Abstract
DNA damage occurs as a by-product of intrinsic cellular processes, like DNA replication, or as a consequence of exposure to genotoxic agents. Organisms have evolved multiple mechanisms to avoid, tolerate, or repair DNA lesions. To gain insight into these processes, we have isolated mutants hypersensitive to DNA-damaging agents in the green alga Chlamydomonas reinhardtii. One mutant, Ble-1, showed decreased survival when it was treated with methyl methanesulfonate (MMS), bleomycin, or hydrogen peroxide (H2O2) but behaved like the wild type when it was exposed to UVC irradiation. Ble-1 carries an extensive chromosomal deletion that includes the gene encoding cytosolic thioredoxin h1 (Trxh1). Transformation of Ble-1 with a wild-type copy of Trxh1 fully corrected the MMS hypersensitivity and partly restored the tolerance to bleomycin. Trxh1 also complemented a defect in the repair of MMS-induced DNA strand breaks and alkali-labile sites. In addition, a Trxh1-β-glucuronidase fusion protein translocated to the nucleus in response to treatment with MMS. However, somewhat surprisingly, Trxh1 failed to correct the Ble-1 hypersensitivity to H2O2. Moreover, Trxh1 suppression by RNA interference in a wild-type strain resulted in enhanced sensitivity to MMS and DNA repair defects but no increased cytotoxicity to H2O2. Thioredoxins have been implicated in oxidative-stress responses in many organisms. Yet our results indicate a specific role of Chlamydomonas Trxh1 in the repair of MMS-induced DNA damage, whereas it is dispensable for the response to H2O2. These observations also suggest functional specialization among cytosolic thioredoxins since another Chlamydomonas isoform (Trxh2) does not compensate for the lack of Trxh1.
Genome integrity and stability, key components in the survival of an organism, are constantly threatened by DNA damage. Endogenous sources of DNA lesions include, among others, replication errors, spontaneous depurination, and alterations caused by reactive oxygen species (ROS) generated during normal metabolism (83). DNA damage can also result from exposure to environmental agents such as UV light, ionizing radiation, and chemical mutagens, including methyl methanesulfonate (MMS), bleomycin, and H2O2 (70). Many DNA lesions, if left unrepaired, can lead to mutations, chromosomal aberrations, aneuploidy, or cell death (22, 58).
A complex cellular system composed of an intricate network of surveillance and repair pathways has evolved to monitor and mend DNA damage. DNA lesions are detected by molecular sensors that signal the delay or arrest of cell cycle progression as well as an array of transcriptional and DNA repair responses (83). DNA repair responses include direct repair, base excision repair (BER), nucleotide excision repair (NER), mismatch repair, and DNA double-strand break (DSB) repair (22, 58). Base excision repairs oxidized, alkylated (usually methylated), or deaminated bases and single-strand breaks (SSBs) (48), whereas NER is the major repair system for removing bulky, helix-distorting DNA lesions (58). However, despite the preferential role of certain systems in the repair of specific lesions, DNA repair pathways are often partly redundant (4, 22, 58). In addition, cells have evolved DNA damage tolerance mechanisms that allow the replicative bypass of base damage, a process called postreplication repair (22, 50, 65).
Organisms have also developed scavenging mechanisms to detoxify genotoxic agents. For instance, ROS are produced continuously as by-products of several metabolic pathways but their toxicity is minimized by a variety of antioxidant systems, some depending on glutathione or thioredoxins (Trxs) for reducing power (44). Trxs contain two redox-active cysteine residues and display two main functions: (i) as a substrate for catalytic enzymes like those involved in the reduction of ribonucleotides, methionine sulfoxide, or peroxides and (ii) as regulators that modulate the activity or other functional properties of interacting proteins, including a variety of signaling and transcription factors (2, 3, 51). Through these activities, Trxs have been implicated in ROS detoxification, redox-sensitive signal transduction, transcriptional activation of stress response genes, and apoptosis (14, 35, 37). Trxs can also modulate the activity of the mammalian apurinic/apyrimidinic endonuclease 1 (APE-1)/Redox factor 1, a multifunctional protein involved in BER (26, 34). However, the effect of thioredoxins in the repair of DNA damage has remained unexplored.
Our understanding of cellular responses to DNA damage is largely derived from genetic and biochemical studies in animal, fungal, and prokaryotic systems (22, 30, 58). By comparison, relatively little is known about DNA repair pathways in plant and algal systems, although an analysis of the completely sequenced Arabidopsis thaliana genome revealed numerous homologs of yeast and mammalian DNA repair genes (1, 5). Photosynthetic organisms may also have evolved novel DNA damage repair and sensing and transduction mechanisms since they face distinct challenges, such as recurring exposure to solar UV radiation and DNA-damaging by-products of photosynthetic metabolism (5). To gain insight into these pathways, we have used the unicellular green alga Chlamydomonas reinhardtii as a model system to isolate insertional mutants sensitive to DNA-damaging agents. We report here the characterization of one such mutant, named Ble-1 for its hypersensitivity to bleomycin.
Ble-1 survival was severely compromised by exposure to MMS or bleomycin, but it behaved like the wild type when it was irradiated with UVC light. Integration of the mutagenic plasmid resulted in a deletion of nearly 60 kb in the Ble-1 genome. Complementation of Ble-1 with cosmid subclones identified the gene encoding one of the cytosolic isoforms of thioredoxin (Trxh1) as responsible for the sensitivity to MMS. Ble-1 is deficient in the repair of MMS-induced strand breaks and alkali-labile abasic sites, and this phenotype was also partly corrected by introduction of a genomic copy of Trxh1. Moreover, strains where Trxh1 expression was suppressed by RNA interference (RNAi) showed MMS hypersensitivity and defects in DNA damage repair. Consistent with a role of Trxh1 in the response to DNA damage, a fusion protein between Trxh1 and Escherichia coli β-glucuronidase (GUS) localized predominantly in the cytosol under normal conditions but redistributed to the nucleus following exposure to several genotoxic agents. Further, the hypersensitivity to MMS of a Saccharomyces cerevisiae trx1 trx2 double mutant was also complemented by ectopic expression of Chlamydomonas Trxh1, provided that it contained an intact redox catalytic site. Our findings indicate (i) a role for Chlamydomonas Trxh1 in DNA repair pathways coping with MMS-induced abasic sites and/or SSBs and (ii) functional specialization of Chlamydomonas cytosolic thioredoxins, since Trxh2 does not compensate for the deficiency in Trxh1.
MATERIALS AND METHODS
Culture conditions, Ble-1 isolation, and genetic analysis.
Unless noted otherwise, C. reinhardtii cells were grown under moderate light in Tris-acetate-phosphate (TAP) medium (28). To isolate insertional mutants hypersensitive to DNA-damaging agents, the wild-type strain CC-124 was transformed by the glass bead procedure with a plasmid containing a mutant form of protoporphyrinogen oxidase (rs-3 marker) conferring resistance to diphenyl ether herbicides (32). Herbicide-resistant transformants were tested for their ability to survive in the presence of 1.5 μg of bleomycin (Invitrogen)/ml or 2.5 mM MMS (Sigma). By using this approach, we recovered a mutant strain, Ble-1, very sensitive to genotoxic agents. For genetic analysis, Ble-1 was crossed to the wild-type strain of the opposite mating type (CC-125) and tetrads were dissected as previously described (27). The phenotype of the meiotic tetrad products was evaluated by spot tests on TAP medium containing 2.5 mM MMS (32). Five-microliter aliquots of appropriately diluted cells were pipetted onto the plates and incubated as previously reported (32).
Plasmid rescue, cosmid library screening, deletion mapping, and sequence analyses.
The genomic sequence flanking one end of the integrated rs-3 marker in Ble-1 was recovered by plasmid rescue in E. coli (32). A 1.5-kb BamHI-NotI fragment from this flanking DNA was used as a probe to screen a Chlamydomonas genomic library (57, 78). Twelve hybridizing cosmid clones were isolated and mapped by restriction enzyme analysis. The longest one (cosmid 1) was cotransformed into Ble-1 together with plasmid pJK7, containing a genetically engineered acetolactate synthase gene conferring resistance to the herbicide sulfometuron methyl (36). However, none of the herbicide-resistant transformants showed complementation of Ble-1's hypersensitivity to genotoxic agents. Moreover, Southern hybridization with a 1.4-kb EcoRI-XhoI fragment from the 3′ end of cosmid 1 (distal to the cloned rs-3 flanking sequence) revealed a large chromosomal deletion in Ble-1. Thus, a combination of genome walking and Southern blot analyses (57, 78) was used to construct a contig of partly overlapping cosmid clones that spanned the deleted region. A subset of these clones, as well as several subclones, was used in complementation assays as described above. Some subclones were also partially sequenced, and putative transcriptional units were identified by searching the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and the Chlamydomonas genome (http://genome.jgi-psf.org/chlre2/chlre2.home.html) databases.
DNA and RNA analyses.
Standard techniques were used to isolate nucleic acids from Chlamydomonas cells (32, 57). Electrophoretic fractionation of nucleic acids, transfer to nylon membranes, and hybridization with 32P-labeled probes were carried out as previously described (32, 57).
Immunoblot analysis.
