Abstract
Transcriptional silencing in Saccharomyces requires specific nucleosome modifications promoted in part by a complex of Sir proteins that binds to the modified nucleosomes. Recent evidence suggests that modifications of both the histone amino termini and the core domain of nucleosomes contribute to silencing. We previously identified histone H4 mutations affecting residues in the core of the nucleosome that yield enhanced silencing at telomeres. Here we show that enhanced silencing induced by these mutations increases the proportion of cells in which telomeres and silent mating-type loci are in the silent state. One H4 mutation affects the expression of a subset of genes whose expression is altered by deletion of HTZ1, which encodes the histone variant H2A.Z, suggesting that the mutation may antagonize H2A.Z incorporation into nucleosomes. A second mutation causes the spread of silencing into subtelomeric regions that are not normally silenced in wild-type cells. Mechanistically, this mutation does not significantly accelerate the formation of silent chromatin but, rather, reduces the rate of decay of the silenced state. We propose that these mutations use distinct mechanisms to affect the dynamic interplay between activation and repression at the boundary between active and silent chromatin.
The heritable features of an organism derive not only from the sequence of their chromosomal DNA but also from the way in which that DNA is packaged by chromatin. Certain regions of genomes are packaged in chromatin that both prevents expression of the underlying DNA and templates its own persistence over multiple cell divisions (and often multiple generations). Such chromatin-based epigenetic repression accounts for such diverse phenomena as X-chromosome inactivation and chromosomal imprinting in mammals, position effect variegation in Drosophila, and transcriptional silencing in fungi.
The budding yeast Saccharomyces cerevisiae exhibits three types of chromatin-based epigenetic repression: mating-type silencing, telomere position effect, and rRNA gene silencing (33). Mating-type genes, which, when present at an expressor locus MAT, determine the mating type of the cell, are permanently repressed when resident at either of two loci, HML or HMR, as a result of packaging in a repressive chromatin. Repression requires small cis-acting sites, or silencers, flanking the two loci and the activity of four genes, SIR1 to SIR4, that participate in encoding the chromatin covering these loci. Similarly, genes residing near telomeres or exogenous genes inserted near telomeres are on average more repressed than the same gene inserted at internal sites on the chromosome (11). Such genes exhibit a two-state behavior, persisting in either an active or inactive state over a number of generations before converting to the opposite state. This epigenetic repression requires telomeric sequences and the corresponding telomeric binding proteins, Ku and Rap1, as well as three of the four SIR genes (SIR2 to SIR4) required for mating-type silencing (22, 26). rDNA silencing is mechanistically related to these two phenomena in that Sir2 and a nucleolar protein, Net1, suppress the expression of RNA polymerase II-dependent genes inserted within the rRNA gene repeat (37, 40). While the mechanistic basis for rRNA gene silencing is less well understood, a simple model for telomere position effect and mating-type silencing is that protein complexes bound to telomeres and to silencers recruit the Sir2-Sir3-Sir4 complex, which both modifies and polymerizes along the chromatin to establish a repressive state (22, 34).
Several lines of evidence indicate that histones, and particularly histone modifications, play a critical role in transcriptional silencing in yeast. Mutations affecting amino terminal residues of histone H4 or histone H3 can abrogate telomeric position effect and mating-type silencing (17, 23, 30). The lysines within the amino termini of histones H3 and H4 are subject to reversible acetylation, and these residues are hypoacetylated in histones of chromatin at telomeres and at silent mating-type loci relative to those at transcriptionally active regions of the genome (5, 41). Sir2 possesses an NAD-dependent deacetylase activity, which probably accounts for hypoactylation of histone H3 and H4 tails at silent domains (14, 38, 42). Sir3 protein binds to peptides corresponding to histone H4 amino-terminal domain, more avidly to the unacetylated than to acetylated forms of the peptide (6). Thus, the Sir complex probably binds more tightly to deacetylated chromatin and the Sir complex bound to this chromatin maintains it in a deacetylated form. This positive-feedback system probably contributes to the epigenetic inheritance of the expression state of silenced chromatin.
Recent observations have implicated a second domain in the nucleosome that contributes to transcriptional silencing. Histone H3 lysine 79 lies on the upper and lower surfaces of the nucleosome core and is subject to methylation by the Dot1 methyltransferase (43). Most nucleosomes in active chromatin are methylated on this residue, while nucleosomes over silent domains are hypomethylated (28). Methylation at this site appears to diminish Sir complex binding to nucleosomes in vivo, and the presence of the Sir complex appears to exclude methylation of this site, another instance of positive feedback that could contribute to inheritance of the expression state of the chromatin. A recent structural analysis of calf thymus nucleosomes demonstrated that histone H4 K59, located on the surface of the nucleosome near H3 K79, is also methylated in vivo (45). As is true of H3 K79, a mutation that converts yeast H4 K59 to alanine reduces mating-type and telomeric silencing (43, 45). Additional genetic studies have highlighted the significance of this nucleosomal surface to transcriptional silencing. Park et al. (31) isolated a number of mutations affecting residues in this nucleosomal domain that abrogated silencing at all three classes of silent loci. This nucleosome domain could constitute an additional interaction site of the nucleosome with the Sir complex, it could participate in interactions between adjacent nucleosomes, or it could facilitate Sir-induced changes in the nucleosome that precludes transcriptional activity.
One of us previously reported the isolation of mutations affecting histone H3 or H4 that enhanced telomeric transcriptional silencing (36). Most of these mutations altered residues in the amino-terminal domains of the histones, and those in the histone H4 amino terminus generally resulted in reduced acetylation of lysine 12. A second class of mutations mapped to residues in the core of the nucleosome in close proximity to the H3 lysine residue subject to methylation and near mutations previously identified as diminishing transcriptional silencing. In this study, we have characterized the effects of mutations within the core of histone H4. These studies suggest that these mutations diminish the effective concentration of Sir proteins required to maintain transcriptional repression, in part by altering the abundance of histone variants in the nucleosome and in part by rendering silent chromatin resistant to decay.
MATERIALS AND METHODS
Plasmids and strains.
Plasmid pYXB10 was derived from pYXB1 (3) by replacing its BstBI fragment with the 1.94-kb AccI-ClaI fragment of the Escherichia coli lacZ sequence. pAR73 was constructed by inserting the SIR3 BamHI fragment of pKAN63 (15) into pUC12. pUC-SIR3 was constructed by ligating the SalI-SIR3-BamHI fragment of pAR73 to pUC19 digested with SalI and BamHI. pUC-sir3-8 was made by replacing the EcoNI-SIR3-XhoI fragment of pUC-SIR3 with the EcoNI-sir3-8-XhoI fragment from pSH135 (13). pUC-SK was made by ligating a BamHI-kanMX2-SacI fragment to pUC-sir3-8 digested with BamHI and ScaI. The kanMX2 module has been previously described (44). pXB001 was made by replacing the BglII fragment of pAR73 with the BamHI-SUP4-o-BamHI fragment from pMB21 (4). pYXB75 was constructed by replacing the NaeI-XhoI fragment of pUC26 (3) with a 950-bp EcoRV-SUP4-o-XhoI fragment. The NaeI-XhoI fragment of pUC26 is part of the BamHI-HML-BamHI fragment carried on pUC26. pDG1-D is a CEN-ARS-TRP1-HHF2 plasmid containing the genomic PstI-HHF2-HindIII fragment. pDG2-D to pDG6-D are identical to pDG1-D, except that they carry the A15T, H75Y, R39K, K12E, and L10P mutant alleles of HHF2, respectively (36). pADH-UCA-III has been described previously (11). Its EcoRI-URA3-TEL-VII-L-SalI fragment can be used to mark the left telomere of chromosome VII.
Strains used in this study are listed in Table 1. All strains were derived in an S288C background either from strain JPY12 (31) or from strain JBD4-7A, the latter being obtained from a cross between strains Y2047b (13) and PKY899 (17). The KpnI-Δhml::SUP4-o-XbaI fragment of plasmid pYXB75 was used to transform JBD4-7A to His+ to generate strain JBXB1. Strain JBXB1 was induced to lose its 2μm plasmid by growth on galactose-containing medium (13), resulting in strain YXB980. Strains YXB94 and YXB102 were constructed by transforming strain YXB980 to canavanine resistance with the BamHI fragment of plasmid pYXB10 or pYXB5 (3), respectively. Strains YXB94-1 to YXB94-6 were constructed by first transforming strain YXB94 to Trp+ with plasmids pDG1-D through pDG6-D and then eliminating the resident URA3-HHF2 plasmid by growth on 5-fluoroorotic acid (FOA). Strains YXB111-YXB116 were constructed in the same manner. Strains YXB111-T to YXB116-T were derived from YXB111 to YXB116, respectively, by transformation to Ura+ with pADH4-UCA-III (a gift from V. A. Zakian) digested with EcoRI and SalI.
