Summary
Currently, platelets for transfusion are stored at room temperature (RT) for 5–7 days with gentle agitation, but this is less than optimal because of loss of function and risk of bacterial contamination. We have previously demonstrated that cold (4°C) storage is an attractive alternative because it preserves platelet metabolic reserves, in vitro responses to agonists of activation, aggregation and physiological inhibitors, as well as adhesion to thrombogenic surfaces better than RT storage. Recently, the US Food and Drug Administration clarified that apheresis platelets stored at 4°C for up to 72 h may be used for treating active haemorrhage. In this work, we tested the hypothesis that cold-stored platelets contribute to generating clots with superior mechanical properties compared to RT-stored platelets. Rheological studies demonstrate that the clots formed from platelets stored at 4°C for 5 days are significantly stiffer (higher elastic modulus) and stronger (higher critical stress) than those formed from RT-stored platelets. Morphological analysis shows that clot fibres from cold-stored platelets were denser, thinner, straighter and with more branch points or crosslinks than those from RT-stored platelets. Our results also show that the enhanced clot strength and packed structure is due to cold-induced plasma factor XIII binding to platelet surfaces, and the consequent increase in crosslinking.
Keywords: Refrigeration, Rheology, Clot strength, Ultrastructure, FXIII
Introduction
Platelets are most critical in preventing bleeding following an injury. Platelet transfusion is the standard of care in case of severe thrombocytopenia, as the platelet levels fall below 10 × 109/l from the normal count of 150–400 × 109/l. Currently, platelets for transfusion are stored at room temperature (RT) for 5 days with agitation, after which they are discarded as per US food and Drug Administration (FDA) guidelines to avoid infectious and inflammatory responses after transfusion (Slichter & Harker 1976; Stroncek & Rebulla 2007). However, RT storage is not ideal as it has been shown to compromise functionality both in vivo and in vitro (‘platelet storage lesion’) and increase the potential risk of microbial contamination (Pidcoke et al. 2013; Reddoch et al. 2014; Rosenfeld et al. 1995). Of note, current prophylactic transfusion practices are aimed at minimising the number of transfusions to avoid potential undesirable immune reactions. The transfusion criterion is based on platelet count (i.e., survival and recovery of transfused platelets) rather than on functional response, despite substantial evidence that platelet dysfunction during RT storage is irreversible. However, platelet functionality is of utmost importance in therapeutic transfusion to acutely bleeding patients, as achieving haemostasis within the first 6 h in traumatic massive bleeding has been associated with improved outcomes (Holcomb et al, 2008; Cap et al, 2012).
Storing platelets at 4°C (4C or cold) is an attractive alternative because cold-stored platelets show better haemostatic response both in vitro and in vivo. Platelets stored at 4°C for 24 h have been shown to decrease in vivo bleeding times by 40% compared to RT-stored platelets (Becker et al. 1973). We and others have shown that cold-stored platelets are in haemostatically primed state, have better preserved metabolic reserves, maintain shear- and agonist-induced aggregation responses, adhesion and aggregation under flow comparable to fresh platelets, respond well to physiological inhibitors, and also form stronger clots (Babic et al. 2007; Evans et al. 2008; Filip & Aster 1978; Montgomery et al. 2012; Reddoch et al. 2014). The only purported disadvantage of cold-stored platelets is the shorter circulation half-life in vivo of 1.8 days compared to 3.8 days for RT-stored platelets (Murphy & Gardner 1969). However, the rapid clearance of cold-stored platelets may in fact be advantageous in actively bleeding patients by reducing the risk of late thrombosis after establishing effective immediate haemostasis.
Efficient haemorrhagic control is attained through the formation of strong and stable clots at the site of injury, and hence an understanding of the clot properties is imperative to fully comprehend the function of this new therapeutic product (Evans et al. 2008). In this work, we have investigated the rheological properties of the clots formed from stored platelets by dynamic mechanical analysis, and established the relationship between these functional changes to underlying structure of the clot network from the morphological analysis of clot microstructure. We also elucidated the contribution of stored platelets to the differences in clot structure and strength by comparing thrombin generation and Factor XIII (FXIII) levels, which regulate fibrin polymerization and crosslinking, respectively.
Materials and methods
Venous blood was drawn from healthy volunteers after signing an informed consent and obtaining written regulatory approval in accordance with the Institutional Review Board (IRB) protocol (IRB #12-227, Office of Research Integrity and Compliance, University of Texas at San Antonio). Similarly, apheresis platelet (AP) units were collected from consented, healthy donors under a protocol reviewed and approved by the US Army Medical Research and Material Command Institutional Review Board and in accordance with the approved protocol.
Platelet preparation
Platelet rich plasma (PRP) was obtained from phlebotomized blood or single/double apheresis platelet (AP) collection, processed and stored as previously described (Montgomery et al, 2012; Nair et al, 2014; Reddoch et al, 2014). For control experiments, AP units were stored in 65% platelet additive solution (PAS, Isoplate, Terumo Inc., Lakewood, CO). Platelet pellets from AP were obtained by centrifuging PRP with PGI2 (Calbiochem, EMD Millipore, Billerica, MA) at 800 x g for 10 min. Platelets were resuspended to 300 × 109 platelets/l in Fresh Frozen Plasma (FFP, South Texas Blood and Tissue Bank, San Antonio, TX) thawed to 37°C, or in PAS or in PPP. Lyophilized platelets (Bio/Data Corporation, Horsham, PA) were used as a control.
Kinetics of clot gelation
Cone-and-plate rheometry combined with dynamic mechanical analysis (DMA) was used to quantify the rheological properties of clots (Weigandt et al. 2012). Small amplitude oscillatory test at low strain (0.5%) and 1 Hz was used to track the evolution of elastic modulus (G′) using Rheoplus (Anton Paar, Germany) for 30 min. In these experiments, fresh or stored PRP mixed with 20 mM CaCl2 was placed on a rheometer plate maintained at 37°C. To prevent evaporation, an immiscible oil layer (Vapor-Lock® liquid vapour barrier, Qiagen, Valencia, CA) was applied along the rim of the exposed surface and the entire set-up was covered with a humidifying chamber. In some experiments, PRP was incubated with 10 μM of final concentration of inhibitors Eptifibatide (San Cruz Biotechnology Inc, Santa Cruz, CA) or Cytochalasin B (Sigma, St. Louis, MO) at 37°C for 10 min and activated with 20 mM CaCl2 prior to testing (Ciborowski & Tomasiak 2009; Siess et al. 1982).
Analysis of clot breakage
Following clotting kinetics measurements, the clots were assayed for their nonlinear rheology (Weigandt et al. 2012). Shear stress applied by the rheometer was progressively increased from 1% to 250% without time constraints until the clot failed. The yield strength values were identified from shear-strain curves as the point where the clots deformed irreversibly. The stress-strain slopes were used to define relative crosslinking density (RCD).
