Abstract
Heterotrimeric G proteins of the Gq/11 family transduce signals from a variety of neurotransmitter and hormone receptors and have therefore been implicated in various functions of the nervous system. Using the Cre/loxP system, we generated mice which lack the genes coding for the α subunits of the two main members of the Gq/11 family, gnaq and gna11, selectively in neuronal and glial precursor cells. Mice with defective gnaq and gna11 genes were morphologically normal, but they died shortly after birth. Mice carrying a single gna11 allele survived the early postnatal period but died within 3 to 6 weeks as anorectic dwarfs. In these mice, postnatal proliferation of pituitary somatotroph cells was strongly impaired, and plasma growth hormone (GH) levels were reduced to 15%. Hypothalamic levels of GH-releasing hormone (GHRH), an important stimulator of somatotroph proliferation, were strongly decreased, and exogenous administration of GHRH restored normal proliferation. The hypothalamic effects of ghrelin, a regulator of GHRH production and food intake, were reduced in these mice, suggesting that an impairment of ghrelin receptor signaling might contribute to GHRH deficiency and abnormal eating behavior. Taken together, our findings show that Gq/11 signaling is required for normal hypothalamic function and that impairment of this signaling pathway causes somatotroph hypoplasia, dwarfism, and anorexia.
The Gq/11 family of heterotrimeric G proteins mediates the cellular effects of numerous neurotransmitter receptors, e.g., the metabotropic glutamate receptor subtypes 1 and 5, the M1 muscarinic-acetylcholine receptor, the 5-HT2 serotonin receptors, or the α1 adrenergic receptor. Gq/11-coupled receptors are also involved in the signal transduction of several hypothalamic peptide hormone receptors, such as the receptors for thyrotropin-releasing hormone (39), gonadotropin-releasing hormone (13), and prolactin-releasing hormones (3). There is an increasing amount of evidence that the release of hypothalamic hormones themselves is also controlled by Gq/11-coupled receptors. Kisspeptins, for example, have been suggested to control the release of gonadotropin-releasing hormone via the Gq/11-coupled receptor GPR54 (14, 20), and the gastrointestinal peptide hormone ghrelin regulates the production of growth hormone-releasing hormone (GHRH) via the hypothalamic growth hormone secretagogue receptor (GHS-R) (12, 22). Gq/11 family G proteins mediate the activation of β isoforms of phospholipase C, resulting in the activation of protein kinase C and intracellular calcium mobilization (2). The Gq/11 family consists of four members, two of which, Gq and G11, are expressed almost ubiquitously in the central nervous system (26). Genetic inactivation of the gnaq gene, which codes for the α subunit of Gq (Gαq), leads to a defect in primary hemostasis (16) and cerebellar ataxia (15). In contrast, mice homozygous for a null allele of the gene coding for Gα11, gna11, did not show any phenotypic abnormalities (17). These defects were relatively mild when one considers the number of potential transmitter systems affected, and this fact is probably due to the high functional redundancy of Gαq and Gα11, which share 88% amino acid sequence identity (24). Indeed, mice lacking both Gαq and Gα11 die at day 10.5 of embryonic development (17). To circumvent this embryonic lethality, we used the Cre/loxP system (18) to generate a mouse line which allows conditional, tissue-specific inactivation of gnaq in constitutively Gα11-deficient mice (36). Postnatal inactivation of Gq/11-mediated signaling in the forebrain by use of the Camkcre4 mouse line was shown to inhibit maternal behavior (35). To investigate the role of Gαq and Gα11 in pre- and postnatal development and the function of the whole nervous system, we used in this study the NestinCre mouse line (31), which causes Cre-mediated recombination in neuronal and glial precursor cells starting at embryonic day 9.5 (40). Interestingly, the Gαq/Gα11 deficiency in descendants of neuronal and glial precursors did not cause gross morphological abnormalities of the developing nervous system but was incompatible with postnatal survival. In addition, we provide evidence that Gq/11-mediated signaling is critically involved in the regulation of hypothalamic GHRH production and consecutive control of somatotroph proliferation.
MATERIALS AND METHODS
Generation of mice lacking Gαq and Gα11 in neuronal precursor cells.
Mice in which the gene coding for Gαq, gnaq, is flanked by loxP sites (gnaqflox) (36) were crossed to the constitutively Gα11-deficient mouse line (17) and to mice which express the recombinase Cre under the control of the Nestin promoter (31, 40). This action led to the generation of mice which lacked one, two, three, or all of the four gnaq/gna11 alleles in derivatives of neuronal and glial precursor cells. Genotyping for the gnaqflox allele, for wild-type and knockout gna11 alleles, and for the Cre transgene was performed as described previously (36).
