Abstract
Multiple models of aberrant emotional processing in depression have been advanced. However, it is unclear which of these models best applies to emotional disturbances in subclinical depressive symptoms. The current study employed a battery of psychophysiological measures and emotional ratings in a picture viewing paradigm to examine whether the underarousal, low positive emotion, heightened negative emotion, or emotion context insensitivity model of emotional dysfunction in subclinical depressive symptoms received greatest support. Postauricular reflex and skin conductance response potentiation for pleasantminus neutral pictures (measuring low positive emotion), overall skin conductance magnitude and LPP amplitude (measuring underarousal), and pleasant minus aversive valence ratings (measuring emotion context insensitivity) and aversive minus neutral arousal ratings (measuring heightened negative emotionality) were all negatively related to depressive symptomatology. Of these, postauricular reflex potentiation and overall LPP amplitude were incrementally associated with depressive symptoms over the other measures. Postauricular reflex potentiation, overall skin conductance magnitude, and aversive minus neutral arousal ratings were incrementally associated with depressive symptomatology after controlling for other symptoms of internalizing disorders. Though no model was unequivocally superior, the low positive emotion and underarousal models received the most support from physiological measures and symptom reports, with self-report data matching patterns consistent with the emotion context insensitivity model.
Keywords: depression, low positive emotion, underarousal, emotion context insensitivity, postauricular reflex, skin conductance
Depression is a common yet debilitating affliction characterized by a disturbance of mood. Common symptoms of depression include anhedonia (inability to experience pleasure and reduced responsiveness to rewards), heightened negative affect, or a combination of the two (American Psychiatric Association, 2013; Pizzagalli, Iosifescu, Hallett, Ratner, & Fava, 2008). Subclinical depression is a significant risk factor for experiencing a major depressive episode (Cuijpers, de Graaf, & van Dorsselaer, 2004; Eaton, Badawi, & Melton, 1995; Lewinsohn, Solomon, Seeley, & Zeiss, 2000) and may be considered as part of the prodromal phase of depression (Eaton et al., 1995). The substantial decrease in quality of life and increased service utilization entailed in subclinical depression (Angst and Merikangas, 1997; Cuijpers et al., 2004; Rapaport and Judd, 1998; Wagner et al., 2000; Wells, Burnam, Rogers, Hays, & Camp, 1992) may result from reductions and elevations of normal-range emotional processes. Though many models of emotional dysfunction have been studied with respect to major depressive disorder, few of these models have been tested in subclinical depression. Early models emphasized the importance of physiological underarousal (Grossberg, 1972). Subsequent work implicated the unique role of low positive emotion in depression, whereas heightened negative emotion represents the emotional dysfunction depression has in common with other internalizing disorders (Clark & Watson, 1991b). Recent theorists have sought to combine findings from prior models into the emotion context insensitivity model of depression (Rottenberg, Gross, & Gotlib, 2005). Although each perspective takes a unique approach to the emotional dysfunctions observed in severe depression, it remains unclear which model best explains less severe forms of depression.
Theoretical Models of Emotional Dysfunction in Depression
Underarousal
Early theories of emotion in depression emphasized the behavioral and physiological underarousal that is a prominent part of depressive symptomatology (Grossberg, 1972). As depicted in Figure 1A, an overall mean shift level down strongly characterizes physiological arousal levels in this model. In the underarousal model, patterns of emotional reactivity are preserved in individuals with depression, though the mean level of response is reduced across all stimulus valences. Thus, depressed individuals exhibit some levels of arousal; however, in comparison to healthy individuals, they show reduced activity across all categories of stimuli. Psychomotor retardation, a form of depressive underarousal characterized by diminished mental and physical activity, is a common component of depressive symptomatology (Nelson & Charney, 1981). Moreover, psychomotor retardation is often associated with other vegetative symptoms like hypersomnia (Mitchell et al., 2001), exemplifying the possible manifestations of underarousal in depression.
Figure 1.
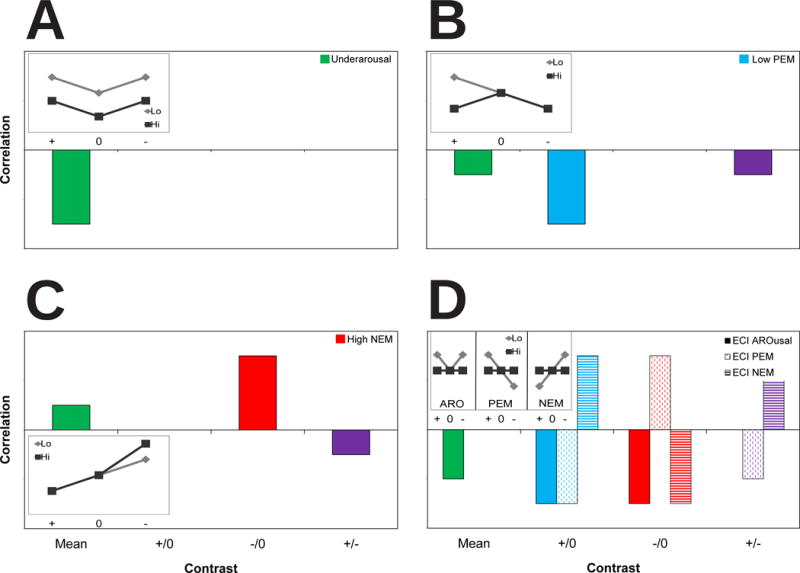
Schematic representation of patterns of results predicted from various models of emotional dysfunction in depression. Each panel represents the pattern of correlations between depressive symptomatology and various emotional contrasts that would support the model depicted. Panel A represents the underarousal model of depression, panel B the low positive emotion (PEM) model, panel C the high negative emotion (NEM) model, and panel D the emotion context insensitivity (ECI) model. Mean = overall mean magnitude (green), +/0 = pleasant minus neutral (blue), −/0 = aversive minus neutral (red), +/− = pleasant minus aversive (purple). To clarify these expected results, each inset diagram depicts the patterns of emotional modulation predicted for depressed (Hi) versus nondepressed (Lo) participants in each model of emotional dysfunction with arbitrary y axis units. + = pleasant, 0 = neutral, − = aversive.
Low positive emotion
The low positive emotion model of depression posits that reduced positive affectivity is crucial in understanding fundamental aspects of emotional reactivity in depression (Clark, Watson, & Mineka, 1994). Low positive emotion distinguishes depression from other internalizing disorders, including anxiety (Clark & Watson, 1991b; Nitschke, Heller, Imig, McDonald, & Miller, 2001). Diurnal and seasonal variations in positive emotion have been implicated in the development of depression (Depue, Krauss, & Spoont, 1987; Clark, Watson, & Leeka, 1989), and dampening of positive emotion is prospectively associated with depressive symptomatology (Raes, Smets, Nels, & Schoofs, 2012). In the low positive emotion model, depressed participants show strong and selective deficits in reactivity to pleasant stimuli specifically rather than to all stimuli; this pattern is depicted in Figure 1B. As a consequence, these deficits may cause weak reductions in the overall response magnitude and differentiation between pleasant and aversive stimuli in depression.
Heightened negative emotion
Low positive emotion accounts for only some of the emotional dysfunction observed in depression. Negative affectivity, a general distress factor shared by internalizing disorders (e.g., anxiety), is central to abnormal emotional reactivity in depression (Clark & Watson, 1991b). Negative affectivity is a multifaceted personality trait (Clark & Watson, 1991a) whose fundamental component is a temperamental sensitivity to aversive stimuli (Tellegen, 1985). Individuals high on this personality trait will exhibit pervasive negative moods ranging from guilt to hostility (Watson & Clark, 1984). Pervasive negative moods enable schemas that serve to negatively distort the processing of emotional stimuli (Beck, 1976; Beck, Rush, Shaw, & Emery, 1979). Specifically, negative moods activate schemas (Scher, Ingram, & Segal, 2005) that facilitate negative emotion when confronted by schema-matching negative stimuli. In the heightened negative emotion model, depressed participants would be expected to have greater reactivity specifically to aversive stimuli compared to controls. Predictions from this model are shown in Figure 1C. The key prediction of this model is that larger reactivity to aversive minus neutral stimuli should be strongly related to depressive symptomatology. Because of the larger reactivity to aversive stimuli, this pattern may also cause weak increases in mean response magnitude and a weakly exaggerated difference in reactivity to pleasant minus aversive stimuli in depression.
Emotion context insensitivity
To integrate data from prior models in favor of a more comprehensive model, theorists formulated a fourth perspective called the emotion context insensitivity (ECI) model. It states that those with depressive symptomatology display reduced emotional reactivity to aversive and pleasant stimuli (Rottenberg et al., 2005). Although loosely similar to previous models, the ECI model is deeply rooted in an evolutionary perspective of depression, which posits that depressed mood is a defensive mechanism promoting disengagement from the environment. Depressed people are thus prevented from engaging in dangerous or life threatening actions during turbulent times (Nesse, 2000). Therefore, diminished motivational activity brought about by negative mood inhibits overall emotional reactivity (Bylsma et al., 2008). In this model, depressed people show similar reactivity as that predicted by the low positive emotion model but the opposite emotional response predicted by the heightened negative emotion model. However, depressive symptomatology should not be related to different levels of reactivity to emotionally neutral stimuli. Thus, the ECI model of depression contrasts against the underarousal model: The ECI model predicts deficits in reactivity only to emotionally evocative stimuli (causing a flatter pattern of reactivity in depressed compared to non-depressed people), whereas the underarousal model predicts deficient reactivity to all stimuli (preserving patterns of emotional reactivity across depressed and non-depressed people).
Predictions from the ECI model are summarized in Figure 1D. Overall, reactivity to pleasant minus neutral and aversive minus neutral stimuli should be strongly reduced in depression, though unlike the underarousal model, reactivity to neutral stimuli should be the same between depressed and non-depressed individuals. The precise pattern these deficits take varies based on whether a particular measure assesses arousal, positive emotion, or negative emotion. Measures of arousal will show negative correlations between depressive symptomatology and reactivity during both pleasant minus neutral stimuli and aversive minus neutral stimuli, leading to a moderately diminished mean response magnitude. In contrast, measures keyed to assess positive emotional valence will display negative correlations between depressive symptomatology and reactivity during pleasant minus neutral stimuli but positive correlations with reactivity during aversive minus neutral stimuli. As a result, measures of positive emotion will also evidence a moderately reduced differentiation in reactivity to pleasant minus aversive stimuli. The reverse pattern will be true for measures keyed to assess negative emotional valence in the ECI model.