Clot ultrastructure using scanning electron microscopy (SEM)
Clots were formed on glass slides with 20 mM calcium at 37°C for 1 h, fixed with 4% formaldehyde for 2 h and stained with 1% Osmium [Electron Microscopy Sciences (EMS), Hatfield, PA] for 30 min. Further, the clots were washed with Zetterquist’s Buffer and then dehydrated with 70%, 95% and 100% Ethyl alcohol (200% absolute alcohol, EMS). Finally the samples were incubated in Hexamethyldisilizane (HMDS) (EMS) for 5 min and allowed to air dry before performing SEM. 8 to 10 images per sample condition was captured and analysed. Morphometric quantification of fibrin density, curvature, length and diameter was carried out using Image J software (National Institutes of Health, Bethesda, MD) and custom written MATLAB codes (Supporting Fig. 1) (Bob 2014; http://dx.doi.org/10.1016/0031-3203(86)90030-0; Katti et al. 2013; Lopes 2007).
Estimation of thrombin generation
Thrombin generation was measured using the calibrated automated thrombogram (CAT) method (Thrombinoscope BV, Maastricht, The Netherlands) as previously described (Hemker et al. 2003). Briefly, 20 μl of PRP reagent (Thrombinoscope BV) containing a final concentration of 0.5 pM and phospholipids or thrombin calibrator were added to each well of a round-bottom 96-well plate followed by the addition of 80 μl of AP platelets in FFP. From the thrombin generation curves, lag times, endogenous thrombin potentials (ETP or area under the thrombin generation curve) and peak thrombin concentrations were analysed using Thrombinoscope software version 5.0.0.742.
Estimation of Factor XIII levels
Platelet protein concentrates were collected for Western blot analysis as described previously (Getz et al. 2010). Activated FXIII (FXIIIa) was probed with primary antibody at 1:1000 dilutions (Rb mAb Factor XIIIa, Abcam, Cambridge, MA,), followed by secondary antibody at 1:5000 dilution (Goat anti rabbit, Santa Cruz Biotechnology). FXIII expression levels were detected using chemiluminescent HRP substrate, and the blots were imaged to obtain the relative band densities (RBD) and quantified using Image J software.
Flow cytometry
Flow cytometry was carried out as described previously (Mitchell et al. 2014). Briefly, PRP was diluted with 2% bovine serum albumin in 1x Hank’s Balanced Salt Solution buffer containing 1 μM PgI2, and treated with Human TrueStain FcXTM for 5 min, followed by fluorescein isothiocyanate (FITC) conjugated FXIIIB antibodies (Zedira GmbH, Darmstadt, Germany) for 45 min at room temperature. The samples were washed and analysed using FACSDIVA software on a Canto I LSR flow cytometer (both from BD Biosciences, San Jose, CA). Aggregates and microparticles in stored samples were gated based on the forward and side scatter with respect to fresh platelets as baseline.
Statistical analysis
The data were analyzed by one-way ANOVA (> 2 groups) for repeated measures with a post-hoc Tukey’s test. Student t-test was used for two-group analyses. The groups were considered statistically significant when the P value was less than 0.05. All data are graphed using Mean ± Standard error of mean. Microsoft Excel (Microsoft Corp, Redmond, WA) was used to manage data and statistical analyses were performed using GraphPad Prism 6 (GraphPad Software Inc. San Diego, CA).
Results
Rheology of clots and the contribution of platelets
We measured the viscoelastic properties of clots by shear rheology. As our goal is to isolate the contribution of platelets to clot properties, all experiments were performed using PRP without red cells. The experiments were performed at low oscillatory strain (<1%) in the linear viscoelastic regime, and as expected, the elastic modulus (G′) was always higher than viscous modulus (G′), indicative of the more elastic than viscous nature of the clot (Supporting Fig. 2). The clotting kinetics, once initiated with calcium, were characterized by a short lag phase of initiation, and a rapid log phase of fibrin polymerization followed by an upper saturation/stationary phase which indicates a stable clot with constant moduli (Fig. 1A).
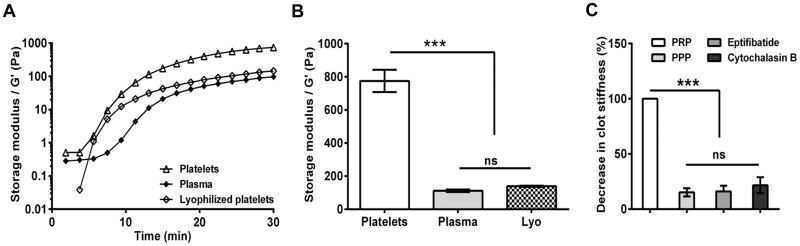
Figure 1. Effect of platelets on clot stiffness.
Rheological properties of evolving clots were investigated using dynamic mechanical analysis at 37°C for 30 min. (A) Representative traces of storage modulus, G′ (clot stiffness) for clots formed from plasma, plasma with platelets (300 ×109 platelets /l) and plasma with lyophilized platelets (300 ×109 platelets /l); (B) Clot stiffness increases by 6-fold with physiological concentration of live platelets in plasma (n=3; *, P<0.05). (C) Platelets failed to form stable clots on blocking glycoprotein IIb/IIIa receptor (Eptifibatide) or actin polymerization (Cytochalasin B). [n=3; ns, not significant (P >0.05); *, P < 0.05; **, P<0.01; ***, P<0.001]. Lyo, lyophilized platelets; PPP, platelet poor plasma; PRP, platelet rich plasma.
The elastic modulus (G′) is the resistance of the clots to reversible deformation under applied stress, and hence is the measure of the stiffness. To delineate the effect of platelets on clot stiffness, we measured the elastic moduli of clots formed from PRP and PPP. The clot stiffness increased by 7-fold due to the presence of platelets in PRP. As a comparison, we measured the clot strength of FFP and lyophilized platelets re-suspended in FFP. We observed that the clot stiffness of FFP was comparable to that of lyophilized platelets re-suspended in FFP. The failure of lyophilized platelets, unlike live platelets, to improve the clot stiffness of FFP, indicates that live, active platelets are imperative to the formation of stiff, strong clots (Fig. 1B). The role of active platelet contraction is further substantiated by blocking either glycoprotein (GP)IIb/IIIa-fibrinogen interactions or actin polymerization using either Eptifibatide or Cytochalasin B, respectively. These treatments resulted in a feeble or no clot, establishing that platelets and their active interaction with the fibrin polymer are essential for optimal clot stiffness (Fig. 1C).
