFC) were identified according to Paxinos and Watson (Paxinos G, 1986) and grossly dissected out.
Measurement of Corticosterone Levels and Indices of Oxidative Stress
Stress-induced release of corticosterone (systemic stress marker) levels was measured in plasma using an enzyme immunoassay (EIA)-based kit (cat # 500655, Cayman Chemical) per the manufacturer’s instructions. The plasma level of 8-isoprostane, a marker of oxidative stress, was also measured using EIA kit (cat # 516351, Cayman Chemical).
Western-Blot Analysis
Brain tissues were homogenized and protein concentration was determined as previously published by us (Vollert et al., 2011). Equal amounts of brain tissue homogenate proteins diluted with 2× laemmli buffer were subjected to SDS-polyacrylamide gel electrophoresis and western blotting as previously published by us (Vollert et al., 2011). Primary antibody dilutions used were as follows; glyoxalase-1 (GLO-1, 1:200, Abcam, Cambridge, MA), glutathione reductase-1 (GSR-1, 1:100, obtained from Dr. Iris Hovatta, Finland), copper-zinc superoxide dismutase (Cu-Zn SOD, 1:1000, EMD Millipore, MA), manganese superoxide dismutase (MnSOD, 1:1000, EMD), and a loading control, β-actin (1:1000, Santacruz Biotechnology, Santa Cruz, CA). The detection of the protein as immunoreactive bands was performed using horseradish peroxidase-conjugated secondary antibody. The images of immunoblots were captured by a Fluorchem 8900 imaging system with intensity of each immunoreactive band determined using Alpha Ease FC 4.0 (Alpha Innotech Corp., San Leandro, CA).
Enzyme Activity Assays
GLO-1 activity (Cat# MAK114, Sigma-Aldrich), total glutathione (GSH), and SOD activity (Cat# 703002, 706002 Cayman) in plasma was measured using EIA kit per the manufacturer’s instructions.
Total Phenolic Content Measurement
To estimate the presence of grape polyphenols in the hippocampus, the total phenolic content was measured spectrophotometrically using Folin Ciocalteu’s method (Iva Juranovic Cindric, 2011).
Cell Culture
The immortalized mouse hippocampal cell line (HT22) was received as a generous gift from Dr. Dave Schubert from the Salk Institute, La Jolla. Materials used for cell growth are mentioned in the supplementary section. HT22 cells were seeded into 6-well plates and divided into 5 groups: control (phosphate-buffered saline, PBS), BSO alone (1 mM in PBS), ethanol alone, resveratrol (R)/quercetin (Q)/kaempferol (K) (1–20 μM) with BSO, and GP (2400–10000 μg/mL) with BSO. BSO was purchased from Sigma-Aldrich (St. Louis, MO). The 1-mM dose of BSO was chosen based on previous studies from our group (Salim et al., 2011b; Salvi et al., 2016). The treatment of BSO or vehicle (DMEM media) was initiated when cells were 60% to 70% confluent. Cells were pretreated with either R/Q/K or GP for 4 hours followed by BSO treatment that lasted 14 hours (supplementary Figure 1B). For mechanistic studies, cells were seeded into 6-well cell culture plates and divided into 4 groups: control (PBS), BSO alone, placebo pretreatment for 4 hours followed by 14 hours of BSO treatment (placebo+BSO) and 14 hours of BSO treatment following 4 hours of GP treatment (GP+BSO). All experiments were conducted at least 3 to 4 times.
HT22 Cell Lysate
Harvesting of HT22 cells were done by trypsinization and cell pellet (approximately 7.5 × 105 cells) was obtained by centrifugation. The cell pellet was homogenized either using PBS or specific assay buffer. The homogenate was subjected to centrifugation at 10000 x g for 15 minutes at 4°C and supernatant was collected. This supernatant was used for determining total antioxidant capacity.
Total Antioxidant Capacity (TAC) Measurement
Measurement of TAC in HT22 cell lysates, plasma, and hippocampus was performed using EIA based kit following the manufacturer’s protocol (catalog no. 709001, Cayman).
Calcium Assay
Calcium assay (catalog no. ab102505, Abcam) was performed using HT22 cell lysates following the manufacturer’s protocol.
Mitochondrial Membrane Potential Measurement by JC-1
HT22 cells were cultured at 1.5 x 104 in a 96-well plate in 200 μL of culture medium. Following the overnight incubation of cells in a humidified environment at 37°C with 10% CO2, mitochondrial membrane potential was analyzed using a kit based assay per the manufacturing protocol (catalog no. 600880, Cayman).
Caspase-3 Assay
Caspase-3 assay (catalog no. ab39401, Abcam) was performed using HT22 cell lysates per following the manufacturer’s protocol.
Statistical Analysis
All values are reported as mean+SEM. Comparisons between groups were made by 1-way ANOVA with subsequent Tukey’s posthoc test where appropriate (GraphPad Software, Inc., San Diego, CA). P<.05 was used to denote statistically significant groups.
Results
SD-Induced Anxiety-Like Behavior Is Reversed and Prevented with GP Treatment
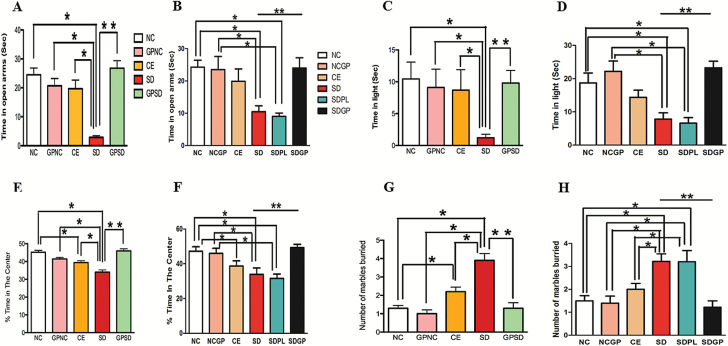
In the EPM test, socially defeated rats spent significantly less time in the open arm of EPM apparatus compared with control rats, an indicator of elevated anxiety-like behavior. GP treatment prevented (F4,45=15.82, P<.0001; Figure 1A) and reversed (F5,51 = 4.51, P=.0017; Figure 1B) SD-induced anxiety-like behavior, suggesting its anxiolytic effect.
