Abstract
Changes in community composition are an important, but hard to predict, effect of climate change. Here, we use a wild-bee study system to test the ability of critical thermal maxima (CTmax, a measure of heat tolerance) to predict community responses to urban heat-island effects in Raleigh, NC, USA. Among 15 focal species, CTmax ranged from 44.6 to 51.3°C, and was strongly predictive of population responses to urban warming across 18 study sites (r2 = 0.44). Species with low CTmax declined the most. After phylogenetic correction, solitary species and cavity-nesting species (bumblebees) had the lowest CTmax, suggesting that these groups may be most sensitive to climate change. Community responses to urban and global warming will likely retain strong physiological signal, even after decades of warming during which time lags and interspecific interactions could modulate direct effects of temperature.
Keywords: urban warming, climate change, bee, pollinator, critical thermal maximum, heat tolerance
1. Introduction
Abundance and geographical ranges of many species have already responded to recent climate change, and these shifts are thought to arise in part from mismatches between environmental temperatures and organisms' physiological tolerances [1,2]. Accordingly, physiological traits such as CTmax, a measure of heat tolerance, are promising predictors of species' sensitivity to environmental warming, particularly for ectotherms [3–5]. In situ changes in community composition are a common effect of warming (e.g. [6,7]), but physiological traits are generally used to explain the distribution and ecology of individual species, or of many species at coarse, global scales [4,5]. Physiological traits have rarely been used to predict community-wide changes, and only in response to short-term warming [3]. In the longer term, direct responses to warming may be complicated by time lags and biotic interactions [8,9], and it remains unclear whether to expect a strong physiological signal in species' relative responses to warming within a given locality.
Here, we address this question using a wild-bee study system. As pollinators, bees provide an essential ecosystem service whose magnitude depends on community composition [10]. Historical data indicate that bee community composition and species distributions are shifting with climate change [11,12], making bees a timely subject for studies of thermal tolerance. We present phylogenetic and ecological correlates of bee thermal tolerance, and a field study using variation in urban heat-island intensity to test the prediction that species with lower CTmax are those whose populations decline the most with warming.
2. Material and methods
For additional details of all methods and analyses, see the electronic supplementary material.
(a). Physiological tolerance
We measured CTmax for 15 common bee species (Agapostemon virescens; Lasioglossum bruneri; Bombus bimaculatus; Megachile campanulae; Bombus griseocollis; Lasioglossum imitatum; Bombus impatiens; Megachile exilis; Halictus ligatus/poeyi; Megachile mendica; Ptilothrix bombiformis; Ceratina calcarata; Megachile rotundata; Xylocopa virginica; Ceratina strenua) collected in urban habitats near our laboratory in Raleigh, NC, USA (35.8° N, 78.68° W). We placed bees individually in 45 ml glass vials, which we heated 0.5°C min−1 in a water bath. We monitored bees every minute and recorded CTmax as the point when postural control was lost [13,14]. All assays were performed from May through August 2014–2015. We pinned and identified tested bees and measured intertegular distance (a proxy for body size) [15]. We categorized each species by social behaviour (social, solitary) and nesting habitat (ground, cavity, stem/wood) using the literature and expert opinion.
(b). Response to warming in the field
We sampled bees at 18 sites (15 residential yards, three parks) that varied in urban heat-island intensity in and around Raleigh. Urban warming generates a temporally stable mosaic of hotter and cooler locations that typically differ by 1–3°C in air temperature [16]. To document heat-island intensity at each site, we installed iButton data loggers (DS1921, Maxim Integrated, San Jose, CA, USA) that recorded temperature hourly from 9 May to 19 July 2015. For analysis, we used mean early-evening temperatures (19.00–21.00) because solar radiation affected daytime air-temperature measurements [17]. We sampled bee communities at each site 11 times over two summers (May–August 2014–2015) using pan traps, vane traps and aerial netting. Hereafter, ‘bee abundance’ refers to the total number of bees collected per species per site.