Measures of Emotional Processes in Clinical and Subclinical Depression
Arousal
Measures of arousal are typically larger during both pleasant and aversive stimuli compared to neutral stimuli. Skin conductance is a peripheral psychophysiological measure of sympathetic nervous activity with diffuse neural correlates (Critchley, Elliott, Mathias, & Dolan, 2000) that is reduced in clinical depression. These reductions are not limited to subclinical or other high-risk depression groups; they also extend across depression subtypes (Ward, Doerr, & Storrie, 1983). Diminished response magnitude consistent with the underarousal model is also believed to be a long-term physiological characteristic of depression. Attenuated skin conductance magnitude in depression is stable between test-retest intervals as short as three weeks (Storrie, Doerr, & Johnson, 1983) and as long as one year (Iacono et al., 1983). The latter study also concluded that antidepressant treatment does not explain these results. This vulnerability is not only present in unipolar depression, but also across other psychopathologies, including bipolar disorder (Lenhart, 1985).
Brain event-related potentials (ERPs) have often been used to assess underarousal in depression (Bruder, Kayser, & Tenke, 2012). The P3, a parietally maximal ERP component peaking at 300–400 ms, has been found to be sensitive to task relevant stimuli and those of motivational significance (Polich & Kok, 1995). Emotionally arousing stimuli provoke larger P3 amplitudes compared to neutral stimuli (Johnston et al., 1986; Kayser et al., 1997; Olofsson, Nordin, Sequiera, & Polich, 2008; Palomba et al., 1997), and P3 amplitude is reduced in depression during a wide variety of auditory and visual ERP paradigms (Bruder et al., 1995; Diner, Holcomb, & Dykman, 1985; Pfefferbaum, Wenegrat, Ford, Roth, & Kopell, 1984; Roth, Pfefferbaum, Kelly, Berger, & Kopell, 1981). However, complex stimuli like photo realistic pictures elicit a subsequent centro-parietal voltage offset termed the late positive potential (LPP) ERP that can overlap the P3 and is also greater during emotional than neutral stimuli (Weinberg, Venables, Proudfit, & Patrick, 2015). The LPP reflects sustained attention to complex arousing stimuli (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). Its amplitude is also negatively associated with depression (Proudfit, Bress, Foti, Kujama, & Klein, 2015), especially for rewarding pictures, though depressed patients with early-onset episodes also have reduced reactivity to rewarding and threatening images compared to patients with adult-onset depression (Weinberg, Perlman, Kotov, & Hajcak, 2016).
Subclinical depression is not associated with reduced overall electrodermal reactivity (Sloan & Sandt, 2010). Instead, it may be associated with a lack of emotional arousal specific to particular emotional valences. Students with subclinical depression show reduced P3 amplitudes to pleasant visual faces compared to healthy controls (Cavanagh & Geisler, 2006). Children’s own subclinical depressive symptoms (Dennis & Hajcak, 2009) and their mothers’ histories of depression (Kujawa, Hajcak, Torpey, Kim, & Klein, 2012) are related to blunted LPP reactivity to aversive pictures and faces, respectively.
Positive emotion
Measures of positive emotion should be largest for pleasant stimuli, smallest during aversive stimuli, and intermediate during neutral stimuli. One such measure is the zygomaticus major EMG, which is involved in smiling (Ekman, Davidson, & Friesen, 1990) and is greater during pleasant than neutral stimuli, particularly those depicting nurturant scenes (Bradley, Codispoti, Cuthbert, & Lang, 2001). Depressed individuals show reduced zygomatic EMG activity when thinking about happy scenarios compared to non-depressed individuals (Schwartz et al., 1976a), and pre-treatment zygomatic EMG magnitudes correlate with treatment response in depressed individuals (Carney, Hong, O’Connell, & Amado, 1981; Greden, Price, Genero, Feinberg, & Levine, 1984). Thus, decreased facial EMG activity during emotional stimuli appears to predict the presence and persistence of depression. Nevertheless, zygomatic EMG is a relatively weak measure of positive emotion for non-nurturant stimuli, particularly for men (Bernat, Patrick, Benning, & Tellegen, 2006; Bradley, Codispoti, Sabatinelli, & Lang, 2001).
Another psychophysiological measure that is greater during pleasant than neutral stimuli is the postauricular reflex, a microreflex behind the ear (Benning, Patrick, & Lang, 2004). Prior investigations consistently report potentiated postauricular reflex responses during pleasant as opposed to neutral or aversive stimuli (Benning, 2011; Hess, Sabourin, & Kleck, 2007; Sandt, Sloan, & Johnson, 2009), indicating its utility as a measure of appetitive emotional experience. Surprisingly, this measure has not been used to investigate low positive emotion in clinical depression. Contrary to the low positive emotion model, emotional postauricular reflex modulation was unrelated to subclinical depression scores in undergraduates (Sloan & Sandt, 2010). Rather, this study found that depressive symptomatology was correlated with reduced overall postauricular reflex magnitude, which is more consistent with the underarousal model. In contrast, individuals with subclinical levels of depression fail to potentiate zygomatic EMG response during pleasant faces compared to those during neutral faces (Sloan, Bradley, Dimoulas, & Lang, 2002), suggesting that this measure may be more sensitive to low positive emotion in subclinical depression.
Negative emotion
Measures of negative emotion are largest for aversive stimuli, smallest for pleasant stimuli, and intermediate for neutral stimuli. The magnitude of the corrugator supercilii EMG, which is typically larger during aversive than neutral emotional states and is associated with brow furrowing, has been used to examine heightened negative emotional expressivity in depression. Individuals with depression tend to show greater corrugator EMG activity while imagining scenes involving anger and scenes from a typical day, whereas non-depressed individuals do not (Schwartz, Fair, Salt, Mandel, & Klerman, 1976a, b; cf. Oliveau & Willmuth, 1979). Pre-treatment corrugator EMG magnitudes correlated with treatment response in individuals with depression (Carney et al., 1981; Schwartz et al., 1978). Thus, clinical depression is marked by heightened negative emotion as assessed through corrugator EMG.
The startle blink is another psychophysiological measure that is associated with increased reactivity during aversive minus neutral stimuli. The startle blink reflex is an involuntary response to an abrupt acoustic probe that is modulated by the emotional valence of a foreground stimulus, acting as a measure of aversive emotional experience. This effect reflects the amygdala’s input into the startle reflex circuit through the nucleus pontis recticularis caudalis (Davis, 2006). Prior investigations confirmed the utility of this affect-startle paradigm in gauging emotional reactivity (Bradley et al., 1990; Bradley et al., 2001), demonstrating the startle reflex is potentiated and attenuated in the presence of aversive and pleasant stimuli, respectively (Vrana, Spence, & Lang, 1988). However, results of multiple startle studies contradict the heightened negative emotional theory; depressed and control participants showed similar startle blink potentiation during aversive images (Allen et al., 1999; Dichter et al., 2004, 2008; Kaviani et al., 2004). In these studies, startle blink magnitudes were less potentiated in severely depressed patients compared to controls, though mildly or moderately depressed patients showed patterns of startle blink modulation comparable to controls. Thus, the bulk of startle blink studies provide psychophysiological evidence for the ECI model in clinical depression (Dichter et al., 2008), reflecting a protective disengagement from aversive stimuli (Nesse, 2000).
With respect to subclinical depression and negative emotion, Sloan et al. (2002) found that both dysphoric and non-dysphoric participants showed similar potentiation of corrugator EMG response during aversive faces compared to those during neutral faces. Dysphoric participants also showed potentiation of corrugator EMG response during pleasant faces compared to those during neutral faces, whereas non-dysphoric participants did not show this pattern of modulation. Some dysphoric undergraduate samples show blunted (Taubitz, Robinson, & Larson, 2013) or absent (Mneimne, McDermut, & Powers, 2008) startle blink potentiation during aversive minus neutral pictures. However, other undergraduate samples provide evidence of heightened or normal startle potentiation in subclinical depression (Larson, Nitschke, & Davidson, 2007; Sloan & Sandt, 2010). Thus, subclinical depression is unlikely to be associated strongly or consistently with psychophysiological measures of negative emotion.
Self-report
Self-report valence ratings have provided evidence for the ECI model of depression. In a study comparing depressed participants to healthy controls, depressed participants exhibited overall blunted emotional reactivity on self-reported affective ratings when exposed to a series of valenced film clips (Kaviani et al., 2004). Furthermore, in the first systematic quantitative review of emotional reactivity in depression, researchers reported data in support of the emotional context insensitivity model (Bylsma et al., 2008). A meta-analysis of 19 studies comparing depressed participants and healthy controls revealed diminished emotional reactivity among participants with depression. In self-report and behavioral measures, depressed patients showed less reactivity in both pleasant and aversive conditions, though there were greater deficits in positive emotion than negative emotion in that meta-analysis. Subsequent work presented evidence that contradicted the emotional context insensitivity model. In daily life events, depressed participants reported potentiated daily negative affect compared to controls (Bylsma, Taylor-Clift, & Rottenberg, 2011), a pattern of findings more congruent with the heightened negative emotion model. Curiously, this model does not appear to have been systematically examined in subclinical depression.
Current Study
In this study, a sample of undergraduates was used to explore which model of emotion in depression is most applicable to individuals with subclinical levels of depressive symptomatology. Participants viewed pleasant, neutral, and aversive emotional pictures while their psychophysiology was recorded. We collected skin conductance responses and LPP amplitude, corrugator and zygomatic EMG, and startle blink and postauricular reflexes during each picture, along with self-reported valence and arousal ratings of each picture. Data from this sample have been reported previously (Aaron & Benning, 2016; Benning, Rozalski, & Klingspon, 2015). However, Aaron and Benning (2016) examined the time course of postauricular reflex modulation, and Benning et al. (2015) focused on ERP measures of emotional picture and startle probe processing as they related to the personality trait of absorption.
The underarousal model of depression predicts that reduced overall reactivity to all stimuli would occur with greater depressive symptomatology, such that mean responses would be lower across all stimuli. If the data followed predictions from the low positive emotion model of depression, depressive symptomatology would be negatively correlated most strongly with contrasts involving pleasant minus neutral pictures. Depression scores positively correlating with contrasts involving aversive minus neutral pictures would be congruent with the heightened negative emotion model. The emotion context insensitivity model would be supported if blunted reactivity to pleasant and aversive pictures minus neutral pictures were observed with no differences in reactivity to neutral pictures themselves. Significant correlates of depressive symptomatology were parsed to examine whether they were associated more with the anhedonic or negative emotional symptoms of depression. These correlates were also entered into regression equations to determine which correlates were incrementally associated with depression after controlling for a) each other and b) symptoms of other internalizing disorders.
Method
Participants
Participants in this study were 90 undergraduates from psychology courses who took part in the study for course credit. Participants were 19.7 years old (SD = 2.09) on average. In this sample, 52% were women; 84% were White, 9% were Black, 4% were Asian, and 4% were Hispanic.
