Abstract
Dopamine (DA) is a neurotransmitter with conserved behavioral roles between invertebrate and vertebrate animals. In addition to its neural functions, in insects DA is a critical substrate for cuticle pigmentation and hardening. Drosophila tyrosine hydroxylase (DTH) is the rate limiting enzyme for DA biosynthesis. Viable brain DA deficient flies were previously generated using tissue selective GAL4-UAS binary expression rescue of a DTH null mutation and these flies show specific behavioral impairments. To circumvent the limitations of rescue via binary expression, here we achieve rescue utilizing genomically integrated mutant DTH. As expected, our DA deficient flies have no detectable DTH or DA in the brain, and show reduced locomotor activity. This deficit can be rescued by L-DOPA/carbidopa feeding, similar to human Parkinson’s disease treatment.
Genetic rescue via GAL4/UAS-DTH was also successful, although this required the generation of a new UAS-DTH1 transgene devoid of most untranslated regions, since existing UAS-DTH transgenes express in the brain without a Gal4 driver via endogenous regulatory elements. A surprising finding of our newly constructed UAS-DTH1m is that it expresses DTH at an undetectable level when regulated by dopaminergic GAL4 drivers even when fully rescuing DA, indicating that DTH immunostaining is not necessarily a valid marker for DA expression. This finding necessitated optimizing DA immunohistochemistry, revealing details of DA innervation to the mushroom body and the central complex. When DA rescue is limited to specific DA neurons, DA does not diffuse beyond the DTH-expressing terminals, such that DA signaling can be limited to very specific brain regions.
Introduction
Dopamine (DA) is a conserved neurotransmitter responsible for controlling voluntary movement (Riemensperger et al. 2011), arousal (Friggi-Grelin, Coulom, et al. 2003; Lebestky et al. 2009), sleep (Nall et al. 2016; Ueno et al. 2012), male courtship behaviors (Liu et al. 2008), learning and reward in Drosophila (Owald & Waddell 2015; Swinderen & Andretic 2011) and vertebrates (reviewed in (Iversen & Iversen 2007)). Its importance for humans is manifested in pathological conditions such as Parkinson’s disease (PD), caused by death of DA neurons via only partly understood mechanisms (Brichta & Greengard 2014; Kalia & Lang 2015).
A variety of PD models have been developed in Drosophila and mice, all of which lead to age dependent loss of variable fractions of DA neurons (Coulom & Birman 2004; Feany & Bender 2000; Meredith & Rademacher 2011; Tieu 2011). In addition, models have been developed in mouse and Drosophila in which the rate limiting tyrosine hydroxylase (TH) enzyme for DA biosynthesis is directly targeted, to better separate the effects of DA loss from factors involved with loss of the DA neurons (Darvas & Palmiter 2009; Henschen et al. 2013; Hnasko et al. 2006; Riemensperger et al. 2011; Zhou & Palmiter 1995).
DA is critical for cuticle sclerotization and melanization in insects (Andersen 2010; Brunet 1980; Wright 1987). Loss of the Drosophila tyrosine hydroxylase gene (DTH, encoded by the ple gene) results in embryonic lethality (Neckameyer & White 1993). Viable lines of brain DA-deficient fruit flies were first generated by Riemensperger et al. 2011, utilizing tissue specific rescue of the DTH null allele ple2 in the hypoderm, but not in the CNS. This was accomplished by taking advantage of the DTH tissue specific alternative splicing mechanism, which results in DTH mRNA and protein isoforms that differ between the CNS and hypoderm (Birman et al. 1994; Friggi-Grelin, Coulom, et al. 2003). Two compensating frameshift (FS+/−) point mutations in the DTH gene (DTHFS+/−) disrupt the translation of the CNS-specific isoform, but preserve the expression of the hypodermal isoform. A UAS-DTHFS+/− transgene expressed under the combined control of TH-Gal4 and DDC-Gal4 fully rescue the lethality of DTH null allele ple2, without any detectable DA in the adult brain (Riemensperger et al. 2011).
Though the approach of Riemensperger et al., (2011) was successful, there are two limitations. First, both TH-Gal4 and DDC-Gal4 drivers are required to achieve hypodermal DA rescue, leading to ectopic expression of TH in DDC-GAL4 expressing serotonin neurons. Second, this initial approach uses the GAL4-UAS binary expression system, greatly complicating further studies requiring additional transgenes, for example, studying the functions of specific DA neurons by restoring DA to selected neuronal subsets. We addressed both these limitations by rescuing ple2 with a 20 kb genomic DTHFS+/− bacterial artificial chromosome (BAC), utilizing endogenous DTH regulatory elements. This strategy eliminates ectopic TH expression in serotonergic neurons and facilitates further genetic manipulations by simplifying the DA-deficient genetic background. Our new DA deficiency model shows locomotor deficits similar to those observed in the previous approach of Riemensperger et al., (2011). We further show that the human Parkinson’s treatment of L-DOPA plus carbidopa works efficiently in Drosophila, indicating mechanistic conservation between the fly model with humans as to passage of biogenic amines through the blood brain barrier. In addition to enabling future behavioral studies, our approach to establish genetic rescue of neural DA in this new background has uncovered several interesting facets of DTH gene, DTH protein post-translational regulation, and DA neuronal localization that have broad implications for studies of catecholamine functions in other systems.
Results
Generation of DA-deficient Drosophila
We used homologous recombination in bacteria to introduce the aforementioned DTHFS+/− mutations into a 20 kb DTH BAC (Fig. S1) (Venken et al. 2006; Venken et al. 2009). After integrating this transgene into the 3rd chromosome docking site attP2, the ple2 mutation was genetically recombined into this strain, with recombinants detected by sequencing (Fig. S2). In parallel, a wild type rescue control strain was generated that contained the parent 20 kb DTH BAC in the ple2 background (DTH ple2).