Cells resuspended in sample buffer (30 mM Tris-HCl [pH 7.9], 1 mM phenylmethylsulfonyl fluoride) were lysed in a French press cell at 1,380 lb/in2. After cellular debris was pelleted by consecutive centrifugations at 20,000 × g for 20 min and 45,000 × g for 45 min, soluble polypeptides were recovered from the supernatant. Samples were standardized for total protein concentration with the bicinchoninic acid assay (62). Next, 100-μg aliquots of proteins were boiled for 3 min in gel loading buffer (10% glycerol, 1.4% sodium dodecyl sulfate, 100 mM dithiothreitol, 30 mM Tris-HCl [pH 6.8]) and fractionated in sodium dodecyl sulfate-15% polyacrylamide gels (57). Electrophoretically separated samples were blotted onto nitrocellulose filters (Amersham) and blocked with Tris-buffered saline-Tween 20 (TBS-T) buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.01% Tween 20) containing 5% nonfat dry milk. Further steps were performed in TBS-T buffer containing 1% milk. Membranes were incubated overnight at 4°C with primary antisera against Chlamydomonas Trxh1 and then reacted for 1 h at room temperature with anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Amersham). Signals were visualized by enhanced chemiluminescence (17).
Phenotypic characterization of Ble-1, complemented strains, and transgenic lines where Trxh1 expression was suppressed by RNAi.
RNAi epi-mutants of Trxh1 were generated as previously described (56). Cell survival upon exposure to MMS or UVC irradiation was examined as already reported (32). To test for hypersensitivity to H2O2 or bleomycin, Chlamydomonas cells were grown to logarithmic phase, serially diluted, and spotted on TAP plates containing 1 mM H2O2 or 1.5 μg of bleomycin/ml. Cell growth was evaluated after 7 to 10 days of incubation under moderate light.
DNA damage repair analysis.
Cells in logarithmic phase were collected by centrifugation, resuspended in TAP medium, and treated with 25 mM MMS for 30 min in the dark (8). Untreated control cells were incubated in TAP medium without MMS (8). Immediately afterwards, aliquots of control and MMS-treated cells were frozen for isolation of DNA corresponding to the zero time points. After three washes with TAP medium to remove unreacted MMS, the remaining cells were allowed to recover in the dark, with moderate shaking, for different time periods and aliquots were frozen as described before (8). Genomic DNA was isolated from the frozen samples, separated by denaturing gel electrophoresis (8), and hybridized with a 32P-labeled probe corresponding to the TOC1 retroelement (32) present at about 15 to 20 copies per haploid nuclear genome. The distribution of radioactivity in each lane was quantified with a phosphorimager and Quantity One software (Amersham). For the analysis of DNA repair by PCR (33), cells were treated with 5 or 10 mM MMS and incubated as described above. Thirty nanograms of genomic DNA was used for the amplification of a 2.1-kb fragment corresponding to the Chlamydomonas Lsm5 (Like Sm 5) gene with primers Mut3-1 (5′-AGAGCTAGGGACCGTGGAGT-3′) and Mut3-2 (5′-TGTTCTCTGTTGCTTGTCTGACG-3′). The number of cycles showing a linear relationship between input DNA and the final product were determined in preliminary experiments. The PCR conditions consisted of 30 cycles at 93°C for 30 s, at 55°C for 30 s, and at 71°C for 180 s. Five-microliter aliquots of each PCR were resolved on 1% agarose gels and visualized by ethidium bromide staining, and signal intensities were quantified with Quantity One software (Bio-Rad). DNA lesion frequency was calculated, assuming a random Poisson distribution, as previously described (11, 33). Under the experimental conditions used (i.e., incubation of concentrated cells in the dark) and without MMS treatment, DNA replication associated with cell cycle progression in the asynchronous Chlamydomonas cultures increased the DNA content by less than 1.5-fold after 24 h (data not shown).
Subcellular localization of a GUS-Trxh1 fusion protein in transiently transformed onion epidermal cells.
The coding sequence of Trxh1 was amplified by PCR from a full-length cDNA in plasmid CrTRXh1 (64) with primers Trx-cod-3′ (5′-CATGCCATGGCCGCGGCGGCGTGCTTGGC-3′, adding an NcoI site) and Trx-cod-5′ (5′-CATGCCATGGGCGGTTCTGTTATTGTG-3′, adding an NcoI site). The Trxh1 PCR fragment was then cloned in frame with the GUS start codon in plasmid pPTN134 (82). The transgenes GUS-Trxh1 and GUS alone were transformed into onion epidermal cells by microprojectile bombardment with DNA-coated tungsten particles (82). After bombardment, cells were allowed to recover for 20 h on regular Murashige and Skoog (MS) medium or on MS medium containing 1 mM MMS, 0.2 mM H2O2, or 0.5 μg of bleomycin/ml. Some epidermal peels, after 20 h of recovery on MS medium, were incubated for 1 h in liquid MS medium containing 8 mM MMS. GUS activity was detected by staining with X-glucuronide, whereas nuclei were identified with propidium iodide (PI) (82). Stained cells were observed by bright-field microscopy for distribution of GUS activity and by epifluorescent microscopy for PI labeling of the nucleus (71, 82).
Plasmid construction for the expression of Chlamydomonas Trxh1 in S. cerevisiae.
EMY63 (MATa ade2-1 ade3-100 his3-11 leu2-3 lys2-801 trp1-1 ura3-1 trx1::TRP1 trx2::LEU2), a yeast strain with both genes encoding cytosolic Trxs deleted, has been previously described (47). EMY63 cells were transformed by the lithium chloride method (24) with constructs for conditional expression of S. cerevisiae Trx1 or C. reinhardtii Trxh1 (CrTrxh1) in both its wild-type and redox-inactive C36S forms (25). Each thioredoxin sequence was amplified from the corresponding cDNA by PCR. The upstream primer allowed introduction of an MluI site and a start codon at the position corresponding to the N terminus of each protein, whereas the downstream primer allowed introduction of a BamHI restriction site after the stop codon. The PCR products were cloned under the control of the yeast Gal1 promoter into the centromere plasmid YCpGal2 containing the URA3 selectable marker (12). Sequence-verified recombinant plasmids were transformed into EMY63, and clones were selected and maintained on standard synthetic minimal medium (lacking uracil) supplemented with 2% glucose as the carbon source. For induction of recombinant Trx expression, 2% galactose was substituted for glucose.
Examination of tolerance of yeast strains to MMS or H2O2.
Transformed EMY63 cells were grown to mid-log phase in glucose medium at 30°C and then diluted and transferred to inducing galactose medium. Growth was continued at 30°C, and cells in mid-log phase were used for tolerance tests. After dilution to an optical density at 600 nm of 0.2, cell growth and survival in the presence of genotoxic agents was evaluated by the halo assay (31). Cells were mixed with galactose top agar and spread on plates to obtain a uniform lawn. Disks containing 10 μl of MMS (1.36 M) or H2O2 (500 mM) were placed on the center of the yeast lawns, and cell growth was monitored after 3 to 5 days of incubation at 30°C.
Flow cytometry analysis.
Yeast cells were grown in synthetic minimal medium (lacking uracil) supplemented with 2% galactose to an optical density at 600 nm of 0.5, centrifuged, and washed in 2 ml of 50 mM Tris-HCl (pH 8). Cells were then fixed in 70% ethanol for 1 h at room temperature, centrifuged, and resuspended in 1 ml of the Tris-HCl buffer containing 1 mg of RNase A/ml. After incubation for 2 h at 37°C, cells were pelleted (12,000 × g, 1 min), resuspended in 1 ml of Tris-HCl buffer containing PI (50 mg/ml), and allowed to stain in the dark at 4°C overnight under mild agitation. Analysis was performed on a fluorescence-activated cell sorter (Vantage; Becton Dickinson, Le pont de Claix, France). Nuclei were excited at 488 nm with an argon laser (Spectra-Physics, Mountain View, Calif.), and FL1-Height and FL1-Area were collected through a band-pass filter allowing light between 620 and 630 nm to reach the detector. Ten thousand nuclei were analyzed per sample. Data were collected with Cellquest software (Becton Dickinson, Mansfield, Mass.) and analyzed with MODFIT (Verity Software House, Inc., Topsham, Maine).
RESULTS
Isolation and genetic analysis of Ble-1.
To identify C. reinhardtii genes involved in the cellular response to DNA damage, we carried out random insertional mutagenesis on the wild-type strain CC-124 (28). Cells from CC-124 were transformed with the rs-3 gene, which encodes a mutated form of protoporphyrinogen oxidase, conferring resistance to diphenyl ether herbicides (32). Herbicide-resistant transformants were then tested by replica plating for their ability to grow on media containing DNA-damaging agents. Since Chlamydomonas is haploid, nonlethal mutations in genes required for DNA damage repair and tolerance will result in reduced survival in the presence of genotoxic agents. By using this approach, we isolated a mutant strain (Ble-1) that is very sensitive to bleomycin and MMS. Ble-1 contained a single, although partly rearranged, copy of the rs-3 plasmid integrated into the nuclear genome (Fig. 1 and data not shown).
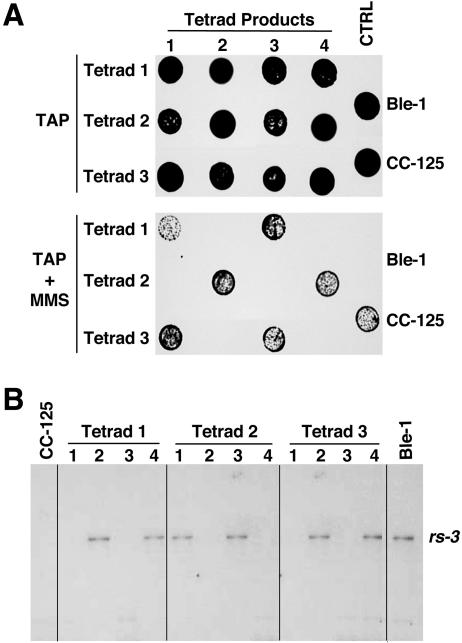
FIG. 1.