Effect of storage temperature on clot stiffness
Next, we evaluated the effect of storage temperature on the contribution of platelets to clot stiffness by storing PRP in autologous plasma for 5 days either at room temperature (RT) or at 4°C (4C). The clotting kinetics and rheological properties were similar to fresh PRP in both RT and 4C PRP in that the clots were “solid-like,” with higher storage (elastic) moduli than loss (viscous) moduli (Supporting Fig. 3). However; the stiffness of the clots from refrigerated PRP was comparable to that from fresh PRP and significantly better than that from PRP stored at RT (Fig. 2A). Further, similar to fresh platelets, blocking GPIIb/IIIa receptor and actin polymerization with Eptifibatide and Cytochalasin, respectively, reduced clot stiffness from stored platelets to undetectable levels (Supporting Fig. 4). As storage may affect both platelets and plasma coagulation factors, we evaluated separately the effect of storage on plasma by estimating the clot stiffness of fresh and stored PPP. We observed that stored PPP samples formed much weaker clots than fresh PPP, probably due to degradation of clotting factors (Fig. 2B) Thus, the data from Fig. 2A and B together reveal that the presence of platelets made a larger difference to clot stiffness in cold storage: the clot stiffness of PRP at 4°C was 20-fold higher than PPP at 4°C, compared to the corresponding increase of 6-fold and 4-fold in fresh and RT-stored samples, respectively.
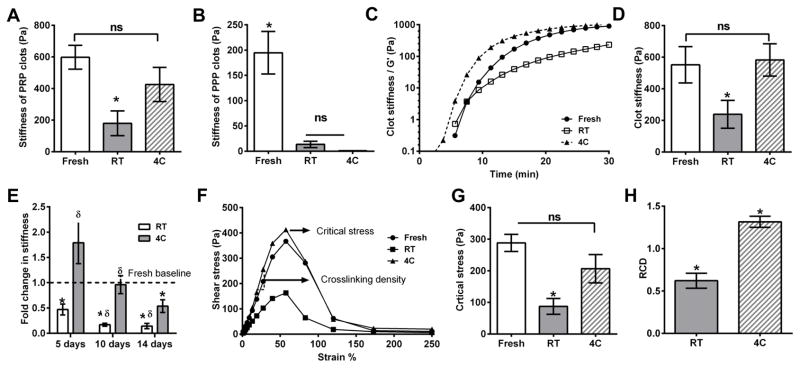
Figure 2. Effect of storage temperature on clot mechanical properties.
Stiffness of clots formed from fresh platelets or platelets stored for 5 days at 4°C (4C) or room temperature (RT) were analysed. (A) Clots from 4C-stored platelet rich plasma (PRP) showed stiffness comparable to that from fresh platelets, and was higher than that from RT-stored PRP; (B) Clots from platelet poor plasma (PPP) stored at either 4°C or RT were much weaker than from fresh PPP (n = 5); (C) Representative rheological traces of fresh/ stored platelets with fresh frozen plasma (FFP); (D) RT-stored platelets form clots with less stiffness, whereas 4C-stored platelets have similar stiffness as fresh platelets (n = 5); (E) Cold storage maintains clot stiffness over longer periods of storage, unlike RT storage. Data normalized to stiffness of fresh platelets (n = 4, * compared to Fresh, δ compared to 5 day RT-stored, P < 0.05); (F) Representative traces from amplitude sweep analysis of pre-formed clots; Clot strength and cross-linking density were quantified as the stress at which the clot deformed irreversibly and the slope of the stress-strain graph in the linear regime, respectively; (G) Cold, but not RT storage maintains clot strength; and (H) Relative crosslinking density (RCD) compared to fresh platelets (= 1.0). Clots from cold-stored platelets have higher cross-linking density (n=5). ns, not significant (P >0.05); *, P<0.05; **, P<0.01.
Given that autologous plasma (PPP) itself degrades during storage, it is imperative to negate the confounding effects of autologous plasma degradation while evaluating the effect of storage temperature on platelet function. To this end, we re-suspended fresh and stored platelets to a physiological concentration (300 × 109/l) in FFP for all of our experiments. The kinetics of clotting of platelets in FFP followed the same pattern as in autologous plasma, and clot stiffness was estimated as described above. The clot stiffness was comparable between fresh and cold-stored platelets but that of RT-stored platelets decreased by 50% (Fig. 2D). Together, these results show that RT storage impairs the contribution of platelets to clot strength, but cold storage maintains, if not even modestly enhances, this contribution.
Considering these promising results, we extended the storage duration beyond the current FDA standard for platelet storage for transfusion from 5 days to 10 and 14 days (Fig. 2E). While storing at RT for 10 and 14 days further reduced the stiffness to <25% of that of fresh platelets, the clot stiffness of platelets stored at 4°C for 10 days was similar to that of fresh platelets, and the clot stiffness at 14 days of storage was >50% of that of fresh platelets. Of note, the clot stiffness of platelets stored at 4°C for 14 days is similar to the current FDA standards for 5 day RT storage (Fig. 2E).
Effect of storage temperature on clot strength and relative crosslinking density (RCD)
The subtle but key difference between stiffness and strength is that the former is a measure of the resistance of the clot to reversible deformation and the latter is the ability of the clot to carry large loads before irreversible deformation. To estimate the contribution of fresh and stored platelets to clot strength, we subjected pre-formed clots to progressively increasing strain from 1% to 250%, i.e., an amplitude sweep, until the clot was deformed irreversibly, to estimate the critical stress (Fig. 2F). Clots formed by fresh and cold-stored platelets have comparable clot strength as deduced from the amplitude sweep analysis; whereas platelets stored at RT formed significantly weaker clots compared to cold-stored 4C platelets (Fig. 2G). The density of crosslinks between individual fibrin fibres is directly proportional to shear modulus in the linear viscoelastic regime characteristic of cross-linked polymers (Flores-Merino et al, 2010; Gent, 2013; Helms et al, 2012). From the slope of stress-strain curves for clots from fresh and stored platelets, we estimated the crosslinking density of the clots from stored platelets relative to that from fresh platelets. As shown in Fig. 2H, the RCD of clots from cold-stored platelets was significantly higher than fresh or RT-stored platelets.
Clot ultrastructure
Given that the biomechanical properties, such as clot stiffness and strength, are strongly dependent on the properties of the individual fibrin fibres and hence on the underlying fibrin structure, we examined the micro-architecture of clots from fresh and stored platelets using SEM (Fig 3A). The representative images show that the morphology of the clots is fundamentally different between fresh and stored platelets. Qualitatively, the clots formed from RT-stored platelets were less dense with thicker, less taut or stretched fibres than those formed from fresh or cold-stored platelets. We quantified various structural attributes of the clots formed from fresh and stored platelets from these images to obtain insights into the molecular mechanisms that govern the complex process of clot formation (Fig. 3B–E).