(c). Data analysis
We characterized each species' response to urban warming as a Poisson regression coefficient describing a loglinear relationship between bee abundance and site temperature. To estimate coefficients for all species jointly, thereby stabilizing estimates for rare species, we fit a hierarchical model in WinBUGS v. 1.4 [18] (see the electronic supplementary material).
Because related species may not be statistically independent, we conducted analyses in a phylogenetic framework. We constructed a maximum-likelihood tree of the 15 species using 10 genes. We then constructed a series of phylogenetic generalized least-squares models to test two hypotheses: (i) CTmax depends on body size, sociality and nesting habitat, and (ii) species' response to urban warming depends on CTmax. For each hypothesis, we constructed four models that differed only in their phylogenetic covariance structures: none, Brownian motion, Pagel's λ and Ornstein–Uhlenbeck. We present the results of the best-fitting models, as determined by Akaike information criterion adjusted for small sample size (AICc). We also examined phylogenetic signal within CTmax by comparing four models of evolution to a phylogenetically independent (white noise) model. Analyses required R (v. 3.1.1) packages ape v. 4.1, geiger v. 2.0.6, nlme v. 3.1 and MuMIn v. 1.15.6 [19–23].
3. Results
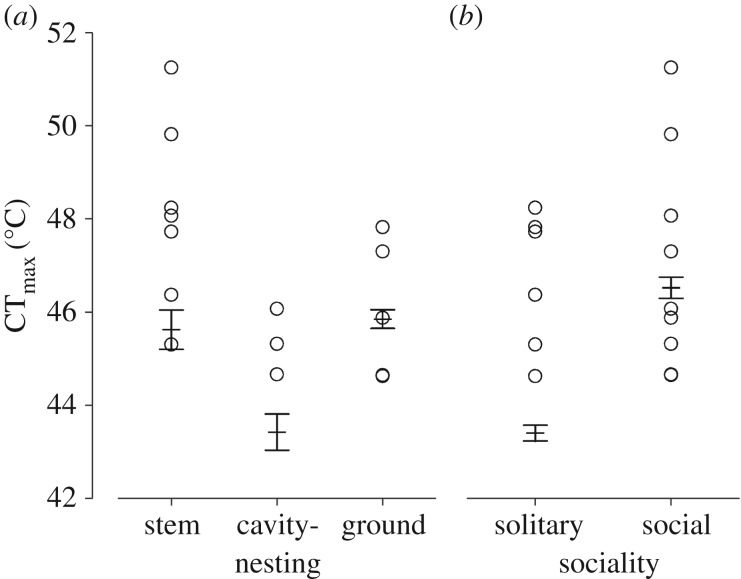
Species' critical thermal maxima (CTmax) ranged from 44.6 to 51.3°C (n = 3–32 per species). We did not detect strong phylogenetic signal in CTmax (see the electronic supplementary material). CTmax covaried with life-history traits but not with body size (table 1). Cavity-nesting species had low heat tolerance, with fitted mean CTmax about 2°C lower than that of stem/wood-nesting or ground-nesting bees after controlling for body size and sociality (figure 1a). For solitary bees, fitted mean CTmax was about 3°C lower than for social species (figure 1b). The best-fitting model incorporated phylogenetic signal using Pagel's λ correction, with λ = −6.35 (95% CI: −6.58, −6.12), indicating negative phylogenetic correlation in model residuals.
Table 1.
Parameter estimates and statistical tests for best-fitting models describing the relationship between CTmax and life-history traits, and between CTmax and population response to warming. (Social and cavity-nesting are the baseline conditions to which others are compared; p-values <0.05 are bold.)
| model | term | coefficient | s.e. | t | p-value |
|---|---|---|---|---|---|
| CTmax ∼ body size + sociality + nesting | intercept | 45.37 | 1.02 | 44.5 | 0.000 |
| body size | −0.14 | 0.25 | −0.6 | 0.574 | |
| nest (ground) | 2.43 | 0.59 | 4.1 | 0.002 | |
| nest (stem) | 2.20 | 0.77 | 2.9 | 0.017 | |
| sociality (solitary) | −3.13 | 0.35 | −8.9 | 0.000 | |
| response to warming ∼ CTmax | intercept | −5.98 | 1.68 | −3.6 | 0.004 |
| CTmax | 0.11 | 0.036 | 3.2 | 0.007 |
Figure 1.