TABLE 1.
Strains used in this study
| Strain | Genotype |
|---|---|
| JBD4-7A | aura3-52 ade2-101 can1-100 trp1Δ901 his3Δ200 his5-1 LEU2-GAL10p-FLP1 hhf1Δ::HIS3 hhf2Δ::LEU2 [CEN4 ARS1 URA3 HHF1] |
| YXB94 | JBD4-7A FRT-E-hml::β-I-FRT [cir0] |
| YXB94-1 | YXB94 [CEN4 ARS1 TRP1 HHF2] |
| YXB94-6 | YXB94 [CEN4 ARS1 TRP1 HHF2L10P] |
| YXB94-5 | YXB94 [CEN4 ARS1 TRP1 HHF2K12E] |
| YXB94-2 | YXB94 [CEN4 ARS1 TRP1 HHF2A15T] |
| YXB94-4 | YXB94 [CEN4 ARS1 TRP1 HHF2R39K] |
| YXB94-3 | YXB94 [CEN4 ARS1 TRP1 HHF2H75Y] |
| YXB102 | JBD4-7A E-FRT-hml::β-FRT-I [cir0] |
| YXB111 | YXB102 [CEN4 ARS1 TRP1 HHF2] |
| YXB116 | YXB102 [CEN4 ARS1 TRP1 HHF2L10P] |
| YXB115 | YXB102 [CEN4 ARS1 TRP1 HHF2K12E] |
| YXB112 | YXB102 [CEN4 ARS1 TRP1 HHF2A15T] |
| YXB114 | YXB102 [CEN4 ARS1 TRP1 HHF2R39K] |
| YXB113 | YXB102 [CEN4 ARS1 TRP1 HHF2H75Y] |
| YXB111-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2] |
| YXB116-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2L10P] |
| YXB115-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2K12E] |
| YXB112-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2A15T] |
| YXB114-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2R39K] |
| YXB113-T | YXB102 TelVIIL::URA3 [CEN4 ARS1 TRP1 HHF2H75Y] |
| JPY12 | ahis3Δ200 leu2Δ1 lys2Δ0 met15Δ0 ura3-167 trp1Δ63 ade2::hisG hht1-hhf1::natMX hht2-hhf2::hygMX RDN1::mURA3/HIS3 RDN1::Ty1-MET15 TELV::ADE2 [CEN4 ARS1 LYS2 HHT1 HHF1] |
| Y3194 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2] |
| Y3195 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2H75Y] |
| Y3197 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2L10P] |
| Y3198 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2K12E] |
| Y3199 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2A15T] |
| Y3200 | JPY12 [CEN4 ARS1 TRP1 HHT2 HHF2R39K] |
| Yex103 | JBD4-7A [CEN4 ARS1 TRP1 HHF2] |
| Yex105 | JBD4-7A [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex242 | JBD4-7A sir1Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2] |
| Yex245 | JBD4-7A sir1Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex126 | JBD4-7A sir4Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2] |
| Yex129 | JBD4-7A sir4Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2H75Y] |
| YXB94-1S | JBD4-7A FRT-E-hml::β-I-FRT sir3Δ::SUP4o [CEN4 ARS1 TRP1 HHF2] |
| Yex083 | JBD4-7A FRT-E-hml::β-I-FRT sir3-8 [CEN4 ARS1 TRP1 HHF2] |
| Yex256 | JBD4-7A hml::URA3 sir3-8 [CEN4 ARS1 TRP1 HHF2] |
| YXB94-3S | JBD4-7A FRT-E-hml::β-I-FRT sir3Δ::SUP4o [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex119 | JBD4-7A FRT-E-hml::β-I-FRT sir3-8 [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex258 | JBD4-7A hml::URA3 sir3-8 [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex257 | JBD4-7A hml::URA3 sir4Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2] |
| Yex261 | JBD4-7A hml::URA3 sir4Δ::KanMX4 [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex263 | JBD4-7A TELIIIL::URA3 (5.5 kb) [CEN4 ARS1 TRP1 HHF2] |
| Yex267 | JBD4-7A TELIIIL::URA3 (4.5 kb) [CEN4 ARS1 TRP1 HHF2] |
| Yex270 | JBD4-7A TELIIIL::URA3 (3.0 kb) [CEN4 ARS1 TRP1 HHF2] |
| Yex271 | JBD4-7A TELIIIL::URA3 (2.2 kb) [CEN4 ARS1 TRP1 HHF2] |
| Yex272 | JBD4-7A TELIIIL::URA3 (1.7 kb) [CEN4 ARS1 TRP1 HHF2] |
| Yex274 | JBD4-7A TELIIIL::URA3 (5.5 kb) [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex279 | JBD4-7A TELIIIL::URA3 (4.5 kb) [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex282 | JBD4-7A TELIIIL::URA3 (3.0 kb) [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex283 | JBD4-7A TELIIIL::URA3 (2.2 kb) [CEN4 ARS1 TRP1 HHF2H75Y] |
| Yex285 | JBD4-7A TELIIIL::URA3 (1.7 kb) [CEN4 ARS1 TRP1 HHF2H75Y] |
Strains Y3194 and Y3195 were constructed by transforming strain JPY12 to Trp+ with plasmid pMP3 or its H75Y allele derivative (36), respectively, followed by elimination of the resident plasmid by growth on α-aminoadipate (αAA; Sigma). Strains Yex103 and Yex105 were constructed from strain JBD4-7A by transformation to Trp+ with plasmids pDG1-D and pDG3-D, followed by elimination of the resident plasmid by growth on FOA. Strains Yex126 and Yex242 were derived from Yex103 by transformation to resistance to G418, using PCR products spanning the KanMX insertion in SIR1 or SIR4 from the haploid yeast deletion strain collection (Open Biosystems). Strains Yex129 and Yex245 were similarly derived from strain Yex105. Strains YXB94-1S and YXB94-3S were derived from strains YXB94-1 and YXB94-3 by transformation to His+ Ade+ with the BamHI-sir3::SUP4o-HindIII fragment of plasmid pXB001. Strains Yex083 and Yex119 were obtained from strains YXB94-1S and YXB94-3S in two steps by first transforming the strains to G418 resistance with Tth111I-digested pUC-SK DNA and then selecting for canavanine resistance and scoring for Ade− His−. Strains Yex256, Yex257, Yex258, and Yex261 were derived from strains Yex083, Yex126, Yex119, and Yex129, respectively, by transformation to Ura+ with EcoRI-HindIII digested pGJ8 DNA (4). Strains Yex263, Yex267, Yex269, Yex270, Yex271, and Yex272 were obtained from strain Yex103, and strains Yex274, Yex279, Yex281, Yex282, Yex283, and Yex285 were obtained from strain Yex105 by transformation to Ura+ with appropriately digested DNA from plasmids pXB165I, pXB167II, pXB175I, pXB176, pXB183, and pXB185 (2). The relevant genotypes of each strain were confirmed by Southern blotting.
Strains were grown on YEPD (1% yeast extract, 2% Bacto Peptone, 2% glucose) or synthetic complete (SC) medium lacking selected amino acids or nucleotide bases or containing FOA or αAA as required (16). Selection for the kanMX marker was performed on YEPD medium containing 200 μg of G418 per ml.
Microarray analysis.
Strains were grown at 30°C to 5 × 106 cells/ml in SC-Trp medium prior to harvesting by centrifugation. Cell pellets were lysed in TRI reagent (Molecular Research Center, Inc., Cincinnati, Ohio) by vortexing with glass beads for 3 mins. After a 5-min incubation at room temperature, 0.1 ml of BCP (1-bromo-3-chloropropane; Molecular Research Center, Inc.) per ml of TRI reagent was added and mixed well by shaking vigorously for 15 s. After centrifugation at 12,000 × g for 15 min at 4°C, the upper (aqueous) phase was removed and precipitated with equal volume of isopropanol. RNA pellets were washed with 75% ethanol, air dried, and dissolved in water. mRNA was purified from the total RNA with Oligotex (Qiagen, Valencia, Calif.).