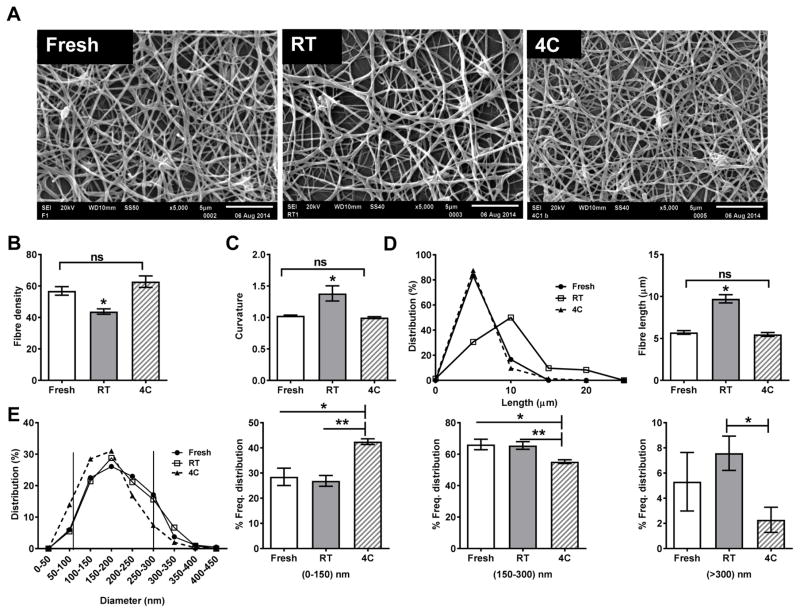
Figure 3. Clot architecture.
Clots were formed from fresh/stored platelets with 20 mM calcium, fixed, stained, dehydrated and analysed by scanning electron microscopy (5000x at 20 kV, Jeol 6610LV; Jeol, Tokyo, Japan). (A) Representative images of clots formed from fresh and stored platelets; (B–E) Quantification of morphology of clots from fresh and stored platelets: (B) Density; (C) Curvature; (D, E) Frequency distribution of fibre lengths and mode (D) and fibre diameters and mode (E). Based on the diameter, the fibres were classified either as thin (<150 nm), intermediate (150–300 nm) or thick (>300 nm). (n=3). ns, not significant (P >0.05); *, P<0.05; **, P<0.01. 4C, platelets stored at 4°C; RT, platelets stored at room temperature.
First, we observed that the fibre density, i.e., number of fibres crossing any given cross-section, was comparable between fresh and cold-stored platelets but those from RT-stored platelets was 25–30% lower. Second, the curved, true length of individual fibres between two branch points in clots formed from fresh and cold-stored platelets had similar length distribution with a median fibre length of 5 μm, while the median length in clots formed from RT-stored platelets was 10 μm with a significant fraction being as long as 20 μm (Fig. 3D). Third, clots from fresh and cold-stored platelets were straighter with a curvature ~1.0 than the clots from RT-stored platelets with a curvature of 1.5, indicating relatively loose ‘spaghetti-like’ organization. Thus, fibres from clots from RT-stored platelets were more wavy and long with fewer branch points (Fig. 3C). Lastly, the distribution of fibre diameters was clustered as three groups: thin (<150 nm), intermediate (150–300 nm) and thick (>300 nm) fibres, respectively (Fig. 3E). Interestingly, the clots formed from fresh and RT-stored platelets had similar, normal distribution but those from cold-stored platelets were skewed towards the shorter range. We observed that clots from cold-stored platelets had ~30% more thin fibres and ~30% fewer thick fibres compared to clots formed from fresh and RT-stored platelets (Fig. 3E). Together, our analysis shows that cold-stored platelets promote denser clots with thinner fibres consisting of more branch points, while RT-stored platelets promote clots that are less dense with fewer branch points.
Thrombin generation in stored platelets
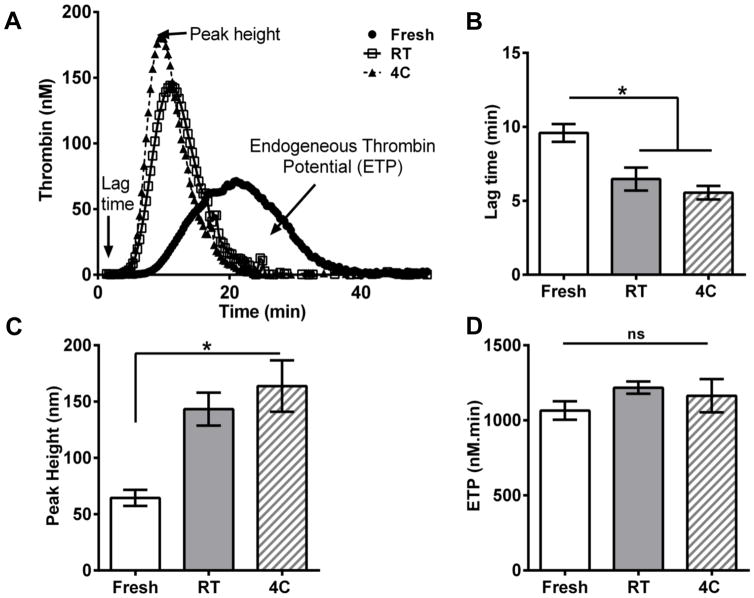
To understand the origin of differences in the clot structure between fresh and stored platelets, we investigated the generation of thrombin, as it is closely associated with fibrin clot morphology. Using the CAT assay, we observed that the thrombin generation profiles were similar for RT- and cold-stored platelets but distinctly different from that of fresh platelets (Fig. 4A). The lag time is a measure of the initiation phase of coagulation, and the lag times of both RT and cold-stored platelets initiate coagulation faster than fresh platelets (Fig. 4B). Peak height is equivalent to the maximum thrombin concentration, and stored platelets generated significantly higher thrombin transiently than fresh platelets (Fig. 4C). The differences seen in stored samples, for lag times and peak heights, may be attributed to increased phosphatidylserine (PS) and FVa expression on the surface of stored platelets (Reddoch et al. 2016). ETP is equivalent to the total amount of thrombin generated during the assay, and similar ETP levels indicate that platelets produce similar amounts of total thrombin despite their storage temperature (Fig. 4D). Thus, although cold-stored and RT-stored platelets have similar thrombin generation profiles, the differences in structure and function suggest that mechanisms other than thrombin-dependent rates of fibrin polymerization may be critical to explaining the observed divergence in clot mechanical properties.
Figure 4. Thrombin generation from fresh and stored platelets.
Thrombin generation in apheresis platelet (AP) pellets resuspended in fresh frozen plasma (150,000/ml) was measured using the calibrated automated thrombogram (CAT) for 50 min. (A) Representative thrombogram from the CAT assay; (B,C) Less lag time and increased peak height for thrombin generation in stored platelets compared to fresh platelets (D) Total thrombin generated over time (ETP) is the same in stored as well as in fresh platelets (n=4).; ns, not significant (P >0.05); *, P<0.05. 4C, platelets stored at 4°C; RT, platelets stored at room temperature.