Hormone levels.
Plasma samples were obtained from 15-day-old mice at the beginning of the dark period. Levels of insulin-like growth factor I (IGF-I) were determined with an OCTEIA rat and mouse IGF-I kit (IDS Inc., Boldon, United Kingdom), ghrelin levels were determined with a rat and mouse ghrelin radioimmunoassay (RIA) kit (Phoenix, Belmont, Calif.), thyroxine levels were determined with a Micro enzyme immunoassay kit (Leinco, St. Louis, Mo.), and adrenocorticotropic hormone (ACTH) levels were determined with an immunometric assay kit (Nichols Institute Diagnostics, San Clemente, Calif.). Corticosterone was measured by RIA in the Steroid Laboratory of the Pharmacology Institute, Heidelberg, Germany. GH and thyroid-stimulating hormone (TSH) were measured by RIA in the laboratory of the National Hormone and Pituitary Program, Torrance, Calif.
Histology.
Mice were deeply anesthetized with pentobarbital (100 mg/kg of body weight) intraperitoneally (i.p.) and perfused with 4% paraformaldehyde (PFA) via the left cardiac ventricle. Brains were postfixed in 4% PFA overnight and then stored in 0.5% PFA at 4°C. For immunohistochemistry, 50-μm-thick vibratome sections were cut and incubated at 4°C with the following antibodies: rabbit anti-c-fos antibody (sc-52; Santa Cruz Biotechnology, Santa Cruz, Calif.) at a 1:20,000 dilution for 3 days, rabbit anti-Gαq/11 antibody (sc-392; Santa Cruz Biotechnology) at a 1:1,000 dilution for 16 h, rabbit anti-GHRH antibody (obtained from F. Talamantes, University of California, Santa Cruz, Santa Cruz, Calif.) at a 1:20,000 dilution for 16 h, and mouse anti-NeuN antibody (Chemicon, Hofheim, Germany) at a dilution of 1:2,000 for 16 h. For staining, we used the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, Calif.). Internal organs and brains were weighed after preparation, and hematoxylin-eosin staining was performed on 5-μm-thick paraffin sections according to standard protocols. For immunofluorescence staining of pituitary somatotroph cells, 5-μm-thick paraffin sections were incubated for 16 h with a rabbit anti-GH antibody (dilution, 1:20,000; obtained from A. F. Parlow) and then with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit antibody (dilution, 1:200; Dianova, Hamburg, Germany) for 2 h. For BrdU labeling, 5-day-old mice were injected intraperitoneally on three consecutive days with 10 μg of GHRH (Phoenix)/kg or with a comparable volume of saline. In the afternoon of the third day, mice were intraperitoneally injected with 10 μg of BrdU/kg of body weight and sacrificed after 2 h. Paraffin sections of the pituitary were stained with anti-BrdU antibody (dilution, 1:100; BD Biosciences, San Diego, Calif.) and FITC-labeled goat anti-rabbit antibody (dilution, 1:200; Jackson ImmunoResearch) for 2 h. For ghrelin immunohistochemistry, 16-μm-thick cryotome sections of the stomach were stained with goat anti-ghrelin antibody (dilution, 1:300; Santa Cruz Biotechnology) and by using a Vectastain Elite ABC kit (Vector Labs). The numbers of c-fos-, NeuN-, ghrelin-, or BrdU-positive cells, as well as the area of GHRH immunoreactivity, were determined with the CellExplorer 2003 program (BioSciTec, Frankfurt, Germany).
Food uptake.
Food uptake during the dark phase was determined in 3-to 4-week-old mice by weighing standard chow before and after the test period. Water was accessible ad libitum. To determine the effect of ghrelin on food intake, mice were injected intraperitoneally with 1 mg of rat ghrelin (Bachem, Heidelberg, Germany) or saline/kg of body weight, and food uptake during the following 3 h was determined every 30 min. In some cases, mice were sacrificed 1 h after ghrelin injection for c-fos immunohistochemistry of their arcuate nuclei (arcN).
Statistics.
Values were expressed as means ± standard errors of the means. Differences between two groups were statistically analyzed by using the unpaired Student's t test. Statistical significance was accepted at a P value of <0.05.