First-strand cDNA was synthesized from mRNA by using high-performance liquid chromatography-purified T7-(dT)24 primer (Genset Corp, San Diego, Calif.) and superscript II reverse transcriptase RT (Invitrogen Corp, Carlsbad, Calif.). Second-strand cDNA was synthesized using DNA ligase (10 U), DNA polymerase I (40 U), and RNase H (2 U) from Invitrogen Corp. Biotin-labeled cRNA was made with the BioArray HighYield RNA transcript-labeling kit (Enzo Diagnostics, Farmingdale, N.Y.) and purified using an RNeasy mini kit (Qiagen). The cRNA was fragmented, mixed with control cRNA cocktail, and hybridized to yeast genome S98 array (Affymetrix Inc., Santa Clara, Calif.) for 16 h in a 45°C oven rotating at 60 rpm. The probe arrays were washed and stained using the GeneChip Fluidics station 400 (Affymetrix Inc.) and scanned at 570 nm with the Agilent GeneArray scanner (Affymetrix Inc.).
We used MicroArray Suite 5.0 software to determine whether the hybridization signal for a gene was reliable and incorporated in our analysis only the measurements that were judged present, which generally included greater than 90% of the gene measurements in any one sample, with greater than 80% of all genes yielding reliable values over all the experiments. All experiments were normalized to the same total signal intensity. All microarray data used in this study can be obtained at http://www.molbio.princeton.edu/labs/broach/microarray.htm.
Kinetic RT-PCR.
Quantification of mRNA levels for genes across chromosome III and for selected telomeric genes was obtained by kinetic reverse transcriptase (RT) PCR analysis as previously described (12). Strains were grown at 30°C to 5 × 106 cells/ml in SC-Trp medium before being harvested by centrifugation for RNA extraction.
Growth of yeast cultures and analysis of DNA circles excised from the HML locus.
Yeast strains were grown in YPR medium (1% yeast extract, 2% Bacto Peptone, 2% raffinose). When needed, galactose was added to YPR cultures at 2%. α-Factor, hydroxyurea, and nocodazole were used at 10 μg/ml, 0.2 M, and 10 μg/ml, respectively. Cells were considered to be in stationary phase when there was no increase in the optical density of the culture during the previous 24-h period and >95% of the cells were unbudded (1). Nucleic acid was isolated from yeast cultures by using the glass bead method (16) and fractionated on agarose gels in 0.5× TPE (45 mM Tris, 45 mM phosphate, 1 mM EDTA [pH 8.0]) supplemented with chloroquine. DNA circles were detected by Southern blotting.
RESULTS
Core mutations of histone H4 enhance Sir-dependent transcriptional repression.
A previously reported genetic screen identified mutants of histone H3 and H4 that were enhanced in telomere position effect (TPE) in a cac1 background, in which TPE is attenuated. Approximately 20% of cells in a cac1 strain in which URA3 had been inserted near the telomere at chromosome VII L are Ura+, due to weak position effect silencing of transcription of the inserted URA3 gene. Smith et al. (36) identified mutations of the histone H3 or H4 gene that decreased the number of Ura+ cells in cultures of such a strain at least 20-fold. Most of the mutations affected residues in the amino-terminal domain of histone H3 or H4, and those in amino-terminal residues of histone H4 noticeably reduced the level of acetylation of lysine 12. Since hypoacetylation of histone H3 and H4 amino termini correlate with silencing, the effect of the mutations on acetylation probably accounts for the observed increase in silencing. In addition to mutations in the amino-terminal domain, the screen returned mutations affecting residues in the core of the nucleosome. These included mutation of histone H3 D77 (to A, G, N, or V) or D81 (to G) or histone H4 R39 (to K) or H75 (to Y). These residues are well removed from the amino-terminal tails and so probably affect silencing in a distinct manner. Accordingly, we have examined the mechanistic basis for the effect of these mutations on silencing.
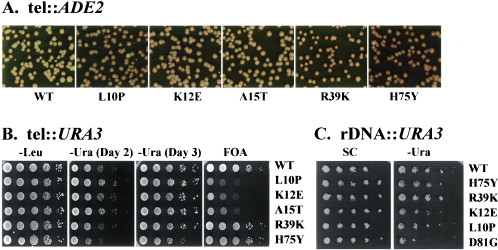
Since the mutants were isolated on the basis of their effect on telomere silencing, we were interested in determining whether these mutations affected other modes of silencing. Accordingly, we examined silencing at mating-type loci and at rRNA genes in the mutant background in addition to the effects on telomeric silencing. As evident from the data in Fig. 1A and as previously reported, all the mutant HHF2 alleles examined yielded enhanced the repression of ADE2 inserted next to the telomere on chromosome V, with the core mutations, HHF2H75Y and HHF2R39K, exhibiting greater enhancement of TPE than the tail mutations. However, the three tail mutations did not exhibit increased TPE when assayed for repression of URA3. The HHF2L10P, HHF2K12E, and HHF2A15T mutations exhibited increased proportions of Ura+ cells and decreased proportions of FOA-resistant cells in a CAC1 strain carrying URA3 inserted at the telomere of chromosome VII L. In contrast, the H4 core mutant HHF2H75Y exhibited an approximate 10-fold reduction in the proportion of Ura+ cells in the same background and HHF2R39K had a modest effect (Fig. 1B). Thus, the core mutants had a significant effect on TPE while the effect of the tail mutants depended on strain background and assay conditions.
FIG. 1.
A mutation in the histone H4 core domain enhances silencing at telomeres but not at rRNA genes. (A) Photographs of colonies from CAC1 ade2 cells carrying ADE2 integrated adjacent to the V R telomere and the indicated HHF2 allele after growth on YEPD plates for 3 days at 30°C followed by incubation at 4°C for 1 week. Reduced expression of ADE2 results in accumulation of red pigment in cells grown on this medium. (B) CAC1 ura3 strains carrying URA3 integrated adjacent to the VII L telomere and the indicated HHF2 allele encoding the sole histone H4 in the cell (strains YXB111-T to YXB116-T) were serially diluted on SC medium lacking the indicated nutrient or containing FOA. Growth is shown after 3 days at 30°C except as indicated. (C) CAC1 ura3 strains carrying URA3 integrated in the rRNA gene locus and the indicated HHF2 allele as the sole histone H4 or H3 (last line) in the cell (same strains as in A) were serially diluted on SC and SC-Ura medium. Growth is shown after 3 days at 30°C.
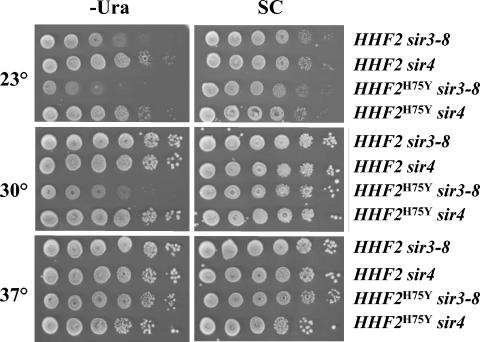
The core mutants also affected silencing at the storage mating-type locus, HML. Expression of α mating-type information at HML renders MATa cells incapable of mating with MATα cells. In a wild-type background, silencing at HML is sufficiently stringent such that all MATa cells are capable of mating. Since an increase in HML silencing would not be observable in a wild-type background, we examined the effects of histone mutations in a sir1 background, in which the efficiency of maintaining silencing is reduced. As is evident in Table 2, the efficiency of mating of the sir1 strain is approximately half that of wild type, due to derepression of HML in many cells (32). The presence of the HHF2H75Y mutation enhanced HML silencing in the sir1 background such that the efficiency of mating was as high as that of wild-type cells. However, the HHF2H75Y mutation was not capable of bypassing the requirement for an active Sir complex, in that the mutation failed to restore mating competence to a sir4 strain. To confirm the effects of histone mutations on HML silencing, we examined the silencing of a reporter gene, URA3, inserted at HML in a strain carrying a temperature-sensitive mutation in SIR3. As is evident in Fig. 2, URA3 inserted at HML in a sir3-8 background is repressed at 23°C and fully derepressed at 30°C, due to a substantial reduction in the amount of Sir3 protein in the cell at the elevated temperature (39). Introduction of the HHF2H75Y mutation fully restored the silencing of URA3 at 30°C, although at 37°C the locus was derepressed in both the wild-type and HHF2H75Y strains. Similarly, HHF2H75Y allele failed to restore silencing to strains carrying deletion of sir4: HHF2H75Y neither restored mating competence to a MATa HMLα sir4 strain nor suppressed URA3 expression in an HML::URA3 sir4 strain. Accordingly, we conclude that the mutant histone cannot exert transcriptional silencing in the absence of Sir proteins but can achieve complete silencing with substantially reduced level of Sir3. We find a similar dependence on an intact silencing apparatus for the enhanced silencing effects of HHF2R39K (data not shown).
TABLE 2.