Bee CTmax as it relates to (a) nesting habitat and (b) sociality. Circles are data (one point per species); whiskers are fitted means ± s.e. from the phylogenetic model, with body size held constant.
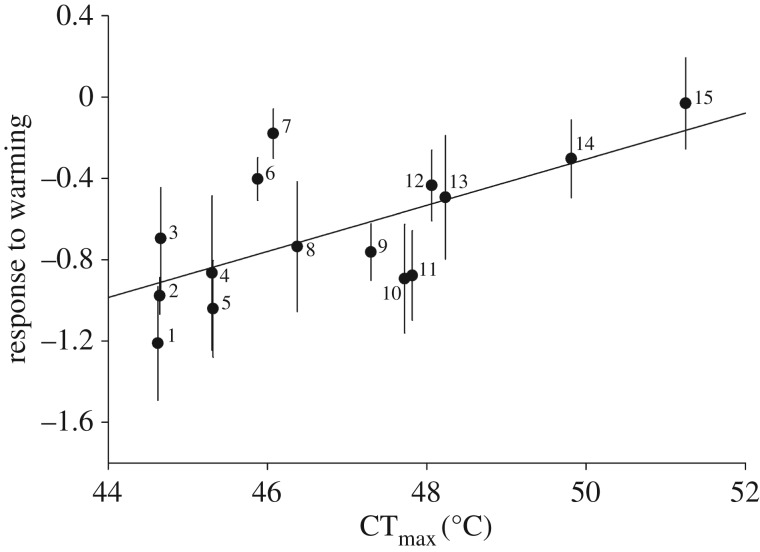
CTmax, in turn, was a strong predictor of population response to urban warming: species with the lowest CTmax were those whose populations declined the most (table 1 and figure 2). The best-fitting model did not incorporate the phylogeny. We refit it with ordinary least squares to determine that r2 = 0.44. The result that species declined at different rates implies that community composition also shifted with warming; see the electronic supplementary material for an explicit test of compositional change.
Figure 2.
CTmax predicts species' responses to warming in the field. Points are species; ‘response to warming’ is a Poisson coefficient (±s.e.) describing change in species' abundance per degree Celsius urban warming. Species are: 1, Agapostemon virescens; 2, Lasioglossum bruneri; 3, Bombus bimaculatus; 4, Megachile campanulae; 5, Bombus griseocollis; 6, Lasioglossum imitatum; 7, Bombus impatiens; 8, Megachile exilis; 9, Halictus ligatus/poeyi; 10, Megachile mendica; 11, Ptilothrix bombiformis; 12, Ceratina calcarata; 13, Megachile rotundata; 14, Xylocopa virginica; 15, Ceratina strenua.
4. Discussion
We show that interspecific variation in CTmax, a measure of physiological heat tolerance, corresponds to community change across steep, local temperature gradients imposed by urban warming. Although urban warming is an imperfect proxy for climate change, it provides a unique opportunity to test predictions about biological effects of long-term warming at temperatures that correspond to those predicted regionally by the end of the century [24,25]. The relationship between CTmax and response to urban warming also suggests a process for community assembly in urban ecosystems. While previous studies have suggested global, latitudinal patterns in ectotherm tolerance to urban warming [26], our results extend the predictive power of CTmax to species' relative responses to warming within local communities.
CTmax may predict which species are at greatest risk from future warming. We show that bees that nest in pre-existing cavities, such as rodent burrows, had the lowest thermal tolerance. All cavity-nesters in our dataset were bumblebees, a group known to be experiencing climate-related range contractions [11]. We thus corroborate the global pattern of bumblebee heat sensitivity at local and organismal scales. Despite bumblebees' membership in the ‘social’ category, social bees were overall more heat tolerant than solitary bees, suggesting that solitary life history may predict climate sensitivity.