Hypersensitivity to MMS cosegregates with the tagging rs-3 marker. (A) Growth and survival of cells on TAP medium or on TAP medium containing 2.5 mM MMS (TAP + MMS). The parental strains and the meiotic products of three tetrads from the cross between the wild-type (CC-125) and mutant Ble-1 strains are shown. Strains grown to logarithmic phase were diluted in TAP medium to 5 × 104 cells per 5 μl, spotted on plates, and incubated as described in Materials and Methods. CTRL, control. (B) Southern blot analysis of the indicated strains. Genomic DNA was digested with HindIII and probed with the pBluescript sequence, the vector backbone of the rs-3 tagging plasmid.
To test whether the mutant phenotypes cosegregated with the rs-3 marker, Ble-1 was crossed with the wild-type strain of the opposite mating type, CC-125. The meiotic tetrad products were examined for survival on medium containing MMS or bleomycin (Fig. 1A and data not shown). Only tetrad products containing the rs-3 gene, as detected by Southern blot hybridization (Fig. 1B), were hypersensitive to MMS (Fig. 1A). In contrast, the tetrad products that did not carry the integrated mutagenic plasmid behaved like the wild type (Fig. 1). The analysis of 10 complete tetrads indicated that hypersensitivity to MMS and bleomycin segregated as a single Mendelian locus genetically linked (within five map units) to the integrated rs-3 marker.
Cloning and identification of the disrupted gene conferring MMS hypersensitivity on Ble-1.
The chromosomal sequence flanking one end of the introduced rs-3 gene was obtained by plasmid rescue (66) and used as a probe to screen a Chlamydomonas genomic library. Although several partly overlapping cosmid clones were isolated, none complemented the Ble-1 mutant phenotype (Fig. 2A, e. g., Cosmid 1). These clones were mapped by restriction enzyme digestion, and the end of the longest one (distal to the cloned rs-3 flanking sequence) was used as a probe for Southern hybridization analysis of Ble-1 and CC-124. Lack of hybridization of this sequence to Ble-1 DNA revealed that integration of the rs-3 marker caused a large deletion in the nuclear genome of the mutant strain (Fig. 2A and data not shown). By using a genome walking approach, we next isolated an overlapping set of cosmids that encompassed the Ble-1 chromosomal deletion (Fig. 2A). The left end of this deletion was precisely defined by sequencing of the chromosome-plasmid junction. The right junction was not sequenced, but restriction enzyme mapping of the cosmid contig together with Southern blot analyses of the wild-type and mutant strains indicated that the deleted chromosomal region spans approximately 60 kb (Fig. 2A and data not shown).
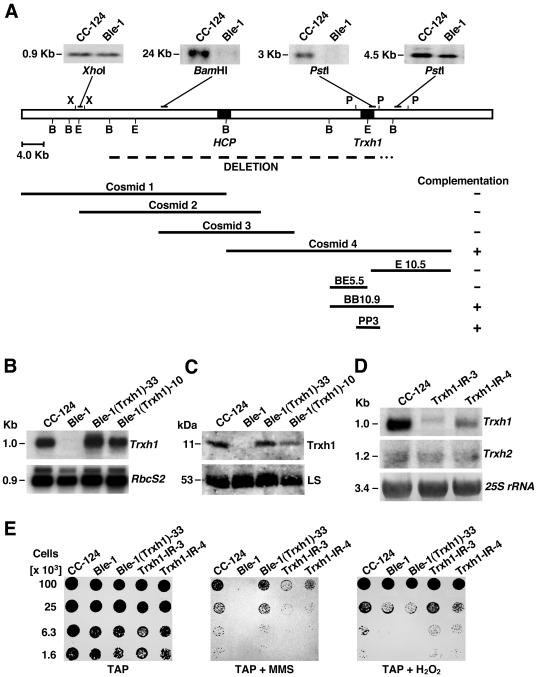
FIG. 2.
Genomic structure of the region affected by integration of the rs-3 marker in Ble-1 and phenotypic defects associated with a lack of cytosolic Trxh1. (A) The open-box diagram represents chromosomal DNA, whereas the dashed line immediately below indicates a deletion of ∼60 kb in Ble-1. The 3′ end of this deletion (dotted line) has not been precisely defined. Genes encoding a hybrid-cluster protein (HCP) and Trxh1 (Trxh1) are depicted by solid boxes. Cosmids and cosmid subclones used for complementation of Ble-1's hypersensitivity to MMS are also indicated. Southern blots of Ble-1 and the wild-type CC-124 strains are shown above the diagram. Genomic DNA was digested with the indicated enzymes and hybridized with different sequences (depicted as solid bars) to examine the deleted chromosomal region. Restriction enzyme sites: B, BamHI; E, EcoRI; P, PstI; X, XhoI. (B) Northern blot analysis of CC-124, Ble-1, and two independent transformants of Ble-1 complemented with a 3-kb PstI fragment containing Trxh1 [Ble-1(Trxh1)-10 and Ble-1(Trxh1)-33]. Total cell RNA was separated by denaturing agarose gel electrophoresis and sequentially probed with the coding sequence of Trxh1 (top panel) and with the coding sequence of the small subunit of the Rubisco gene (RbcS2, bottom panel) as a control for equal loadings in the lanes. (C) Immunoblot analysis of total soluble proteins, from the indicated strains, probed with polyclonal antibodies raised against Chlamydomonas Trxh1 (top panel) or the Rubisco holoenzyme (bottom panel). LS, large subunit of Rubisco. (D) RNA gel blot analysis of CC-124 and two independent strains where Trxh1 expression was suppressed by RNAi (Trxh1-IR-3 and Trxh1-IR-4). Total cell RNA was fractionated and sequentially hybridized with the Trxh1 probe (top panel), the coding sequence of the cytosolic thioredoxin h2 gene (Trxh2) (middle panel), and a fragment of the 25S rRNA gene (bottom panel) as a control for comparable sample loadings. (E) Growth and survival of the indicated strains on TAP medium or on TAP medium containing 2.5 mM MMS (TAP + MMS) or 1 mM hydrogen peroxide (TAP + H2O2).
To identify the gene(s) responsible for the hypersensitivity of Ble-1 to DNA-damaging agents, individual cosmid clones were tested for their ability to complement the mutant phenotypes. Ble-1 cells were cotransformed with each cosmid clone and with plasmid pJK7, encoding resistance to the herbicide sulfometuron methyl (36). Herbicide resistance transformants were then examined for their survival on medium containing MMS or bleomycin. This analysis showed that cosmid 4 complemented the hypersensitivity of Ble-1 to MMS but had only a partial effect on restoring bleomycin tolerance (Fig. 2A and 3). To define precisely the gene required for this phenotypic correction, several cosmid 4 subclones were cotransformed into Ble-1. In addition, partial sequence analysis of cosmid 4 revealed that it included the Trxh1 gene, which encodes cytosolic thioredoxin h1. Therefore, we also tested the complementation capability of a 3-kb PstI fragment exclusively containing Trxh1 (64). In all cases, transformation of Ble-1 with fragments that included a full-length Trxh1 gene reversed the MMS hypersensitivity, but the survival defect upon exposure to bleomycin was only partly corrected (Fig. 2A and 3).
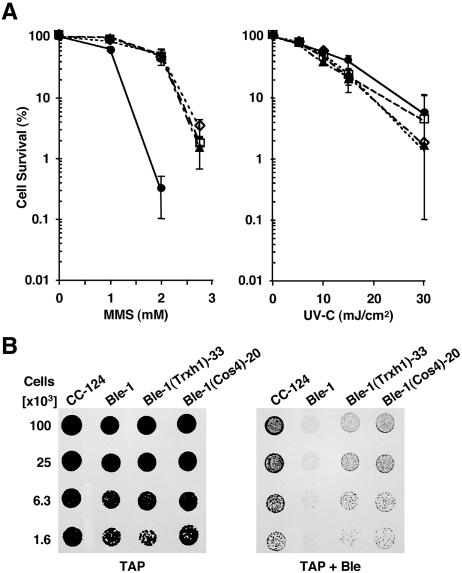
FIG. 3.
Effect of genotoxic agents on the survival of the mutant (Ble-1), the wild-type strain (CC-124), and transformants of Ble-1 containing the 3-kb Trxh1 fragment [Ble-1(Trxh1)-33] or all of cosmid 4 [Ble-1(Cos4)-20]. (A) The panels show the survival of cells grown on TAP medium containing increasing concentrations of MMS or exposed to increasing levels of UVC irradiation. Each graph point represents the mean (± standard deviation) of results of nine replicates (three independent experiments). Where the error bars are not visible, they are smaller than the symbols [□, CC-124; •, Ble-1; ▴, Ble-1(Cos4)-20; ⋄, Ble-1(Trxh1)-33]. (B) Growth and survival of the indicated strains on TAP medium with (TAP + Ble) or without (TAP) 1.5 μg of bleomycin/ml.