For each measure, varying numbers of participants had scoreable data. SAM ratings data were excluded from three participants because they gave average valence ratings more than 2.5 SD above the mean or arousal ratings more than 2.5 SD below the mean, leaving 87 participants in these analyses. Three participants were lost for each psychophysiological measure due to equipment failure, leaving 87 participants with valid corrugator and zygomatic EMG data. One participant had excessively noisy postauricular EMGs, and three participants did not have mean postauricular reflexes above 2 μV, leaving 83 participants with valid postauricular data. A total of 83 participants had valid startle blink data, with four excluded because their mean startle blink reflex magnitude was less than 2 μV. Eight participants had EEG activity exceeding 100 μV at Cz on more than 30% of the trials, leaving 79 participants with valid EEG data. Finally, because a module for recording skin conductance response (SCR) was added to the psychophysiological equipment after data from the first 22 participants were collected, SCR was available for 65 participants.
Stimuli
The acoustic noise probe was a bilateral 50 ms, 105 dB white noise probe with nearly instantaneous rise time; probes were presented 3000, 4000, or 5000 ms after the onset of most pictures. A total of 55 pictures from the International Affective Picture System (IAPS; CSEA-NIMH, 1999) were used in the experiment.1 Maximally intense exemplars of pleasant and aversive picture contents depicting stimuli that are directly or indirectly related to an organism’s survival were included. All picture contents were gender balanced on dimensions of normatively rated valence (median t(6) between men and women = 0.35, p = .735) and arousal (median t(6) between men and women = 0.13, p = .897).
The first three pictures (IAPS numbers 4650, 7080, and 9252) were probed at the beginning of the experiment to habituate abnormally large initial reflex magnitudes (Graham, 1979); data from these pictures were not analyzed. Probes were not presented during four pictures during the experiment (IAPS numbers 2220, 5460, 7233, and 8485); instead, probes were presented during the inter-trial interval (ITI) after these pictures to reduce the predictability of the startle probes.
A total of eight run orders were used in this study. Four different serial positions of the pictures were used, with appropriate stimulus substitutions made for women and men in the study to keep normative ratings of valence and arousal of each category of stimuli essentially identical between women and men. In each run order, no more than two pictures of the same valence occurred contiguously, and pictures of the same content did not follow each other. Startle probes were presented only once during each picture.
Self-Report Measures of Internalizing Psychopathology
Generalized Anxiety Disorder Inventory (GADI; Argyropoulos et al., 2007)
The GADI consists of 18 items that assess the cognitive-emotional, somatic, and sleep-related symptoms of generalized anxiety disorder (GAD). Each item is rated on a Likert scale from 1 (a little of the time) to 4 (most of the time). In the initial validation studies of this instrument, patients with GAD had higher scores on this inventory than either controls or patients with other anxiety disorders. Coefficient α for GADI total scores was .87 in this sample (M = 39.3, SD = 8.63).
Obsessive-Compulsive Inventory – Revised (OCI-R; Foa et al., 2002)
The OCI-R comprises 18 items that comprehensively assess obsessive-compulsive symptomatology. Each item is rated on a Likert scale from 1 (not at all) to 5 (extremely). Scores on this inventory adequately discriminate non-anxious controls from patients with obsessive-compulsive disorder, though they are also correlated strongly with self-reported symptoms of depression. Coefficient α for OCI-R total scores was .86 in this sample (M = 32.3, SD = 8.85).
Fear Survey Schedule – III (FSS-III; Arrindell, Emmelkamp, & van der Ende, 1984)
The 52-item FSS-III was designed to provide a comprehensive assessment of phobic symptomatology, with separate subscales developed for various diagnostically relevant classes of fears. Each item is rated on a Likert scale from 1 (not at all) to 5 (very much). Coefficients α for the subscales of the FSS-III were .69 (fears of sex and aggression, 8 items M = 14.2, SD = 3.63), .81 (agoraphobia, 13 items, M = 21.0, SD = 5.51), and .86 (social phobia, 13 items, M = 33.5, SD = 7.73; blood-injection-injury phobia, 12 items, M = 26.0, SD = 7.68; and fear of harmless animals, 6 items, M = 12.0, SD = 4.61).
Symptom Checklist – 14 (SCL-14; Lipman, Covey, & Shapiro, 1979)
The 14 items empirically identified as somatization items on the Hopkins Symptom Checklist-90 (Derogatis, Lipman, & Covey, 1973) were administered to provide a measure of experiencing wide-ranging and diffuse physical symptoms consistent with somatization disorder. Each item is rated on a Likert scale from 1 (not at all) to 5 (extremely). Coefficient α for SCL-14 total scores was .87 in this sample (M = 22.5, SD = 6.68).
Self-rating Depression Scale (SDS; Zung, 1965)
The SDS was developed to provide a comprehensive assessment of the symptoms of depression. Each of the 20 items is rated on a Likert scale from 1 (a little of the time) to 4 (most of the time). Relative to the Beck Depression Inventory, the Center for Epidemiological Studies – Depression scale, and the Hamilton Rating Scales for Depression, the SDS has essentially equal coverage of the anhedonic and negative affective symptoms of depression, though at the expense of coverage of the somatic symptoms of depression (Shafer, 2006). Coefficient α for SDS total scores was .78 in this sample (M = 34.3, SD = 7.15). This level of symptomatology is at the median (50th percentile) of what would be expected in a large, representative sample of the general population (Crawford et al., 2011). Coefficients α for the anhedonia and negative affect factors were .74 and .68, respectively.
Psychophysiological Recordings
All physiological channels were recorded using reusable Ag/AgCl electrodes and sampled at 2000 Hz with a Neuroscan SynAmps2 bioamplifier at DC with a 500 Hz lowpass filter to avoid aliasing of the physiological signals. Postauricular electrodes were placed according to locations PAM 5 and PINNA 2 detailed in O’Beirne and Patuzzi (1999), and recordings of the EMG activity in the postauricular muscles were taken from each ear. Startle blink magnitude was recorded from the orbicularis oculi muscle underneath the right eye, zygomatic EMG was recorded from the right cheek, and corrugator EMG was recorded from above the right eyebrow. These EMG electrodes had a 4 mm interior diameter. EEG was collected using a Neuroscan Quik-Cap, and SCR was collected from the thenar and hypothenar eminences with 8 mm inner diameter electrodes connected to a UFI 2701 BioDerm skin conductance meter. Impedances were kept below 10 kΩ for all measures.
Procedure
Participants completed a consent form after being escorted into the laboratory. They then completed the study questionnaires in the order noted above using a computer keyboard as the electrodes were attached. Once hookups were completed, participants were told to follow the directions on the screen, keeping as still as possible at all times. Participants were instructed to watch each picture the entire time it appeared on the 1280×1024 pixel resolution 19” LED monitor 1 m away and to keep their gaze directed toward the fixation cross whenever no picture was on the screen. In addition, participants were told that they could simply ignore the brief noises they would hear through the headphones. They were then presented with the three habituation pictures and given a demonstration of the ratings procedure they would use to rate each picture. After the demonstration, participants attended to each stimulus in the sequence determined by the run order.
Pictures were preceded by a 3 s baseline consisting of a blank screen with a fixation point; each was presented for 6 s, followed by a 3 s recovery period, during which the ITI startle probes were presented halfway through. After each trial, participants completed valence and arousal ratings of their current emotional state using the Self-Assessment Manikin (SAM; Bradley & Lang, 1994) via computer keyboard. The ratings were followed by a blank screen lasting 3 s to allow participants to prepare for the next picture. After participants viewed and rated all pictures, they were debriefed and compensated.
Data Reduction
Offline, startle blink and postauricular EMGs were epoched from 100 ms pre-probe onset to 250 ms post-probe onset. Postauricular EMGs were not filtered further, and startle blink EMGs were bandpass filtered from 28–250 Hz (Blumenthal et al., 2005). Startle blink and postauricular EMGs were then rectified; startle blink data were additionally smoothed with a single-pole recursive infinite impulse lowpass 5th order Butterworth filter with a 10 ms time constant. Corrugator and zygomatic EMGs were epoched from 1050 ms pre-picture onset to 6050 ms post-picture onset, then they were highpass filtered at 20 Hz before rectification (van Boxtel, 2001). Skin conductance data were epoched similar to the corrugator and zygomatic EMG data before being lowpass filtered at 20 Hz. EEG data were referenced to linked mastoids before being epoched from 250 ms pre-picture onset to 1550 ms post-picture onset. An ocular artifact correction was applied (Semlitsch, Anderer, Schuster, & Presslich, 1986) to correct for blinks before data were lowpass filtered at 20 Hz. All filters were applied at 24 dB/octave; including 50 ms of data at both ends of each epoch permitted filter artifacts to be discarded before data of interest were analyzed.
Because the postauricular reflex is a microreflex, postauricular muscle activity was assessed using aggregate rectified waveforms. Postauricular EMG activity to noise probes was averaged across all pictures of a given valence, yielding average waveforms comprising 16 trials. In each aggregation, postauricular reflex magnitudes were assessed as the peak EMG activity occurring 8–35 ms after noise probe onset minus the mean 50 ms pre-probe EMG baseline activity (Sloan & Sandt, 2010). LPP amplitude was assessed using similar aggregate waveforms at Cz (the site with the strongest effects related to depression in this study; see supplemental Figure S1 and Table S3 for more details) as the mean activity 500–800 ms after picture onset minus the mean 200 ms pre-picture baseline activity (Benning et al., 2015). For these measures, valid data from at least 8 out of 16 trials per valence were required for a participant to be included in further analyses. Prior to signal averaging, trials were excluded if baseline activity exceeded 100 μV.
Startle blink reflexes were scored on a trial by trial basis as the maximum smoothed activity 30–120 ms after noise probe onset minus the mean 50 ms pre-probe EMG baseline activity (Blumenthal et al., 2005). Rectified corrugator and zygomatic EMGs were scored trial by trial as the mean activity during the 6 s picture presentation minus the mean activity 1 s before picture presentation (Bradley et al., 2001). Skin conductance magnitude was scored as the peak activity 900–4000 ms after picture onset minus the mean skin conductance level 1 s before picture onset (Bradley et al., 2001). Within each of these measures, trials were excluded if baseline activity exceeded 3 SD above the mean. For all non-ERP measures, negative peaks were set to 0 and included in the analyses (Bradley et al., 2001). Furthermore, there were no corrections in the EMG or SCR data for startle probe presentation, as probed and non-probed trials do not typically differ in their modulations by emotion (Bradley et al., 2001).
Data Analysis
All statistical analyses were conducted in SPSS 22.0, and a critical α of .05 was used for all comparisons. Our data and analytic script are included as supplemental files to enhance reproducibility. We examined the effects of picture valence on each measure through a series of repeated measures MANOVAs. Significant effects of valence were followed up using polynomial contrasts. In each case, the linear contrast assessed whether responses during pleasant and aversive pictures differed from each other. The quadratic contrast examined whether responses during both pleasant and aversive pictures differed from those during neutral pictures. Furthermore, internal consistency reliabilities for each psychophysiological measure were computed using Cronbach’s alpha (coefficient α) following Aaron and Benning (2016). Reliabilities for mean responses were computed across pleasant, neutral, and aversive valences; reliabilities for pleasant minus neutral and aversive minus neutral responses were computed across each of the four contents within a valence minus the overall response during neutral pictures. The latter two reliabilities assessed the internal consistency of each measure’s emotional modulation after removing variance associated solely with each measure’s mean scores, which the first reliability coefficient captured (see supplemental Table S4 for reliabilities of each valence for each measure).