Our results strongly suggest that species' physiology shapes community composition, even after decades of warming during which time lags and interspecific interactions could modulate direct effects of temperature. Although major reviews have compiled heat tolerance data for hundreds of ectotherms (e.g. [4]), CTmax has rarely been measured for bees (but see [27]), and most ecological communities remain poorly represented. Filling this data gap will be an important step towards improved predictions of species composition in urban and future climates.
Supplementary Material
Acknowledgements
Holly Menninger, Sally Thigpen and volunteer homeowners facilitated site selection and research permissions. Tyson Wepprich built the heat-ramping apparatus. John Ascher, Adrian Carper, Sheila Colla, Sam Droege, Joel Gardner, Jason Gibbs and Leif Richardson shared knowledge of bee sociality and nesting. Sam Droege and Jason Gibbs identified bees. Nicole Bissonnette, Bobby Chanthammavong, Catherine Croft, Laura Daly, Samantha Dietz, Karly Dugan, Morgan Duncan, Anna Holmquist and Danielle Schmidt assisted with data collection.
Ethics
We used standard protocols for field collection and thermal tolerance assays, and minimized handling time and stress of individuals. Ethics committee approval was not required. Permissions to conduct fieldwork were granted by NC State University, City of Raleigh Department of Parks, Recreation and Cultural Resources and volunteer homeowners.
Data accessibility
Files for bee abundance, site temperatures, CTmax and 10-gene alignment are available from Dryad: http://dx.doi.org/10.5061/dryad.34dk0 [28].
Authors' contributions
A.L.H. and S.D.F. designed the study, A.L.H. collected data, M.M.L.-U. and E.Y. analysed data, E.Y. drafted the manuscript, all authors revised and approved the manuscript and are accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by an Agriculture and Food Research Initiative Competitive Grant (2013-02476) from the USDA National Institute of Food and Agriculture to S.D.F. and E.Y.; and by Cooperative Agreement No. G11AC20471 and G13AC00405 from the United States Geological Survey to Robert R. Dunn and S.D.F.
References
- 1.Walther G-R. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024. ( 10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helmuth B, Kingsolver JG, Carrington E. 2005. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 67, 177–201. ( 10.1146/annurev.physiol.67.040403.105027) [DOI] [PubMed] [Google Scholar]
- 3.Diamond SE, Nichols LM, McCoy N, Hirsch C, Pelini SL, Sanders NJ, Ellison AM, Gotelli NJ, Dunn RR. 2012. A physiological trait-based approach to predicting the responses of species to experimental climate warming. Ecology 93, 2305–2312. ( 10.1890/11-2296.1) [DOI] [PubMed] [Google Scholar]
- 4.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley LB. 2008. Linking traits to energetics and population dynamics to predict lizard ranges in changing environments. Am. Nat. 171, E1–E19. ( 10.1086/523949) [DOI] [PubMed] [Google Scholar]
- 6.Youngsteadt E, Ernst AF, Dunn RR, Frank SD. 2016. Responses of arthropod populations to warming depend on latitude: evidence from urban heat islands. Glob. Change Biol. 23, 1436–1447. ( 10.1111/gcb.13550) [DOI] [PubMed] [Google Scholar]
- 7.Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. ( 10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 8.Bertrand R, Lenoir J, Piedallu C, Riofrío-Dillon G, De Ruffray P, Vidal C, Pierrat J-C, Gégout J-C. 2011. Changes in plant community composition lag behind climate warming in lowland forests. Nature 479, 517–520. ( 10.1038/nature10548) [DOI] [PubMed] [Google Scholar]