HHF2H75Y enhances silencing at silent mating-type locia
| Gene | No. of diploids (mating efficiency)b
|
||
|---|---|---|---|
| SIR1 SIR4 | sir1 | sir4 | |
| HHF2 | 0.53 (1.0) | 0.37 (0.70) | <10−4 (0) |
| HHF2H75Y | 0.46 (0.88) | 0.65 (1.2) | <10−4 (0) |
MATa test cells of the indicated HHF2 and SIR genotype were mixed with MATα tester cells (17α) and allowed to form diploids. The number of initial MATa cells and the number of diploids formed were determined by plating on YEPD and SD, respectively. More than 104 cells were tested for each strain.
The number of diploids as a fraction of the initial number of MATa haploids is presented, with the mating efficiency relative to the isogenic HHF2 SIR1 SIR4 strain shown in parentheses.
FIG. 2.
HHF2H75Y enhances but does not bypass Sir protein function. Strains carrying URA3 integrated at HML, the indicated HHF2 allele, and either sir3-8 or a deletion of SIR4 (strains Yex256, Yex257, Yex258, and Yex261) were serially diluted on SC-Ura or SC plates and grown at the indicated temperature for 3 days.
We addressed whether core histone mutations affected silencing at the rRNA gene locus by examining the expression of various reporter genes inserted in the rRNA gene locus in wild-type and mutant histone backgrounds. URA3 inserted within the rDNA cluster exhibits reduced expression due to Sir2-dependent silencing of RNA polymerase II transcribed genes resident in the rDNA cluster, an effect observed in our strain. However, this repression was not altered in strains carrying either the HHF2H75Y or the HHF2R39K mutation (Fig. 1C). Similarly, we found that expression of MET15 inserted in the rRNA gene locus in this strain showed no difference in expression in wild-type versus HHF2H75Y mutant background (data not shown). Thus, although the HHF2H75Y and HHF2R39K mutations promote enhanced TPE and mating-type silencing, the mutation does not alter rDNA silencing, even in the same strain in which these other effects are manifest.
We asked whether the HHF2H75Y allele affected silencing indirectly through alteration in the gene expression of components of the silencing apparatus. To do so, we performed RT-PCR analysis to measure transcript levels of a number of genes whose products contribute to silencing. As is evident in Table 3, the transcript levels for HMLα2, HHF2, SIR3, and SIR4 are unchanged or increased by less than 50% in an HHF2H75Y strain relative to an isogenic HHF2 strain. As a control, we found that increasing the gene dosage of HHF2, SIR2, SIR3, or SIR4 by a factor of 2 had no detectable effect on repression of ADE2 inserted next to chromosome V telomere. Thus, the effects of this mutation on repression probably do not involve changes in levels of the silencing machinery.
TABLE 3.
HHF2H75Y does not affect the expression of silencer proteins
| Gene | Relative expressiona |
|---|---|
| ACT1 | 1.0 |
| HMLα2 | 1.6 |
| HHF2 | 1.4 |
| SIR3 | 1.16 |
| SIR4 | 1.2 |
Expression levels were determined by RT-PCR as described in Materials and Methods and are presented as the expression level in HHF2H75Y relative to that in an isogenic HHF2 strain, normalized in both strains to the level of expression of ACT1.
The HHF2H75Y and HHF2R39K alleles disproportionately affect telomere-proximal genes.
To address whether HHF2 core mutations affect transcription in general or specifically enhance silencing, we examined global expression changes in strains carrying the HHF2H75Y or HHF2R39K mutation. RNA was prepared from HHF2, HHF2H75Y, and HHF2R39K strains, labeled, and hybridized to Affymetrix chips containing oligonucleotides corresponding to most yeast genes. Analysis of data from three separate experiments is presented in Table 4. Most genes were expressed at essentially the same level in all three strains. Twenty-seven genes were consistently expressed in the HHF2H75Y strain at levels at least twofold lower than in the wild-type strain, while no genes were consistently increased in their expression. A small set of genes was consistently repressed in the HHF2R39K strain. However, in this strain a significant number of genes exhibited enhanced expression relative to the wild type. Except for two gene, YOL162W and YOL164W, which were activated in the HHF2R39K strain and repressed in the HHF2H75Y strain, all the genes whose expression was affected by HHF2R39K were unaffected by HHF2H75Y and vice versa. Thus, HHF2H75Y and HHF2R39K affect different genes and elicit distinct transcriptional responses. HHF2R39K caused both repression and derepression, while the HHF2H75Y allele provoked only increased repression and both mutations affected only a limited number of genes.
TABLE 4.
Histone core mutations predominantly affect the expression of telomere-proximal genesa
| Gene | No. of genesc
|
||||
|---|---|---|---|---|---|
| Totalb |
HHF2H75Y
|
HHF2R39K
|
|||
| Repressed | Induced | Repressed | Induced | ||
| All | 5,149 | 27 | 0 | 23 | 130 |
| Telomere proximal | 158 | 25 | 0 | 6 | 16 |
| −Log Pd | 151 | NAe | 9.8 | 9.0 | |
RNA samples prepared from isogenic HHF2, HHF2H75Y, and HHF2R39K strains were hybridized in three separate experiments to yeast genome S98 arrays and expression levels were determined as described in Materials and Methods.
The total number of unique genes determined to be expressed at a detectable level in wild-type cells as determined by Affymetrix software is indicated (All), as is the number of those expressed genes that reside within 20 kb of a telomere (telomere proximal).
The number of genes whose expression level in the HHF2H75Y or HHF2R39K strain was at least twofold lower (Repressed) or twofold higher (Induced) than that in the HHF2 strain in all experiments is indicated, as is the number of those repressed or induced genes that reside within 20 kb of a telomere.
The negative log of the probability that the concentration of repressed or induced genes in the subtelomeric regions occurred by chance. R2 values for expression data for all genes from different experiments with the same strain were all greater than 0.95.
NA, not applicable.
As noted in Table 4, a disproportionate number of genes affected by the core mutations reside within 20 kb of telomeres. Essentially all the genes exhibiting reduced expression in the HHF2H75Y strain are telomeric. In contrast, the majority of genes affected by HHF2R39K are not located near telomeres, although even in this strain more telomeric genes were affected than would be predicted by chance. These results reinforce the distinction between the effects of the two core mutations, suggesting that they affect silencing through distinct mechanisms. Regardless of the mechanism, though, both mutations affected a disproportionate number of telomeric genes.
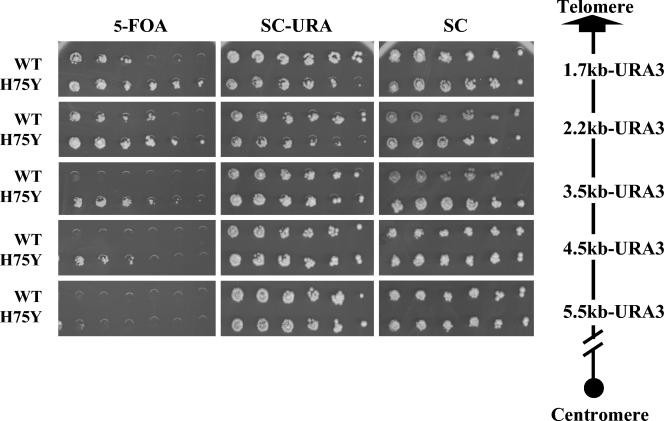
One difficulty with the Affymetrix microarray analysis is that many genes near telomeres are normally expressed at relatively low levels, rendering measurements of further repression of such genes beyond the limits of detection by hybridization to an Affymetrix chip. Accordingly, we used two additional methods to determine whether genes near telomeres were significantly affected by the HHF2H75Y mutation. In one experiment, we inserted URA3 at different distances from the left arm of chromosome III and then examined the repression of the inserted locus in isogenic wild-type and HHF2H75Y strains. As is evident from the results in Fig. 3, the HHF2H75Y mutation resulted in increased repression up to 5.5 kb from the end of the chromosome but had no apparent effect beyond that. Thus, the mutation appears to enhance the level of repression within a gradient extending inward from the end of the chromosome, although repression did not extend significantly beyond that region.
FIG. 3.
HHF2H75Y enhances silencing in a gradient extending inward from a telomere. Strains containing URA3 integrated at the indicated distance from the right end of chromosome III and carrying either HHF2 or HHF2H75Y (see Table 1) were serially diluted onto SC medium with or without uracil or containing FOA. Growth is shown after 3 days at 30°C.