Immunohistochemistry confirmed that DTH is not detectable in brains from DTHFS+/− ple2 (hereafter referred to as ‘DA-deficient’) flies, whereas DTH ple2 flies show the normal pattern of TH-immunoreactive (TH IR) neurons (Figs. 1A, 2B). As expected, DA-deficient flies have no detectable DA in brain extracts as assayed by high performance liquid chromatography (HPLC) (Hardie & Hirsh 2006) (Fig. 1B).
Fig. 1.
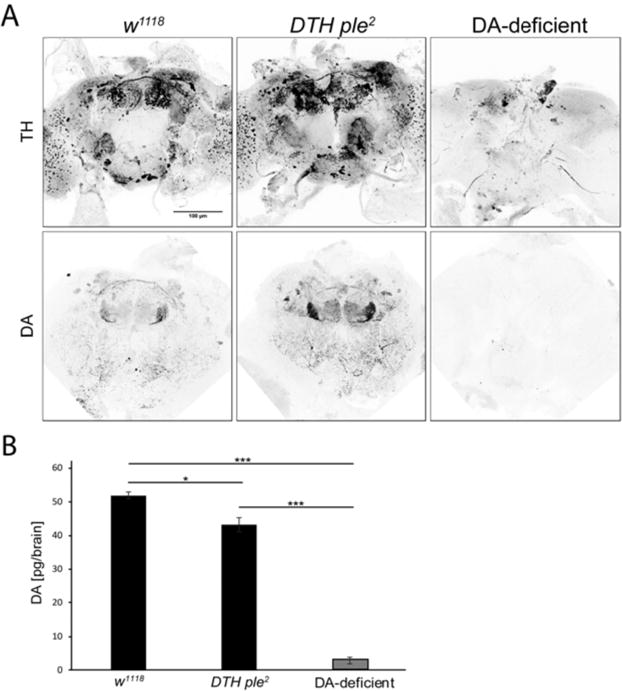
DTH and DA in w1118, DTH ple2, and DA-deficient flies. (A) DTH and DA Immunoreactivity (IR) in adult brain. DTH ple2 flies show normal patterns of DTH and DA IR, whereas DA-deficient flies show no detectable signals. DA IR shows strong innervation of mushroom body subregions and central complex. The few remaining TH positive cells in the DA-deficient brain are superficially located, and most likely represent antibody cross-reactivity because they do not show DA IR. (B) Analysis of brain DA by HPLC in adult brain extracts. DTH ple2 brains show near-normal levels of DA, whereas DA-deficient brains show <5% normal levels of DA, with the residual peak most likely caused by an unknown electroactive compound that can comigrate with DA (Riemensperger et al, 2011). [***P<1E-10, *P<0.01, one-way ANOVA (Bonferroni-corrected), error bars represent SEM, n=6 to 10].
Fig. 2.
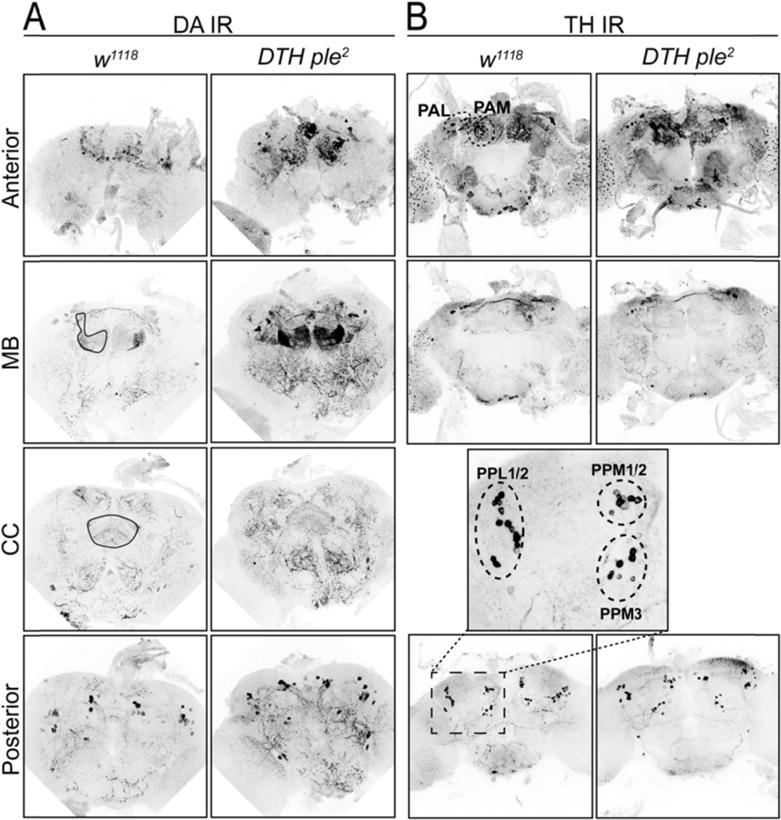
DA IR (A) and TH IR (B) reveals details of mushroom body (MB) and central complex (CC) DA innervation. TH localizes primarily to cell bodies. DA localizes to cell bodies and to synaptic projections innervating mushroom body and central complex, showing discrete localization to MB subregions. Images are maximum intensity projections of selected brain Z-projection regions.
Individual DA neurons innervate distinct subregions of the mushroom body (MB) and the central complex (CC), regions involved in learning and memory (Mao et al. 2009; Pech et al. 2013) and regulating locomotion and sleep/wake (Kong et al. 2010; Liu et al. 2012), respectively. In course of this study we optimized DA immunohistochemistry (Budnik & White 1988; Riemensperger et al. 2011), which allowed us to directly visualize DA innervation in these release sites (Fig. 1A, 2A, supplementary videos 1, 2 and 3), as well as in DA cell bodies, with a distinct pattern similar to DTH immunohistochemistry (Fig. 2A,B, supplementary video 1, Table S1). However, DA IR shows vastly enhanced levels at DA terminals in MB and CC relative to DTH IR.