- 9.Suttle K, Thomsen MA, Power ME. 2007. Species interactions reverse grassland responses to changing climate. Science 315, 640–642. ( 10.1126/science.1136401) [DOI] [PubMed] [Google Scholar]
- 10.Winfree R, JW Fox, Williams NM, Reilly JR, Cariveau DP. 2015. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 18, 626–635. ( 10.1111/ele.12424) [DOI] [PubMed] [Google Scholar]
- 11.Kerr JT, et al. 2015. Climate change impacts on bumblebees converge across continents. Science 349, 177–180. ( 10.1126/science.aaa7031) [DOI] [PubMed] [Google Scholar]
- 12.Bartomeus I, Ascher JS, Gibbs J, Danforth BN, Wagner DL, Hedtke SM, Winfree R. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl Acad. Sci. USA 110, 4656–4660. ( 10.1073/pnas.1218503110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: data to support the onset of spasms as the definitive end point. Can. J. Zool. 75, 1553–1560. ( 10.1139/z97-782) [DOI] [Google Scholar]
- 14.Lighton JR, Turner RJ. 2004. Thermolimit respirometry: an objective assessment of critical thermal maxima in two sympatric desert harvester ants, Pogonomyrmex rugosus and P. californicus. J. Exp. Biol. 207, 1903–1913. ( 10.1242/jeb.00970) [DOI] [PubMed] [Google Scholar]
- 15.Cane JH. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kans. Entomol. Soc. 60, 145–147. [Google Scholar]
- 16.Gaffin S, et al. 2008. Variations in New York City's urban heat island strength over time and space. Theor. Appl. Clim. 94, 1–11. ( 10.1007/s00704-007-0368-3) [DOI] [Google Scholar]
- 17.Meineke E, Youngsteadt E, Dunn RR, Frank SD. 2016. Urban warming reduces aboveground carbon storage. Proc. R. Soc. B 283, 20161574 ( 10.1098/rspb.2016.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegelhalter D, Thomas A, Best N, Lunn D. 2007. WinBUGS User Manual Version 1.4.3, MRC Biostatistics Unit.
- 19.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2016. Linear and nonlinear mixed effects models. See http://CRAN.R-project.org/package=nlme.
- 21.Barton K. 2016. MuMIn: multi-model inference. See https://CRAN.R-project.org/package=MuMIn.
- 22.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. ( 10.1093/bioinformatics/btm538) [DOI] [PubMed] [Google Scholar]
- 23.R Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 24.Farrell C, Szota C, Arndt SK. 2015. Urban plantings: ‘Living laboratories’ for climate change response. Trends Plant Sci. 20, 597–599. ( 10.1016/j.tplants.2015.08.006) [DOI] [PubMed] [Google Scholar]
- 25.Youngsteadt E, Dale AG, Terando AJ, Dunn RR, Frank SD. 2015. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Change Biol. 21, 97–105. ( 10.1111/gcb.12692) [DOI] [PubMed] [Google Scholar]
- 26.Chown SL, Duffy GA. 2015. Thermal physiology and urbanization: perspectives on exit, entry and transformation rules. Funct. Ecol. 29, 902–912. ( 10.1111/1365-2435.12478) [DOI] [Google Scholar]
- 27.Oyen KJ, Giri S, Dillon ME. 2016. Altitudinal variation in bumble bee (Bombus) critical thermal limits. J. Therm. Biol. 59, 52–57. ( 10.1016/j.jtherbio.2016.04.015) [DOI] [PubMed] [Google Scholar]
- 28.Hamblin AL, Youngsteadt E, López-Uribe MM, Frank SD. 2017. Data from: Physiological thermal limits predict differential responses of bees to urban heat-island effects. Dryad Digital Repository. ( 10.5061/dryad.34dk0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hamblin AL, Youngsteadt E, López-Uribe MM, Frank SD. 2017. Data from: Physiological thermal limits predict differential responses of bees to urban heat-island effects. Dryad Digital Repository. ( 10.5061/dryad.34dk0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Files for bee abundance, site temperatures, CTmax and 10-gene alignment are available from Dryad: http://dx.doi.org/10.5061/dryad.34dk0 [28].