Figure 1.
Examination of anxiety-like behavior using elevated plus maze (A-B), light-dark (C-D), open field (E-F), and marble burrow (G-H) tests in the control or socially defeated (SD) rats with/without grape powder (GP) treatment after protective protocol (A, C, E, G) and reversal protocol (B, D, F, H). Rats subjected to SD spent significantly less time in the open arm of the EPM, lit area of the LD box, and center area of the open field arena that was protected and reversed with GP treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDPL, SD rats treated with placebo; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD/SDPL, P < .05. Values are mean ± SEM, n = 10 to 12 rats/group.
In the LD test, we observed that NC, GPNC, CE, and NCGP rats spent similar time in the lit compartment while SD rats spent significantly less time in the lit area. Similar to the EPM test, the GP treatment before (F4,44 = 2.419, P=.0421; Figure 1C) and after (F5,46=7.22, P<.0001; Figure 1D) SD significantly increased the time spent in the lit area, suggesting its protective and reversal effects in mitigating SD-induced anxiety-like behavior.
In the OFT, SD rats spent an average of 34.04 ± 1.21 percent time in the center of the arena, which is significantly shorter than the control groups. SD rats treated with GP spent a significantly greater percentage of time in the center of the arena, suggesting its anxiolytic effects (F4,39=19.02, P<.0001, Figure 1E; F5,48 = 5.92, P=.0002, Figure 1F).
In the MB test, SD rats buried more marbles than control groups. Interestingly, control exposure rats exhibited anxiety-like behavior as indicated by the increased number of marbles buried compared with the naïve control group. Additionally, similar to what was observed in the EPM, LD, and OF, the MB data showed that GP treatment in SD rats protected (F4,45 = 19.31, P < .0001, Figure 1G) and reversed (F5,47 = 8.21, P<.0001, Figure 1H) anxiety-like behavior as demonstrated by a marked decrease in number of marbles buried. Overall, these data suggest that SD-induced anxiety-like behavior was protected and reversed by GP treatment.
SD-Induced Depression-Like Behavior Is Reversed and Prevented with GP Treatment
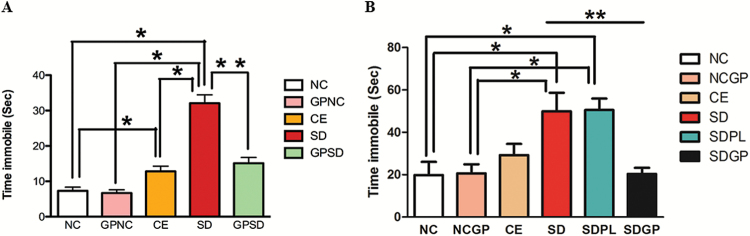
In the forced swim test, the SD rats exhibited significantly higher immobility compared with control groups. Interestingly, control exposure rats exhibited higher immobility than the naïve control group. The GP-treated SD rats showed significantly decreased immobility that was similar to that of control groups (F4,45 = 43.04, P<.0001, Figure 2A; F5,48 = 5.36, P=.0005, Figure 2B). These data suggest an antidepressant-like effect of GP in SD rats.
Figure 2.
Examination of depression-like behavior using forced swim test in control or socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to SD spent significantly increased amount of time immobile compared with control groups that was protected (A) and reversed (B) with GP treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDPL, SD rats treated with placebo; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD/SDPL, P < .05. Values are mean ± SEM, n = 10 to 12 rats/group.
SD-Induced Impaired Short- and Long-Term Memory Are Reversed and Prevented with GP Treatment
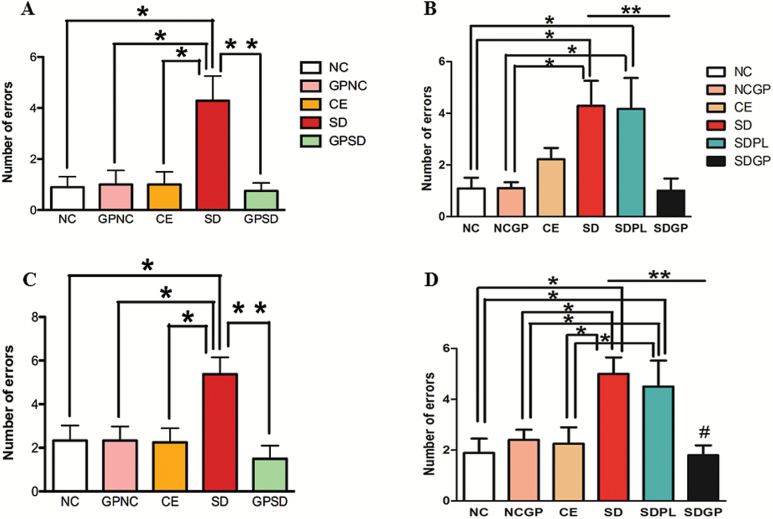
In the short-term memory (STM) test, performed 30 minutes after the end of the 12th learning trial, SD rats made significantly more errors than control groups. Three weeks of GP treatment significantly reduced the number of errors in both protection (F4,38 = 6.36, P=.0005; Figure 3A) and reversal (F5,47 = 6.21, P=.0002; Figure 3B) protocols. Similar results were obtained in the long-term memory (LTM) test, which was performed 24 hours after the 12th learning trial. SD rats made an average of 5.37 ± 0.77 errors in the LTM task that was significantly higher compared with all other control groups. GP treatment significantly improved their LTM as indicated by decreased errors made in the LTM task (F4,38=4.76, P=.0034, Figure 3C; F5,46 = 5.56, P=.0004, Figure 3D). It is noteworthy that GP treatment failed to improve performance in control rats, as both the control groups with and without GP treatment performed similarly on the STM and LTM tasks. However, GP treatment significantly enhanced STM and LTM of SD rats.