Next, in the sample as a whole, partial correlations (controlling for trait absorption)2 were computed for each measure between depressive symptomatology and mean psychophysiological response magnitude or picture rating along with emotional modulation of each psychophysiological measure or rating. Modulation scores were computed as scores for pleasant pictures minus scores for neutral pictures, scores for aversive pictures minus scores for neutral pictures, and scores for pleasant pictures minus scores for aversive pictures.
We also conducted a number of follow-up analyses. To examine whether significant correlates were more strongly associated with the heightened negative emotion common among all internalizing disorders or the low positive emotion specific to depression, we correlated each measure significantly related to depression with the Anhedonia and Negative Affect factors of the SDS separately. To determine which measures were incrementally related to depressive symptomatology after controlling for other internalizing symptomatology, a regression analysis predicting depression scores from the measures significantly related to depression was conducted. Missing values for each psychophysiological measure were replaced with the mean for that measure in this analysis, which used one-tailed tests because the directions of the expected effects were established in the partial correlations described above. To examine the specificity of the relationships found above to depressive symptomatology alone, significant correlations were followed up with regression analyses predicting responses from the measures of internalizing psychopathology described above. Across all regressions, coefficients featured variance inflation factors (VIF) less than 3, which is substantially below the VIF value of 10 that typically indicates potentially problematic multicollinearity (Belsley, Kuh, & Welsch, 1980; see supplemental Table S1 for the full correlation matrix for all of these analyses).
Results
Manipulation Checks
Figure 2 depicts the main effect of valence on each measure in this study. Both psychophysiological measures of arousal performed as expected. SCR magnitude was modulated by picture valence, F(2,63) = 4.20, p = .019, ηp2 = .12. It was greater during both pleasant and aversive pictures compared to neutral pictures, quadratic F(1,64) = 8.51, p = .005, ηp2 = .12, but it did not differ during pleasant and aversive pictures, linear F(1,64) = 0.80, p = .374, ηp2 = .01. Coefficient α for mean SCR magnitude was .94, SCR pleasant-neutral α was .34, and SCR aversive-neutral α was .45. Likewise, LPP amplitude was modulated by picture valence, F(2,77) = 36.0, p < .001, ηp2 = .48. It was greater during both pleasant and aversive pictures compared to neutral pictures, quadratic F(1,78) = 69.2, p < .001, ηp2 = .47, but it did not differ during pleasant and aversive pictures, linear F(1,78) = 0.03, p = .855, ηp2 = .00. Coefficient α for mean LPP amplitude at CZ was .91, LPP pleasant-neutral α was .40, and LPP aversive-neutral α was .51.
Figure 2.
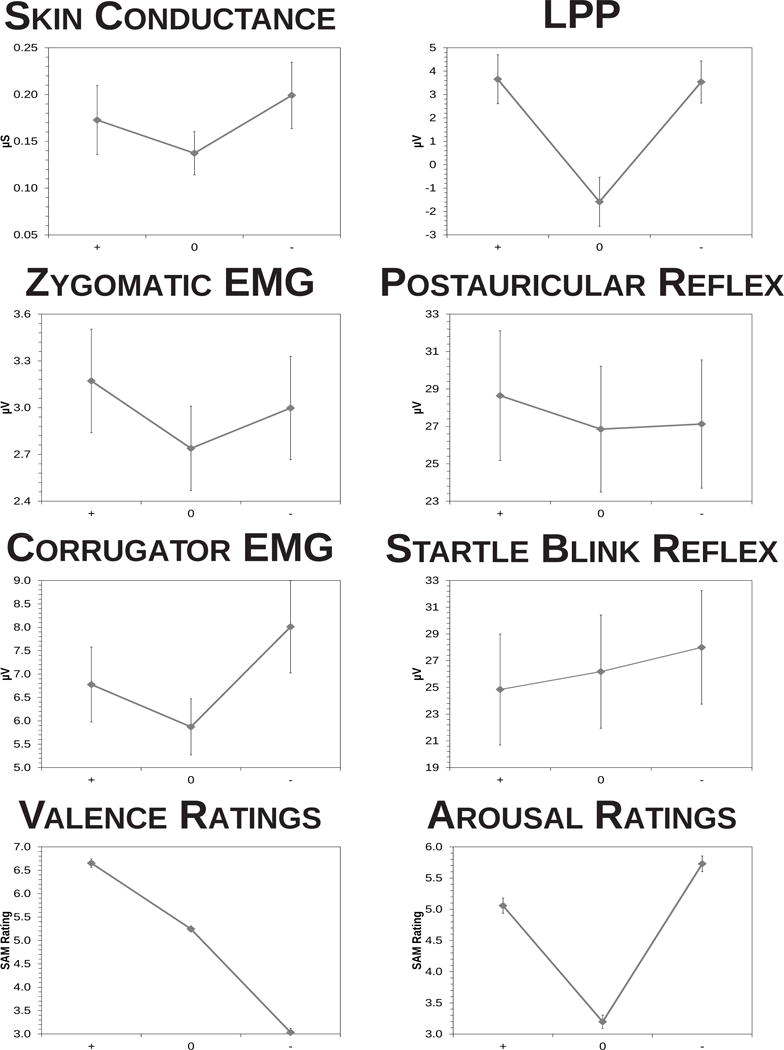
Main effects of valence on all measures in this study. Error bars represent the standard error of the mean. + = pleasant, 0 = neutral, − = aversive.
One psychophysiological measure of positive emotion performed as expected, but another did not. Postauricular reflex magnitude was modulated by picture valence, F(2,81) = 4.80, p = .011, ηp2 = .11. It was greater during pleasant than aversive pictures, linear F(1,82) = 3.95, p = .050, ηp2 = .05, and it was intermediate during neutral pictures, quadratic F(1,82) = 3.23, p = .076, ηp2 = .04. Coefficient α for mean postauricular reflex magnitude was .99, postauricular pleasant-neutral α was .30, and postauricular aversive-neutral α was .48. Conversely, zygomatic EMG magnitude was not modulated by picture valence, F(2,85) = 2.73, p = .071, ηp2 = .06. Coefficient α for mean zygomatic magnitude was .89, zygomatic pleasant-neutral α was .11, and zygomatic aversive-neutral α was .36.
Both psychophysiological measures of negative emotion were affected by emotional pictures as expected. Startle blink reflex magnitude was modulated by picture valence, F(2,81) = 8.09, p = .001, ηp2 = .17. It was greater during aversive than pleasant pictures, linear F(1,82) = 16.4, p < .001, η2p = .17, and it was intermediate during neutral pictures, quadratic F(1,82) = 0.09, p = .762, ηp2 = .00. Coefficient α for mean startle blink magnitude was .99, startle blink pleasant-neutral α was .60, and startle blink aversive-neutral α was .29. Likewise, corrugator EMG magnitude was modulated by picture valence, F(2,85) = 6.15, p = .003, ηp2 = .13. It was greater during aversive than pleasant pictures, linear F(1,81) = 10.7, p = .002, ηp2 = .11, though it was also larger during emotional than during neutral pictures, quadratic F(1,86) = 4.16, p = .045, ηp2 = .05. Coefficient α for mean corrugator magnitude was .90, corrugator pleasant-neutral α was .31, and corrugator aversive-neutral α was .46.
Scores on both self-report measures of emotion were strongly influenced by emotional pictures. SAM valence ratings were modulated by picture valence, F(2,85) = 280, p < .001, ηp2 = .87. They were greater for pleasant than aversive pictures, linear F(1,86) = 565, p < .001, ηp2 = .87, though neutral pictures were rated slightly more akin to pleasant pictures than aversive pictures, quadratic F(1,86) = 38.0, p < .001, ηp2 = .31. Consistent with the negative correlation between ratings of positive and negative affective adjective ratings (e.g., −.43 in Tellegen, Watson, & Clark, 1999), coefficient α for mean valence ratings was −.22, largely because the correlation between valence ratings of pleasant and aversive pictures was −.41 in this study. However, αs for contents within each valence were substantially higher (.73 for pleasant, .76 for neutral and aversive). The valence rating pleasant-neutral α was .71, and the valence rating aversive-neutral α was .84. SAM arousal ratings were also modulated by picture valence, F(2,85) = 171, p < .001, ηp2 = .80. They were greater for pleasant and aversive pictures compared to neutral pictures, quadratic F(1,86) = 324, p < .001, ηp2 = .79, and aversive pictures were rated as more arousing than pleasant pictures, linear F(1,86) = 37.4, p < .001, ηp2 = .30. Coefficient α for mean arousal ratings was .70, arousal rating pleasant-neutral α was .81, and arousal rating aversive-neutral α was .89.
Correlations and Congruence with Models of Emotional Dysfunction in Depression
For each measure shown in a panel of Figure 3, negative correlations solely in the first column support the underarousal model. Negative correlations primarily in the second column support the low positive emotion model. Positive correlations primarily in the third column support the heightened negative emotion model. The emotion context insensitivity model would be supported by correlations indicative of reduced emotional reactivity in the second and third columns combined with either a) the corresponding correlation in the fourth column (for measures of emotional valence) or b) a negative correlation in the first column (for measures of emotional arousal). To illustrate these findings, inset panels depict participants with an SDS total score more than 1 SD above the sample mean (corresponding to a score of 42 or more; n = 14) compared to all other participants (n = 73). Similar cutpoints have identified clinically depressed participants with at least mild levels of symptomatology in previous studies (Fountoulakis et al., 2001; Zung, 1973; see supplemental Table S2 for further details).
Figure 3.