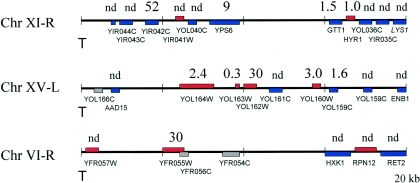
In a second series of experiments, we used RT-PCR to obtain more precise measurements of transcript levels from genes near the ends of several chromosomes and entirely across chromosome III. First, we analyzed the expression of genes resident near the telomeres of chromosomes 6, 11, and 15, regions shown by Affymetrix analysis to be affected by the HHF2H75Y allele. As is evident from the results presented in Fig. 4, six of seven of the genes in these regions identified by Affymetrix hybridization as being significantly repressed in the HHF2H75Y strain relative to the wild type exhibited reduced transcription by RT-PCR. This repression depended on a functional silencing apparatus, since the levels of expression of all the genes analyzed were essentially identical (less than 50% difference in levels) in a sir4 HHF2 strain and in an isogenic sir4 HHF2H75Y strain (data not shown). Thus, HHF2H75Y-enhanced repression is mediated through Sir-dependent silencing.
FIG. 4.
HHF2H75Y represses the expression of telomere-adjacent genes. Diagrammed are 20 kb of telomere-proximal regions of the indicated chromosome (telomere positioned on the left), on which are located all the identified open reading frames (those on the Watson strand in blue, those on the Crick strand in red, and questionable genes in gray) along with their gene designations. The number above each gene represents the ratio of mRNA levels for that gene in strain Yex103 (HHF2) versus that in Yex105 (HHF2H75Y) as determined by RT-PCR (see Materials and Methods). nd, not determined.
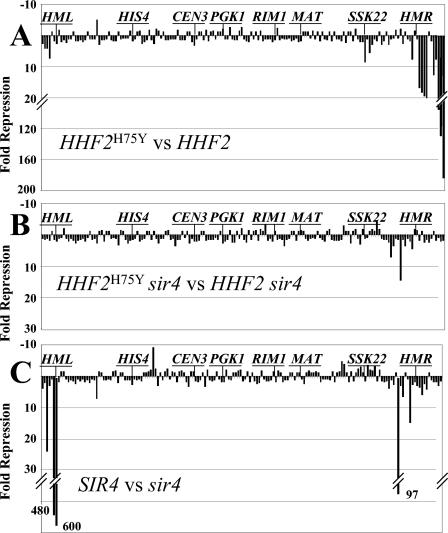
To obtain a more detailed picture of the global transcriptional effects of the HHF2H75Y allele, we measured the expression of every gene on chromosome III in isogenic HHF2H75Y and wild-type strains by RT-PCR. The results of this analysis are presented in Fig. 5A as the expression of each gene in the HHF2H75Y strain relative to that in the isogenic wild-type strain. As is evident, few genes in the interior of the chromosome were affected by the mutation. Only two internal genes (SSK22 and ERS1, respectively 74 and 70 kb from the right end) were repressed by more than threefold in the mutant and only one gene (YCL046) was induced by more than threefold. In contrast, genes near the ends of the chromosome were substantially repressed in the HHF2H75Y background relative to the wild type. All four genes adjacent to the left end of the chromosome were repressed three- to eightfold in the mutant background. More dramatically, of the 13 genes adjacent to the right end of the chromosome (extending from the end of the chromosome to one gene beyond HMR), 9 were repressed between 8- and 175-fold, 2 (HMRa1 and HMRa2) could not be measured (due to expression from MATa1 and MATa2 in the cell), and 1 was a pseudogene (YCL102W-A-A). Thus, only one gene in this 26-kb telomere-proximal domain was not repressed by the presence of the HHF2 mutation. All of the repression exerted by HHF2H75Y results from enhanced Sir-dependent silencing: deletion of SIR4 from the isogenic HHF2 and HHF2H75Y strains completely eliminated differential repression by the mutation, not only in the subtelomeric domains but also at SSK22 and ERS1 (Fig. 5B). From these results, we conclude that the HHF2H75Y allele stimulates the Sir-dependent silencing predominantly, if not exclusively, of genes located adjacent to telomeres.
FIG. 5.
HHF2H75Y predominantly enhances Sir-dependent repression of telomere adjacent genes on chromosome III. The relative mRNA levels were determined by RT-PCR for every detectable gene on chromosome III in four strains: HHF2 SIR4 (Yex103), HHF2H75Y SIR4 (Yex105), HHF2 sir4 (Yex126), and HHF2H75Y sir4 (Yex129). The ratios of mRNA expression for every gene in pairs of strains (presented as the fold repression of expression in the first strain relative to that in the second) are plotted as a function of the relative position of each gene along chromosome III from the left arm (left) to the right arm. The positions of several specific genes are noted on the graphs. (A) Yex105 versus Yex103; (B) Yex129 versus Yex126; (C) Yex103 versus Yex126.
To address whether the HHF2H75Y allele caused de novo silencing of genes or simply enhanced existing silencing, we examined whether genes affected by the HHF2H75Y mutation on chromosome III were subject to Sir-dependent silencing in the absence of HHF2H75Y. To do so, we measured the expression of all genes on chromosome III in isogenic SIR4 and sir4 strains. The results of this analysis are shown in Fig. 5C. As expected, mutation of SIR4 resulted in substantial derepression of HML (HMR derepression could not be measured due to expression from MATa). However, few other genes were affected. The five genes bracketing HMR were derepressed 3- to 15-fold by inactivation of SIR4, and the four genes adjacent to the telomere on the left arm of chromosome III were derepressed 2- to 24-fold. In addition, one gene (YCR090C) was derepressed almost 100-fold by deletion of SIR4, although the expression of this gene in wild-type cells was so low (two orders of magnitude below the repressed HML locus) as to render the relative measurement unreliable. Reciprocally, the five genes immediately adjacent to the right end of the chromosome that were significantly repressed by the HHF2H75Y allele were not affected by deletion of SIR4. Thus, most of the genes affected by the HHF2H75Y mutation lay adjacent to silent domains but were not subject to Sir-dependent repression in the wild-type background. Rather, the HHF2H75Y allele predominantly extends existing silent domains into adjacent regions not previously subject to silencing.
The HHF2H75Y allele affects maintenance but not establishment of silent chromatin.
We probed the effects of histone H4 mutants on the structure and dynamics of silent chromatin by using a topological assay. We previously showed that heterochromatin induced by the silencing apparatus imposed a different topology on DNA across the HML locus than does chromatin associated with the active form of the locus. Specifically, heterochromatin caused an increase in the negative superhelical density of a circular DNA molecule obtained by in vivo excision of the HML locus from the chromosome of a SIR+ versus a sir strain, which reflects either an increased nucleosome density across the locus in the SIR+ background or an increase in the extent of DNA wrapping around individual nucleosomes within the locus (3, 7). We further showed that circles excised from the HML locus that spanned both the coding region and associated silencers maintained the silent state indefinitely during cell growth whereas excised HML circles lacking their silencers lost the silent state as cells progress through the cell cycle (3).
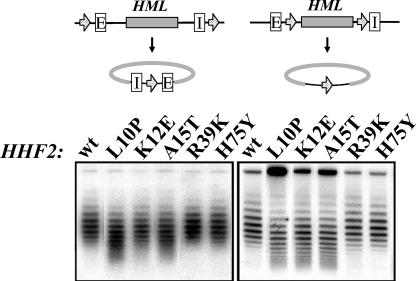
We applied this DNA topology assay to analyze the effects of specific histone mutations on the structure of silent chromatin. We constructed strains carrying HML bracketed by FRT recombination sites (positioned to either include or exclude the silencers following excision [Fig. 6 ]), the FLP recombinase under the control of the galactose-inducible GAL10 promoter, and either the wild-type HHF2 allele or one of the mutant alleles. These strains were grown to mid-log phase and induced to excise HML by addition of galactose. DNA isolated from these strains 2 h after galactose addition was fractionated on chloroquine-agarose gels and probed for HML DNA. As is evident from the results of this analysis presented in Fig. 6, all three histone tail mutants destabilized the silent chromatin on HML circles lacking silencers and two of the three caused a slight decondensation of HML-circles bearing silencers. This decondensation was not observed for circles excised from cells in stationary phase (Fig. 7), suggesting that decondensation resulted only during cell cycle progression, a conclusion confirmed by the results presented below. In contrast, HML circle excised from strains containing the wild-type HHF2 allele or HHF2H75Y or HHF2R39K alleles exhibited identical topological profiles. Thus, these two mutations did not affect either nucleosome density or the extent of DNA wrapping around nucleosome across the silenced HML locus. Similarly, the topologies of HML circles isolated from wild-type and mutant strains in a sir4 background (data not shown) were identical. Thus, the mutations do not affect chromatin structure over active genes. These data on the stability of silent chromatin as assayed by DNA topology correlated well with silencing as assayed by telomere position effect, with histone tail mutants generally destabilizing silencing by either assay while the two core mutants maintained silencing. Further, the enhanced silencing obtained with HHF2H75Y is not a consequence of measurable differences in the structure or topology of the silent or active chromatin.