The binding of plasma FXIII to cold-stored platelets
Given that clots formed from cold-stored platelets are significantly thinner with more branch points than those from RT-stored platelets, we surmised that stored platelets influence the mechanism of fibrin crosslinking. Factor XIII is a transglutaminase enzyme present in platelets and plasma, that catalyses the ligation of fibrin molecules within clot fibres, thus remarkably increasing the clot density, strength and stiffness. We tested the hypothesis that platelet-associated FXIII may be an important contributor to the observed differences between storage conditions.
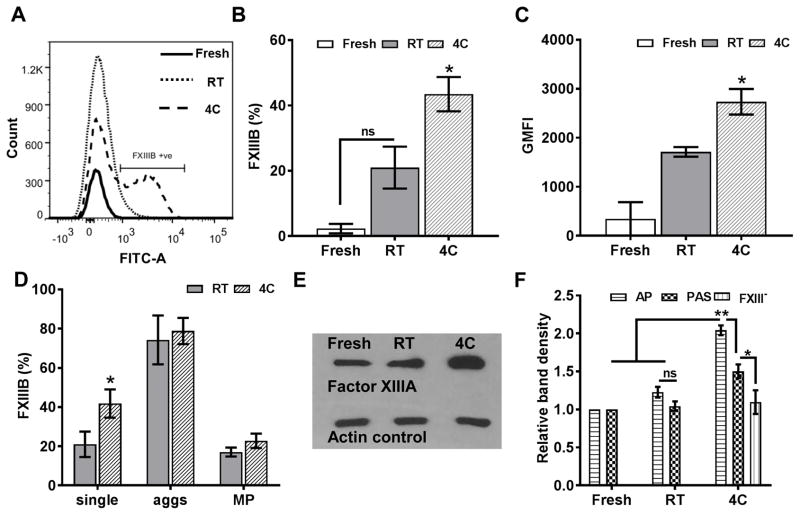
To this end, our flow cytometry results show that FXIII levels were significantly higher in cold-stored platelets than in fresh or RT-stored platelets (Fig. 5A–D). In particular, we used antibody directed against the inhibitory FXIIIB subunit because FXIIIB subunit is present only in plasma FXIII but is absent in intracellular platelet FXIII, which is exclusively the catalytic FXIIIA subunit. We observed a 40% increase in FXIIIB expression and a subsequent increase in geometric mean fluorescence intensity in cold-stored platelets (Fig. 5B, C), with a higher expression of FXIIIB in aggregates and microparticles (Fig. 5D). Interestingly, we found significantly higher FXIIIB than FXIIIA on cold-stored platelets consistent with the higher molar ratio (2:1) of FXIIIB in plasma (data not shown). We also confirmed that cold-stored platelets had 2-fold higher FXIII levels than RT-stored platelets using antibody directed against FXIIIA subunit on platelet lysate (Fig. 5E). To confirm the source of FXIII as exogenous plasma and not intracellular storage in platelets, we estimated the FXIII levels in platelets stored in PAS consisting of only 35% plasma or FXIII-free plasma as storage solutions. Plasma dilution reduced the amount of FXIII bound to cold-stored platelets by 50% or down to the same levels as RT-stored platelets (Fig. 5F). Finally, we confirmed that cold-induced activation may not trigger the release of intracellular FXIII stores because thrombin receptor activating peptide-activation of platelets in FXIII-free buffer did not show an increase in FXIII levels (Supporting Fig. 5). Together, our results show that cold storage induces the binding of plasma FXIII to platelet surfaces.
Figure 5. Cold induced plasma factor XIII (FXIII) binding.
Platelets were stained with anti-FXIIIB antibodies to confirm the source of FXIII binding in cold-stored platelets.(A) Representative histogram of FXIIIB expression in stored vs. fresh platelets (B) Increased FXIIIB positive platelets compared to fresh platelets at baseline (C) Geometric mean fluorescence (GMFI) indicates more FXIIIB binding on individual cold-stored platelet surfaces (D) FXIIIB expression in aggregates and microparticles in stored platelets implies fibrinogen-mediated binding; FXIIIA was probed in immunoblots. (E) Representative immunoblot showing increased relative density of FXIIIA content in cold-stored platelets (F) Decreased FXIII binding in PAS storage compared to AP (n=4). ns, not significant (P >0.05); *, P<0.05; **, P<0.01. 4C, platelets stored at 4°C; aggs, aggregates; AP, apheresis platelets; MP, microparticles; PAS, platelet additive solution; RT, platelets stored at room temperature.
The contribution of FXIII to clot rheology
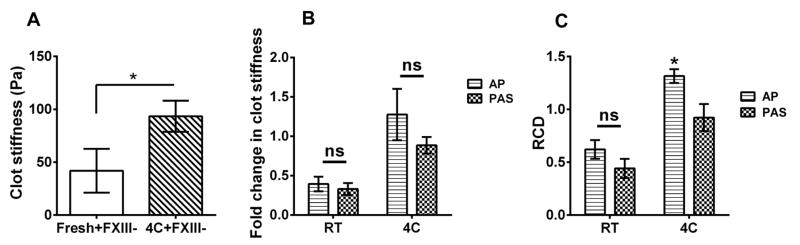
Having confirmed the binding of plasma FXIII to platelets during cold storage, we isolated the effect of FXIII-binding on clot properties. First, we evaluated the stiffness of clots formed from fresh or cold-stored platelets in FXIII-deficient plasma. As expected, the clot stiffness was considerably lower than in normal plasma (775 ±66.7 vs.51.46 ±11.96) (Fig. 1A vs Fig 6A). Interestingly, platelets stored at 4°C for 5 days produced a clot with significantly (2-fold) higher stiffness compared to those produced from fresh platelets (Fig. 6A). This data suggests that platelet-bound FXIII can supplement plasma FXIII in producing stronger clots. Next, cold storage of platelets in plasma produced clots with modestly higher stiffness and significantly higher crosslinks than from platelets stored in PAS (Fig. 6B, C). This data, together with the results from FXIII binding during cold storage, demonstrates that the exogenous plasma FXIII may contribute to the superior properties of the clot in cold-stored platelets compared to RT-stored platelets.
Figure 6. Effect of FXIII on clot mechanics.
Rheological analysis was carried out on stored platelets stored in PAS. (A, B) Cold-stored platelets in PAS have similar clot stiffness and crosslinking density to fresh platelets (fresh = 1.0, n=4). (C) Cold-stored platelets in autologous plasma form clots in FXIII-deficient plasma with higher stiffness than fresh platelets (n=4). ns, not significant (P >0.05); *, P<0.05. 4C, platelets stored at 4°C; AP, apheresis platelets; PAS, platelet additive solution; RCD, relative crosslinking density; RT, platelets stored at room temperature.