Figure 3.
Examination of memory using radial-arm water maze (RAWM) memory test in control or socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to SD exhibited impaired short (A-B) and long-term memory (C-D) compared with control groups that was protected (A, C) and reversed (B, D) with GP treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDPL, SD rats treated with placebo; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD/SDPL, P < .05. Values are mean ± SEM, n = 10 to 12 rats/group.
SD-Induced Increased Plasma Levels of Corticosterone and 8-Isoprostane Are Reversed and Prevented with GP Treatment
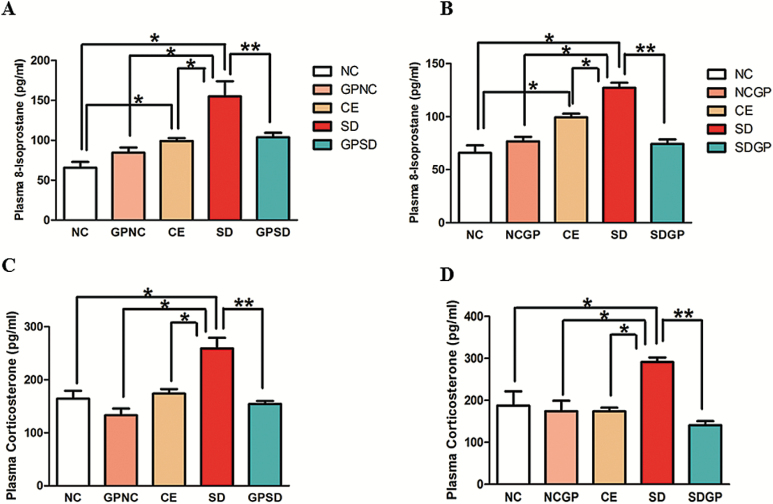
SD rats exhibited increased levels of plasma corticosterone compared with control groups. Interestingly, control exposure rats also exhibited increased levels of plasma corticosterone compared with the naïve control group. GP treatment significantly decreased SD-induced increase in plasma corticosterone levels (F4,13=12.25, P<.0002, Figure 4A; F4,15=7.93, P=.0012, Figure 4B).
Figure 4.
Analysis of plasma corticosterone and 8-isoprostane in control or socially defeated rats treated with/without grape powder. Rats subjected to social defeat showed marked increase in plasma levels of 8-isoprostane (A, B) and corticosterone (C, D) as compared to control groups that was protected and reversed with grape powder treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD, P < .05. Values are mean ± SEM, n = 4 to 5 rats/group.
Furthermore, plasma levels of 8-isoprostanes (marker of oxidative stress) were found to be significantly higher in SD rats compared with control groups. Interestingly, control exposure rats also had increased oxidative stress as indicated by increased levels of plasma 8-isoprostane compared with the naïve control group. Furthermore, 3 weeks of GP treatment significantly diminished plasma 8-isoprostane levels in SD rats (F4,14 = 10.75, P=.0003; Figure 4C, F4,14 = 25.19, P<.0001, Figure 4D).
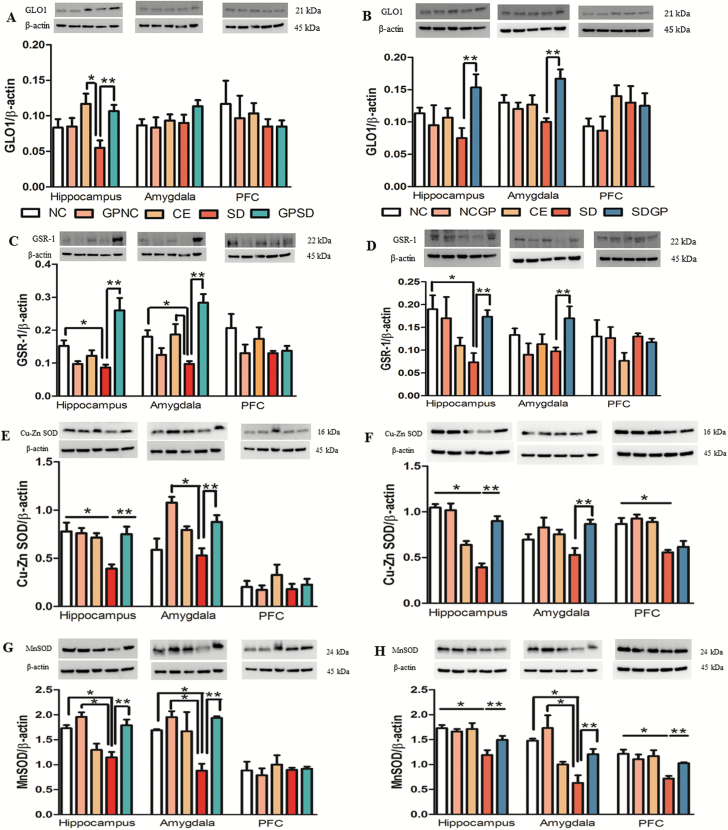
Examination of Beneficial Effects of GP Treatment on the Levels of Antioxidant Enzymes in the Hippocampus, Amygdala, and Prefrontal Cortex of Rats
Protein expression levels of antioxidant enzymes, GLO-1, GSR-1, Cu-Zn SOD, and Mn SOD were examined in the hippocampus, amygdala, and PFC of controls and SD rats treated with/without GP. While GSR-1, Cu-Zn SOD, and Mn SOD protein expression significantly decreased in the hippocampus of SD rats, a decreased trend was observed for GLO-1 expression. GP treatment prevented SD-induced decreased GLO-1 (F14,34 = 4.26, P=.0225, Figure 5A), GSR-1 (F14,37 = 6.52, P=.0003, Figure 5C), Cu-Zn SOD (F14,36 = 16.75, P=.0065, Figure 5E), and Mn SOD (F14,35 = 9.77, P=.0011, Figure 5G) in the hippocampus of SD rats. While GP treatment protected from SD-induced decreased expression of GSR-1, Cu-Zn SOD, and Mn SOD in the amygdala of SD rats, a similar trend was observed in amygdalar expression of GLO-1. No significant protective effect of GP was observed in the PFC of SD rats.