DA-deficient flies have reduced locomotor activity, and respond to L-DOPA/carbidopa treatment
The DA-deficient flies show the expected phenotype of spontaneous locomotor hypoactivity (Fig. 3B) (Riemensperger et al. 2011). To determine whether these hypoactive flies might retain functional levels of DA, we fed them 3-iodotyrosine (3-IY), a DTH inhibitor that when fed to wild type flies leads to a significant decrease in locomotor activity (Neckameyer 1996; Riemensperger et al. 2011). As shown in Fig. 3A,B, the DTH ple2 flies are sensitive to 3-IY feeding, but the DA-deficient flies are insensitive, indicating that the remaining activity in these flies is not dependent on trace levels of DTH activity.
Fig. 3.
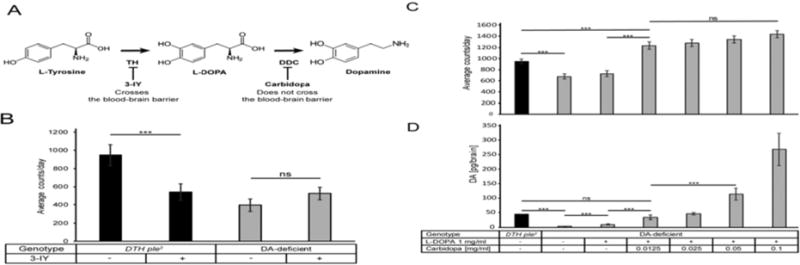
Pharmacological or genetic modulation of DA biosynthesis affects spontaneous locomotor activity. (A) DA biosynthesis pathway with inhibitors used in this study. (B) Locomotion of DTH ple2 flies (black bars) is inhibited by the DTH inhibitor 3-IY. DA-deficient flies (gray) are hypoactive, similar to DTH ple2 flies treated with 3IY. Their locomotor activity is not further reduced by 3-IY, indicating that residual activity is not affected by trace levels of DA synthesis [***P<0.001, one-way ANOVA (Bonferroni-corrected), error bars represent SEM, n=16]. (C) L-DOPA/Carbidopa feeding rescues (C) behavioral hypoactivity and (D) brain DA of DA-deficient flies in a dose dependent manner. Drugs were included in fly food and present throughout the experiment. Locomotor activity averaged over 5 days of feeding [***P<5E-04, one-way ANOVA (Bonferroni-corrected), error bars represent SEM, n=32]. HPLC [***P<1E-03, one-way ANOVA (Bonferroni-corrected), error bars represent SEM, n=5]
We next attempted pharmacological rescue of the low locomotor activity phenotype in the DA-deficient flies. Including the dopamine precursor L-DOPA (L-3,4-dihydroxyphenylalanine) in the food resulted in no significant increase in locomotor activity and only a partial rescue of DA (Fig. 3A,C). This differs from findings of (Riemensperger et al. 2011), but that study examined different behaviors and selected explicitly for flies consuming the food. The efficacy of oral L-DOPA therapy in human Parkinson’s patients is increased by simultaneous dosing with carbidopa, a DOPA decarboxylase (DDC) inhibitor (Neha Singh 2007). Carbidopa functions by preventing L-DOPA conversion to DA in the periphery, thus allowing more L-DOPA to cross the blood-brain barrier (BBB) (Seeberger & Hauser 2007). To determine whether a similar mechanism might hold in Drosophila, we added increasing concentrations of carbidopa to the L-DOPA containing food. As shown in Fig. 3C, the lowest concentration of carbidopa, 0.0125 mg/ml, results in complete rescue of locomotor activity and brain DA levels (Fig. 3D), with increasing doses of carbidopa resulting in supra-normal DA levels, with a concomitant graded increase in locomotor activity. However, all combinations of L-DOPA/carbidopa resulted in locomotor activity levels significantly greater than wild type (Fig. 3C). These observations suggest that the Drosophila BBB functions similarly to that in higher animals, with permeability to L-DOPA but not to DA. Lack of BBB permeability to physiological levels of DA is indicated by the finding that adult flies contain significant levels of DA in the hemolymph (Zhao et al., 2010). If the BBB were permeable to DA, it would not be possible to generate flies lacking brain DA.
Undriven UAS-DTH constructs restore DA biosynthesis
A major motivation for constructing this DA-deficient background was to allow neuron-selective rescue of DA synthesis using GAL4 lines that drive UAS-DTH expression in specific subsets of DA neurons. In the course of initiating these studies, we performed the routine control of examining brain DA of UAS-DTHg transgene (Fig. 4A) in the absence of a GAL4 driver in the DA-deficient background. We found a most unexpected result, namely that the undriven UAS-DTHg (Friggi-Grelin, Iché, et al. 2003; Riemensperger et al. 2011) expresses normal DA levels, as assessed by HPLC (Fig. 4B). The most likely explanation for this result is that neuronal expression of DTHg is driven via intra-genic transcriptional regulatory elements, a result consistent with the location of elements required for neuronal DTH expression (Friggi-Grelin, Coulom, et al. 2003; Liu et al. 2012). Other available UAS-DTH constructs also showed partial DA rescue. UAS-DTH1 (Friggi-Grelin, Iché, et al. 2003) removes most DTH introns, retaining only introns 3 and 4, yet this undriven construct still rescues DA to ~50% of normal levels (Fig. 4B). Undriven UAS-DTH2 (True et al. 1999) also rescued significant amounts of DA (Fig. 4B).
Fig. 4.