Discussion
The quality and functionality of stored platelets is vital in platelet transfusion. In this work, we investigated the effect of storage on the mechanical properties of clots formed by fresh and stored platelets, and elucidated the contribution of platelets by analysing the underlying structural and molecular mechanisms. We have previously shown that cold storage better preserves the haemostatic response of platelets to agonists and inhibitors of activation than RT storage (Nair et al. 2014; Reddoch et al. 2016; Reddoch et al. 2014). Together with our previous results, the current work is of high clinical relevance, particularly in the context of the recent approval of cold-stored platelets for the treatment of active haemorrhage.
Effective haemorrhage control is solely dependent upon the strength and stability of the fibrin network. Clot stiffness is directly related to the elastic energy stored in the fibrin fibres; and the strength refers to the end of elastic behaviour and onset of the breakage of fibrin fibres. Clots deform reversibly at small strains (1%), which is akin to their ability to resist deformation to nominal forces due to blood flow, while the irreversible deformation at large strains (200%) is a measure of the ability to dam active haemorrhage. Cold-stored platelets contributed substantially more than RT-stored platelets to both clot stiffness and to clot strength (2.5fold), suggesting the superior haemostatic properties of cold-stored platelets. Consistent with previous studies on fresh platelets, our data shows that the presence of active, but not lyophilized, platelets significantly increases the clot stiffness compared to plasma (Glover et al. 1975). As a comparison, the stiffness of plasma clots or clots containing lyophilized platelets was as low as that of common “jello” (1.5% w/v gelatin) at 37°C (data not shown). It may be instructive to relate clot stiffness/strength from rheology to the more familiar maximum amplitude (MA) from thromboelastography (TEG) or maximum clot firmness from rotational thromboelastometry (ROTEM®) as both quantities relate the ultimate stability of the clot. Our previous MA measurements of the clots formed by stored platelets showed a trend similar to those of G′ observed in this study, i.e., RT platelets formed clots with significantly reduced stiffness compared to cold-stored platelets (65.2± 2.2 vs.58.9± 2.4 mm; 582.7 ±102.7 vs. 283.8± 88 Pa) (Reddoch et al. 2014). The G′ measurements may be a better manifestation of the effect of platelet storage on clot elasticity given that TEG employs autologous plasma unlike FFP used in these studies, and our data (Fig 1) demonstrates that plasma degrades during storage.
The quantifiable structural attributes of fibrin polymers including curvature, density, diameter, branch points and cross-links may be uniquely correlated to the mechanical properties of the clots (Weisel 2004). The fibres are under constant tension and, as a result of this, they appear very straight, and straighter fibres have better clot strength because of increased flexural stiffness (Lam et al. 2011; Varjú et al. 2011). In contrast, increased curvature makes these fibres wavier, and increased curve length implies that these long fibres are formed at the expense of branch points, and hence less connectivity between the individual fibres of the network (Petersen & Suenson 1991). Dense fibrin networks store more energy and resist deformation forces. The detailed ultra-structural analysis presented in this work shows that the underlying form governs the observed function, namely, the denser, straighter fibres in clots from cold-stored platelets provide stiffer and stronger clots vis-à-vis thinner, less dense fibres formed from RT-stored platelets that render the clots less stiff and weaker. In addition to contributing to the mechanical properties (Lord 2011; Veklich et al. 1998), fibres with taut conformation, higher density and increased branches are less susceptible to fibrinolysis, probably due to lower permeability of lytic enzymes (Longstaff et al. 2013; Lord 2011; Varjú et al. 2011; Wufsus et al. 2013).
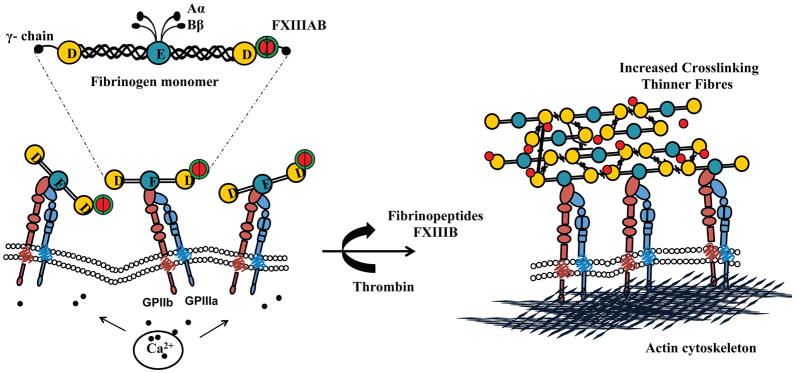
Clot formation is a complex process, which may be defined by three key steps: linear polymerization of fibrinogen to protofibrils mediated by thrombin, lateral aggregation and crosslinking of the protofibrils to form a 3D fibrin network, and compaction due to active platelet contraction. The presence of substantially higher levels of FXIII on the surface of cold-stored platelets may contribute to increased clot stiffness. FXIII is a key transglutaminase enzyme in the platelet contractility apparatus, as well as a modulator of fibrin structure. While the importance of free plasma FXIII in mediating fibrin cross-linking and lateral aggregation of fibres is well documented, the role of platelet-bound FXIII is not clear. Platelet-bound FXIII is believed to support adhesion to collagen and von Willebrand factor, induce activation signalling (Magwenzi et al. 2011) and also modulate clot retraction (Kasahara et al. 2010). Our studies on the mechanism of cold-induced FXIII binding to platelet surface shows a novel role for platelet-bound FXIII, i.e., cross-linking the fibrin fibres, which decreases the fibrin thickness and increases the clot stiffness by ~4-fold compared to uncross-linked fibres, and reduced fibrinolysis (Carr & Hermans 1978; Ferry et al. 1951; Gerth et al. 1974; Hethershaw et al. 2014; Roberts et al. 1973; Ryan et al. 1999a; Ryan et al. 1999b; Standeven et al. 2007; Yeromonahos et al. 2010). Our results indicate that the increase in platelet-associated FXIII on cold-stored platelets is due to plasma FXIII. Both forms of plasma FXIII, i.e., A2B2 and B2 have been found to be complexed with the circulating fibrinogen in plasma in a 2:1 ratio (Byrnes et al. 2016; Katona et al. 2014; Yorifuji et al. 1988). Our group has recently shown that the surface of cold-stored platelets contains activated GPIIb/IIIa, which suggests that plasma FXIII may probably be binding to the surface directly and also through fibrinogen.(Getz et al. 2016) Thus, we propose that the surface of cold platelets is uniquely poised, not only for the initiation and propagation of fibrin polymerization by rapid thrombin generation, but also conducive for crosslinking to form thinner fibres due to the presence of high local concentrations of fibrinogen and FXIIIA2 on cold platelet surfaces (Fig. 7). The surface of cold platelets thus not only provides a rich catalytic surface for thrombin generation but also a nucleation site for the assembly and crosslinking of fibrin.