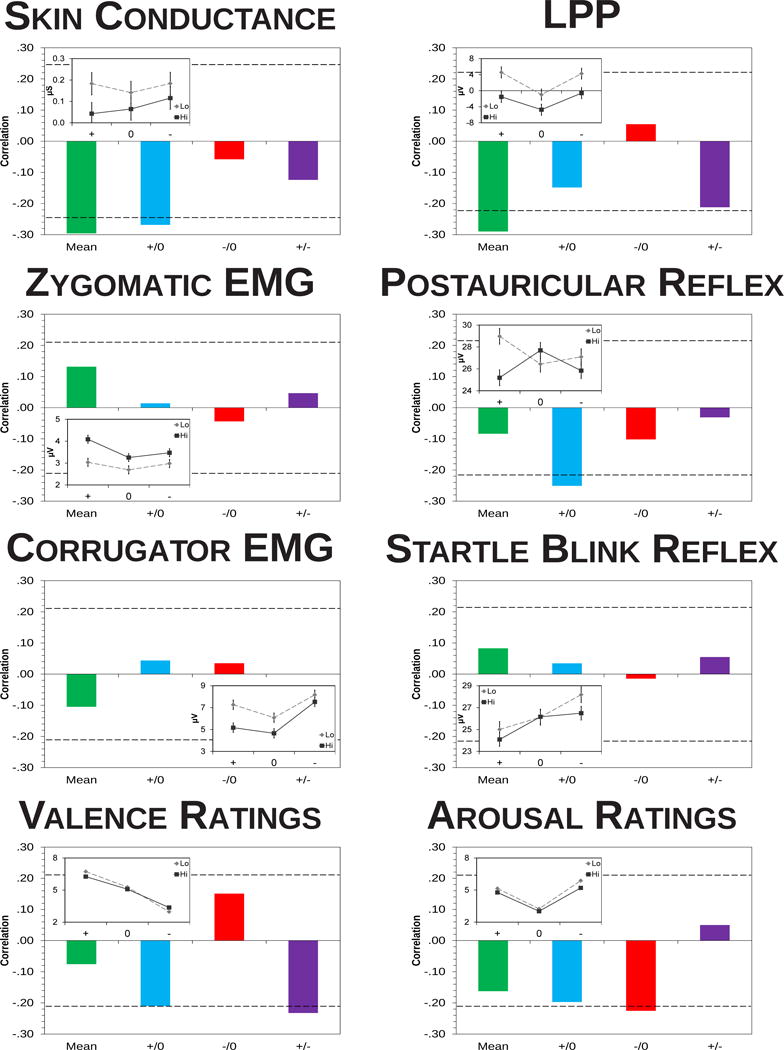
Correlations of total depressive symptomatology with the mean and emotional modulation of each measure. The patterns of correlations in each panel may be compared with those depicted in Figure 1 to ascertain which models of emotional dysfunction in depression are supported for a given measure. Correlations outside the dashed lines are significant at an α level of .05. Mean = overall mean magnitude (green), +/0 = pleasant minus neutral (blue), −/0 = aversive minus neutral (red), +/− = pleasant minus aversive (purple). n = 65 for skin conductance response, n = 79 for LPP amplitude, n = 83 for postauricular and startle blink reflexes, n = 87 for zygomatic and corrugator EMGs and for pleasantness and arousal ratings. Given the α level of .05, there were more significant correlations than would be expected by chance alone, binomial test p = .005. To clarify these results, each inset diagram displays the patterns of emotional reactivity for participants 1 SD above the mean in depressive symptoms (Hi) compared to all other participants (Lo). Error bars represent the error term of the Valence × Group interaction.
Supporting the underarousal model, mean SCR magnitude, rp(61) = −.30, p = .019, and mean LPP amplitude, rp(75) = −.29, p = .011, during all pictures were negatively related to depression symptoms. During pleasant minus neutral pictures, skin conductance magnitude, rp(61) = −.27, p = .033, and postauricular reflex magnitude, rp(79) = −.25, p = .021, were negatively related to depressive symptomatology. Overall, the patterns of correlations with self-report scores were nearly identical to those predicted by the emotion context insensitivity model. However, only valence ratings for pleasant minus aversive pictures, rp(84) = −.23, p = .031, and arousal ratings for aversive minus neutral pictures, rp(84) = −.23, p = .037, were significantly correlated with depression scores.
No contrasts involving zygomatic EMG, corrugator EMG, or startle blink were significant. The lack of relationships of depression symptoms with zygomatic EMG activity was particularly unsurprising given the lack of zygomatic EMG valence modulation in this sample.
Relationships with Factors of Depressive Symptomatology
Table 1 gives the correlations with factors of depressive symptomatology (Shafer, 2006) of each measure that was significantly correlated with depression total scores.3 Mean LPP amplitude and both skin conductance measures were associated with anhedonia. Mean skin conductance, postauricular reflex potentiation during pleasant minus neutral pictures, and valence ratings for pleasant minus aversive pictures were correlated with the negative emotion symptoms of depression.
Table 1.
Correlations between Factors of Depressive Symptomatology and Experimental Measures
| Measure | Anhedonia | Negative Affect |
|---|---|---|
| Skin conductance: mean magnitude | −.28* | −.30* |
| Skin conductance: pleasant > neutral | −.30* | −.19 |
| LPP: mean amplitude | −.27* | −.18 |
| Postauricular reflex: pleasant > neutral | −.20 | −.25* |
| Valence ratings: pleasant > aversive | −.18 | −.25* |
| Arousal ratings: aversive > neutral | −.20 | −.13 |
Note. n = 65 for skin conductance response, n = 79 for LPP amplitude, n = 83 for postauricular reflexes, n = 87 for emotion ratings.
p < .05.
Incremental Validity Analyses
To demonstrate which contrasts were incrementally associated with depressive symptoms above and beyond the others in this study, Table 2 displays the standardized coefficients from regressions predicting total depression symptoms from all measures that were significantly associated with these symptoms. Reduced mean LPP amplitude and postauricular reflex magnitude during pleasant minus neutral pictures remained as predictors, indicating that they alone contributed uniquely to the assessment of depression symptoms. However, it is reasonable to assume that the multicollinearity between the two SCR contrasts (r = .54) removed a substantial portion of variance from each of these contrasts in the regressions.
Table 2.
Standardized Regression Coefficients of Experimental Measures as Predictors of Depression Total Scores
| Measure | All Predictors | Only Mean SCR Contrast | Only +/0 SCR Contrast |
|---|---|---|---|
| Skin conductance: mean magnitude | −.16(.13) | −.18*(.10) | – |
| Skin conductance: pleasant > neutral | −.04(.13) | – | −.15(.10) |
| LPP: mean amplitude | −.18*(.10) | −.18*(.10) | −.18*(.10) |
| Postauricular reflex: pleasant > neutral | −.18*(.10) | −.18*(.10) | −.18*(.10) |
| Valence ratings: pleasant > aversive | −.08(.13) | −.09(.13) | −.12(.11) |
| Arousal ratings: aversive > neutral | −.05(−.13) | −.05(−.13) | −.07(−.11) |
Note. n = 65 for skin conductance response, n = 79 for LPP amplitude, n = 83 for postauricular reflexes, n = 87 for emotion ratings. Standard errors of the coefficients are displayed in parentheses. +/0 = pleasant minus neutral.
p < .05 (one-tailed).
Therefore, two additional regressions were conducted, dropping one SCR contrast or the other from the analysis. In both regressions, mean LPP amplitude, postauricular reflex potentiation during pleasant minus neutral pictures, and valence ratings of pleasant minus aversive pictures were included. In this set of analyses, mean SCR magnitude (but not SCR magnitude during pleasant minus neutral pictures) emerged as an incrementally valid predictor of depressive symptomatology along with reduced mean LPP amplitude and postauricular reflex potentiation during pleasant minus neutral pictures.
Specificity to Depression
To show whether the significant contrasts were specifically related to depression symptoms above and beyond other internalizing symptoms, Table 3 gives the standardized coefficients from regressions predicting each experimental measure from the assessments of internalizing symptomatology. Most contrasts remained associated with depression symptoms after controlling for other internalizing symptomatology. Skin conductance magnitude during pleasant minus neutral pictures, mean LPP amplitude, and valence ratings during pleasant minus aversive pictures were no longer associated with depressive symptoms. Mean LPP amplitude was positively predicted by social phobia, whereas postauricular reflex magnitude during pleasant minus neutral pictures was negatively predicted by social phobia. Mean skin conductance magnitude was negatively predicted by fears related to sex and aggression.
Table 3.
Standardized Regression Coefficients of Depression and Other Internalizing Symptomatology as Predictors of Experimental Measures
| Measure | SDS | Social Phobia | Agora-phobia | BII Phobia | Sex and Aggression | Harmless Animals | GADI | OCI-R | SCL-14 |
|---|---|---|---|---|---|---|---|---|---|
| Skin conductance: mean magnitude | −.38*(.14) | .19(.14) | .23(.14) | .19(.14) | −.28*(.14) | −.08(.15) | .05(.17) | .05(.13) | −.09(.17) |
| Skin conductance: pleasant > neutral | −.26(.15) | .17(.15) | .11(.14) | .01(.15) | −.03(.15) | .02(.16) | .06(.17) | −.01(.13) | −.17(.17) |
| LPP: mean amplitude | −.18(.14) | .31*(.14) | .15(.14) | −.16(.14) | −.04(.14) | .18(.15) | −.24(.16) | .11(.12) | −.21(.19) |
| Postauricular reflex: pleasant > neutral | −.31*(.14) | −.33*(.14) | −.18(.14) | −.06(.14) | .05(.14) | −.08(.15) | .10(.17) | .07(.13) | .29(.17) |
| Valence ratings: pleasant > aversive | −.18(.14) | .14(.14) | .24(.14) | −.14(.14) | .11(.14) | .17(.15) | −.22(.17) | −.09(.13) | −.11(.17) |
| Arousal ratings: aversive > neutral | −.33*(.14) | .17(.14) | .10(.14) | −.26(.14) | .00(.14) | .14(.15) | −.13(.17) | .11(.13) | .13(.17) |
Note. n = 65 for skin conductance response, n = 79 for LPP amplitude, n = 83 for postauricular reflexes, n = 87 for emotion ratings. SDS = Zung Self-rating Depression Scale, BII = blood-injection-injury, GADI = Generalized Anxiety Disorder Inventory, OCI-R = Obsessive Compulsive Inventory – Revised, SCL-14 = Symptom Checklist – 14 somatization items. Standard errors of the coefficients are displayed in parentheses.
p < .05.
Discussion
In this study, four models of deviant emotional processing in depression were tested in subclinical depression using multiple psychophysiological and self-report measures. Though no model won unequivocal support from the data, the results of this study were most consistent with the low positive emotion and underarousal models. Reduced potentiation of the postauricular reflex and SCR to pleasant minus neutral pictures was associated with depressive symptomatology, and these measures were also most congruent with the low positive emotion model. Conversely, reduced overall SCR magnitude and LPP amplitude was associated with depressive symptomatology, even after controlling for other measures or symptoms of other internalizing disorders in the case of mean SCR magnitude. Reduced differentiation for valence ratings of pleasant minus aversive pictures represented the sole contrast associated with depression symptoms for the emotion context insensitivity model, though the patterns of valence and arousal picture ratings were highly congruent with this model. There was no evidence supporting the heighted negative emotion model of subclinical depression; indeed, the results for arousal ratings for aversive minus neutral pictures ran counter to this model.