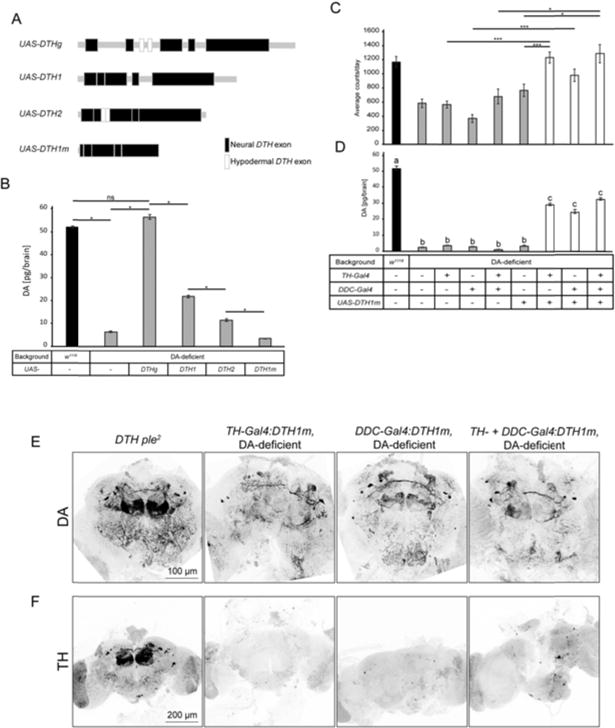
Neuron selective rescue of DA biosynthesis. (A) UAS constructs tested for neuron selective restoration of DTH expression and DA. (B) Undriven UAS-DTHg, UAS-DTH1, and UAS-DTH2 rescue DA biosynthesis in the DA-deficient background without a Gal4 driver. Only UAS-DTH1m does not rescue DA biosynthesis [*P<7E-03, one-way ANOVA (Bonferroni-corrected), error bars represent SEM], n=3]. (C) TH-Gal4 and/or DDC-Gal4 driven UAS-DTH1m rescues normal locomotor activity [***P<1E-6, *P<0.006, one-way ANOVA (Bonferroni-corrected), error bars represent SEM], and (D) brain DA [a vs b P<1E-5, a vs c and b vs c P<1E-3, one-way ANOVA (Bonferroni-corrected), error bars represent SEM, n=32]. (E) TH-and/or DDC-GAL4 driven UAS-DTH1m rescues normal pattern of adult brain DA, in spite of the fact that the DTH1 protein is almost undetectable by TH immunohistochemistry (F).
To address this issue, we generated constructs containing even less non-coding DTH sequences. A minimal construct, DTH1m, that removes almost all DTH non-coding sequences, retaining only 12 bp of 5′ UTR containing the Kozak sequence (Fig. 4A), yielded the desired result, in that it fails to rescue any detectable DA in the DA-deficient background in the absence of a GAL4 driver (Fig. 4B). TH-Gal4 and/or DDC-Gal4;UAS-DTH1m mediated rescue in the DA-deficient background restores normal locomotion (Fig. 4C), 50–60% normal DA level measured by HPLC (Fig. 4D), and brain DA immunohistochemistry (Fig. 4E).
Surprisingly, DTH immunoreactivity is drastically decreased in flies in which UAS-DTH1m is driven by the single or combined action of TH-Gal4 and Ddc-Gal4 (Fig. 4F, S3), even though this transgene is capable of rescuing significant DA levels in the DA-deficient background (Fig. 4D, 4E). Images of the posterior DA-neurons of TH-GAL4/DDC-Gal4:DTH1m rescued brains show very faint TH IR in cell bodies (Fig. S3). Since the DTH protein is subject to feedback inhibition by DA (Vié et al. 1999), low level expression of DTH1 in a DA-deficient neuron should result in a highly active/phosphorylated DTH1 protein that would remain fully active until intracellular DA levels approach normal. We tested if DTH phosphorylation, potentially modifying the epitope recognized by the antibody, could be responsible for the lack of TH IR in TH-GAL4/DDC-Gal4:DTH1m rescued flies, by increasing brain DA levels with L-DOPA/carbidopa feeding (Fig S3). We found no effect of this treatment on TH IR. Since mRNA expression levels of the neural DTH1 isoform are normal in this genotype (Fig. S4) the most likely explanation for the low TH IR expressed from UAS-DTH1m is poor translatability of the mRNA caused by severe pruning of the untranslated regions.
The low level of DTH1 expression detected by our TH IR protocol indicates a potential discordance between TH IR and the presence of DA, providing the initial rationale to optimize detection of DA IR. Budnik and White (Budnik & White 1988) detected DA in cell bodies in whole mount larval CNS preparations of Drosophila. Riemensperger et al. (2011) adapted this procedure to whole mount adult brain, but signal to noise was low, with little to no DA IR detected in DA neuron terminals. With our optimized protocol (see Methods) we have been able to achieve high signal to noise with extensive DA IR observed in terminals and essentially no signal remaining in the adult brains of the DA-deficient flies (Fig. 1A, supplementary videos 1, 2 and 3).
DA biosynthesis can be strictly limited to selected mushroom body subdomains
We used the split-Gal4 expression system (Luan et al. 2006; Pfeiffer et al. 2010) with UAS-DTH1m to rescue DA in small subsets of DA neurons. Activation domain (AD) drivers overlapping with subsets of DA neurons were combined with TH-DBD and DDC-DBD Gal4 drivers (Aso, Hattori, et al. 2014) (Fig. 5, S5, Table 1). In a separate experiment, these driver combinations were used to drive mCD8-GFP expression in a wild type DA background, which allowed mapping of DA neurons relative to TH IR (Fig 5, S5).
Fig. 5.