To evaluate further whether Trxh1 was required for tolerance to genotoxic agents, we examined its expression in the wild-type strain and the mutant Ble-1 strain, as well as two strains complemented with the 3-kb PstI fragment, Ble-1(Trxh1)-10 and Ble-1(Trxh1)-33. Northern blot analysis of total RNA revealed no detectable Trxh1 transcripts in Ble-1, whereas one complemented strain, Ble-1(Trxh1)-33, had RNA levels similar to those of the wild type (Fig. 2B). The other complemented strain, Ble-1(Trxh1)-10, had slightly reduced amounts of Trxh1 mRNA in comparison with CC-124 (Fig. 2B). Corresponding variations in Trxh1 protein levels were observed among these strains by immunoblot assay of total protein extracts probed with an anti-Trxh1 antibody (Fig. 2C). Interestingly, the Trxh1 expression level in independently complemented strains negatively correlated with their sensitivity to MMS (data not shown). Moreover, if Trxh1 is required for tolerance to MMS exposure, Trxh1 suppression by RNAi should result in a phenotype similar to that of Ble-1. To test this hypothesis, we transformed wild-type Chlamydomonas cells with inverted repeat constructs designed to produce double-stranded RNA homologous to Trxh1 (56). In several independent transformants, Trxh1 transcript levels were specifically down-regulated, whereas mRNA amounts for the closely related cytosolic thioredoxin h2 gene (Trxh2) (40) remained unperturbed (Fig. 2D, Trxh1-IR-3 and Trxh1-IR-4). Like Ble-1, these RNAi strains (Trxh1 epi-mutants) were hypersensitive to MMS treatment (Fig. 2E). However, they showed only mild survival defects when they were grown on bleomycin-containing medium (data not shown). These results, taken together, suggested a role for the cytosolic Trxh1 isoform in the cellular response to certain genotoxic agents such as MMS. Yet, Trxh1 did not fully complement the defect in the survival of Ble-1 when it was exposed to bleomycin or H2O2 (see below). Therefore, we hypothesize that disruption of another yet-to-be-identified gene(s) within the 60-kb deletion is responsible for the latter phenotypes.
Effect of genotoxic agents on cell survival and DNA damage repair in Ble-1, the Trxh1-complemented strains, and the Trxh1 RNAi-induced epi-mutants.
To gain insight into the molecular role of Trxh1, we exposed cells to a variety of genotoxic agents causing different kinds of DNA lesions and requiring distinct pathways for their repair. In all cases, we compared the survival of the wild-type CC-124 strain, the Ble-1 mutant, a strain complemented with cosmid 4 [Ble-1(Cos4)-20], and a strain complemented with the Trxh1-containing 3-kb PstI fragment [Ble-1(Trxh1)-33]. All strains behaved similarly to the wild type when they were irradiated with UVC light and allowed to recover under nonphotoreactivating conditions (Fig. 3A). In contrast, Ble-1 was very sensitive to treatment with MMS and this phenotype was nearly fully complemented by ectopic expression of Trxh1 (Fig. 3A). In addition, as already discussed, strains where Trxh1 was suppressed by RNAi displayed hypersensitivity to MMS (Fig. 2E). Ble-1 survival was also compromised by exposure to H2O2 or bleomycin (Fig. 2E and 3B). However, bleomycin sensitivity was only partly reversed by transformation with a wild-type copy of Trxh1 (Fig. 3B), whereas the defect in H2O2 tolerance was not corrected (Fig. 2E). Conversely, down-regulation of Trxh1 expression by RNAi caused only mild hypersensitivity to bleomycin (data not shown) and did not affect survival in the presence of H2O2 (Fig. 2E). Somewhat surprisingly, given the known role of thioredoxins in the oxidative-stress response (14, 35, 38), these findings indicated that Trxh1 plays a key role in cellular protection against MMS and, to some extent, bleomycin but has no apparent effect on the tolerance to H2O2.
To test whether Trxh1 is required for the repair of MMS-induced DNA damage, cells were briefly treated with this chemical and then allowed to recover for different periods of time. The extent of induced or residual DNA damage was evaluated by alkaline gel electrophoresis (8). Under these conditions, both SSBs and DSBs as well as alkali-labile sites (i.e., abasic sites) arising from BER (21) are detectable by the enhanced electrophoretic mobility of fragmented DNA. Examination of DNA isolated immediately after the treatment revealed similar extents of MMS-induced nuclear DNA damage in the wild-type, Ble-1, and Ble-1(Trxh1)-33 strains (Fig. 4). However, after incubation in drug-free medium, Ble-1 cells repaired DNA strand breaks very slowly in comparison with the wild type, as indicated by the smaller average molecular mass of DNA molecules at each time point (Fig. 4). The complemented strain, Ble-1(Trxh1)-33, showed an intermediate phenotype (Fig. 4). This partial correction of the DNA repair capacity differs from the nearly full reversal of the survival defect in the same strain when it is exposed to MMS (Fig. 3A).
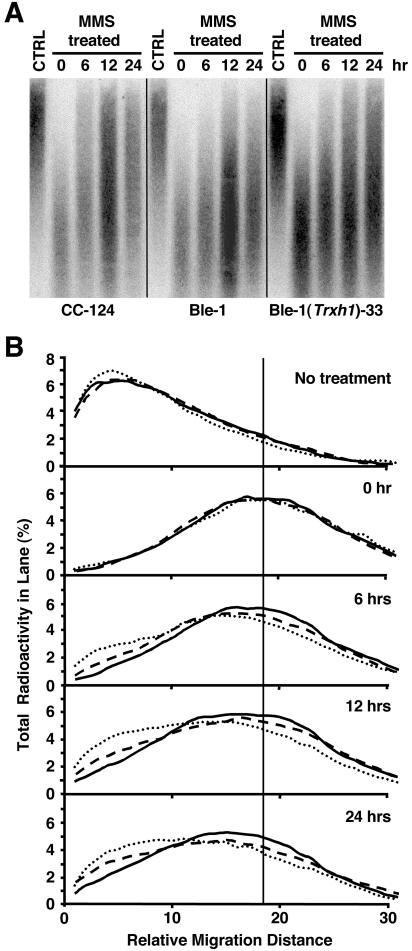
FIG. 4.
Analysis of nuclear DNA repair after MMS-induced damage in the wild-type, Ble-1, and Ble-1(Trxh1)-33 strains. (A) Southern blot showing DNA repair after exposure to 25 mM MMS for 30 min. Genomic DNA was isolated from untreated control cells (CTRL) and MMS-treated cells either immediately after the treatment (0 h) or after recovery in the absence of MMS for 6, 12, or 24 h. The DNA was separated by alkaline gel electrophoresis and hybridized with a sequence corresponding to the TOC1 transposable element. (B) In the same kind of experiment described above, the relative distribution of radioactivity in each lane (indicative of the DNA mass distribution) was analyzed with a phosphorimager and plotted as a function of migration distance. Graphs represent the average of results of two independent experiments. CC-124 is indicated by dotted lines, Ble-1 is indicated by solid lines, and Ble-1(Trxh1)-33 is indicated by dashed lines. The vertical solid line indicates the average molecular mass of damaged DNA (similar in all strains) immediately after MMS treatment.
MMS induces high levels of N-methylpurines and secondary lesions resulting from DNA damage processing and replication, such as SSBs and DSBs (21). We speculate that, at the higher MMS concentration (25 mM) used to cause DNA damage in the repair experiments (in comparison with the concentrations employed to test for cell survival), a greater proportion of MMS-induced lesions corresponds to secondary DSBs. Indeed, at relatively high concentrations, MMS behaves as a radiomimetic agent in a manner similar to that of DSB-inducing bleomycin (43). Hence, considering also that Ble-1 shows hypersensitivity to bleomycin and that this phenotype is only partly corrected by the expression of Trxh1, Ble-1 is likely defective in the repair of DSBs, but this deficiency is not a consequence of the lack of Trxh1.
We also tested the role of Trxh1 in the repair of DNA lesions induced by a lower concentration of MMS, one comparable to that employed in the survival experiments (see Material and Methods), by using a semiquantitative PCR approach (11, 33). The ability of a DNA fragment to support PCR amplification is an indicator of its in vivo intactness, since DNA sequences containing DNA polymerase-blocking or -terminating lesions will not be amplified in this assay (11, 33). However, removal of DNA lesions through DNA repair will enhance the template integrity of genomic DNA, enabling more PCR amplification. The MMS treatment generated similar levels of DNA lesions in the wild-type and Trxh1 RNAi epi-mutants (Fig. 5), suggesting that Trxh1 does not play a major role in the detoxification of MMS and prevention of alkylation DNA damage. In contrast, the strains where Trxh1 expression was suppressed by RNAi showed a lower rate of DNA repair (measured as the recovery of amplification signal at 6 and 12 h after treatment) in comparison with the wild type (Fig. 5). It is unlikely that the increase in DNA amplification over time results predominantly from the replication of intact DNA molecules since, under the conditions used, even in the absence of treatment with genotoxic agents, measured DNA replication was less than 1.5-fold after 24 h (data not shown). Therefore, our results suggest that Chlamydomonas Trxh1 is required, either directly or indirectly (see discussion), for the repair of alkali-labile abasic sites and/or SSBs induced by treatment with alkylating agents.
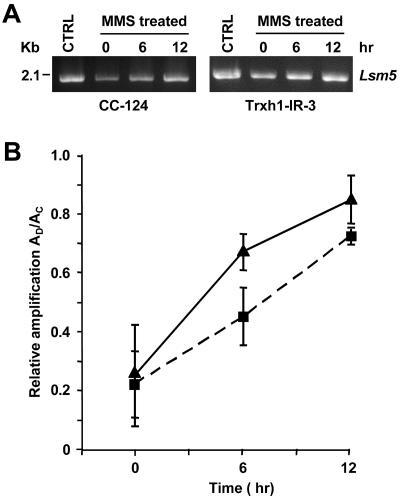
FIG. 5.