Emotion in Subclinical and Clinical Depression
Our results suggest better continuity between subclinical and clinical depression in central and autonomic measures of arousal than previous studies of subclinical depression. We found that mean LPP amplitude and skin conductance magnitude were negatively associated with subclinical depressive symptomatology, mirroring previous findings with P3 (Bruder et al., 1995; Diner et al., 1985; Pfefferbaum et al., 1984) and skin conductance (Iacono et al., 1983; Ward et al., 1983) in depressed participants. Furthermore, consistent with our findings for subclinical depression, depressed patients have specific skin conductance deficits to pleasant pictures (Margada & Hansenne, 2009). However, our results contrast against previous P3 work in subclinical depression (Cavanaugh & Geisler, 2006), which indicated that P3 was a better marker of low positive emotion than underarousal in subclinical depression. Also unlike our study, previous work on skin conductance in subclinical depression has not found evidence of underarousal using that measure (Sloan & Sandt, 2010).
Though the postauricular reflex has yet to be used in studies of clinical depression, our findings with this reflex also conflict with those from the other study that used this reflex in subclinical depression. In this study, potentiation of the postauricular reflex during pleasant minus neutral pictures was most consistently and uniquely related to subclinical depression. These results contrast against those in Sloan and Sandt (2010), in which reduced mean postauricular reflex magnitude was most strongly related to subclinical depression scores. Their findings would suggest that underarousal in subclinical depression, not reduced positive emotion, would be best measured through the postauricular reflex. The sample in that study was similar in composition to this sample and the two experimental procedures were nearly identical, suggesting that these factors were not responsible for the differences observed between the two studies. Furthermore, our sample’s mean level of depressive symptoms (50th percentile; 95% CI: 44th – 55th percentile) was similar to theirs (58th percentile; 95% CI: 48th – 68th percentile) according to population-based norms (Crawford et al., 2011) for the SDS and 21-item version of the Depression Anxiety Stress Scales (DASS; Lovibond & Lovibond, 1995) used in their study, respectively. In our study, the SDS had the same pattern of correlations with positive emotionality (r = −.26) and negative emotionality (r = .31) on the Multidimensional Personality Questionnaire (Patrick, Curtin, & Tellegen, 2002) as did the DASS Depression scale with trait positive affectivity (r = −.48) and negative affectivity (r = .59) in a large-scale study (Henry & Crawford, 2005). Thus, the differences in the constructs assessed by these depression scales deserve further exploration, as their items do not load together strongly in joint confirmatory factor analyses, particularly the SDS anhedonia items (Taylor et al., 2005).
Contrary to Sloan et al. (2002), neither corrugator nor zygomatic EMGs correlated with depressive symptomatology. These results are also incongruent with Schwartz et al. (1976a), who found potentiated corrugator and diminished zygomatic EMG activity in depression. However, the type of emotion-eliciting stimuli employed by Schwartz et al. (1976a) may account for the discrepancy between our studies. Schwartz et al. (1976a) asked participants to envision scenes of either anger or happiness, whereas this study required participants to view IAPS pictures. Additional research suggests that depressed individuals can vividly imagine aversive impending scenes but not pleasant scenes (Holmes, Lang, Moulds, & Steele, 2008). Therefore, facial EMGs may be better measures of low positive emotion in depression during mental imagery than during picture viewing, due in part to difficulties depressed individuals may have imagining pleasant scenarios. Nevertheless, the lack of valence modulation of the zygomatic EMG in this study vitiates making strong conclusions about the utility of this measure in understanding subclinical depressive symptoms.
Following previous work (Bylsma et al., 2008), self-reported valence and arousal ratings matched the emotion context insensitivity model of depression well. However, the psychophysiological measures in this study did not, indicating that subclinical depression may preserve emotional reactivity compared to clinical depression. This study found no evidence supporting the heightened negative emotion model of subclinical depression. Consistent with the broader literature, our participants with subclinical levels of depressive symptomatology had similar levels of fear-potentiated startle as those without depression or with mild depressive symptoms (Dichter et al., 2008; Kaviani et al., 2004; Larson et al., 2007). Thus, the startle blink reflex may not be the best measure of amygdala-related dysfunction in low-grade depression. The strongest evidence for heightened negative emotion in depression comes from startle potentiation during pleasant minus neutral pictures in individuals with depression that is severe (Allen et al., 1999) or recurrent (Vaidyanathan, Melo, Malone, Burwell, & Iacono, 2014). However, this finding is not consistent across the literature (Dichter et al., 2004, 2008; Kaviani et al., 2004), and further work is needed to explore the participant or symptom characteristics associated with heightened startle blink potentiation in depression.
Low Positive Emotion and Underarousal as Diatheses to Depression
This study implicates underarousal and low positive emotion as core emotional components of subclinical depression, whereas previous studies have emphasized genetic (for a review, see Levinson, 2006) and cognitive (Ingram, Miranda, & Segal, 1998) predispositions for depression. Low positive emotionality may be a temperamental precursor or risk factor for depression (Shankman, Tenke, Bruder, Durbin, Hayden, & Klein, 2005) and depressotypic cognitions (Hayden, Klein, Durbin, Olino, 2006). Furthermore, low reward sensitivity is associated with the first onset of a major depressive episode in adolescent girls (Bress, Foti, Kotov, Klein, & Hajcak, 2013). Similarly, a broad cortical underarousal characterizes depression (Jaworska, Blier, Fusee, & Knott, 2012) even before the disorder manifests itself fully (Grin-Yatsenko, Baas, Ponomarev, & Kropotov, 2011). Further highlighting the role of underarousal in depression, smaller skin conductance responses also correlate specifically with depression rather than other internalizing disorders (Argyle, 1991). Depressed children of depressed mothers show reduced skin conductance responses compared to children of mothers with anxiety disorders or no psychopathology (Waters, Peters, Forrest, & Zimmer-Gembeck, 2014). Thus, depressive underarousal affects both measures, even though the brain networks that generate skin conductance responses (thalamus, medial orbital gyri, and right supramarginal gyrus; Anders, Lotze, Erb, Grodd, & Birbaumer, 2004) and LPP (occipito-temporal junction, insula, amygdala, hippocampus, temporal poles, and left orbital frontal cortex; Liu, Huang, McGinnis-Deweese, & Keil, 2012) during picture viewing do not overlap strongly.
If underarousal and low positive emotion are key components of depression, interventions that ameliorate these two deficits simultaneously may prove particularly efficacious in treating depression. For instance, behavioral activation treatments, which increase participants’ frequency of partaking in pleasant activities, increase well-being and positive emotion above and beyond providing symptom relief equally well for people with clinical or subclinical depression (Mazzucchelli, Kane, & Rees, 2010). Such interventions may also ameliorate the psychomotor slowing and lack of energy that are symptoms of underarousal in depression (Salamone, Correa, Mingote, Weber, & Farrar, 2006). Conversely, stimulant-like drugs (but not traditional psychostimulant medications) show promise in treating the fatigue and sleepiness specifically that are implicated in depressive underarousal (Malhi et al., 2016). Such drugs may ameliorate patterns of cortical activity in depression that are consistent with both underarousal and low positive emotionality (Bruder et al., 1997).
Limitations and Future Directions
As is typical in psychophysiological work, psychometric limitations were present in this study. The power available to detect effects in this study varied substantially across contrasts as a function of the number of participants with interpretable scores on each measure, and the zygomatic EMG essentially had no power to detect these relationships, as it was not modulated by picture valence. Nevertheless, the measure with the lowest ostensible power – skin conductance – was the only measure to have multiple significant contrasts in this study. Because this was an exploratory study, we used analytic strategies that maximized our power to detect relationships between depressive symptomatology and measures of emotion, and we found more relationships of emotion with depression than chance alone would predict. The follow-up analyses reported in Tables 2 and 3 were designed in part to examine which findings were robust when controlling for multiple confounds. Even though the reduced postauricular reflex potentiation and overall skin conductance results were robustly associated with depression across these analyses, there is no guarantee that they were not false positive (Type I) errors. Future studies would benefit from more focal contrasts derived from this study to confirm these results, especially when combined with more stringent α levels to control Type I error rates.
The reliabilities for mean psychophysiological responses were excellent, indicating that each participant has a relatively stable raw response magnitude or amplitude across valences. However, the reliability for raw postauricular and startle blink reflex potentiation in this dataset was less than that for within-subject standardized data in this sample (Aaron & Benning, 2016). Reliabilities were also lower than desirable for many measures in this study, though the results for startle blink modulation were better than those obtained for picture viewing in previous studies (Kaye, Bradford, & Curtin, 2016). Further work should determine the optimal methods for creating reliable psychophysiological measures of positive and negative emotion.
Participants only completed self-reports of psychopathology in this study, which limits its generalizability to the construct of depression as a whole. Future studies should employ multiple assessments of depression, including clinical ratings of depressive symptoms, to assess whether these results would replicate across various measures of depression, differing in their coverage of anhedonia and other depressive symptomatology. Likewise, even though our sample had a substantial range of subclinical depressive symptomatology, the demographics of further samples should be broadened beyond college students to ensure the generalizability of these results to a larger population. Other studies of depressed undergraduates found evidence of emotion context insensitivity after picture offset (Moran, Mehta, & Kring, 2012), indicating that future studies would benefit from considering how long the deficits in positive emotion last after stimuli are no longer displayed. With respect to broader models of positive emotion (Berridge & Kringelbach, 2008), we also assessed only the “liking” component of positive emotional processing in depression. Additional laboratory studies of the “wanting” (e.g, Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009) and “learning” (e.g., Pizzagalli et al., 2008) components of positive emotion would be fruitful to examine the generality of deficits in positive emotion in depression.
Future studies should investigate multiple methods of eliciting different kinds of positive emotion in the laboratory. For instance, deficits in emotional reactivity to films inducing pride, which is difficult to instantiate with pictures, appear to be uniquely associated with depressive symptomatology (Gruber, Ovies, Keltner, & Johnson, 2011). Such stimuli may overcome the well-established difficulties in obtaining modulation of the zygomatic EMG by emotional pictures (Bernat et al., 2006; Bradley et al., 2001), which was evident in our study. Furthermore, though this study implicates reduced positive emotion as a diathesis for depression, it did not follow up participants longitudinally to determine whether those participants who had reduced positive emotion subsequently developed depression as rated by clinicians. Prospective studies in samples at risk of developing depression could examine whether deficits in positive emotion uniquely predict the onset of depression, as deficits in positive emotion appear to lengthen the course of depressed episodes (Morris, Blysma, & Rottenberg, 2009).
Nevertheless, this study demonstrated the unique contribution of low positive emotion and emotional underarousal to subclinical levels of depression, even after accounting for symptoms of other internalizing disorders (cf. Beck et al., 2001). Furthermore, we found emotion context insensitivity and heightened negative emotion received limited to no support, respectively. The findings suggest that the psychophysiological measures like the postauricular reflex and skin conductance response may be sensitive markers for emotional reactivity in depression. These measures could become valuable tools for investigating emotional deficiencies, particularly positive affective states, in psychopathology. Overall, results of this study set the stage for studies of the potential continuity between normal functioning and subclinical and clinical depression using behavioral and biological measures (Ingram, Siegle, & Steidtmann, 2014), consistent with the Research Domain Criteria approach to psychopathology (Woody & Gibb, 2015).