Figure 5.
Examination of GLO-1, GSR-1, Cu-Zn SOD, and Mn SOD protein levels in the hippocampus, amygdala, and prefrontal cortex (PFC) of control or socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to SD showed marked decrease in the expression of these enzymes, which was protected (A, C, E, G) and reversed (B, D, F, H) with GP treatment. The upper panels are representative blots of GLO-1, GSR-1, Cu-Zn SOD, Mn SOD, and lower panels are protein loading control β-actin, respectively. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD, P < .05. Values are mean ± SEM, n = 4 to 5 rats/group.
In the case of reversal protocol, SD exposure resulted in decreased GSR-1, Cu-Zn SOD, and Mn SOD expression in hippocampus compared with control groups and a nonsignificant decreased trend of hippocampal GLO-1 expression. GP treatment was able to reverse SD-induced decreased GLO-1 (F14,35 = 4.25, P=.028, Figure 5B), GSR-1 (F14,37 = 2.16, P=.030, Figure 5D), Cu-Zn SOD (F14,31 = 10.31, P<.0001, Figure 5F), and Mn SOD (F14,36 = 6.98, P=.006, Figure 5H) protein expression in the hippocampus and the amygdala of rats subjected to SD. While no significant reversal effect of GP in PFC was observed for GLO-1, GSR-1, and Cu-Zn SOD expression, Mn SOD expression in PFC was successfully reversed with GP treatment.
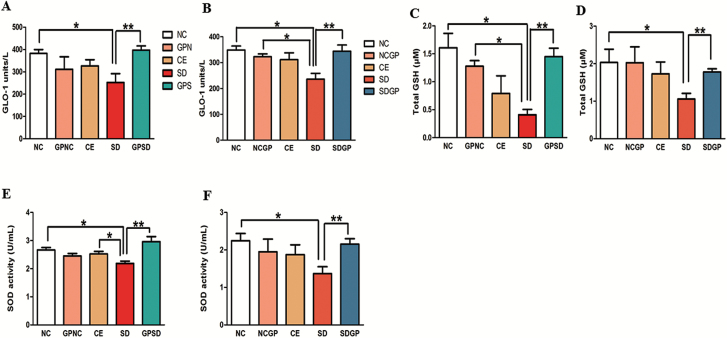
Examination of Beneficial Effects of GP Treatment on GLO-1, Total GSH, and SOD Activity in Plasma
SD exposure in rats resulted in marked decrease in plasma activity of key antioxidant enzymes such as GLO-1, GSH, and SOD. GP treatment increased social defeat-induced decreased GLO-1 (F4,13 = 4.26, P=.0046, Figure 6A; F4,13 = 4.007, P=.0248, Figure 6B), total GSH (F4,14 = 5.66, P=.0063, Figure 6C; F4,13 = 2.803, P=.038, Figure 6D), and SOD activity (F4,15 = 6.46, P=.0031, Figure 6E; F4,14 = 1.999, P=.041, Figure 6F) in plasma. These data suggest that SD-induced decreased activity of key antioxidants in plasma was reversed and protected by GP treatment.
Figure 6.
Examination of GLO-1, GSH, and SOD enzyme activity in the plasma of socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to social defeat showed marked decrease in the activity of these antioxidant enzymes, which was protected (A, C, E) and reversed (B, D, F) with GP treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD, P < .05. Values are mean ± SEM, n = 4 to 5 rats/group.
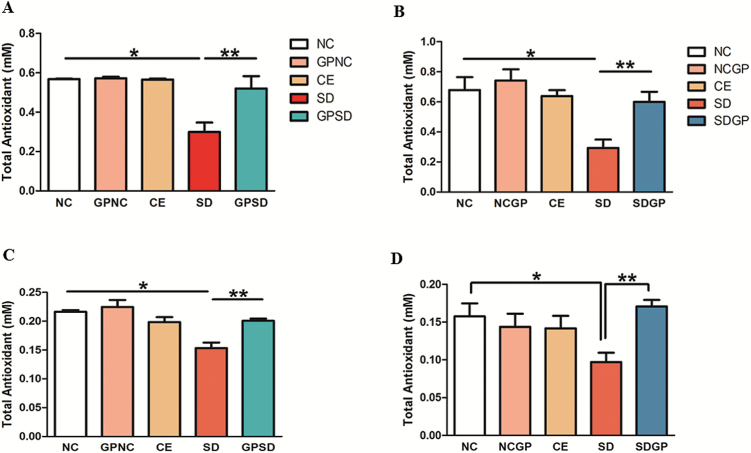
Examination of Beneficial Effects of GP Treatment on Total Antioxidant Capacity in Plasma and Hippocampus
Marked decrease in TAC in plasma and hippocampus was observed in rats exposed to social stress. GP treatment replenished the TAC in both plasma (F4,14 = 14.74, P<.0001, Figure 7A; F4,15 = 6.91, P=.0023, Figure 7B) and the hippocampus (F4,10 = 11.37, P=.001, Figure 7C; F4,10 = 3.48, P=.049, Figure 7D).