Repair of MMS-induced DNA lesions in the wild type (CC-124) and a Trxh1 RNAi epi-mutant (Trxh1-IR-3) as examined by semiquantitative PCR. (A) Amplification of a 2.1-kb genomic DNA fragment (Lsm5) by using, as the template, DNA isolated from cells immediately after treatment with 10 mM MMS (0 h) or after allowing the cells to recover for 6 or 12 h in the absence of MMS. Untreated control cells (CTRL) were also analyzed. The amplified products were resolved by agarose gel electrophoresis and stained with ethidium bromide. (B) Relative amplification of the 2.1-kb fragment calculated by dividing the amount of amplification from damaged samples (AD) by the amount of amplification from nondamaged controls (AC). Each graph point represents the mean (± standard error) of results of three independent experiments. Symbols: ▴, CC-124; ▪, Trxh1-IR-3.
Relocalization of a Trxh1-GUS fusion protein to the nucleus in cells exposed to genotoxic agents.
The Chlamydomonas Trxh1 isoform does not contain a canonical nuclear localization signal, and it has been assumed to localize to the cytosol (64). However, if Trxh1 were directly involved in DNA repair, it would be expected to localize to the nucleus and/or to redistribute to the nucleus in response to treatment with genotoxic agents. To test this hypothesis, we examined the subcellular partitioning of a fusion polypeptide consisting of the Trxh1 coding sequence linked to the N terminus of the E. coli GUS protein. A transgene expressing GUS alone was used as a control, since this 68-kDa polypeptide is largely excluded from the nucleus (71). Both constructs were placed under the control of the Cauliflower mosaic virus 35S promoter and introduced into onion epidermal cells by particle bombardment. Onion epidermal peels were used as a transient gene expression system because the large, transparent (chlorophyll-less) cells facilitate the imaging of subcellular structures (71). In addition, we and others have previously demonstrated the correct subcellular localization of Chlamydomonas fusion proteins in this system (79, 82).
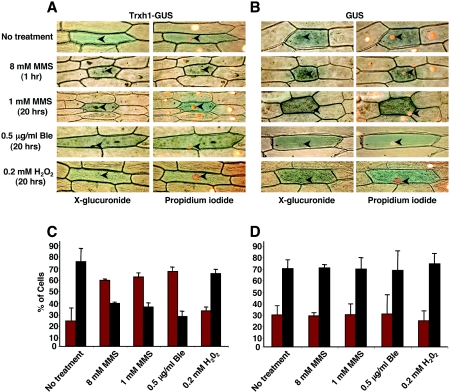
After particle bombardment, the onion peels were incubated for 18 to 20 h on MS medium with or without MMS, bleomycin, or H2O2. In other cases, epidermal peels were incubated overnight on MS and then transferred for a short period to medium containing genotoxic agents. The subcellular distribution of the expressed proteins was determined histochemically by the X-glucuronide assay (71). Regardless of treatment, the GUS polypeptide was predominantly localized in the cytoplasm of onion cells (Fig. 6B and D). Likewise, in the absence of genotoxic agents or upon treatment with H2O2, the Trxh1-GUS protein was largely found in the cytosol (Fig. 6A and C). In contrast, after treatment with MMS or bleomycin, the fusion polypeptide showed dual localization, in both the nucleus and the cytoplasm, in the majority of the examined cells (Fig. 6A and C and data not shown). Thus, consistent with a role of Trxh1 in DNA repair, our data indicated that the protein relocalizes to the nucleus in response to certain DNA-damaging agents.
FIG. 6.
Subcellular distribution of GUS and GUS-Trxh1 fusion proteins in transiently transformed onion epidermal cells. Polypeptides were localized histochemically by the X-glucuronide assay. (A) Representative cellular staining patterns corresponding to GUS-Trxh1. Onion epidermal peels bombarded with DNA-coated tungsten particles were incubated on MS medium alone (No treatment) or with the indicated concentrations of MMS, bleomycin (Ble), or hydrogen peroxide (H2O2). Tissues were simultaneously analyzed by X-glucuronide staining (left panels, blue color) and nucleus-specific PI staining (right panels, orange color). Nuclei are indicated with arrowheads. (B) Representative staining patterns in onion epidermal cells transiently transformed with GUS and treated as described for panel A. Panels stained with X-glucuronide (left) and PI (right) are shown. (C) Frequency analysis of GUS-Trxh1 subcellular distribution under the indicated treatments. Transformed cells were classified as showing exclusive cytoplasmic localization (black bars) or dual, nuclear, and cytoplasmic localization (red bars) of GUS activity. The results show the averages (± standard deviations) of results of three independent experiments (300 cells analyzed per treatment). (D) Frequency analysis of GUS subcellular localization under the indicated treatments as described for panel C.
Functional complementation of a S. cerevisiae trx1 trx2 double mutant by expression of C. reinhardtii Trxh1.
Budding yeast contains two genes encoding cytoplasmic thioredoxins (Trx1 and Trx2), which are dispensable during normal growth conditions (47). However, deletion of both Trx genes results in hypersensitivity to oxidative agents and defects in the cell cycle, particularly a prolonged S phase (47, 69). Trx functions as a reductant for ribonucleotide reductase, an essential enzyme in deoxyribonucleotide biosynthesis (69), but the alterations in the cell cycle do not result from reduced levels of deoxyribonucleotides (46). In fact, glutaredoxin can also function as an electron donor for ribonucleotide reductase (54) and the thioredoxin and glutathione-glutaredoxin systems appear to have overlapping functions, since only one is required for S. cerevisiae viability (16, 68). The actual role of Trxs in the yeast cell cycle has remained elusive, but we hypothesize that lack of cytosolic Trxs may result in increased DNA damage by endogenously produced ROS and/or a slower repair of spontaneous DNA lesions that activate the S-phase DNA damage checkpoint, preventing entry into mitosis (42, 67).
Little is known about a potential function of yeast Trxs in response to monofunctional alkylating agents, although Trx2 and the thioredoxin reductase 1 gene are transcriptionally activated by MMS treatments (10, 35). Therefore, we examined whether the S. cerevisiae trx1 trx2 double mutant was hypersensitive to a variety of genotoxic agents and whether Chlamydomonas Trxh1 could complement the phenotypic deficiencies, suggestive of evolutionary conservation of function. S. cerevisiae cells with both Trx1 and Trx2 deleted showed defects in survival (although to different degrees) when they were exposed to MMS or H2O2 (Fig. 7A). Expression of Chlamydomonas Trxh1 from a yeast-replicating vector, under the control of a galactose-inducible promoter, resulted only in reversion of MMS hypersensitivity. However, a mutant form of Chlamydomonas Trxh1 where a cysteine residue in the redox-active site was replaced by serine (C36S) (25) failed to complement this phenotype (Fig. 7A). In flow cytometry analysis of asynchronous cultures, the yeast trx1 trx2 mutant also displays a considerably lengthened S phase that becomes apparent by a marked decrease in the proportion of cells having a G1 (1N) or, to a lower degree, G2 (2N) DNA content (47). Expression of Chlamydomonas Trxh1 partly corrected this deficiency in cell cycle progression, provided that the protein contained the wild-type cysteine residues in its catalytic site (Fig. 7B). Thus, these results suggest functional conservation between budding yeast and Chlamydomonas cytosolic thioredoxins as key components in the cellular response to alkylating DNA-damaging agents. Moreover, a redox-active Cys-X-X-Cys site is necessary for this function.
FIG. 7.
Phenotypic complementation of an S. cerevisiae trx1 trx2 double mutant (EMY63) by expression of Chlamydomonas Trxh1. (A) Expression of Trxh1, under the control of a galactose-inducible promoter in a centromeric plasmid restored the tolerance of the mutant to MMS to the same extent that transformation with S. cerevisiae Trx1 did (left panel). In contrast, Trxh1 did not complement the hypersensitivity to H2O2 (right panel). Yeast cells were transformed with the empty plasmid (YCpGal2), with S. cerevisiae Trx1 (Yeast Trx1), with Chlamydomonas Trxh1 (CrTrxh1), or with a mutant form of Trxh1 lacking a catalytically active cysteine (CrTrxh1 C36S). Cells were plated, forming a lawn, and exposed to a gradient of MMS or H2O2 concentrations, established by diffusion from a centrally placed disk containing 1.36 M MMS or 500 mM H2O2. The size of the halo where cell growth was suppressed was used as an estimation of the sensitivity to genotoxic agents. The results show the averages (± standard deviations) of three to five independent experiments. (B) Relative DNA content in asynchronous cultures of transformed trx1 trx2 mutant cells. Yeast cells of the indicated transformants were grown to logarithmic phase, stained with PI, and analyzed by flow cytometry. Relative fluorescence intensity (DNA content) is plotted against the number of counted events (Nuclei counts). Relative DNA contents corresponding to 1N (G1) and 2N (G2) are indicated.
DISCUSSION
Thioredoxins are small (∼12 kDa), ubiquitous proteins with thiol:disulfide oxidoreductase activity and a consensus WC(G/P)PC active site. In their reduced state, Trxs reduce disulfide bridges in target polypeptides and thereby modulate the activity of proteins involved in a variety of cellular processes (7, 23, 37). Eukaryotes often contain multiple cytosolic thioredoxin isoforms (38, 41, 53)—for instance, S. cerevisiae and the unicellular green alga C. reinhardtii contain two each (2, 51)—but the functional specificity or redundancy of these proteins is for the most part unknown (23, 38). In mammals and yeast, Trxs are clearly involved in the response to oxidative stress via ROS-scavenging mechanisms and modulation of the activity of signaling and transcription factors (14, 35, 37). Plant and Chlamydomonas cytosolic Trxs also interact with ROS-detoxifying enzymes, such as ascorbate peroxidase, catalase, glutathione peroxidase, peroxiredoxins, and superoxide dismutase (3, 23, 41, 80). However, only Arabidopsis Trxh5 has been implicated in vivo in a response to oxidative stress, whereas other Trxh genes are not induced by oxidative agents (38, 53). Moreover, expression studies of Arabidopsis and differential complementation of thioredoxin-deficient phenotypes in yeast have suggested functional specialization among plant cytosolic Trxs (38, 45, 53). Yet, this issue has been difficult to address experimentally because mutants deficient in individual Trxs often do not show any obvious phenotype, an effect attributed to compensation by other thioredoxins and/or glutaredoxins (38).