RESULTS
We used the NestinCre mouse line (31) to generate mice which lacked the G protein α subunit Gαq selectively in neuronal and glial precursor cells, and we then mated these mice with the constitutively Gα11-deficient mouse line (17). This procedure resulted in the generation of mice which lacked one, two, three, or all four gnaq/gna11 alleles in neuronal and glial precursor cells. To prove that Cre expression leads to the recombination of the gnaqflox allele and thereby to a loss of Gαq protein, we performed Western blot experiments with extracts from the brains of newborn mice by using an antibody directed against Gαq/11 (Fig. 1a). In the brains of NestinCre+/− gnaqflox/flox gna11−/− mice (i.e., no gnaq or gna11 alleles were left), neither Gαq nor Gα11 protein was detectable, whereas NestinCre+/− gnaqflox/wt gna11−/− (i.e., one gnaq allele is intact) or NestinCre+/− gnaqflox/flox gna11+/− mouse brains (i.e., one gna11 allele is intact) lacked only Gα11 or Gαq, respectively. These results show that the NestinCre-mediated recombination of gnaqflox alleles indeed leads to a loss of Gαq protein in the nervous system.
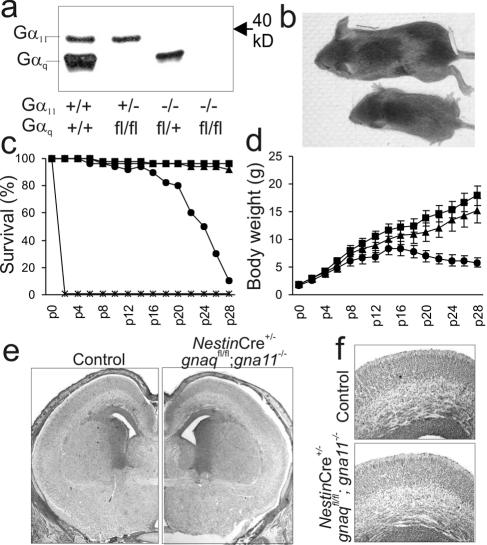
FIG. 1.
The survival of Nestin-Gαq/11-deficient mice depends on the number of remaining gnaq/gna11 alleles. (a) Western blot for Gαq/11 proteins in brain extracts (10 μg per lane) from newborn mice with different genotypes. +, wild-type allele; fl, recombined floxed allele; −, null allele. (b) Reduced body length of a mouse with only one remaining gna11 allele (bottom) compared to a control littermate (top) at postnatal day 20. (c and d) Survival rate (c) and postnatal weight gain (d) of NestinCre+/− gnaqflox/flox gna11−/− mice (asterisks), NestinCre+/− gnaqflox/flox gna11+/− mice (circles), NestinCre+/− gnaqflox/wt gna11−/− mice (triangles), or control mice (squares) (24 to 30 mice per group). p, postnatal day. (e) Representative hematoxylin-eosin stainings of frontal brain sections at embryonic day 18.5 from a control animal (left) and a NestinCre+/− gnaqflox/flox gna11−/− mouse (right) (magnification, ×2.5). (f) Tenfold magnification of the mouse sensory cortices shown in panel e.
Matings between triple-heterozygous mice (NestinCre+/− gnaqflox/wt gna11+/−) showed that mice of all genotypic combinations were born at expected frequencies (data not shown). However, the postnatal survival of a newborn strongly depended on the number of intact gnaq/gna11 alleles (Fig. 1c). Normal numbers of newborns in which all four gnaq/gna11 alleles were inactivated were born without obvious malformations, but they did not take up rhythmic breathing after delivery. Accordingly, the lungs of these animals were only partially inflated (data not shown). Neither histological nor basic immunohistochemical analysis of the brains of these animals revealed major structural abnormalities (Fig. 1e and f and data not shown). The peripheral nervous system, including the phrenic nerve, was histologically normal in each mouse. Initial reflexes towards tactile stimuli and basal cardiovascular function seemed to be normal, suggesting that inefficient respiration caused the deaths of these newborns.
Mice with at least one intact gnaq allele or two intact gna11 alleles were viable and fertile. Of note, mice lacking both gnaq alleles showed an ataxic gait comparable to the one seen in constitutively Gαq-deficient mice (16), whereas animals with a single intact gnaq allele were normal in this respect. In contrast, mice with only one gna11 allele were strongly retarded in their somatic growth (Fig. 1b and d) and died as underweight dwarfs between the third and sixth postnatal weeks (Fig. 1c).