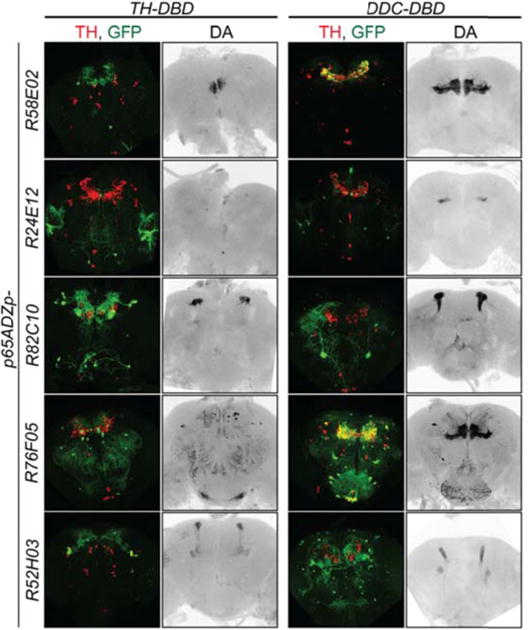
DA does not diffuse nor is recycled in distant neurons and its biosynthesis and signaling can be limited to specific MB neurons. Patterns of Split-Gal4 DA neuron subsets mapped with UAS-GFP (green) on a DTH/DA-competent background coimmunostained for TH (red); maximum intensity projections of whole brain. DA-rescue in subsets of DA-neurons using Split-Gal4 system on a DA-deficient background show innervation limited to specific MB neurons; DA immunohistochemistry (grey); maximum intensity projections of MB planes. Even though the Split-Gal4 expression system is highly specific, we found some off-target neurons expressing UAS-GFP that do not overlap with TH IR and do not show DA IR in rescued brains (Fig. 5). We also noticed a surprising coverage of the PPL1 DA neurons by the DDC-DBD driver (Table 1), which was absent from the DDC-Gal4 pattern (Aso et al., 2012).
Table 1. Split-Gal4 combinations targeting DA-neurons.
Number of targeted neurons within each cluster.
| p65ADZp- | TH-DBD | DDC-DBD |
|---|---|---|
| R58E02 | ~10 PAM | ~75 PAM |
| R24E12 | No DA neurons | 10 PAM |
| R82C10 5 | PPL1 | 3 PPL1 |
| R76F05 | 1 PAL, ~4 PAM, 4 PPM1/2, 2 PPM3, 1 PPL1, 1 SP1 | 2 PAL, ~45 PAM, 3 PPM1/2, 1 PPM3, 2 PPL1, 1 SP1 |
| R52H03 | 1 PPM3, 3 PPL1 | 1 PPM3, 4 PPL1 |
The neurons detected in this experiment by DA IR innervate subregions of the mushroom body (Fig. 5), showing high spatial specificity. Furthermore, there is no detectable diffusion of DA that would lead to DAT dependent reuptake to projections from nearby neurons. Of particular interest are the split R58E02 and R76F05 GAL4 driver combinations that target subsets of the PAM neurons, comprising ~110 small medially localized cell bodies. The R58E02/TH split GAL4 drivers target ~10 of these neurons, whereas the R58E02/DDC drivers target ~75. The former targets PAM DA neurons at the medial tip of the horizontal lobes, apparently the β2β2′γ5 domain, whereas the latter targets the entirety of the horizontal lobes. This subset of PAM neuron cell bodies appears to be highly intermingled and in rather close proximity to other PAM neurons (Aso, Hattori, et al. 2014). Since this cluster of ~110 PAM neuron cell bodies is roughly 40 μm in diameter, we consider it unlikely that somatic release/reuptake of DA is occurring (Rice & Patel 2015) within the PAM neuronal cluster.
Discussion
We developed a Drosophila strain that lacks DA in the brain. This new model reproduces hypoactivity phenotypes of the previous model (Hirsh et al. 2010; Riemensperger et al. 2011), which resemble those observed with pharmacological DTH inhibition using 3-IY. A combination of drugs used in Parkinson’s disease treatment was able to rescue this behavioral hypoactivity. In Riemensperger et al. (2011), L-DOPA feeding alone was able to rescue negative geotaxis behavior. However, the two studies use different feeding protocols and behavioral assays, making comparisons difficult.
We were able to restore DA biosynthesis in selected neurons using a new UAS-DTH1 construct that lacked regulatory elements contained in previously constructed UAS-DTH transgenes which allowed DTH expression even in the absence of a GAL4 driver. The UAS-DTH1m transgene we developed shows no basal expression, allows full rescue of DA and locomotor activity. Paradoxically, the TH gene product from UAS-DTH1m is almost undetectable by TH IR. The simplest explanation for this unexpected finding is that this transgene must be translated at a very low level due to severe pruning of 5′ and 3′ untranslated regions. Since the DTH1 protein encoded by this construct is subject to regulation by feedback inhibition by DA (Vié et al. 1999), expression of low levels of DTH1 protein in a DA depleted neuronal environment would result in a phosphorylated protein with high TH activity, allowing efficient restoration of normal DA levels. This discordance between DA vs TH IR is of broad interest given the common use of TH IR as a catecholaminergic marker in vertebrate neuronal systems where TH is similarly regulated (Vié et al. 1999).
Roles for specific subsets of DA neurons and their respective MB innervation regions in learning and memory (reviewed in (Aso, Sitaraman, et al. 2014; Waddell 2013; Waddell 2016)) and locomotion control (Agrawal & Hasan 2015; Kong et al. 2010; Riemensperger et al. 2013) have been determined using conditionally regulated silencing and activating genes, and by utilizing live Ca2+ imaging. In particular, these studies show that subsets of PAM neurons and their innervation to the MB horizontal lobes mediate either aversive or appetitive learning, and that subsets of PPL1 neurons can convey an aversive signal. Striking examples are shown by Hige et al. (2015), who demonstrate that optogenetic stimulation of DA neurons innervating particular MB subdomains, leads to long term depression of MB output neurons (MBONs) only if the DA-neurons are innervating the same MB subdomain. The implication of these studies is that DA released to specific regions of the MB horizontal lobes does not diffuse to adjacent domains. Here we show this directly using our improved methodology for DA IR.
Although the Split-Gal4 expression system is highly specific, we found some off-target neurons expressing UAS-GFP that do not overlap with TH IR and do not show DA IR in rescued brains (Fig. 5). We suspect that these ectopic neurons could result from extremely low level expression of each driver in these neurons, amplified by the high sensitivity of split-GAL4 followed by GFP detection.