While conducting a screen for Chlamydomonas mutants hypersensitive to DNA-damaging agents, we isolated a strain (Ble-1) with a large genomic deletion that included Trxh1. Ble-1 showed hypersensitivity to treatment with MMS, bleomycin, or H2O2. The strain's poor tolerance to MMS and, to a lower degree, its deficient survival in the presence of bleomycin were complemented by transformation with a wild-type copy of Trxh1. In contrast, the hypersensitivity to H2O2 was not rescued by the ectopic expression of Trxh1. Conversely, in wild-type cells, suppression of Trxh1 expression by RNAi resulted in reduced survival upon exposure to MMS and, to a lower extent, bleomycin but no enhanced sensitivity to H2O2. These findings indicated that (i) an unidentified gene(s) within the deleted chromosomal region of Ble-1 is likely responsible for part of the bleomycin and virtually all of the H2O2 hypersensitivity and (ii) Trxh1 is essential for the cellular response to certain DNA-damaging agents, including monofunctional alkylating chemicals such as MMS, but it is not required for coping with H2O2-induced oxidative stress. Consistent with these observations, C. reinhardtii Trxh1 expression is not enhanced by exposure to H2O2 or diamide (39). Moreover, Chlamydomonas Trxh1 cannot correct the hypersensitivity to H2O2 of a yeast trx1 trx2 double mutant.
To gain insight into the molecular mechanism(s) uniquely dependent on Trxh1 (Fig. 8), we examined the phenotypic defects associated with a lack of this protein upon exposure to an array of DNA-damaging agents. UVC irradiation leads primarily to the formation of bulky pyrimidine dimers (61). In contrast, MMS generates mainly N7-methylguanine and N3-methyladenine, but DNA lesion processing and replication of damaged templates can produce SSBs and DSBs as secondary lesions (15, 59). Bleomycin consists of a mixture of glycopeptides that intercalate between DNA bases and generate an activated oxygen species (most likely a hydroxyl radical) that causes SSBs and DSBs (77). We found that neither Ble-1 nor Trxh1-suppressed RNAi strains were hypersensitive to UVC irradiation. Thus, since UV-induced pyrimidine dimers are mended by direct repair and/or NER (5, 22, 58), Trxh1 does not appear to be required for the correct function of these pathways (Fig. 8). Similarly, Trxh1 does not seem to be necessary for the homologous recombination or nonhomologous end-joining pathways (19, 58) because, although Ble-1 appears to be defective in the repair of DSBs generated by bleomycin as well as exposure to high concentrations of MMS, Trxh1 does not complement this defect. This interpretation is also supported by the mild hypersensitivity to bleomycin of the Trxh1-suppressed RNAi strains. An unidentified gene(s) within the deleted chromosomal region of Ble-1 is likely responsible for the putative defect in DSB repair and the much greater sensitivity to bleomycin of this mutant.
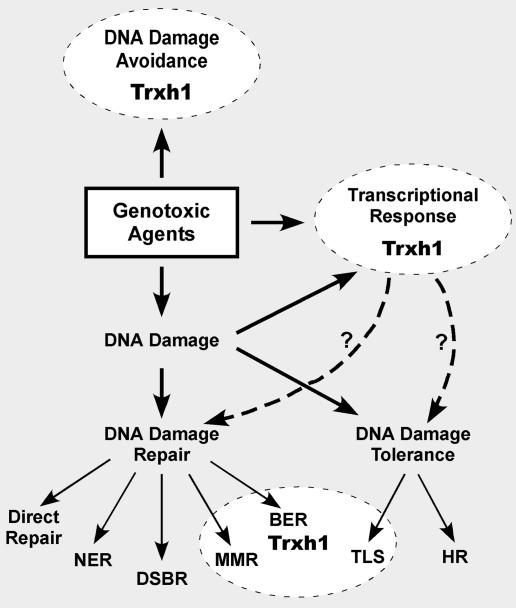
FIG. 8.
Proposed role(s) of Chlamydomonas Trxh1 in the response to DNA-damaging agents. A function of Trxh1 in DNA damage avoidance (detoxification of genotoxic agents) and the transcriptional activation of stress response genes is inferred from the well-documented role of cytosolic thioredoxins in other eukaryotes. Our results suggest a specialized function of Chlamydomonas Trxh1 in the repair of alkylation-induced DNA damage (see the text for details). DSBR, DNA DSB repair; HR, homologous recombination; MMR, mismatch repair; TLS, translesion DNA synthesis.
Both Ble-1 and the RNAi strains showed significant defects in survival when they were exposed to MMS, and transformation of Ble-1 with a wild-type copy of Trxh1 complemented the hypersensitivity to MMS and partly restored the capacity to repair MMS-induced DNA damage. Moreover, based on heterologous complementation in S. cerevisiae, a wild-type Trx redox site appears to be necessary for this function. Enhanced MMS-induced cytotoxicity in Chlamydomonas lacking Trxh1 may be due to a defect in the detoxification of this genotoxic agent. However, at least in vitro, MMS preferentially modifies lysine and histidine protein residues rather than cysteines (the critical amino acids in the Trx active site) (49). Further, glutathione, which is present at a millimolar concentration in cells, has been implicated in the detoxification of alkylating electrophiles in eukaryotes (6, 9, 73, 75). In addition, Chlamydomonas Trxh1 RNAi epi-mutants do not show an enhanced level of MMS-induced DNA lesions (Fig. 5), as would be expected for strains with a defect in a scavenging function.
In a number of organisms, the predominant pathway involved in mending MMS-induced DNA lesions is BER (4, 21, 55). Therefore, the phenotypes of the mutant and RNAi strains strongly suggest that Trxh1 is required, directly or indirectly, for BER (Fig. 8). Yet a role for Trxh1 in BER might seem counterintuitive since this pathway is also expected to participate in the repair of H2O2-generated oxidized bases (18, 21, 58), but a lack of Trxh1 does not result in hypersensitivity to H2O2 in Chlamydomonas. However, in mammals, BER is carried out by several distinct subpathways. BER is commonly initiated by a DNA N-glycosylase that removes a damaged base to form an apurinic/apyrimidinic (AP) site (48). This is followed by strand scission by APEs, DNA resynthesis, and ligation (4, 13, 58). In mammalian cells, short-patch BER is dependent on DNA polymerase β (Polβ) whereas long-patch BER requires Polβ or DNA Polδ/Polɛ (13, 58). The choice of repair subpathway is at least in part determined by the nature of the DNA N-glycosylase and the nature of the resultant AP site (13, 58). Interestingly, short-patch BER is the favored pathway for the repair of MMS-induced N-methylpurines, while long-patch BER preferentially repairs oxidation-mediated base loss (21). Mouse cells deficient in Polβ are hypersensitive to a variety of monofunctional alkylating chemicals but much less sensitive to other DNA-damaging agents, including H2O2 (21, 63). These phenotypes are remarkably similar to those of Trxh1-defective Chlamydomonas. Moreover, short-patch BER is likely operative in C. reinhardtii since the homologs of key enzymes in this pathway, mammalian APE 1/Redox factor 1 (APE-1/Ref-1) and Polβ, are encoded by the Chlamydomonas genome (http://genome.jgi-psf.org/chlre2/chlre2.home.html).
Consistent with a role for Trxh1 in DNA damage repair, in transient-expression assays, a Trxh1-GUS fusion protein redistributed to the nucleus after treatment with certain genotoxic agents. Similarly, wheat Trxh proteins localized predominantly to the nuclei of aleurone and scutellum cells in maturating seeds, a feature that has been correlated with oxidative stress in these tissues (60). Exposure to UV light, ionizing radiation, or alkylhydroxyperoxides also increases the translocation of Trx from the cytoplasm to the nucleus in mammalian cells (14, 72, 74), but a potential role of Trx in the repair of DNA lesions has not been examined. Mammalian Trx has been implicated mostly in modulating the transcriptional activity of a number of factors, in some cases in association with APE-1/Ref-1, and in the activation of stress response genes (20, 29, 52, 74). APE-1/Ref-1 is a multifunctional protein that possesses both DNA repair and redox-regulatory activities (20), and it can control BER by regulating the assembly, function, and/or expression of other enzymes in this pathway (52, 76). Some APE-1/Ref-1 activities can be modulated posttranslationally by direct interaction with thioredoxin (26, 34).
Thioredoxins have been implicated in genotoxic stress responses mostly via their role in damage prevention, by providing reducing power to detoxifying enzymes and/or by regulating their expression (14, 51, 81). Although we cannot entirely rule out a scavenging function for Chlamydomonas Trxh1 in the response to MMS treatment, our results indicate that a lack of Trxh1 results in the defective repair of alkylation-induced DNA lesions. We propose that Chlamydomonas Trxh1 could have at least two, not mutually exclusive, roles in DNA damage repair (Fig. 8). Trxh1 may modulate DNA repair activities by direct interaction with BER components, such as the Chlamydomonas APE-1/Ref-1 homolog. Alternatively, Trxh1 may regulate the expression of BER enzymes by controlling the activity of redox-dependent transcription factors. Although we have emphasized the potential role of Trxh1 in BER, since this is the primary mechanism for removal of DNA lesions that cause minor helix distortion (4, 13, 58), Trxh1 may also function in mismatch repair and/or translesion DNA synthesis (Fig. 8). Nonetheless, our findings demonstrate an essential requirement for Chlamydomonas Trxh1 in DNA damage repair. Moreover, they also indicate functional specialization among Chlamydomonas cytosolic thioredoxins since Trxh2 does not compensate for this role.