Figure 7. Mechanism of FXIII-mediated cross linking in cold-stored platelets.
(A) Cold storage leads to Intracellular calcium leakage in platelets, resulting in shape change and activation of GPIIb/IIIa receptor through calcium inside-out signalling. Fibrinogen (complexed with FXIII on γ chain) in stored autologous plasma binds to GPIIb/IIIa (B) Upon activation, thrombin cleaves fibrinopeptides to convert fibrinogen monomer to fibrin, as well as FXIIIB to activate FXIIIA. Platelets act as nucleation site for fibrin polymerization, and FXIIIA mediates γ–dimer (longitudinal and transverse), γ-tetramer, α-polymer, αγ-hybrid crosslinks (covalent bonds) in laterally aggregated fibrin polymer (soft clot) to form a strong clot (hard clot) with thinner fibres. D, fibrinopeptide D; E, fibrinopeptide E; GP, glycoprotein.
In summary, this work provides insights into the haemostatic efficacy of platelets to form stable clots and supports the notion that cold-stored platelets may be a better alternative to the current standard of care, especially when treating acute bleeding where immediate haemostasis is the priority. Furthermore, these platelets provide greater clot strength than RT-stored platelets, which may be beneficial in patients who need to be maintained at higher blood pressure to support transfusion and avoid ‘popped clot phenomenon’ (Dutton et al. 2002). Clot microstructure analyses demonstrated that cold-stored platelets form denser clots with thinner fibres and more crosslinks, which provides a rationale for the superior mechanical properties. Finally, we demonstrate that an increase in platelet-associated FXIII may contribute to crosslinking and hence superior mechanical properties during cold storage. Of note, the rapid clearance of cold-stored platelets means that the utilization of their maximum haemostatic potential for acute response, with clot strength comparable to that of fresh platelets, is can be accomplished with a reduced risk of late thrombosis.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the US Army Institute of Surgical Research blood bank for collection of apheresis units, as well as Ashwati Cheeniyil and Barbara Hunter for technical assistance. This study was supported by a contract from the Department of Defense (W81XWH-13-P-0146 to AKR), and partially by a grant from the NIH (HL112629 to AKR) and AHA (14PRE19580011 to KMR).
Footnotes
Author contributions
PMN, APC, and AKR designed research, and analysed and interpreted data. PMN performed the experiments. PMN, APC and AKR wrote and critically reviewed the manuscript; SP wrote MATLAB codes and contributed to SEM image analysis. SFD and RKM performed FXIII experiments; KMR carried out CAT assay and analysed the data. HFP assisted with experimental design, data analysis and interpretation.
Disclaimers
The opinions or assertions expressed herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Department of the Army or the US Department of Defense.
Conflict of Interest Disclosures
The authors declare no conflict of interest.
References
- Babic AM, Josefsson EC, Bergmeier W, Wagner DD, Kaufman RM, Silberstein LE, Stossel TP, Hartwig JH, Hoffmeister KM. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47:442–451. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion. 1973;13:61–68. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- Bob [Last accessed August 2015];Kittler-Illingworth Thresholding. 2014 Available at: http://www.mathworks.com/matlabcentral/fileexchange/45685-kittler-illingworth-thresholding.
- Byrnes JR, Wilson C, Boutelle AM, Brandner CB, Flick MJ, Philippou H, Wolberg AS. The interaction between fibrinogen and zymogen FXIII-A2B2 is mediated by fibrinogen residues gamma390–396 and the FXIII-B subunits. Blood. 2016;128:1969–1978. doi: 10.1182/blood-2016-04-712323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr ME, Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules. 1978;11:46–50. doi: 10.1021/ma60061a009. [DOI] [PubMed] [Google Scholar]
- Cap AP, Spinella PC, Borgman MA, Blackbourne LH, Perkins JG. Timing and location of blood product transfusion and outcomes in massively transfused combat casualties. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S89–S94. doi: 10.1097/TA.0b013e318260625a. [DOI] [PubMed] [Google Scholar]
- Ciborowski M, Tomasiak M. The in vitro effect of eptifibatide, a glycoprotein IIb/IIIa antagonist, on various responses of porcine blood platelets. Acta Pol Pharm. 2009;66:235–242. [PubMed] [Google Scholar]
- Dutton RP, Mackenzie CF, Scalea TM. Hypotensive resuscitation during active hemorrhage: impact on in-hospital mortality. J Trauma. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- Evans PA, Hawkins K, Lawrence M, Williams RL, Barrow MS, Thirumalai N, Williams PR. Rheometry and associated techniques for blood coagulation studies. Medical Engineering & Physics. 2008;30:671–679. doi: 10.1016/j.medengphy.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ferry JD, Miller M, Shulman S. The conversion of fibrinogen to fibrin. VII. Rigidity and stress relaxation of fibrin clots, eff. of calcium. Arch Biochem Biophys. 1951;34:424–436. doi: 10.1016/0003-9861(51)90021-5. [DOI] [PubMed] [Google Scholar]
- Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4°C and 22°C. J Lab Clin Med. 1978;91:618–624. [PubMed] [Google Scholar]
- Flores-Merino MV, Chirasatitsin S, Lopresti C, Reilly GC, Battaglia G, Engler AJ. Nanoscopic mechanical anisotropy in hydrogel surfaces. Soft matter. 2010;6:4466–4470. doi: 10.1039/C0SM00339E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent AN. Rubber Elasticity: Basic Concepts and Behavior. In: Mark JE, Erman B, Roland CM, editors. The Science and Technology of Rubber. 4. Chapter 1. Academic Press; Waltham, MA, USA: 2013. pp. 1–26. [Google Scholar]
- Gerth C, Roberts WW, Ferry JD. Rheology of fibrin clots. II. Linear viscoelastic behavior in shear creep. Biophys Chem. 1974;2:208–217. doi: 10.1016/0301-4622(74)80046-3. [DOI] [PubMed] [Google Scholar]
- Getz TM, Dangelmaier CA, Jin J, Daniel JL, Kunapuli SP. Differential phosphorylation of myosin light chain (Thr)18 and (Ser)19 and functional implications in platelets. J Thromb Haemost. 2010;8:2283–2293. doi: 10.1111/j.1538-7836.2010.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz TM, Montgomery RK, Bynum JA, Aden JK, Pidcoke HF, Cap AP. Storage of platelets at 4°C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56:1320–1328. doi: 10.1111/trf.13511. [DOI] [PubMed] [Google Scholar]
- Glover CJ, McIntire LV, Brown CH, 3rd, Natelson EA. Dynamic coagulation studies: influence of normal and abnormal platelets on clot structure formation. Thromb Res. 1975;7:185–198. doi: 10.1016/0049-3848(75)90135-8. [DOI] [PubMed] [Google Scholar]
- Helms CC, Ariëns RA, Uitte de Willige S, Standeven KF, Guthold M. α-α Cross-Links Increase Fibrin Fiber Elasticity and Stiffness. Biophysical Journal. 2012;102:168–175. doi: 10.1016/j.bpj.2011.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- Hethershaw EL, Cilia La Corte AL, Duval C, Ali M, Grant PJ, Ariens RA, Philippou H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J Thromb Haemost. 2014;12:197–205. doi: 10.1111/jth.12455. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, Williams KL, Park MS. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- Kasahara K, Souri M, Kaneda M, Miki T, Yamamoto N, Ichinose A. Impaired clot retraction in factor XIII A subunit-deficient mice. Blood. 2010;115:1277–1279. doi: 10.1182/blood-2009-06-227645. [DOI] [PubMed] [Google Scholar]
- Katona E, Penzes K, Csapo A, Fazakas F, Udvardy ML, Bagoly Z, Orosz ZZ, Muszbek L. Interaction of factor XIII subunits. Blood. 2014;123:1757–1763. doi: 10.1182/blood-2013-10-533596. [DOI] [PubMed] [Google Scholar]
- Katti DS, Pal A, Pandya SG. Image based structural characterization of fibrous materials. 8571267. US Patent. 2013 http://patft.uspto.gov/netacgi/nph-Parser?Sect2=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/search-bool.html&r=1&f=G&l=50&d=PALL&RefSrch=yes&Query=PN/8571267.