Figure 7.
Examination of total antioxidant capacity (TAC) in the plasma and hippocampus of control or socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to SD showed marked decrease in the TAC, which was protected (A, plasma; C; hippocampus) and reversed (B, plasma; D, hippocampus) with GP treatment. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD, P < .05. Values are mean ± SEM, n = 4 to 5 rats/group.
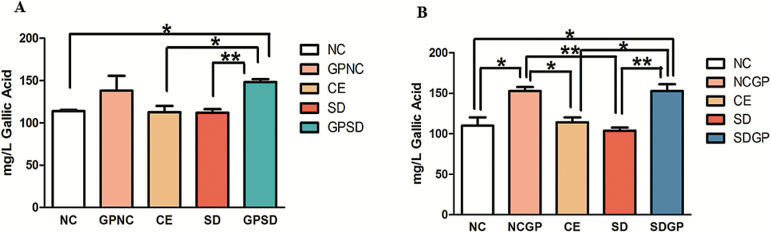
Examination of Total Phenolic Content in the Hippocampus
To confirm the presence of grape phenols and polyphenols in the brain, total phenolic content assay was performed. Groups treated with vehicle had similar basal levels of phenolic content in the hippocampus (Figure 8A-B). However, GP treatment led to a significant increase in the total phenolics in the hippocampus (GPSD: F4,10 = 3.618, P=.0451; SDGP: F4,10 = 11.43, P=.0009). These data suggest that grape polyphenols reach the rodent brain by crossing the blood-brain barrier.
Figure 8.
Examination of total phenolic content (TPC) in the hippocampus of control or socially defeated (SD) rats treated with/without grape powder (GP). Rats subjected to GP treatment showed marked increase in the total phenolic content of hippocampus in both protection (A) and reversal (B) protocol. NC, naïve control; GPNC, naïve control rats pretreated with GP; CE, control exposure; GPSD, socially defeated rats pretreated with GP; NCGP, GP-treated naïve control rats; SDGP, SD rats treated with GP. *Significantly different from NC, GPNC/NCGP, and CE; **significantly different from SD, P < .05. Values are mean ± SEM, n = 4 to 5 rats/group.
Assessment of Beneficial Effects of Resveratrol, Quercetin, and Kaempferol in Protecting BSO-Induced Decreased TAC in HT22 Cells
In this study, prooxidant BSO treatment significantly decreased TAC in HT22 cells. Decrease in TAC was prevented with GP treatment. Similarly, resveratrol at 1- and 5-μM concentrations also prevented BSO-induced decreased TAC (F11,24=13.21, P<.0001; supplementary Figure 2A). These data suggest that resveratrol was effective at the lowest concentration in maintaining TAC levels. In addition, the ability of quercetin and kaempferol in protecting BSO-induced decreased TAC was investigated. While quercetin was found to be effective at 5-, 10- and 20-μM concentrations (F11,27 = 3.94, P=.0018, supplementary Figure 2B), kaempferol failed to protect BSO-induced decreased TAC (1, 5, 10, and 20 μM) concentrations (F11,27 = 19.96, P<.0001; supplementary Figure 2C). The protective effect of quercetin was comparable with that of GP treatment. Overall, these data suggest that resveratrol and quercetin were effective in preventing TAC levels from declining upon induction of oxidative insult. TAC levels were normalized with resveratrol and quercetin treatment.
Mechanistic Insights into Protective Effect of GP in Simulated Model of Oxidative Stress Using HT22 Cells
BSO treatment led to marked increase in calcium levels in HT22 cells, while it was significantly decreased and normalized with GP treatment (F5,12 = 10.78, P=.0004, supplementary Figure 3A). Furthermore, mitochondrial membrane potential is an indicator of cell health or injury (Gogvadze et al., 2006). BSO treatment lowered mitochondrial membrane potential. GP pretreatment reestablished mitochondrial membrane potential (F5,12 = 40.13, P<.0001; supplementary Figure 3B).
Fourteen hours of BSO treatment led to increase in the levels of cytosolic cytochrome-c. Four hours of GP treatment prior to BSO treatment prevented release of cytochrome-c (F7,16 = 87.21, P<.0001; supplementary Figure 3C). Similarly, 14 hours of BSO treatment increased caspase-3 activity in BSO-treated cells. BSO-induced increased activity of caspase-3 was absent in the group treated for 4 hours with GP prior to BSO treatment (F5,12 = 6.52, P=.0038; supplementary Figure 3D).
Discussion
SD-induced anxiety- and depression-like behaviors as well as cognitive impairments were reversed and prevented with GP treatment. And, concomitant normalization of SD-induced elevation in systemic and neuronal oxidative stress was observed. Hypothalamic-pituitary-adrenal axis hyperactivity indicated by elevated corticosterone levels also was noted. This not only suggests beneficial effects of GP on SD-induced behavioral and cognitive deficits, but also implies that beneficial effects of GP could be attributed to its antioxidant properties. This seems particularly plausible considering previously reported beneficial effects of grapes and grape components such as resveratrol on behavioral and cognitive deficits (Sonmez et al., 2007; Singleton et al., 2010; Ge et al., 2015; Gocmez et al., 2016). We also have reported a protective role of GP in 3 different models of direct or indirect induction of oxidative stress (Allam et al., 2013; Patki et al., 2013b; Solanki et al., 2015). These are interesting results, but revealing the mechanistic basis for this protective effect is important especially considering present results in a translationally relevant animal model of social stress.