FIG. 6.
Silencing-enhancing histone H4 substitutions in the amino-terminal tail but not in the core-destabilize silent chromatin. (Top) The HML alleles in strains analyzed below. The E and I silencers are indicated. Arrows denote the position and orientation of FRT sites. Flp1-induced recombination results in excision in vivo of the diagrammed circular DNA containing HML. (Bottom) Strains carrying the HML allele indicated at the top of the figure and the HHF2 allele indicated above each lane were grown to early log phase in YPR medium. The cultures were then incubated in the presence of galactose for 2.5 h before the cells were collected for DNA isolation. DNA was fractionated by agarose gel electrophoresis in the presence of 18 μg of chloroquine per ml. (Left) Strains YXB94-1 to YXB94-6. (Right) strains YXB111 to YXB116. wt, wild type.
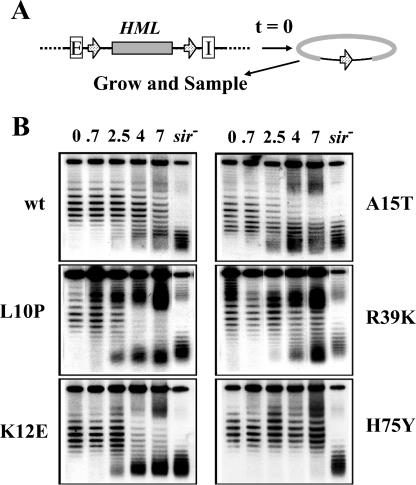
FIG. 7.
HHF2H75Y increases the stability of silent chromatin. (A) Experimental design. Strains carrying the HHF2 allele indicated in panel B and the diagrammed HML locus were grown to stationary phase and then induced to excise a circular DNA carrying HML without associated silencers. The strains were then diluted into fresh media, and samples were removed at various times following growth of the culture: (B). DNA samples from cells removed from cultures at the indicated times were fractionated by agarose gel electrophoresis in the presence of 18 μg of chloroquine per ml. DNA from stationary-phase cells of the sir4 derivative of each of the strains was included in the final lane of each gel (sir). wt, wild type.
To address more precisely the process affected by histone mutants, we examined the dynamics of silent chromatin formation and persistence at the silent loci in mutant versus wild-type cells. Each strain was grown to stationary phase, and HML circles were excised. The cells were then shifted to fresh synthetic medium, and the fate of the HML circles was analyzed during subsequent cell growth. In the wild-type strain, as expected, silencer-free HML circles gradually converted from the silent state to the active state (indicated by the decrease in the number of topoiosomers with high negative supercoiling and the increase in the number of topoisomeres with lower negative supercoiling) (Fig. 7B). The three mutations affecting the histone tail all accelerated this process, while the HHF2R39K mutation had little affect on this decay process. In contrast, the HHF2H75Y mutation significantly delayed the decondensation of silent chromatin on the silencer-free circle. At 7 h of growth, when half of the excised HML circles had converted to the decondensed state in wild-type cells, essentially no conversion of the excised circles to the decondensed state had occurred in the HHF2H75Y strain. Even after 20 h, no decondensed circles were evident in the HHF2H75Y strain (data not shown). Thus, the presence of the mutant histone delayed the loss of silencing following separation of the silent locus from its silencers.
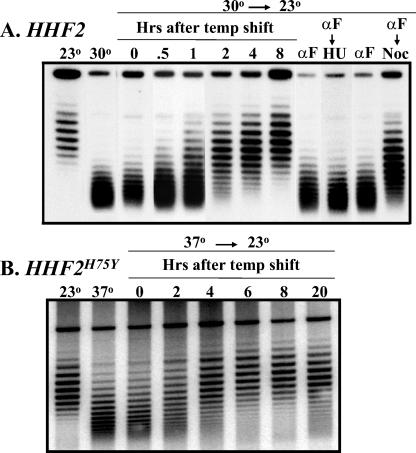
We also examined the establishment of silencing by monitoring the topological state of silencer-bearing HML circles following reactivation of the silencing machinery in a sir3 temperature-sensitive strain. Excision of a silencer-bearing HML circle from a sir3-8 HHF2 strain grown at 30°C yielded circles with the lower supercoiled density characteristic of active chromatin. Growing this strain with the decondensed excised circle at 23°C led to the gradual acquisition of the increased supercoil density characteristic of the silenced state (Fig. 8A). Thus, silencing could be established across an extrachromosomal HML locus following reactivation of the silent apparatus by shifting a sir3 temperature-sensitive strain to the permissive temperature. Establishment required the presence of silencers on the circles (data not shown) and progression through the cell cycle. Cells with excised circles arrested at G1 by α-factor and then shifted to the permissive temperature in the presence of α-factor failed to establish silencing of the locus on the circle. Furthermore, as previously shown in a different experimental paradigm, progress from G1 to S phase was insufficient for establishment but progression from G1 to mitosis allowed establishment (10, 18, 21, 25). Thus, monitoring the topological state of an excised silencer-bearing circle following a shift of a sir3 temperature-sensitive strain provides a means of monitoring the kinetics of establishment of silencing.
FIG. 8.
HHF2H75Y does not affect the rate of establishment of silencing. Strains carrying HHF2 (A) or HHF2H75Y (B) and the HML allele diagrammed in the upper left section of Fig. 6 were grown at 23°C (left lane) or at an elevated temperature (next to left lane) in SC medium and shifted to galactose medium to induce excision of the HML circle. In addition, cells were grown at the indicated elevated temperature, induced to excise the HML circle, shifted to 23°C, and sampled at the indicated times (lanes 0 to 8). Finally, cells were grown at the elevated temperature and arrested by addition of α-factor (αF). They were then induced to excise the HML circle and, after removal of a sample for analysis (lanes αF in panel A) were shifted to media lacking α-factor but containing either hydroxyurea (HU) or nocodazole (Noc). DNA was isolated from all samples and fractionated by agarose gel electrophoresis in the presence of 18 μg of chloroquine per ml.
We performed the same experiment with a HHF2H75Y strain. The strain was pregrown at 37°C, the silencer-bearing HML circle was excised, and the culture was shifted to 23°C. As is evident in Fig. 8, the kinetics for establishment of silencing in this mutant background was essentially identical to that seen with wild-type cells. In addition, the requirement for passage through S phase was retained in the mutant strain (data not shown). Thus, while HHF2H75Y reduces the rate of decay of silent chromatin, it does not affect the rate of establishment.
DISCUSSION
We have demonstrated that mutations affecting residues in the core domain of histone H4 can enhance Sir-dependent transcriptional silencing at silent mating-type loci and telomeres. Previous analysis of mutations isolated in this screen for histone alleles with enhanced silencing focused on mutations causing amino acid changes in the amino-terminal domains of H3 and H4. The H4 amino-terminal mutations isolated in this screen were associated with significantly lowered levels of lysine 12 acetylation in vivo, suggesting that predisposing nucleosomes to this hypoacetylated state enhanced the likelihood that telomeric nucleosomes would assume a silenced configuration. We found that the core mutations enhance silencing to a significantly greater extent and in more contexts than do the tail mutations, although both sets of mutations primarily promoted enhanced silencing of subtelomeric regions of the genome (36). Neither set of the mutations bypasses the requirement for Sir proteins for silencing; rather, the core mutations appear to allow a limited number of silencing proteins to silence more efficiently and more extensively.