We suspect that a nervous system developing in the absence of DA may develop compensatory mechanisms, and potentially altered neuronal processes, such that the dopamine deficient brain may not be perfectly comparable with a wild type brain architecture, but we have so far not detected any such abnormalities. Our pharmacological rescue with L-DOPA/carbiodopa combination may allow to generate a conditional model with DA synthesized from L-DOPA supplemented in a food present throughout the development, and DA-deficiency generated later in life by withdrawal from drugs.
Death of DA-neurons in the substantia nigra pars compacta is a major hallmark of Parkinson’s disease, directly responsible for the locomotor symptoms. Currently, Parkinson’s therapies are mostly limited to alleviating symptoms by dopamine replacement. It has been known for decades that up to 80% of substantia nigra DA neurons degenerate in a human brain prior to expression of the locomotor symptoms of Parkinson’s disease (Hirsch et al. 1988; Hornykiewicz 1966), although more recent studies suggest that only ~30% of substantia nigra DA neurons and around 50-60% of their axon terminals have been lost when motor signs first appear (Cheng et al. 2010). Compensatory signaling mechanisms likely play roles in maintaining functions of the nervous system during this period, and a viable brain DA-deficient model that allows sophisticated genetic manipulations will be a valuable research tool in elucidating these mechanisms.
Materials and Methods
Generation of the 20 kb DTHFS+/− BAC construct
We generated a genetically recombineered BAC containing the DTHFS+/− (Riemensperger et al. 2011), starting with the BAC plasmid CH322-134F22 (Venken et al. 2006; Venken et al. 2009), containing the wild type DTH embedded within a 20 kb segment of genomic DNA (3L: 6,701,493 to 6,721,400), obtained from P[acman] Resources (Venken et al. 2006; Venken et al. 2009). The DTHFS+/− BAC was constructed by recombineering a 2,471 bp DNA fragment (synthesized by GeneScript (Piscataway, NJ, USA)) containing portions of DTH exons 4 and 5 containing the DTHFS+/− mutations (Riemensperger et al. 2011), and the neomycin (Neo) resistance marker flanked by loxP sites located within DTH intron 4 (Fig. S1), with a 7 kb DTHFS+/− sequence in a pUAST vector, generating the plasmid pUAST DTH FS+/− Neo. Next, the 8.8 kb DTHFS+/− Neo fragment from pUAST DTHFS+/− Neo was recombineered with the wild type DTH BAC CH322-134F22 to generate DTHFS+/− Neo BAC. The neomycin resistance cassette was then excised by Cre recombinase, leaving one loxP site in the intron between exons 4 and 5. The site of the intronic loxP insertion was chosen to avoid any conserved sequences when comparing the intronic region with the comparable sequences of other Drosophilids. The 20 kb DTHFS+/− BAC construct was validated by restriction digestion, Sanger sequencing, and 454 Sequencing. It was then site specifically integrated into attP2 site on the 3rd chromosome (Bischof et al. 2007) by Rainbow Transgenic Flies (Camarillo, CA). The recombineering host strain was SW106 E.coli, NCI-Frederick (Frederick, MD) (Warming et al. 2005)
Generation of the UAS-DTH1m construct
We generated UAS-DTH1m, an intronless DTH1 (neuronal isoform) cDNA with minimal 5′ and 3′ untranslated regions. This was accomplished by PCR amplification of genomic DNA isolated from a transgenic fly containing UAS-DTH1.C. This is an intronless DTH1, but with extensive 5′ and 3′ UTRs. Primers were designed (see below) that amplified this sequence as a 1,539 bp DTH1m sequence, with 12 bp 5′UTR and no 3′ UTR. This fragment was cloned into EcoRI and XbaI restriction sites in a pUAST plasmid using the homologous recombination reagent, In-Fusion HD (Clontech). The following primers were used for PCR amplification, vector homology regions underlined and initiating ATG and the termination codons in boldface:
DTHcDNAForEcoRI: 5′-AGGGAATTGGGAATTACACAAAAAAAAATGATGGCCG-3′
DTHcDNARevXbaI: 5′-ACAAAGATCCTCTAGTTAGAACGGGCGTCGCAACTTG-3′
The construct was validated by Sanger sequencing and site-specifically integrated into the 2nd chromosome attP40 site (Bischof et al. 2007) by Genetic Services (Sudbury, MA).
HPLC
HPLC with electrochemical detection (Decade, Antec, Leyden) was performed as per Hardie & Hirsh (2006), using a mobile phase consisting of 50 mM citrate/acetate, pH 4.5, 1 mM decyl sulfonic acid, 0.1 mM EDTA, and 7% acetonitrile modifying agent.
qPCR
RNA was isolated from 10 brains per genotype using RNeasy Mini Kit (QIAGEN). Reverse transcription was carried out using SuperScript® VILO™ Master Mix (Invitrogen) and qPCR reactions were run on an Applied Biosystems® 7500 Fast Real-Time PCR machine. Expression was quantitated using a relative ΔΔCt method with internal control primers specific for Ef1β using 7500 Software v2.3 (Applied Biosystems). Primers specific for the DTH transcript are isoform specific, detecting only the neural DTH1 isoform. DTH CNS For primer is specific for the 2nd DTH exon whereas DTH CNS Rev is complementary to the 2nd–5th exon-exon junction. EF1β For primer also spans an exon-exon junction. Primer sequences: DTH CNS For: CAGCCTGGTGGATGATGCCCG; DTH CNS Rev: GTGAGACCGTAATCATTTGCCTTGC; EF1β For: ACATCAGCGGATATACTCCCAGCA; EF1β Rev: CCAGCGGGCCACGTTCACAT.