Acknowledgments
We thank Byeong-ryool Jeong and Karin Van Dijk for thoughtful discussions. We also thank Sarbani Chakraborty, M. P. Doutriaux, and G. Noctor, as well as members of the lab, for critical reading of the manuscript and Michel Hours for technical assistance in the flow cytometry experiments.
This work was supported by grant GM62915 from the National Institutes of Health.
REFERENCES
- 1.Arabidopsis Genome Initiative 2000. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408:796-815. [DOI] [PubMed] [Google Scholar]
- 2.Åslund, F., and J. Beckwith. 1999. The thioredoxin superfamily: redundancy, specificity, and gray-area genomics. J. Bacteriol. 181:1375-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balmer, Y., W. H. Vensel, C. K. Tanaka, W. J. Hurkman, E. Gelhaye, N. Rouhier, J. P. Jacquot, W. Manieri, P. Schurmann, M. Droux, and B. B. Buchanan. 2004. Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. Proc. Natl. Acad. Sci. USA 101:2642-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boiteux, S., and M. Guillet. 2004. Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amsterdam) 3:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Britt, A. B. 1999. Molecular genetics of DNA repair in higher plants. Trends Plant Sci. 4:20-25. [DOI] [PubMed] [Google Scholar]
- 6.Britten, R. A., J. A. Green, and H. M. Warenius. 1992. Cellular glutathione (GSH) and glutathione-S-transferases (GST) activity in human ovarian tumor biopsies following exposure to alkylating agents. Int. J. Radiat. Oncol. Biol. Phys. 24:527-531. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, B. B., P. Schurmann, P. Decottignies, and R. M. Lozano. 1994. Thioredoxin: a multifunctional regulatory protein with a bright future in technology and medicine. Arch. Biochem. Biophys. 314:257-260. [DOI] [PubMed] [Google Scholar]
- 8.Cerutti, H., A. M. Johnson, J. E. Boynton, and N. W. Gillham. 1995. Inhibition of chloroplast DNA recombination and repair by dominant negative mutants of Escherichia coli RecA. Mol. Cell. Biol. 15:3003-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasseaud, L. F. 1979. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv. Cancer Res. 29:175-274. [DOI] [PubMed] [Google Scholar]
- 10.Chen, D., W. M. Toone, J. Mata, R. Lyne, G. Burns, K. Kivinen, A. Brazma, N. Jones, and J. Bahler. 2003. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14:214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, K. H., F. M. Yakes, D. K. Srivastava, R. K. Singhal, R. W. Sobol, J. K. Horton, B. Van Houten, and S. H. Wilson. 1998. Up-regulation of base excision repair correlates with enhanced protection against a DNA damaging agent in mouse cell lines. Nucleic Acids Res. 26:2001-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, G. M., D. E. Stone, and S. I. Reed. 1990. Stoichiometry of G protein subunits affects the Saccharomyces cerevisiae mating pheromone signal transduction pathway. Mol. Cell. Biol. 10:510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dianov, G. L., K. M. Sleeth, I. I. Dianova, and S. L. Allinson. 2003. Repair of abasic sites in DNA. Mutat. Res. 531:157-163. [DOI] [PubMed] [Google Scholar]
- 14.Didier, C., I. Kerblat, C. Drouet, A. Favier, J. C. Beani, and M. J. Richard. 2001. Induction of thioredoxin by ultraviolet-A radiation prevents oxidative-mediated cell death in human skin fibroblasts. Free Radic. Biol. Med. 31:585-598. [DOI] [PubMed] [Google Scholar]
- 15.Doetsch, P. W., N. J. Morey, R. L. Swanson, and S. Jinks-Robertson. 2001. Yeast base excision repair: interconnections and networks. Prog. Nucleic Acid Res. Mol. Biol. 68:29-39. [DOI] [PubMed] [Google Scholar]
- 16.Draculic, T., I. W. Dawes, and C. M. Grant. 2000. A single glutaredoxin or thioredoxin gene is essential for viability in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 36:1167-1174. [DOI] [PubMed] [Google Scholar]
- 17.Durrant, I. 1990. Light-based detection of biomolecules. Nature 346:297-298. [DOI] [PubMed] [Google Scholar]
- 18.Evans, M. D., and M. S. Cooke. 2004. Factors contributing to the outcome of oxidative damage to nucleic acids. Bioessays 26:533-542. [DOI] [PubMed] [Google Scholar]
- 19.Featherstone, C., and S. P. Jackson. 1999. DNA double-strand break repair. Curr. Biol. 9:R759-R761. [DOI] [PubMed] [Google Scholar]
- 20.Flaherty, D. M., M. M. Monick, and G. W. Hunninghake. 2001. AP endonucleases and the many functions of Ref-1. Am. J. Respir. Cell Mol. Biol. 25:664-667. [DOI] [PubMed] [Google Scholar]
- 21.Fortini, P., B. Pascucci, F. Belisario, and E. Dogliotti. 2000. DNA polymerase β is required for efficient DNA strand break repair induced by methyl methanesulfonate but not by hydrogen peroxide. Nucleic Acids Res. 28:3040-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedberg, E. C., L. D. McDaniel, and R. A. Schultz. 2004. The role of endogenous and exogenous DNA damage and mutagenesis. Curr. Opin. Genet. Dev. 14:5-10. [DOI] [PubMed] [Google Scholar]
- 23.Gelhaye, E., N. Rouhier, and J. P. Jacquot. 2004. The thioredoxin h system of higher plants. Plant Physiol. Biochem. 42:265-271. [DOI] [PubMed] [Google Scholar]
- 24.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyer, A., P. Decottignies, S. Lemaire, E. Ruelland, E. Issakidis-Bourguet, J. P. Jacquot, and M. Miginiac-Maslow. 1999. The internal Cys-207 of sorghum leaf NADP-malate dehydrogenase can form mixed disulphides with thioredoxin. FEBS Lett. 444:165-169. [DOI] [PubMed] [Google Scholar]
- 26.Gros, L., A. A. Ishchenko, H. Ide, R. H. Elder, and M. K. Saparbaev. 2004. The major human AP endonuclease (Ape1) is involved in the nucleotide incision repair pathway. Nucleic Acids Res. 32:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, E. H. 2001. Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52:363-406. [DOI] [PubMed] [Google Scholar]
- 28.Harris, E. H. 1989. The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory. Academic Press, San Diego, Calif. [DOI] [PubMed]
- 29.Hirota, K., M. Matsui, S. Iwata, A. Nishiyama, K. Mori, and J. Yodoi. 1997. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA 94:3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochegger, H., E. Sonoda, and S. Takeda. 2004. Post-replication repair in DT40 cells: translesion polymerases versus recombinases. Bioessays 26:151-158. [DOI] [PubMed] [Google Scholar]
- 31.Issakidis-Bourguet, E., N. Mouaheb, Y. Meyer, and M. Miginiac-Maslow. 2001. Heterologous complementation of yeast reveals a new putative function for chloroplast m-type thioredoxin. Plant J. 25:127-135. [DOI] [PubMed] [Google Scholar]
- 32.Jeong B.-R., D. Wu-Scharf, C. Zhang, and H. Cerutti. 2002. Suppressors of transcriptional transgenic silencing in Chlamydomonas are sensitive to DNA-damaging agents and reactivate transposable elements. Proc. Natl. Acad. Sci. USA 99:1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinowski, D. P., S. Illenye, and B. Van Houten. 1992. Analysis of DNA damage and repair in murine leukemia L1210 cells using a quantitative polymerase chain reaction assay. Nucleic Acids Res. 20:3485-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley, M. R., and S. H. Parsons. 2001. Redox regulation of the DNA repair function of the human AP endonuclease Ape1/ref-1. Antioxid. Redox. Signal. 3:671-683. [DOI] [PubMed] [Google Scholar]
- 35.Koerkamp, M. G., M. Rep, H. J. Bussemaker, G. P. Hardy, A. Mul, K. Piekarska, C. A. Szigyarto, J. M. De Mattos, and H. F. Tabak. 2002. Dissection of transient oxidative stress response in Saccharomyces cerevisiae by using DNA microarrays. Mol. Biol. Cell 13:2783-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovar, J. L., J. Zhang, R. P. Funke, and D. P. Weeks. 2002. Molecular analysis of the acetolactate synthase gene of Chlamydomonas reinhardtii and development of a genetically engineered gene as a dominant selectable marker for genetic transformation. Plant J. 29:109-117. [DOI] [PubMed] [Google Scholar]
- 37.Laloi, C., K. Apel, and A. Danon. 2004. Reactive oxygen signalling: the latest news. Curr. Opin. Plant Biol. 7:323-328. [DOI] [PubMed] [Google Scholar]
- 38.Laloi, C., D. Mestres-Ortega, Y. Marco, Y. Meyer, and J. P. Reichheld. 2004. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 134:1006-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemaire, S., E. Keryer, M. Stein, I. Schepens, E. Issakidis-Bourguet, C. Gérard-Hirne, M. Miginiac-Maslow, and J.-P. Jacquot. 1999. Heavy-metal regulation of thioredoxin gene expression in Chlamydomonas reinhardtii. Plant Physiol. 120:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaire, S. D., V. Collin, E. Keryer, A. Quesada, and M. Miginiac-Maslow. 2003. Characterization of thioredoxin y, a new type of thioredoxin identified in the genome of Chlamydomonas reinhardtii. FEBS Lett. 543:87-92. [DOI] [PubMed] [Google Scholar]