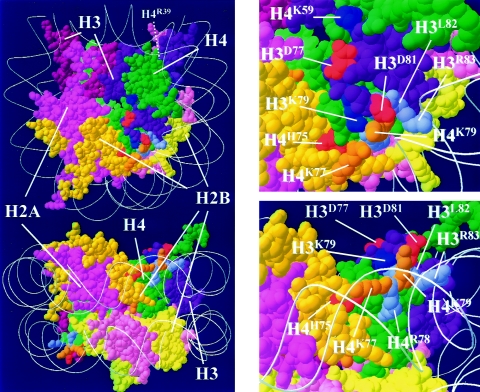
The histone H4 mutations characterized in this study join a list of mutations within the nucleosome core domain that influence silencing (Fig. 9). However, these mutations define at least three distinct domains. Histone H3 residues D77 and D81 (red residues on histone H3 in Fig. 9), whose mutation can lead to enhanced silencing (36), lie on the surface of the nucleosome contiguous to residue H3K79 (dark blue), methylation of which by the Dot1 methyltransferase probably diminishes the association of Sir proteins with nucleosomes. Mutation of the Dot1 methylation site, HHT1K79A, reduces telomeric and mating-locus silencing substantially and rRNA gene silencing to a significantly lesser extent, presumably due to redistribution of Sir proteins to other sites in the genome (43). A second residue in this vicinity, H4K59, is also subject to methylation, at least in nucleosomes isolated from bovine thymus, and mutation of the residue to alanine in yeast reduces silencing at telomeres and silent mating-type loci (45). Two other lysine residues in this region of the nucleosome, H4K77 and H4K79 (orange in Fig. 9), are subject to acetylation, at least in nucleosomes from bovine thymus (45). Mutation of H4K79 as well as those indicated in light blue in Fig. 9 cause diminished silencing at all three silent domains: telomeres, mating-type loci, and rDNA (31). These residues, while near H3K79, are less exposed to the surface of the nucleosome; rather, they comprise a contact domain with the adjacent DNA. Finally, residue H4H75, whose mutation to tyrosine causes enhanced silencing at telomeres and silent mating-type loci but not at rDNA, lies buried within the nucleosomal core, at the interface between histones H4 and H2B. Similarly, H4R39 also lies at the interior of the nucleosome, at the interphase between histones H3 and H4 (not visible in Fig. 9). Thus, several residues within the nucleosomal core affect transcriptional silencing, although they fall into at least three discrete domains on the basis both of their positions within the nucleosome and their spectrum of effects on different modes of silencing.
FIG. 9.
Mutations in the nucleosome core affect silencing. Solid rendering of the Van der Waal radii of the yeast nucleosomal core with amino acid side chains and with encircling DNA represented as a ribbon trace of the phosphodiester backbone. Molecular color scheme: H2A, pink; H2B, yellow; H3, purple; H4, green; residues subject to methylation, dark blue; residues homologous to those acetylated in bovine nucleosomes, orange; residues whose mutation enhance silencing, red; residues whose mutation diminish silencing, light blue.
In the nucleosome, the histone core complex is maintained as an octamer by two types of protein-protein interactions. One type is the strong interaction between H3 and H4 within the tetramer [H3-H4]2 and between H2A and H2B within the two dimer subunits [H2A-H2B]. The other type is the weaker interaction between the [H3-H4]2 tetramer and the two [H2A-H2B] dimer subunits. The interfaces between the centrally located tetramer and the flanking dimers are formed from both fold and nonfold elements and contain a number of tyrosine residues in two distinct groupings. In the first group, H4Y72 and H4Y88 interact with one of the flanking [H2A-H2B] dimers while H4Y98 in the second group interacts with the other dimer. In the first group, the histone fold part of H4 interacts with the histone fold part of H2B from a [H2A-H2B] dimer to form a four-helix bundle. Although a number of residues from both H4 and H2B contribute to this interface, one of the most striking features of the interface is the large hydrophobic domain generated by the contacts between three tyrosines: H4Y72, H4Y88, and H2BY83. Genetic analysis of histone H4 confirmed the essential nature of the tetramer-dimer interactions mediated by the tyrosine residues (35). Since H4H75 resides in the same histone fold as H4Y72 and H4Y88, the H75Y mutation introduces another tyrosine to one of the tetramer-dimer interfaces critical for nucleosome function. This extra tyrosine at 75 may join H4Y72, H4Y88, and H2BY83 to form a stronger hydrophobic domain, strengthening the tetramer-dimer interface. The resulting enhanced tetramer-dimer interface could either affect the chromatin-remodeling activity or diminish the ease of exchange of H2A for H2A.Z (see below), either of which might account for the phenotypic consequences of the mutation.
As a means of addressing how the core mutations affect silencing, we used a topological assay for silent chromatin to monitor the kinetics of decay in vivo of silent chromatin following its separation from an associated silencer locus. In wild-type cells, excision of silent chromatin from its associated silencer results in a stochastic decay over one to two generations from repressed to active chromatin. We found that the presence of the HHF2H75Y mutation significantly delays this transition from repressed to active chromatin such that no decay in repression of the excised chromatin was observed over more that four generations of growth. In contrast, we found that the rate of formation of silent chromatin, following reactivation of a mutant Sir3 protein, was essentially the same in wild-type and HHF2H75Y mutant cells. This indicates that the mutation stabilizes silent chromatin but does not appear to facilitate its formation. This could account for the increased silencing imparted to HML in a sir1 background, since a decrease in the rate of decay of repressed chromatin would lead to an overall increase in the proportion of cells in which the locus was in the repressed state.
We also found that the HHF2H75Y mutation appears to expand existing regions of silencing into adjacent chromatin not subject to silencing in wild-type cells. We found little evidence that the mutation promoted de novo formation of silencing at sites within the genome separate from existing silent domains. Given that this mutation reduces the rate of decay of silencing rather than enhancing its rate of formation, we might conclude that the mutation causes a normally dynamic interchange between silencing and chromatin activation in regions adjacent to preexisting silent chromatin to freeze into a silenced configuration. That is, heterochromatin might normally extend transiently outward from existing regions of stable silencing but such transient extensions would usually be unstable and would rapidly decay back to active chromatin. The mutant histone would stabilize these transient extensions, rendering them more permanent domains of heterochromatin. This is consistent with a recent view of the boundary between heterochromatin and active chromatin as the site of a dynamic interplay between activities promoting heterochromatin and those promoting active chromatin (8, 9, 29). Furthermore, this would suggest that one means of restricting heterochromatin spread is achieved by disrupting newly formed heterochromatin rather than blocking its formation.
We have entertained several explanations for the molecular basis of the effects of HHF2R39K and HHF2H75Y on silencing function. First, the mutations could enhance binding of Sir proteins to nucleosomes. An interaction domain on the surface of the nucleosomal core has been hypothesized on the basis of the mutations of the H3 and H4 core region, noted above, that reduce silencing and the fact that methylation of H3H79 precludes silencing (Fig. 9) (31, 43). Silencing-enhancing mutations at H3 D77 and D81 may be explained by an effect on the binding of Sir proteins to nucleosome. However, since the H4R39K and H4H75Y substitutions do not lie on the surface of the nucleosome, these substitutions would probably not significantly alter the conformation of the nucleosomal face or enhance interaction with Sir proteins.
A second explanation is that the histone H4 mutations could inhibit the deposition of histone H2A.Z into nucleosomes. A multienzyme complex catalyzes an ATP-dependent exchange of H2A/H2B dimers in nucleosome cores for variant H2A.Z-H2B dimers, inserting the H2A.Z variant at regions of the genome adjacent to silent domains (19, 20, 24, 27). Strains lacking HTZ1, the gene encoding H2A.Z, exhibit increased, Sir-dependent repression of genes adjacent to telomeres. Thus, H2A.Z appears to confine or antagonize Sir-dependent silencing. Nonetheless, deletion of HTZ1 promotes not only repression of genes adjacent to silent domains but also activation of a number of genes elsewhere in the genome (27). In contrast, from our microarray analysis, HHF2H75Y causes no transcriptional activation of any gene in the genome. Thus, the phenotypes of HHF2H75Y and loss of HTZ1 are not entirely coincident. In contrast, the pattern of expression changes in the HHF2R39K mutation significantly overlaps that resulting from deletion of HTZ1. Of the 154 genes we found to be affected by HHF2R39K, 33 were previously determined to be affected by deletion of HTZ1 (P = 10−9 [see reference 27 and the supplemental material]). Thus, HHF2R39K may enhance silencing by excluding H2A.Z.
Another possible explanation for the phenotype of HHF2H75Y mutants is that the H4H75Y substitution facilitates a conformational change in the nucleosome that converts Sir complex binding into transcriptional repression. For instance, this substitution could redirect adjacent residues (such as H4R78) to make more substantial contact with the surrounding DNA. Consistent with this hypothesis, the loss of silencing activity associated with mutation of these adjacent residues (light blue in Fig. 9) and their location within the nucleosome suggest that their primary role in silencing is through binding to DNA rather than to the Sir complex. Alternatively, the mutation could stabilize interaction between the H3-H4 tetramer and the H2A-H2B dimer, stabilizing the nucleosome against chromatin remodeling activity and precluding transcriptional activation or elongation. Experiments are under way to distinguish among these possible models.
Supplementary Material
Acknowledgments
We thank Alan Rose; Jef Boeke, Michael Grunstein, and Virginia Zakian for strains and plasmids; Ying Wang for advice on Affymetrix microarrays; and Jef Boeke and Karl Zawadski for valuable discussions.