Immunohistochemistry
DTH immunohistochemistry
Adult brains were dissected in ice-cold PBS, fixed for 35 min in 4% paraformaldehyde at room temperature, and processed by standard procedures at room temperature (Vinayak et al 2013). DTH was detected using a rabbit polyclonal anti-TH, diluted 1:40, (AB152, Millipore/Chemicon)
DA immunohistochemistry, adapted from (Budnik & White 1988)
Solutions and reagents
Fix: 1.25% glutaraldehyde (Sigma G5882, Lot #SLBG1666V), 1% Na metabisulfite in PBS; PM: PBS + 1%_ Na2S2O5; NaBH4: 1% NaBH4 in PBS; PMBT: 5 ml PM + 5mg BSA + 15 μl Triton X-100; Antibody: mouse monoclonal anti-DA diluted 1:40 (MAB5300 Millipore/Chemicon, Lot #2580700). The 25% glutaraldehyde stock solution, supplied under nitrogen gas, was divided into 20 μl aliquots in an anaerobic glove box and stored anaerobically at −20C until use.
Procedure
Adult brains were dissected in ice cold PBS and processed with all subsequent steps at 4°C unless noted. Fixation in 1.25% glutaraldehyde was for 3-4h, followed by 3×20 min washes in PM, and reduction for 40 min (22°C) in freshly prepared 1% NaBH4. Brains were then washed 2×20 min in PM, 20 min in PMBT, and then overnight with PMBT containing 1% goat serum. This solution was replaced with primary antibody solution and incubated for 24h, then washed 3×20 min in PBT (22°C). Incubation in secondary antibody solution was for 24h, followed by 2×20 min washes in PBT. This solution was replaced with fresh PBT for an overnight wash, rinsed in 2×5 min in PBS and mounted in 60% 2,2-thiodiethanol (Costantini et al. 2015). Confocal imaging was performed on a Leica SP5 × WLL Multispectral confocal microscope in the Keck Imaging Facility (University of Virginia). Images within individual figures were captured and processed with identical sensitivity/gain/contrast settings.
Flies and locomotor activity analysis
Male adult flies of indicated genotypes were 3–5 days post eclosion at initiation of behavioral analyses. Flies were raised on 12:12 LD light schedule at 25°C, 60% relative humidity.
Fly locomotor activity was monitored in Trikinetics activity monitors (Waltham, MA) for 5 days at 12:12 light:dark cycle, with 2 μW/cm2 intensity green LED light, at 22°C, 60% relative humidity. 16 to 32 male flies were used per condition, and activity monitor tubes contained a plug of fly food at one end. Data was analyzed using Clocklab software (Coulbourne Instruments, Whitehall, PA, USA) and Microsoft Excel.
Drug feeding
3-Iodo-L-tyrosine (3-IY) (I8250, Sigma) and L-DOPA (D9628, Sigma) were mixed directly into melted fly food at final concentrations of 1.25 mg/ml and 1 mg/ml, respectively. S-(−)-Carbidopa (C1335, Sigma) was dissolved in 1 ml of water at the 10× final concentration and mixed with 9 ml of melted fly food. The Trikinetics activity monitor tubes were loaded with a plug of food at one end, with drug feeding throughout the course of the experiment.
Statistical Analyses
All statistical comparisons were performed using ANOVA, with post hoc corrections for multiple comparisons using a Bonferroni correction. Sample numbers are given in relevant figure legends.
Supplementary Material
Acknowledgments
The laboratory of JH was supported by NIH R01 GM GM84128. The laboratory of SB was supported by research grants from the Fédération pour la Recherche sur le Cerveau and the Fondation de France. We thank Magali Torres for technical help with the original DTH FS+/− and DTH1 constructs.
References
- Agrawal T, Hasan G. Maturation of a central brain flight circuit in Drosophila requires Fz2/Ca2+ signaling. eLife. 2015;4:e07046. doi: 10.7554/eLife.07046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SO. Insect cuticular sclerotization: A review. Insect Biochemistry and Molecular Biology. 2010;40(3):166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, et al. Mushroom body output neurons encode valence and guide memory-based action selection in Drosophila. eLife. 2014;3 doi: 10.7554/eLife.04580. Available at: http://elifesciences.org/lookup/doi/10.7554/eLife.04580 [Accessed April 1, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife. 2014;3 doi: 10.7554/eLife.04577. Available at: http://elifesciences.org/lookup/doi/10.7554/eLife.04577 [Accessed April 1, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birman S, et al. A novel and major isoform of tyrosine hydroxylase in Drosophila is generated by alternative RNA processing. The Journal of Biological Chemistry. 1994;269(42):26559–26567. [PubMed] [Google Scholar]
- Bischof J, et al. An optimized transgenesis system for Drosophila using germ-line-specific ϕC31 integrases. Proceedings of the National Academy of Sciences. 2007;104(9):3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta L, Greengard P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: an update. Frontiers in Neuroanatomy. 2014;8 doi: 10.3389/fnana.2014.00152. Available at: http://journal.frontiersin.org/article/10.3389/fnana.2014.00152/abstract [Accessed April 6, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet PCJ. The metabolism of the aromatic amino acids concerned in the cross-linking of insect cuticle. Insect Biochemistry. 1980;10(5):467–500. [Google Scholar]
- Budnik V, White K. Catecholamine-containing neurons in Drosophila melanogaster: Distribution and development. Journal of Comparative Neurology. 1988;268(3):400–413. doi: 10.1002/cne.902680309. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Annals of Neurology. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulom H, Birman S. Chronic Exposure to Rotenone Models Sporadic Parkinson’s Disease in Drosophila melanogaster. The Journal of Neuroscience. 2004;24(48):10993–10998. doi: 10.1523/JNEUROSCI.2993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini I, et al. A versatile clearing agent for multi-modal brain imaging. Scientific Reports. 2015;5:9808. doi: 10.1038/srep09808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restriction of dopamine signaling to the dorsolateral striatum is sufficient for many cognitive behaviors. Proceedings of the National Academy of Sciences. 2009;106(34):14664–14669. doi: 10.1073/pnas.0907299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404(6776):394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Journal of Neurobiology. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Iché M, Birman S. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. genesis. 2003;35(4):260–269. doi: 10.1002/gene.1082. [DOI] [PubMed] [Google Scholar]