Several important observations were noted in this study. First, we not only observed heightened oxidative stress and diminished total antioxidant capacity peripherally but also in the brain of SD rats, and GP treatment normalized elevated oxidative stress in specific brain regions, particularly the hippocampus. Interestingly, total phenolic content was enhanced in the hippocampus with GP treatment suggesting successful blood brain barrier penetration of GP. Second, GLO-1, total GSH, and SOD antioxidant enzyme activity in plasma were found to be decreased in SD rats, which were replenished with GP treatment. Third, we noted higher vulnerability of the hippocampus and the amygdala to SD-induced oxidative stress and breakdown of antioxidant defense system, which was normalized with GP treatment.
Basically, antioxidant protein expression levels were examined in 3 key brain regions, namely hippocampus, amygdala, and the PFC, regions considered susceptible to oxidative stress and implicated in anxiety, depression, and cognitive impairments (Mathew and Charney, 2008; Patki et al., 2013a; Solanki et al., 2015). Protein expression levels of GLO-1 and GSR-1 were reduced in the hippocampus and amygdala of SD rats, which was reversed but not prevented in amygdala with GP treatment. Furthermore, Cu-Zn SOD and Mn SOD expression were significantly decreased in SD rats, which were restored in the hippocampus and the amygdala of SD rats. Interestingly, the expression of these proteins did not change in the PFC of SD rats except for Mn SOD. The rationale for such regional differences is not clearly understood, but important insights could be gained from what is known about the hippocampal dentate gyrus-cornu ammonis 3 (DG-CA3) system, which is known to regulate structural plasticity, regenerative/remodeling capacity as well as neurogenesis factors such as brain-derived neurotrophic factor (Popov and Bocharova, 1992). It has also been suggested that the pyramidal cells of CA1 and CA3 and granule cells of DG are highly susceptible to oxidative damage (Vornov et al., 1998; Sarnowska, 2002). Thus, SD-induced oxidative damage of DG-CA function may diminish cell proliferation, impair remodeling capacity, alter structural plasticity, and disrupt neurogenesis, simultaneously impairing the antioxidant defense system by reducing total antioxidant capacity and decreasing antioxidant enzyme function. Collectively, this disturbs normal synaptic neurotransmission. These events offer an attractive explanation for SD-induced behavioral and cognitive impairment, and GP potentially by mitigating oxidative stress protected and reversed SD-induced behavioral and cognitive deficits.
While beneficial effects of GP are clearly observed, 2 questions emerged. First, which components of GP are responsible for beneficial effects? Second, by what mechanism does GP exert the beneficial effects? To answer these questions, a series of experiments were performed in HT22 cell line by simulating elevated oxidative stress as observed in SD rats. Grapes are rich in polyphenols including anthocyanins, resveratrol, quercetin, and kaempferol. Among grape polyphenols, resveratrol, quercetin, and kaempferol have potent antioxidant activity (Graf et al., 2005; Bouayed and Bohn, 2010). Neuroprotective effects of resveratrol, quercetin, and kaempferol also have been reported (Ndiaye et al., 2005; Curin et al., 2006; Albani et al., 2009). Therefore, we tested the effects of these 3 pure synthetic compounds on prooxidant (BSO)-induced oxidative stress using our previously published concentrations (Salvi et al., 2016). Results were compared with GP treatment. The dose of polyphenols and GP was chosen based on the pilot studies performed in our laboratory and also by the others (Fukui et al., 2010; E.J. Yang et al., 2014). BSO-induced decreased total antioxidant capacity was normalized with 1 μM resveratrol and 5 μM quercetin that was similar to GP treatment, suggesting its antioxidant effects. This is in agreement with other studies where resveratrol and quercetin were found to be protective against glutamate-induced oxidative stress (Fukui et al., 2010; E.J. Yang et al., 2013). Interestingly, in the present study, resveratrol was found to be most effective at the lowest concentration but not at higher concentrations in protecting BSO-induced decreased total antioxidant capacity. A dose-dependent decrease in the ability of resveratrol to protect against BSO-induced oxidative stress could be attributed to its potential toxicity as evident by increased number of detached cells/nonviable cells. Pertinent to this, concentration-dependent inhibition of cell viability by resveratrol has been reported in HT22 cells (Hsieh, 2009; Zhou et al., 2009). It is likely that resveratrol-induced apoptosis at 20-µM concentration diminishes total antioxidant capacity. Grape polyphenols are known to induce phase-II antioxidant enzymes via facilitating the binding between nuclear factor erythroid 2–related factor 2 and antioxidant response element in the promoter region of several antioxidant genes (J. Yang and Xiao, 2013). Perhaps resveratrol and quercetin increased total antioxidant capacity upon oxidative insult partly due to their ability to induce phase-II antioxidant enzymes. While others have reported neuroprotective effects of kaempferol against oxidative stress, in our study, kaempferol failed to protect and maintain total antioxidant capacity of HT22 cells from oxidative insult at these concentrations. This observation could be partly due to the different mode of induction of oxidative stress. While others have used glutamate (E. J. Yang et al., 2014), we have used BSO to induce oxidative stress. At higher concentrations, glutamate is neurotoxic and induces oxidative stress via increasing intracellular reactive oxygen species generation with a concomitant decrease in total antioxidant capacity and regulation of expression of apoptosis-inducing factor and MAPK (Vyas et al., 2013; E.J. Yang et al., 2014). Kaempferol was found to protect HT22 cells from glutamate-induced apoptosis. In contrast, in our model, BSO exerts its effects via disrupting glutathione synthesis (Marengo et al., 2008). Impaired glutathione synthesis results in poor detoxification of H2O2, leading to its accumulation and toxicity in the cell (Dunning et al., 2013). Therefore, appropriate functioning of the glutathione system is likely to play a critical role in maintaining redox homeostasis. Perhaps kaempferol failed to reinstate BSO-induced glutathione deficit in our model and therefore did not exert any beneficial effects. Furthermore, others have reported neuroprotective effects of kaempferol at the dose of 25 to 50 μM that was higher than what we have used (1 to 20 μM). Overall, these data suggest that beneficial effects of GP could be attributed to resveratrol and quercetin.