- 41.Lemaire, S. D., B. Guillon, P. Le Marechal, E. Keryer, M. Miginiac-Maslow, and P. Decottignies. 2004. New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 101:7475-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longhese, M. P., M. Clerici, and G. Lucchini. 2003. The S-phase checkpoint and its regulation in Saccharomyces cerevisiae. Mutat. Res. 532:41-58. [DOI] [PubMed] [Google Scholar]
- 43.Mirzayans, R., M. Liuzzi, and M. C. Paterson. 1988. Methylmethanesulfonate-induced DNA damage and its repair in cultured human fibroblasts: normal rates of induction and removal of alkali-labile sites in xeroderma pigmentosum (group A) cells. Carcinogenesis 9:2257-2263. [DOI] [PubMed] [Google Scholar]
- 44.Mittler, R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7:405-410. [DOI] [PubMed] [Google Scholar]
- 45.Mouaheb, N., D. Thomas, L. Verdoucq, P. Monfort, and Y. Meyer. 1998. In vivo functional discrimination between plant thioredoxins by heterologous expression in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95:3312-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller, E. G. 1995. A redox-dependent function of thioredoxin is necessary to sustain a rapid rate of DNA synthesis in yeast. Arch. Biochem. Biophys. 318:356-361. [DOI] [PubMed] [Google Scholar]
- 47.Muller, E. G. 1991. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J. Biol. Chem. 266:9194-9202. [PubMed] [Google Scholar]
- 48.Nilsen, H., and H. E. Krokan. 2001. Base excision repair in a network of defence and tolerance. Carcinogenesis 22:987-998. [DOI] [PubMed] [Google Scholar]
- 49.Paik, W. K., P. DiMaria, S. Kim, P. N. Magee, and P. D. Lotlikar. 1984. Alkylation of protein by methyl methanesulfonate and 1-methyl-1-nitrosourea in vitro. Cancer Lett. 23:9-17. [DOI] [PubMed] [Google Scholar]
- 50.Plosky, B. S., and R. Woodgate. 2004. Switching from high-fidelity replicases to low-fidelity lesion-bypass polymerases. Curr. Opin. Genet. Dev. 14:113-119. [DOI] [PubMed] [Google Scholar]
- 51.Powis, G., and W. R. Montfort. 2001. Properties and biological activities of thioredoxins. Annu. Rev. Pharmacol. Toxicol. 41:261-295. [DOI] [PubMed] [Google Scholar]
- 52.Raffoul, J. J., D. C. Cabelof, J. Nakamura, L. B. Meira, E. C. Friedberg, and A. R. Heydari. 2004. Apurinic/apyrimidinic endonuclease (APE/REF-1) haploinsufficient mice display tissue-specific differences in DNA polymerase β-dependent base excision repair. J. Biol. Chem. 279:18425-18433. [DOI] [PubMed] [Google Scholar]
- 53.Reichheld, J.-P., D. Mestres-Ortega, C. Laloi, and Y. Meyer. 2002. The multigenic family of thioredoxin h in Arabidopsis thaliana: specific expression and stress response. Plant Physiol. Biochem. 40:685-690. [Google Scholar]
- 54.Rietsch, A., and J. Beckwith. 1998. The genetics of disulfide bond metabolism. Annu. Rev. Genet. 32:163-184. [DOI] [PubMed] [Google Scholar]
- 55.Robertson, K. A., H. A. Bullock, Y. Xu, R. Tritt, E. Zimmerman, T. M. Ulbright, R. S. Foster, L. H. Einhorn, and M. R. Kelley. 2001. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 61:2220-2225. [PubMed] [Google Scholar]
- 56.Rohr, J., N. Sarkar, S. Balenger, B.-R. Jeong, and H. Cerutti. 2004. Tandem inverted repeat system for selection of effective transgenic RNAi strains in Chlamydomonas. Plant J. 40:611-621. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Sancar, A., L. A. Lindsey-Boltz, K. Unsal-Kacmaz, and S. Linn. 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 73:39-85. [DOI] [PubMed] [Google Scholar]
- 59.Sedgwick, B. 2004. Repairing DNA-methylation damage. Nat. Rev. Mol. Cell Biol. 5:148-157. [DOI] [PubMed] [Google Scholar]
- 60.Serrato, A. J., and F. J. Cejudo. 2003. Type-h thioredoxins accumulate in the nucleus of developing wheat seed tissues suffering oxidative stress. Planta 217:392-399. [DOI] [PubMed] [Google Scholar]
- 61.Sinha, R. P., and D. P. Hader. 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1:225-236. [DOI] [PubMed] [Google Scholar]
- 62.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 63.Sobol, R. W., J. K. Horton, R. Kuhn, H. Gu, R. K. Singhal, R. Prasad, K. Rajewsky, and S. H. Wilson. 1996. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 379:183-186. [DOI] [PubMed] [Google Scholar]
- 64.Stein, M., J. P. Jacquot, E. Jeannette, P. Decottignies, M. Hodges, J. M. Lancelin, V. Mittard, J. M. Schmitter, and M. Miginiac-Maslow. 1995. Chlamydomonas reinhardtii thioredoxins: structure of the genes coding for the chloroplastic m and cytosolic h isoforms; expression in Escherichia coli of the recombinant proteins, purification and biochemical properties. Plant Mol. Biol. 28:487-503. [DOI] [PubMed] [Google Scholar]
- 65.Sutton, M. D., and G. C. Walker. 2001. Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proc. Natl. Acad. Sci. USA 98:8342-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tam, L. W., and P. A. Lefebvre. 1995. Insertional mutagenesis and isolation of tagged genes in Chlamydomonas. Methods Cell. Biol. 47:519-523. [DOI] [PubMed] [Google Scholar]
- 67.Tercero, J. A., M. P. Longhese, and J. F. Diffley. 2003. A central role for DNA replication forks in checkpoint activation and response. Mol. Cell 11:1323-1336. [DOI] [PubMed] [Google Scholar]
- 68.Trotter, E. W., and C. M. Grant. 2003. Non-reciprocal regulation of the redox state of the glutathione-glutaredoxin and thioredoxin systems. EMBO Rep. 4:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trotter, E. W., and C. M. Grant. 2002. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 46:869-878. [DOI] [PubMed] [Google Scholar]
- 70.Tuteja, N., M. B. Singh, M. K. Misra, P. L. Bhalla, and R. Tuteja. 2001. Molecular mechanisms of DNA damage and repair: progress in plants. Crit. Rev. Biochem. Mol. Biol. 36:337-397. [DOI] [PubMed] [Google Scholar]
- 71.Varagona, M. J., R. J. Schmidt, and N. V. Raikhel. 1992. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. Plant Cell 4:1213-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watson, W. H., and D. P. Jones. 2003. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 543:144-147. [DOI] [PubMed] [Google Scholar]
- 73.Watson, W. H., X. Yang, Y. E. Choi, D. P. Jones, and J. P. Kehrer. 2004. Thioredoxin and its role in toxicology. Toxicol. Sci. 78:3-14. [DOI] [PubMed] [Google Scholar]
- 74.Wei, S. J., A. Botero, K. Hirota, C. M. Bradbury, S. Markovina, A. Laszlo, D. R. Spitz, P. C. Goswami, J. Yodoi, and D. Gius. 2000. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res. 60:6688-6695. [PubMed] [Google Scholar]
- 75.Wilhelm, D., K. Bender, A. Knebel, and P. Angel. 1997. The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol. Cell. Biol. 17:4792-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong, D., and B. Demple. 2004. Modulation of the 5′-deoxyribose-5-phosphate lyase and DNA synthesis activities of mammalian DNA polymerase β by apurinic/apyrimidinic endonuclease 1. J. Biol. Chem. 279:25268-25275. [DOI] [PubMed] [Google Scholar]
- 77.Wozniak, K., M. Arabski, E. Malecka-Panas, J. Drzewoski, and J. Blasiak. 2004. DNA damage in human colonic mucosa cells induced by bleomycin and the protective action of vitamin E. Cell. Mol. Biol. Lett. 9:31-45. [PubMed] [Google Scholar]
- 78.Wu-Scharf, D., B.-R. Jeong, C. Zhang, and H. Cerutti. 2000. Transgene and transposon silencing in Chlamydomonas reinhardtii by a DEAH-box RNA helicase. Science 290:1159-1162. [DOI] [PubMed] [Google Scholar]
- 79.Xiang, Y., J. Zhang, and D. P. Weeks. 2001. The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 98:5341-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamazaki, D., K. Motohashi, T. Kasama, Y. Hara, and T. Hisabori. 2004. Target proteins of the cytosolic thioredoxins in Arabidopsis thaliana. Plant Cell Physiol. 45:18-27. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida, T., S. Oka, H. Masutani, H. Nakamura, and J. Yodoi. 2003. The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxid. Redox. Signal. 5:563-570. [DOI] [PubMed] [Google Scholar]
- 82.Zhang, C., D. Wu-Scharf, B.-R. Jeong, and H. Cerutti. 2002. A WD40-repeat containing protein, similar to a fungal co-repressor, is required for transcriptional gene silencing in Chlamydomonas. Plant J. 31:25-36. [DOI] [PubMed] [Google Scholar]
- 83.Zhou, B. B., and S. J. Elledge. 2000. The DNA damage response: putting checkpoints in perspective. Nature 408:433-439. [DOI] [PubMed] [Google Scholar]