Supplementary Material
Acknowledgments
This research was supported in part by grant MH093692 from the National Institute of Mental Health.
We wish to acknowledge Stephany M. Molina, Leiszle Lapping-Carr, and Vincent Rozalski for their helpful comments on drafts of this manuscript. Portions of this research were presented at the 49th and 53rd annual meetings of the Society for Psychophysiological Research.
Footnotes
The following IAPS pictures comprised each content category. Pleasant content: adventure: 5623, 8034, 8180, 8210; nurturant: (1811, 2071, 2160, 2340/1463, 1722, 2341, 2655); erotic: 4640, 4660, 4680, (4255/4572); food: 7200, 7230, 7260, 7460. Neutral content: buildings: 5731, 7180, 7490, 7491; humans: 2190, 2393, 2870, 2890; landscapes: 5120, 5390, 5740, 9210; objects: 7002, 7004, 7034, (7031/7038). Aversive content: disgust: 9342, 9520, 9560, 9830; mutilation: (3051, 3061, 9253, 9420/9042, 9265, 9440, 9490); threat: 6250, 6260, 9630, (6243/6190); victim: 6570, 9920, (6312, 6540/6530, 6561). Pictures not in parentheses were presented to participants of either gender; pictures within parentheses to the left of the slash were presented only to men, and those within parentheses to the right of the slash were presented only to women. These substitutions ensured that each content had similar normative valence and arousal ratings between men and women.
In this sample, trait absorption correlated .05 with SDS total scores, −.05 with SDS anhedonia, and .16 with negative affect (all ps > .13), indicating that the effects in this sample were independent of those reported in Benning et al. (2015). Furthermore, the pattern of results was identical in zero-order correlations that did not control for absorption.
It is possible that excluding from Table 1 all the non-significant contrasts reported in Figure 3 might have obscured their relationships with anhedonia or negative affect in depression specifically. However, none of the measures and contrasts excluded from Table 1 were significantly correlated with the anhedonia factor of depression (|r|s <.21, ps > .05). The negative affect factor of depression was correlated with LPP amplitude for pleasant minus aversive pictures, r = −.31, p = .006. No other correlations were significant with the negative affect factor, |r|s < .23, ps > .05. Thus, based on the binomial test with an α level of .05, there were not significantly more correlations found with the negative affect factor in the 26 excluded contrasts than would be expected by chance alone, p = .736.
Contributor Information
Stephen D. Benning, Department of Psychology, University of Nevada, Las Vegas
Belel Ait Oumeziane, Department of Psychological Sciences, Purdue University.
References
- Aaron RV, Benning SD. Postauricular reflexes elicited by soft acoustic clicks and loud noise probes: Reliability, prepulse facilitation, and sensitivity to picture contents. Psychophysiology. 2016;53(12):1900–1908. doi: 10.1111/psyp.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NB, Trinder J, Brennen C. Affective startle modulation in clinical depression: preliminary findings. Biological Psychiatry. 1999;46(4):542–550. doi: 10.1016/S0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: A response-related fMRI study. Human Brain Mapping. 2004;23(4):200–209. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J, Merikangas K. The depressive spectrum: diagnostic classification and course. Journal of Affective Disorders. 1997;45(1):31–40. doi: 10.1016/s0165-0327(97)00057-8. [DOI] [PubMed] [Google Scholar]
- Argyle N. Skin conductance levels in panic disorder and depression. Journal of Nervous and Mental Disease. 1991;179(9):563–566. doi: 10.1097/00005053-199109000-00008. [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Ploubidis GB, Wright TB, Palm ME, Hood SD, Nash JR, Potokar JP. Development and validation of the Generalized Anxiety Disorder Inventory (GADI) Journal of Psychopharmacology. 2007;21(2):145–152. doi: 10.1177/0269881107069944. [DOI] [PubMed] [Google Scholar]
- Arrindell WA, Emmelkamp PMG, van der Ende J. Phobic dimensions: I. Reliability and generalizability across samples, gender, and nations: The Fear Survey Schedule (FSS-III) and Fear Questionnaire (FQ) Advances in Behavior Research and Therapy. 1984;6(4):207–254. doi: 10.1016/0146-6402(84)90001-8. [DOI] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. Madison, CT: International Universities Press; 1976. [Google Scholar]
- Beck AT, Rush J, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford; 1979. [Google Scholar]
- Beck R, Perkins TS, Holder R, Robbins M, Gray M, Allison SH. The cognitive and emotional phenomenology of depression and anxiety: Are worry and hopelessness the cognitive correlates of NA and PA? Cognitive Therapy and Research. 2001;25:829–838. doi: 10.1023/A:1012983726272. [DOI] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE. Regression diagnostics: Identifying influential data and sources of collinearity. Ipswich, MA: Wiley; 1980. [Google Scholar]
- Benning SD. Postauricular and superior auricular reflex modulation during emotional pictures and sounds. Psychophysiology. 2011;48(3):410–414. doi: 10.1111/j.1469-8986.2010.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Lang AR. Emotional modulation of the post-auricular reflex. Psychophysiology. 2004;41(3):426–432. doi: 10.1111/j.1469-8986.00160.x. [DOI] [PubMed] [Google Scholar]
- Benning SD, Rozalski V, Klingspon KL. Trait absorption is related to enhanced emotional picture processing and reduced processing of acoustic noise probes. Psychophysiology. 2015;52(10):1409–1415. doi: 10.1111/psyp.12468. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42(1):1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1(3):300–319. doi: 10.1037/1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Startle reflex modification: Emotion or attention? Psychophysiology. 1990;27(5):513–522. doi: 10.1111/j.1469-8986.1990.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50(1):74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JW, McGrath PJ, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41(9):939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. Event-related brain potentials in depression: Clinical, cognitive and neurophysiologic implications. The Oxford Handbook of Event-Related Potential Components. 2012:563–592. doi: 10.1093/oxfordhb/9780195374148.013.0257. [DOI] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event‐related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32(4):373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28(4):676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Taylor-Clift A, Rottenberg J. Emotional reactivity to daily events in major and minor depression. Journal of Abnormal Psychology. 2011;120(1):155–167. doi: 10.1037/a0021662. [DOI] [PubMed] [Google Scholar]
- Carney RM, Hong BA, O’Connell MF, Amado H. Facial electromyography as a predictor of treatment outcome in depression. British Journal of Psychiatry. 1981;138(6):485–489. doi: 10.1192/bjp.138.6.485. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Geisler MW. Mood effects on the ERP processing of emotional intensity in faces: A P3 investigation with depressed students. International Journal of Psychophysiology. 2006;60(1):27–33. doi: 10.1016/j.ijpsycho.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Leeka J. Diurnal variation in the positive affects. Motivation and Emotion. 1989;13(3):205–234. doi: 10.1007/BF00995536. [DOI] [Google Scholar]
- Clark LA, Watson D. General affective dispositions in physical and psychological health. In: Snyder CR, Forsyth DR, editors. Handbook of social and clinical psychology: The health perspective. New York: Pergamon Press; 1991a. pp. 221–245. [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991b;100(3):316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103(1):103–116. doi: 10.1037/0021-843X.103.1.103. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Cayley C, Wilson PH, Lovibond PF, Hartley C. Percentile norms and accompanying interval estimates from an Australian general adult population sample for self-report mood scales (BAI, BDI, CRSD, CES-D, DASS, DASS-21, STAI-X, STAI-Y, SRDS, and SRAS) The Australian Psychologist. 2011;46(1):3–14. doi: 10.1111/j.1742-9544.2010.00003.x. [DOI] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and maintenance of galvanic skin conductance responses: A functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20(8):3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. Journal of Affective Disorders. 2004;79(1):71–79. doi: 10.1016/s0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(2):95–111. doi: 10.1016/S030-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: A neurophysiological marker for emotional regulation in children. Journal of Child Psychology and Psychiatry. 2009;50(11):1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR. A two-dimensional threshold model of seasonal bipolar affective disorder. In: Magnusson D, Ohman A, editors. Psychopathology: An Interactional Perspective. San Diego, CA: Academic Press; 1987. pp. 95–123. [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90 – An outpatient psychiatric rating scale: Preliminary report. Psychopharmacology Bulletin. 1973;9(1):13–25. [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41(3):433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ. The chronometry of affective startle modulation in unipolar depression. Journal of Abnormal Psychology. 2008;117(1):1–15. doi: 10.1037/0021-843X.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diner BC, Holcomb PJ, Dykman RA. P300 in major depressive disorder. Psychiatry Research. 1985;15(3):175–184. doi: 10.1016/0165-1781(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Badawi M, Melton B. Prodromes and precursors: Epidemiologic data for primary prevention of disorders with slow onset. The American Journal of Psychiatry. 1995;152(7):967–972. doi: 10.1176/ajp.152.7.967. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58(2):342–353. doi: 10.1037/0022-3514.58.2.342. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM. The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychological Assessment. 2002;14:485–496. doi: 10.1037/1040-3590.14.4.485. [DOI] [PubMed] [Google Scholar]
- Fountoulakis KN, Iacovides A, Samoulis S, Kleanthous S, Kaprinis SG, St Kaprinis G, Bech P. Reliability, validity, and psychometric properties of the Zung depression rating scale. BMC Psychiatry. 2001;1 doi: 10.1186/1471-244X-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grin-Yatsenko VA, Baas I, Ponomarev VA, Kropotov JD. Electroencephalograms of patients with initial signs of depression: Independent components analysis. Human Physiology. 2011;37(1):39–48. doi: 10.1134/S0362119710051019. [DOI] [Google Scholar]
- Grossberg S. A neural theory of punishment and avoidance, II: Quantitative theory. Mathematical Biosciences. 1972;15(3):253–285. doi: 10.1016/0025-5564(72)90038-7. [DOI] [Google Scholar]
- Gruber J, Ovies C, Keltner D, Johnson SJ. A discrete emotions approach to positive emotion disturbance in depression. Cognition & Emotion. 2011;25(1):40–52. doi: 10.1080/02699931003615984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Positive emotionality at age P3 predicts cognitive styles in 7-year-old children. Development and Psychopathology. 2006;18(2):409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- Hess U, Sabourin G, Kleck RE. Postauricular and eyeblink startle responses to facial expressions. Psychophysiology. 2007;44(3):431–435. doi: 10.1111/j.1469-8986.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Lykken DT, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB. Electrodermal activity in euthymic unipolar and bipolar affective disorders: A possible marker for depression. Archives of General Psychiatry. 1983;40(5):557–565. doi: 10.1001/archpsyc.1983.01790050083010.. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive vulnerability to depression. New York: Guilford; 1998. [Google Scholar]
- Ingram RE, Siegle GJ, Steidtmann D. Methodological issues in the study of depression. In: Gotlib IH, Hammon CL, editors. Handbook of depression. 3rd. New York: Guilford; 2014. pp. 45–63. [Google Scholar]