Finally, GP treatment normalized BSO-induced increase in cellular Ca2+ concentrations. Similar to this, others have reported protective effects of grape components such as resveratrol and kaempferol in glutamate-induced increased Ca2+ concentrations (Fukui et al., 2010; E.J. Yang et al., 2014). Cellular Ca2+ signals are vital for various physiological processes, cell injury, and apoptosis (Smaili et al., 2000). Increased Ca2+ concentrations result in mitochondrial dysfunction leading to impaired mitochondrial membrane potential and opening of mitochondrial permeability transition pore (Carraro and Bernardi, 2016). In congruence with the existing knowledge, in this study BSO treatment induced mitochondrial dysfunction as demonstrated by impaired mitochondrial membrane potential in HT22 cells. And mitochondrial impairment was prevented with GP treatment. Furthermore, mitochondrial intermembrane space is occupied by cytochrome c. Several stress signals including oxidative stress are known to induce release of cytochrome c. Once in the plasma, cytochrome c regulates the activation of apoptosis-inducing factor-1, which serves as a precursor for activation of caspases. Activation of caspases is marked as a signal for cell death (Garrido et al., 2006). Interestingly, in this study, GP seemed to prevent BSO-induced elevation in Ca2+ levels, mediated mitochondrial impairment and subsequently prevented cell death.
To summarize, our behavioral, biochemical, and in-vitro findings indicate that GP treatment reversed and prevented social defeat-induced behavioral and cognitive deficits potentially via inhibiting oxidative stress pathway of cell death as depicted in supplementary Figure 4. And, beneficial effects of GP could be attributed to resveratrol and quercetin. Thus, daily moderate GP consumption may serve as a useful adjuvant therapy for psychological stress-induced anxiety and depression.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Statement of Interest
None.
Supplementary Material
Acknowledgments
The authors thank the California Table Grape Commission for providing us with the GP and especially Courtney Romano for her help with prompt delivery arrangements.
This work was supported by National Institutes of Health grant (2R15MH093918-02) and University of Houston start-up funds awarded to Samina Salim.
References
- Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. (2009) The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1–42) peptide. J Neurochem 110:1445–1456. [DOI] [PubMed] [Google Scholar]
- Allam F, Dao AT, Chugh G, Bohat R, Jafri F, Patki G, Mowrey C, Asghar M, Alkadhi KA, Salim S. (2013) Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. J Nutr 143:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton H. (1994) Guidelines for the rational use of benzodiazepines. When and what to use. Drugs 48:25–40. [DOI] [PubMed] [Google Scholar]
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. (2004) Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol 19:437–454. [DOI] [PubMed] [Google Scholar]
- Behl C, Trapp T, Skutella T, Holsboer F. (1997) Protection against oxidative stress-induced neuronal cell death--a novel role for RU486. Eur J Neurosci 9:912–920. [DOI] [PubMed] [Google Scholar]
- Bouayed J, Bohn T. (2010) Exogenous antioxidants: double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro M, Bernardi P. (2016) Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 60:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CC, McIntosh MK. (2011) Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Ann Rev Nutr 31:155–176. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. (2007) Psychological stress and disease. JAMA 298:1685–1687. [DOI] [PubMed] [Google Scholar]
- Curin Y, Ritz MF, Andriantsitohaina R. (2006) Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem 4:277–288. [DOI] [PubMed] [Google Scholar]
- Dunning S, Ur Rehman A, Tiebosch MH, Hannivoort RA, Haijer FW, Woudenberg J, van den Heuvel FA, Buist-Homan M, Faber KN, Moshage H. (2013) Glutathione and antioxidant enzymes serve complementary roles in protecting activated hepatic stellate cells against hydrogen peroxide-induced cell death. Biochim Biophys Acta 1832:2027–2034. [DOI] [PubMed] [Google Scholar]
- Ferguson JM. (2001) SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry 3:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Choi HJ, Zhu BT. (2010) Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med 49:800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433. [DOI] [PubMed] [Google Scholar]
- Ge JF, Xu YY, Li N, Zhang Y, Qiu GL, Chu CH, Wang CY, Qin G, Chen FH. (2015) Resveratrol improved the spatial learning and memory in subclinical hypothyroidism rat induced by hemi-thyroid electrocauterization. J Endocrinol 62:927–938. [DOI] [PubMed] [Google Scholar]
- Gocmez SS, Gacar N, Utkan T, Gacar G, Scarpace PJ, Tumer N. (2016) Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol Learn Mem 131:131–136. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. (2006) Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta 1757:639–647. [DOI] [PubMed] [Google Scholar]
- Graf BA, Milbury PE, Blumberg JB. (2005) Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food 8:281–290. [DOI] [PubMed] [Google Scholar]
- Gudex C. (1991) Adverse effects of benzodiazepines. Soc Sci Med 33:587–596. [DOI] [PubMed] [Google Scholar]
- Hollis F, Kabbaj M. (2014) Social defeat as an animal model for depression. ILAR J 55:221–232. [DOI] [PubMed] [Google Scholar]
- Hsieh TC. (2009) Uptake of resveratrol and role of resveratrol-targeting protein, quinone reductase 2, in normally cultured human prostate cells. Asian J Androl 11:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iva Juranovic Cindric M, Michaela Z, Gerhard S, Rusak G. (2011) Sample preparation methods for the determination of the antioxidant capacity of apple juices. Croat Chem Acta 84:435–438. [Google Scholar]
- Joseph JA, Shukitt-Hale B, Willis LM. (2009) Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr 139:1813S–1817S. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. (1997) Social stress in rats and mice. Acta Physiol Scand Suppl640:69–72. [PubMed] [Google Scholar]