This work was supported by National Institutes of Health grants GM43893 (to D.E.G.), HG1736 (to M.J.H.), and GM45840 (to J.R.B.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Aparicio, O. M., and D. E. Gottschling. 1994. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 8:1133-1146. [DOI] [PubMed] [Google Scholar]
- 2.Bi, X. 2002. Domains of gene silencing near the left end of chromosome III in Saccharomyces cerevisiae. Genetics 160:1401-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi, X., and J. R. Broach. 1997. DNA in transcriptionally silent chromatin assumes a distinct topology that is sensitive to cell cycle progression. Mol. Cell. Biol. 17:7077-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi, X., B. M., G.-J. Shei, and J. R. Broach. 1999. The Yeast HML I silencer defines a heterochromatin domain boundary by directional establishment of silencing. Proc. Natl. Acad. Sci. USA 96:11934-11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braunstein, M., A. B. Rose, S. G. Holmes, C. D. Allis, and J. R. Broach. 1993. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 7:592-604. [DOI] [PubMed] [Google Scholar]
- 6.Carmen, A. A., L. Milne, and M. Grunstein. 2002. Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J. Biol. Chem. 277:4778-4781. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, T. H., Y. C. Li, and M. R. Gartenberg. 1998. Persistence of an alternate chromatin structure at silenced loci in the absence of silencers. Proc. Natl. Acad. Sci. USA 95:5521-5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheutin, T., A. J. McNairn, T. Jenuwein, D. M. Gilbert, P. B. Singh, and T. Misteli. 2003. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299:721-725. [DOI] [PubMed] [Google Scholar]
- 9.Festenstein, R., S. N. Pagakis, K. Hiragami, D. Lyon, A. Verreault, B. Sekkali, and D. Kioussis. 2003. Modulation of heterochromatin protein 1 dynamics in primary mammalian cells. Science 299:719-721. [DOI] [PubMed] [Google Scholar]
- 10.Fox, C. A., A. E. Ehrenhofer-Murray, S. Loo, and J. Rine. 1997. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science 276:1547-1551. [DOI] [PubMed] [Google Scholar]
- 11.Gottschling, D. E., O. M. Aparicio, B. L. Billington, and V. A. Zakian. 1990. Position effect at S. cerevisiae telomeres: reversible repression of pol II transcription. Cell 63:751-762. [DOI] [PubMed] [Google Scholar]
- 12.Holland, M. J. 2002. Transcript abundance in yeast varies over six orders of magnitude. J. Biol. Chem. 277:14363-14366. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, S., and J. R. Broach. 1996. Silencers are required for inheritance of the repressed state in yeast. Genes Dev. 10:1021-1032. [DOI] [PubMed] [Google Scholar]
- 14.Imai, S. I., C. M. Armstrong, M. Kaeberlein, and L. Guarente. 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795-798. [DOI] [PubMed] [Google Scholar]
- 15.Ivy, J. M., A. J. S. Klar, and J. B. Hicks. 1986. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol. Cell. Biol. 6:688-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y:
- 17.Kayne, P. S., U.-J. Kim, M. Han, J. R. Mullen, F. Yoshizaki, and M. Grunstein. 1988. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell 55:27-39. [DOI] [PubMed] [Google Scholar]
- 18.Kirchmaier, A. L., and J. Rine. 2001. DNA replication-independent silencing in S. cerevisiae. Science 291:646-650. [DOI] [PubMed] [Google Scholar]
- 19.Kobor, M. S., S. Venkatasubrahmanyam, M. D. Meneghini, J. W. Gin, J. L. Jennings, A. J. Link, H. D. Madhani, and J. Rine. 2004. A protein complex containing the conserved Swi2/Snf2-related ATPase Swrlp deposits histone variant H2A.Z into euchromatin. PLoS Biol 2:E131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krogan, N. J., M. C. Keogh, N. Datta, C. Sawa, O. W. Ryan, H. Ding, R. A. Haw, J. Pootoolal, A. Tong, V. Canadien, D. P. Richards, X. Wu, A. Emili, T. R. Hughes, S. Buratowski, and J. F. Greenblatt. 2003. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol. Cell 12:1565-1576. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y. C., T. H. Cheng, and M. R. Gartenberg. 2001. Establishment of transcriptional silencing in the absence of DNA replication. Science 291:650-653. [DOI] [PubMed] [Google Scholar]
- 22.Luo, K., M. A. Vega-Palas, and M. Grunstein. 2002. Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 16:1528-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Megee, P. C., B. A. Morgan, B. A. Mittman, and M. M. Smith. 1990. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science 247:841-845. [DOI] [PubMed] [Google Scholar]
- 24.Meneghini, M. D., M. Wu, and H. D. Madhani. 2003. Conserved histone variant H2A.Z protects euchromatin from the ectopic spread of silent heterochromatin. Cell 112:725-736. [DOI] [PubMed] [Google Scholar]
- 25.Miller, A., and K. Nasymth. 1984. Role of DNA replication in the repression of silent mating type loci in yeast. Nature 312:247-251. [DOI] [PubMed] [Google Scholar]
- 26.Mishra, K., and D. Shore. 1999. Yeast Ku protein plays a direct role in telomeric silencing and counteracts inhibition by rif proteins. Curr. Biol. 9:1123-1126. [DOI] [PubMed] [Google Scholar]
- 27.Mizuguchi, G., X. Shen, J. Landry, W. H. Wu, S. Sen, and C. Wu. 2004. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303:343-348. [DOI] [PubMed] [Google Scholar]
- 28.Ng, H. H., D. N. Ciccone, K. B. Morshead, M. A. Oettinger, and K. Struhl. 2003. Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: a potential mechanism for position-effect variegation. Proc. Natl. Acad. Sci. USA 100:1820-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oki, M., and R. T. Kamakaka. 2002. Blockers and barriers to transcription: competing activities? Curr. Opin. Cell Biol. 14:299-304. [DOI] [PubMed] [Google Scholar]
- 30.Park, E.-C., and J. W. Szostak. 1990. Point mutations in the yeast histone H4 gene prevent silencing of the silent mating type locus HML. Mol. Cell. Biol. 10:4932-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, J. H., M. S. Cosgrove, E. Youngman, C. Wolberger, and J. D. Boeke. 2002. A core nucleosome surface crucial for transcriptional silencing. Nat. Genet. 32:273-279. [DOI] [PubMed] [Google Scholar]
- 32.Pillus, L., and J. Rine. 1989. Epigenetic inheritance of transcription states in S. cerevisiae. Cell 59:637-647. [DOI] [PubMed] [Google Scholar]
- 33.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481-516. [DOI] [PubMed] [Google Scholar]
- 34.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 13:2207-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santisteban, M. S., G. Arents, E. N. Moudrianakis, and M. M. Smith. 1997. Histone octamer function in vivo: mutations in the dimer-tetramer interfaces disrupt both gene activation and repression. EMBO J. 16:2493-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, C. M., Z. W. Haimberger, C. O. Johnson, A. J. Wolf, P. R. Gafken, Z. Zhang, M. R. Parthun, and D. E. Gottschling. 2002. Heritable chromatin structure: mapping “memory” in histones H3 and H4. Proc. Natl. Acad. Sci. USA 99(Suppl. 4): 16454-16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, J. S., and J. D. Boeke. 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 11:241-254. [DOI] [PubMed] [Google Scholar]
- 38.Smith, J. S., C. B. Brachmann, I. Celic, M. A. Kenna, S. Muhammad, V. J. Starai, J. L. Avalos, J. C. Escalante-Semerena, C. Grubmeyer, C. Wolberger, and J. D. Boeke. 2000. A phylogenetically conserved NAD+ dependent protein deacetylase activity in the Sir2 protein family. Proc. Natl. Acad. Sci. USA 97:6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone, E. M., C. Reifsnyder, M. McVey, B. Gazo, and L. Pillus. 2000. Two classes of sir3 mutants enhance the sir1 mutant mating defect and abolish telomeric silencing in Saccharomyces cerevisiae. Genetics 155:509-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straight, A. F., W. Shou, G. J. Dowd, C. W. Turck, R. J. Deshaies, A. D. Johnson, and D. Moazed. 1999. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97:245-256. [DOI] [PubMed] [Google Scholar]
- 41.Suka, N., Y. Suka, A. A. Carmen, J. Wu, and M. Grunstein. 2001. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8:473-479. [DOI] [PubMed] [Google Scholar]
- 42.Tanner, K. G., J. Landry, R. Sternglanz, and J. M. Denu. 2000. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl. Acad. Sci. USA 97:14178-14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Leeuwen, F., P. R. Gafken, and D. E. Gottschling. 2002. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell 109:745-756. [DOI] [PubMed] [Google Scholar]
- 44.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, L., E. E. Eugeni, M. R. Parthun, and M. A. Freitas. 2003. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma 112:77-86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.