- Hardie SL, Hirsh J. An improved method for the separation and detection of biogenic amines in adult Drosophila brain extracts by high performance liquid chromatography. Journal of Neuroscience Methods. 2006;153(2):243–249. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Henschen CW, Palmiter RD, Darvas M. Restoration of Dopamine Signaling to the Dorsal Striatum Is Sufficient for Aspects of Active Maternal Behavior in Female Mice. Endocrinology. 2013;154(11):4316–4327. doi: 10.1210/en.2013-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hige T, et al. Heterosynaptic Plasticity Underlies Aversive Olfactory Learning in Drosophila. Neuron. 2015;88(5):985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334(6180):345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hirsh J, et al. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Current Biology: CB. 2010;20(3):209–214. doi: 10.1016/j.cub.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, et al. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proceedings of the National Academy of Sciences. 2006;103(23):8858–8863. doi: 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-Hydroxytyramine) and Brain Function. Pharmacological Reviews. 1966;18(2):925–964. [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends in Neurosciences. 2007;30(5):188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Lang AE. Parkinson’s disease. The Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PloS One. 2010;5(4):e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky TJ, et al. Two Different Forms of Arousal in Drosophila are Independently and Oppositely Regulated by the Dopamine D1 Receptor DopR via Distinct Neural Circuits. Neuron. 2009;64(4):522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, et al. Two Dopaminergic Neurons Signal to the Dorsal Fan-Shaped Body to Promote Wakefulness in Drosophila. Current Biology. 2012;22(22):2114–2123. doi: 10.1016/j.cub.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, et al. Increased Dopamine Level Enhances Male–Male Courtship in Drosophila. The Journal of Neuroscience. 2008;28(21):5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, et al. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52(3):425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, et al. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Rademacher DJ. MPTP Mouse Models of Parkinson’s Disease: An Update. Journal of Parkinson’s disease. 2011;1(1):19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall AH, et al. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Scientific Reports. 2016;6:20938. doi: 10.1038/srep20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer WS. Multiple Roles for Dopamine in Drosophila Development. Developmental Biology. 1996;176(2):209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. Journal of Neurogenetics. 1993;8(4):189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- Neha Singh VP. Advances in the treatment of Parkinson’s disease. Progress in neurobiology. 2007;81(1):29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Owald D, Waddell S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Current Opinion in Neurobiology. 2015;35:178–184. doi: 10.1016/j.conb.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech U, et al. Localization of the Contacts Between Kenyon Cells and Aminergic Neurons in the Drosophila melanogaster Brain Using SplitGFP Reconstitution. Journal of Comparative Neurology. 2013;521(17):3992–4026. doi: 10.1002/cne.23388. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, et al. Refinement of Tools for Targeted Gene Expression in Drosophila. Genetics. 2010;186(2):735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Patel JC. Somatodendritic dopamine release: recent mechanistic insights. Phil Trans R Soc B. 2015;370(1672):20140185. doi: 10.1098/rstb.2014.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemensperger T, et al. A Single Dopamine Pathway Underlies Progressive Locomotor Deficits in a Drosophila Model of Parkinson Disease. Cell Reports. doi: 10.1016/j.celrep.2013.10.032. Available at: http://www.sciencedirect.com/science/article/pii/S2211124713006165 [Accessed November 22, 2013] [DOI] [PubMed]
- Riemensperger T, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberger LC, Hauser RA. Optimizing bioavailability in the treatment of Parkinson’s disease. Neuropharmacology. 2007;53(7):791–800. doi: 10.1016/j.neuropharm.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Swinderen BV, Andretic R. Dopamine in Drosophila: setting arousal thresholds in a miniature brain. Proceedings of the Royal Society of London B: Biological Sciences. 2011;278(1707):906–913. doi: 10.1098/rspb.2010.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K. A Guide to Neurotoxic Animal Models of Parkinson’s Disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1):a009316–a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True JR, et al. Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Current Biology. 1999;9(23):1382–1391. doi: 10.1016/s0960-9822(00)80083-4. [DOI] [PubMed] [Google Scholar]
- Ueno T, et al. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nature Neuroscience. 2012 doi: 10.1038/nn.3238. Available at: http://www.nature.com/neuro/journal/vaop/ncurrent/full/nn.3238.html [Accessed October 19, 2012] [DOI] [PubMed]
- Venken KJT, et al. P[acman]: A BAC Transgenic Platform for Targeted Insertion of Large DNA Fragments in D. melanogaster. Science. 2006;314(5806):1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- Venken KJT, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature Methods. 2009;6(6):431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vié A, et al. Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. The Journal of Biological Chemistry. 1999;274(24):16788–16795. doi: 10.1074/jbc.274.24.16788. [DOI] [PubMed] [Google Scholar]
- Waddell S. Neural Plasticity: Dopamine Tunes the Mushroom Body Output Network. Current Biology. 2016;26(3):R109–R112. doi: 10.1016/j.cub.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Waddell S. Reinforcement signalling in Drosophila; dopamine does it all after all. Current Opinion in Neurobiology. 2013;23(3):324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, et al. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Research. 2005;33(4):e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TR. The genetics of biogenic amine metabolism, sclerotization, and melanization in Drosophila melanogaster. Advances in Genetics. 1987;24:127–222. [PubMed] [Google Scholar]
- Zhao Y, et al. Corazonin Neurons Function in Sexually Dimorphic Circuitry That Shape Behavioral Responses to Stress in Drosophila. PLOS ONE. 2010;5(2):e9141. doi: 10.1371/journal.pone.0009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