- Liu J, Wang A, Li L, Huang Y, Xue P, Hao A. (2010) Oxidative stress mediates hippocampal neuron death in rats after lithium-pilocarpine-induced status epilepticus. Seizure 19:165–172. [DOI] [PubMed] [Google Scholar]
- Marengo B, De Ciucis C, Verzola D, Pistoia V, Raffaghello L, Patriarca S, Balbis E, Traverso N, Cottalasso D, Pronzato MA, Marinari UM, Domenicotti C. (2008) Mechanisms of BSO (L-buthionine-S,R-sulfoximine)-induced cytotoxic effects in neuroblastoma. Free Radic Biol Med 44:474–482. [DOI] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O’Donnell JM. (2008) Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharm Exp Ther 326:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S, Charney D. (2008) Anxiety and depression: leading edge of therapy. Mt Sinai J Med, New York 75:171–173. [DOI] [PubMed] [Google Scholar]
- Meerlo P, van den Hoofdakker RH, Koolhaas JM, Daan S. (1997) Stress-induced changes in circadian rhythms of body temperature and activity in rats are not caused by pacemaker changes. J Biol Rhythms 12:80–92. [DOI] [PubMed] [Google Scholar]
- Morre DM, Morre DJ. (2006) Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett 238:202–209. [DOI] [PubMed] [Google Scholar]
- Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB. (2005) Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J 19:455–457. [DOI] [PubMed] [Google Scholar]
- Oliveira-Dos-Santos AJ, Matsumoto G, Snow BE, Bai D, Houston FP, Whishaw IQ, Mariathasan S, Sasaki T, Wakeham A, Ohashi PS, Roder JC, Barnes CA, Siderovski DP, Penninger JM. (2000) Regulation of T cell activation, anxiety, and male aggression by RGS2. Proc Natl Acad Sci USA 97:12272–12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Solanki N, Atrooz F, Allam F, Salim S. (2013a) Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 1539:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, Asghar M, Jafri F, Bohat R, Alkadhi KA, Salim S. (2013b) Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female Wistar rats. PloS One 8:e74522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1986) The rat brain stereotaxic coordinates. 6th ed Academic Press. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. (1992) Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience 48:53–62. [DOI] [PubMed] [Google Scholar]
- Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. (2010) Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res 208:545–552. [DOI] [PubMed] [Google Scholar]
- Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, Vu A. (2011a) Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res 1404:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Asghar M, Taneja M, Hovatta I, Wu YL, Saha K, Sarraj N, Hite B. (2011b) Novel role of RGS2 in regulation of antioxidant homeostasis in neuronal cells. FEBS Lett 585:1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi A, Patki G, Khan E, Asghar M, Salim S. (2016) Protective effect of tempol on buthionine sulfoximine-induced mitochondrial impairment in hippocampal derived HT22 cells. Oxid Med Cell Longev 2016:5059043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnowska A. (2002) Application of organotypic hippocampal culture for study of selective neuronal death. Folia Neuropathol 40:101–106. [PubMed] [Google Scholar]
- Shi J, Yu J, Pohorly JE, Kakuda Y. (2003) Polyphenolics in grape seeds-biochemistry and functionality. J Med Food 6:291–299. [DOI] [PubMed] [Google Scholar]
- Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. (2010) Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma 27:1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaili SS, Hsu YT, Youle RJ, Russell JT. (2000) Mitochondria in Ca2+ signaling and apoptosis. J Bioenerg Biomembr 32:35–46. [DOI] [PubMed] [Google Scholar]
- Solanki N, Alkadhi I, Atrooz F, Patki G, Salim S. (2015) Grape powder prevents cognitive, behavioral, and biochemical impairments in a rat model of posttraumatic stress disorder. Nutr Res 35:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers JM, Goldner EM, Waraich P, Hsu L. (2006) Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry 51:100–113. [DOI] [PubMed] [Google Scholar]
- Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. (2007) Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett 420:133–137. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. J Psychopharmacol 130:203–212. [DOI] [PubMed] [Google Scholar]
- Trindade E, Menon D, Topfer LA, Coloma C. (1998) Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. Can Med Assoc J 159:1245–1252. [PMC free article] [PubMed] [Google Scholar]
- Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. (2011) Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res 224:233–240. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Park J, Thomas AG. (1998) Regional vulnerability to endogenous and exogenous oxidative stress in organotypic hippocampal culture. Exp Neurol 149:109–122. [DOI] [PubMed] [Google Scholar]
- Vyas P, Kalidindi S, Chibrikova L, Igamberdiev AU, Weber JT. (2013) Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J Agric Food Chem 61:7769–7776. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, Fenech M, Zhou XF. (2009) Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer’s disease mouse. Neurotox Res 15:3–14. [DOI] [PubMed] [Google Scholar]
- Yang EJ, Kim GS, Kim JA, Song KS. (2013) Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn Mag 9:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EJ, Kim GS, Jun M, Song KS. (2014) Kaempferol attenuates the glutamate-induced oxidative stress in mouse-derived hippocampal neuronal HT22 cells. Food Funct 5:1395–1402. [DOI] [PubMed] [Google Scholar]
- Yang J, Xiao YY. (2013) Grape phytochemicals and associated health benefits. Crit Rev Food Sci Nutr 53:1202–1225. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y, Toledo RT. (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem 52:255–260. [DOI] [PubMed] [Google Scholar]
- Zhou R, Fukui M, Choi HJ, Zhu BT. (2009) Induction of a reversible, non-cytotoxic S-phase delay by resveratrol: implications for a mechanism of lifespan prolongation and cancer protection. Br J Pharmacol 158:462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.