- Jaworska N, Blier P, Fusee W, Knott V. Alpha power, alpha asymmetry, and anterior cingulate cortex activity in depressed males and females. Journal of Psychiatry Research. 2012;46(11):1483–1491. doi: 10.1016/j.psychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston V, Miller D, Burleson M. Multiple P3s to emotional stimuli and their theoretical significance. Psychophysiology. 1986;23(6):684–694. doi: 10.1111/j.1469-8986.1986.tb00694.x. [DOI] [PubMed] [Google Scholar]
- Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. Journal of Affective Disorders. 2004;83(1):21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53(8):1241–1255. doi: 10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke C, Nordby H, Hammerborg D, Hugdahl K, Erdmann G. Event-related potential (ERP) asymmetries to emotional stimuli in a visual half-field paradigm. Psychophysiology. 1997;34(4):414–426. doi: 10.1111/j.1469-8986.1997.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53(2):207–215. doi: 10.1111/j/11469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patters of affective response in dimensions of anxiety and depression. Emotion. 2007;7(1):182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Lenhart RE. Lowered skin conductance in a subsyndromal high-risk depressive sample: Response amplitudes versus tonic levels. Journal of Abnormal Psychology. 1985;94(4):649–652. doi: 10.1037/0021-843X.94.4.649. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biological Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109(2):345. doi: 10.1037/e323132004-004. [DOI] [PubMed] [Google Scholar]
- Lipman RS, Covi L, Shapiro AK. The Hopkins Symptom Checklist (HSCL): Factors derived from the HSCL-90. Journal of Affective Disorders. 1979;1:9–24. doi: 10.1016/0165-0327(79)90021-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A. Neural substrate of the late positive potential in emotional processing. Journal of Neuroscience. 2012;32(42):14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd. Sydney: Psychology Foundation; 1995. [Google Scholar]
- Malhi GS, Byrow Y, Bassett D, Boyce P, Hopwood M, Lyndon W, Murray G. Stimulants for depression: On the up and up? Australian and New Zealand Journal of Psychiatry. 2016;50(3):203–207. doi: 10.1177/0004867416634208. [DOI] [PubMed] [Google Scholar]
- Mardaga S, Hansenne M. Autonomic aspect of emotional response in depressed patients: Relationships with personality. Clinical Neurophysiology. 2009;39(4):209–216. doi: 10.1016/j.neucli.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli TG, Kane RT, Rees CS. Behavioral activation interventions for well-being: A meta-analysis. Journal of Positive Psychology. 2010;5(2):105–121. doi: 10.1080/17439760903569154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PB, Wilhelm K, Parker G, Austin MP, Rutgers P, Malhi GS. The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. Journal of Clinical Psychiatry. 2001;62(3):212–216. doi: 10.4088/JCP.v62n0314a. [DOI] [PubMed] [Google Scholar]
- Mneimne M, McDermut W, Powers AS. Affective ratings and startle modulation in people with nonclinical depression. Emotion. 2008;8(4):552–559. doi: 10.1037/a0012827. [DOI] [PubMed] [Google Scholar]
- Moran EK, Mehta N, Kring AM. Emotional responding in depression: Distinctions in the time course of emotion. Cognition and Emotion. 2012;26(7):1153–1175. doi: 10.1080/02699931.2011.638909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BH, Blysma LM, Rottenberg J. Does emotion predict the course of major depressive disorder? A review of prospective studies. British Journal of Clinical Psychology. 2009;48(3):255–273. doi: 10.1348/014466508X396549. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Charney DS. The symptoms of major depressive illness. American Journal of Psychiatry. 1981;138(1):1–13. doi: 10.1176/ajp.138.1.1. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Archives of General Psychiatry. 2000;57(1):14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25(1):1–22. doi: 10.1023/A:1026485530405. [DOI] [Google Scholar]
- Oliveau D, Willmuth R. Facial muscle electromyography in depressed and nondepressed hospitalized subjects: A partial replication. American Journal of Psychiatry. 1979;136:548–550. [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequiera H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba D, Angrilli A, Mini A. Visual evoked potentials, heart rate responses and memory to emotional pictorial stimuli. International Journal of Psychophysiology. 1997;27(1):55–67. doi: 10.1016/S0167-8760(97)00751-4. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037/1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Wenegrat BG, Ford JM, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. II. Dementia, depression and schizophrenia. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1984;59(2):104–124. doi: 10.1016/0168-5597(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biological Psychology. 1995;41(2):103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN. Depression and event-related potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology. 2015;4:110–113. doi: 10.1016/j.copsyc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F, Smets J, Nelis S, Schoofs H. Dampening of positive affect prospectively predicts depressive symptoms in non-clinical samples. Cognition & Emotion. 2012;26(1):75–82. doi: 10.1080/02699931.2011.555474. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Judd LL. Minor depressive disorder and subsyndromal depressive symptoms: functional impairment and response to treatment. Journal of Affective Disorders. 1998;48(2):227–232. doi: 10.1016/s0165-0327(97)00196-1. [DOI] [PubMed] [Google Scholar]
- Roth WT, Pfefferbaum A, Kelly AF, Berger PA, Kopell BS. Auditory event-related potentials in schizophrenia and depression. Psychiatry Research. 1981;4(2):199–212. doi: 10.1016/0165-1781(81)90023-8. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114(4):627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus accumbens dopamine and the forebrain circuitry involved in behavioral activation and effort-related decision making: Implications for understanding anergia and psychomotor slowing in depression. Current Psychiatry Reviews. 2006;2(2):267–280. doi: 10.2174/15734000776875914. [DOI] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive responding with the postauricular reflex. Psychophysiology. 2009;46(3):491–497. doi: 10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25(4):487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Fair PL, Mandel MR, Salt P, Mieske M, Klerman GL. Facial electromyography in the assessment of the improvement in depression. Psychosomatic Medicine. 1978;40:355–360. doi: 10.1097/00006842-197806000-00008. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Fair PL, Salt P, Mandel MR, Klerman GL. Facial expression and imagery in depression: An electromyographic study. Psychosomatic Medicine. 1976a;38(5):337–347. doi: 10.1097/00006842-197609000-00006. [DOI] [PubMed] [Google Scholar]
- Schwartz GE, Fair PL, Salt P, Mandel MR, Klerman GL. Facial muscle patterning to affective imagery in depressed and nondepressed subjects. Science. 1976b;192:489–491. doi: 10.1126/science.1257786. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17(1):85–98. doi: 10.1017/S0954579405050054. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49(7):624–636. doi: 10.1016/S0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/S0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Bradley MM, Dimoulas E, Lang PJ. Looking at facial expressions: Dysphoria and facial EMG. Biological Psychology. 2002;60(2):79–90. doi: 10.1016/S0301-0511(02)00044-3. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Sandt AR. Depressed mood and emotional responding. Biological Psychology. 2010;84(2):368–374. doi: 10.1016/j.biopsycho.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Storrie MC, Doerr HO, Johnson MH. Skin conductance characteristics of depressed subjects before and after therapeutic intervention. The Journal of Nervous and Mental Disease. 1981;169(3):176–179. doi: 10.1097/00005053-198103000-00004. [DOI] [PubMed] [Google Scholar]
- Taubitz LE, Robinson JS, Larson CL. Modulation of the startle reflex across time by unpleasant pictures distinguishes dysphoric from non-dysphoric women. International Journal of Psychophysiology. 2013;87(2):124–129. doi: 10.1016/j.ijpsycho.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R, Lovibond PF, Nicholas MK, Cayley C, Wilson PH. The utility of somatic items in the assessment of depression in patients with chronic pain: Comparison of the Zung Self-Rating Depression Scale and the Depression Anxiety Stress Scales in chronic pain and clinical and community samples. Clinical Journal of Pain. 2005;21:91–100. doi: 10.1097/00002508-200501000-00011. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale, NJ: Erlbaum; 1985. pp. 681–796. [Google Scholar]
- Tellegen A, Watson D, Clark LA. On the dimensional and hierarchical structure of affect. Psychological Science. 1999;10(4):297–303. doi: 10.1111/1467-9280.00157. [DOI] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the EEfRT? The Effort Expenditure for Rewards Task as an objective measure of motivation and anhedonia. PLoS ONE. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Welo EJ, Malone SM, Burwell SJ, Iacono WG. The effects of recurrent episodes of depression on startle responses. Psychophysiology. 2014;51(1):103–109. doi: 10.1111/psyp.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology. 2001;38(1):22–34. doi: 10.1111/1469-8986.3810022. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–491. doi: 10.1037/0021-843X.97.4.487. [DOI] [PubMed] [Google Scholar]
- Wagner HR, Burns BJ, Broadhead WE, Yarnall KSH, Sigmon A, Gaynes BN. Minor depression in family practice: functional morbidity, co-morbidity, service utilization and outcomes. Psychological Medicine. 2000;30(06):1377–1390. doi: 10.1017/s0033291799002998. [DOI] [PubMed] [Google Scholar]
- Ward NG, Doerr HO, Storrie MC. Skin conductance: A potentially sensitive test for depression. Psychiatry Research. 1983;10(4):295–302. doi: 10.1016/0165-1781(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Waters AM, Peters RM, Forrest KE, Zimmer-Gembeck M. Fear acquisition and extinction in offspring of mothers with anxiety and depressive disorders. Developmental Cognitive Neuroscience. 2014;7:30–42. doi: 10.1016/j.dcn.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin. 1984;96(3):465–490. doi: 10.1037/0033-2909.96.3.465. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Chmielewski M, McDade-Montez EA, Koffel E, Naragon K, Stuart S. Further validation of the IDAS: Evidence of convergent, discriminant, criterion, and incremental validity. Psychological Assessment. 2008;20(3):248–259. doi: 10.1037/a0012570. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Perlman G, Kotov R, Hajcak G. Depression and reduced neural response to images: Distinctions from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology. 2016;125(1):26–39. doi: 10.1037/abn0000118. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfit GH, Patrick CJ. Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social, Cognitive, and Affective Neuroscience. 2015;10(3):424–434. doi: 10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KB, Burnam MA, Rogers W, Hays R, Camp P. The course of depression in adult outpatients: results from the Medical Outcomes Study. Archives of General Psychiatry. 1992;49(10):788–794. doi: 10.1001/archpsyc.1992.01820100032007. [DOI] [PubMed] [Google Scholar]
- Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into depression research. Current Opinion in Psychology. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zung WWK. A self-rating depression scale. Archives of General Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- Zung WWK. From art to science: The diagnosis and treatment of depression. Archives of General Psychiatry. 1973;29:328–337. doi: 10.1001/archpsyc.1973.04200030026004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