- Lam WA, Chaudhuri O, Crow A, Webster KD, Li TD, Kita A, Huang J, Fletcher DA. Mechanics and contraction dynamics of single platelets and implications for clot stiffening. Nat Mater. 2011;10:61–66. doi: 10.1038/nmat2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C, Varju I, Sotonyi P, Szabo L, Krumrey M, Hoell A, Bota A, Varga Z, Komorowicz E, Kolev K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem. 2013;288:6946–6956. doi: 10.1074/jbc.M112.404301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes D. [last accessed August 2015];Anisotropic Diffusion (Perona & Malik) 2007 Available from: http://www.mathworks.com/matlabcentral/fileexchange/14995-anisotropic-diffusion--perona---malik-
- Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011;31:494–499. doi: 10.1161/ATVBAHA.110.213389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwenzi SG, Ajjan RA, Standeven KF, Parapia LA, Naseem KM. Factor XIII supports platelet activation and enhances thrombus formation by matrix proteins under flow conditions. J Thromb Haemost. 2011;9:820–833. doi: 10.1111/j.1538-7836.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JL, Lionikiene AS, Fraser SR, Whyte CS, Booth NA, Mutch NJ. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood. 2014;124:3982–3990. doi: 10.1182/blood-2014-06-583070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion. 2012;53:1520–1530. doi: 10.1111/j.1537-2995.2012.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability--deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094–1098. doi: 10.1056/NEJM196905152802004. [DOI] [PubMed] [Google Scholar]
- Nair PM, Pidcoke HF, Cap AP, Ramasubramanian AK. Effect of cold storage on shear-induced platelet aggregation and clot strength. J Trauma Acute Care Surg. 2014;77:S88–93. doi: 10.1097/TA.0000000000000327. [DOI] [PubMed] [Google Scholar]
- Petersen LC, Suenson E. Effect of plasminogen and tissue-type plasminogen activator on fibrin gel structure. Fibrinolysis. 1991;5:51–59. [Google Scholar]
- Pidcoke HF, McFaul SJ, Ramasubramanian AK, Parida BK, Mora AG, Fedyk CG, Valdez-Delgado KK, Montgomery RK, Reddoch KM, Rodriguez AC, Aden JK, Jones JA, Bryant RS, Scherer MR, Reddy HL, Goodrich RP, Cap AP. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion. 2013;53(Suppl 1):137S–149S. doi: 10.1111/trf.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk C, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4 °C and 22 °C. Shock. 2014;41(Suppl 1):54–61. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddoch KM, Montgomery RK, Rodriguez AC, Meledeo MA, Pidcoke HF, Ramasubramanian AK, Cap AP. Endothelium-Derived Inhibitors Efficiently Attenuate the Aggregation and Adhesion Responses of Refrigerated Platelets. Shock. 2016;45:220–227. doi: 10.1097/SHK.0000000000000493. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Lorand L, Mockros LF. Viscoelastic properties of fibrin clots. Biorheology. 1973;10:29–42. doi: 10.3233/bir-1973-10105. [DOI] [PubMed] [Google Scholar]
- Rosenfeld BA, Herfel B, Faraday N, Fuller A, Braine H. Effects of storage time on quantitative and qualitative platelet function after transfusion. Anesthesiology. 1995;83:1167–1172. doi: 10.1097/00000542-199512000-00006. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Mockros LF, Stern AM, Lorand L. Influence of a natural and a synthetic inhibitor of factor XIIIa on fibrin clot rheology. Biophys J. 1999a;77:2827–2836. doi: 10.1016/S0006-3495(99)77114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999b;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W, Lapetina EG, Cuatrecasas P. Cytochalasins inhibit arachidonic acid metabolism in thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982;79:7709–7713. doi: 10.1073/pnas.79.24.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichter SJ, Harker LA. Preparation and Storage of Platelet Concentrates. Transfusion. 1976;16:8–12. doi: 10.1046/j.1537-2995.1976.16176130842.x. [DOI] [PubMed] [Google Scholar]
- Standeven KF, Carter AM, Grant PJ, Weisel JW, Chernysh I, Masova L, Lord ST, Ariens RA. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximizes clot stiffness. Blood. 2007;110:902–907. doi: 10.1182/blood-2007-01-066837. [DOI] [PubMed] [Google Scholar]
- Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- Varjú I, Sótonyi P, Machovich R, Szabó L, Tenekedjiev K, Silva MMCG, Longstaff C, Kolev K. Hindered dissolution of fibrin formed under mechanical stress. Journal of Thrombosis and Haemostasis. 2011;9:979–986. doi: 10.1111/j.1538-7836.2011.04203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veklich Y, Francis CW, White J, Weisel JW. Structural Studies of Fibrinolysis by Electron Microscopy. Blood. 1998;92:4721–4729. [PubMed] [Google Scholar]
- Weigandt KM, White N, Chung D, Ellingson E, Wang Y, Fu X, Pozzo DC. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103:2399–2407. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Wufsus AR, Macera NE, Neeves KB. The hydraulic permeability of blood clots as a function of fibrin and platelet density. Biophys J. 2013;104:1812–1823. doi: 10.1016/j.bpj.2013.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromonahos C, Polack B, Caton F. Nanostructure of the fibrin clot. Biophys J. 2010;99:2018–2027. doi: 10.1016/j.bpj.2010.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji H, Anderson K, Lynch GW, Van de Water L, McDonagh J. B protein of factor XIII: differentiation between free B and complexed B. Blood. 1988;72:1645–1650. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.