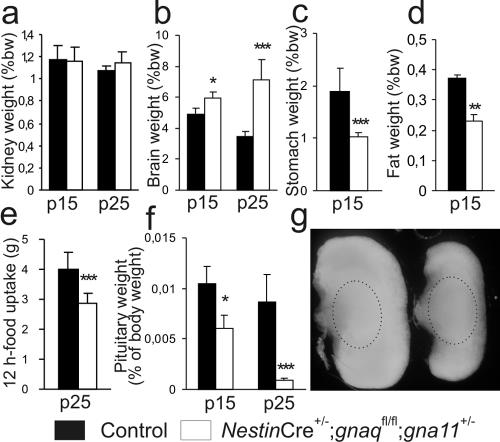
Growth retardation in mice with a single gna11 allele was first detectable between postnatal days 5 and 9 (Fig. 1d). During the following weeks, the body weights increased only slowly, peaked between the second and third weeks, and finally decreased again (Fig. 1d), usually leading to the deaths of the animals between the third and sixth weeks (Fig. 1c). Except for their small size and slightly retarded motor development, these animals appeared to be normal, i.e., they did not show obvious behavioral abnormalities or signs of illness. Neither the removal of normal littermates to decrease competition for the dam's milk nor the presence of easily accessible extra food changed the final outcome. To determine whether the growth retardation and premature deaths of mice with only one intact gna11 allele could be attributed to the failure of a specific organ system, we performed macroscopic and microscopic analyses of their central nervous systems and internal organs. Brains, hearts, lungs, kidneys, livers, thyroids, and adrenal glands did not show major morphological abnormalities (data not shown). Analyses of organ weight/body weight ratios showed that kidneys and hearts were of normal sizes relative to the body weights of the mice (Fig. 2a and data not shown), whereas the relative brain weights were increased (Fig. 2b). In contrast, the relative weights of the stomach and of retrorenal fat deposits were decreased (Fig. 2c and d), and this was probably due to reduced food uptake (Fig. 2e). Most strikingly, the pituitaries were much too small in relation to body weights, especially at older ages, and this circumstance was due mainly to the reduced size of the anterior pituitary in each mouse, since the posterior pituitary was of normal size (Fig. 2f and g).
FIG. 2.
Organ weights and food uptake of wild-type mice and mice with only one intact gna11 allele (NestinCre+/− gnaqflox/flox gna11+/−). (a to d, f) Organ weights as percentages of body weight (%bw) for kidneys (a), brains (b), stomachs (c), retrorenal fat deposits (d), and pituitaries (f) at postnatal days 15 (p15) and 25 (p25) (five to six mice per group). (e) Food uptake in 3-week-old mice (five to six mice per group). *, P < 0.05; **, P < 0.01; ***, P < 0.005. (g) Pituitaries from a control mouse (left) and an animal with only one intact gna11 allele (right) at postnatal day 15. Borders of the posterior pituitary are indicated by hatched ovals.
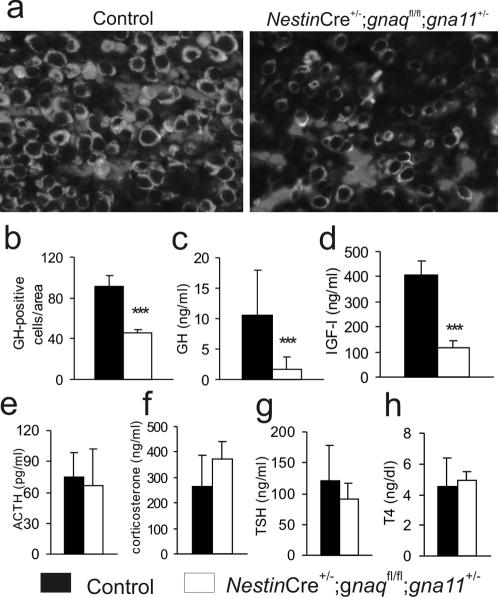
As somatic growth is regulated mainly through secretion of GH from somatotroph cells of the anterior pituitary, we reasoned that the observed dwarfism might be due to impaired somatotroph function. Immunofluorescence staining showed that the densities of GH-positive cells were reduced to about 50% of that of the control cells in pituitaries from 2-week-old mice (Fig. 3a and b). This reduced density of GH-positive cells, together with the massive reduction of anterior pituitary volume (Fig. 2f and g), leads to GH deficiency, with plasma GH levels at 15% of the normal level (Fig. 3c). Consistent with this, IGF-I levels were reduced to 30% of normal levels in 2-week-old animals (Fig. 3d). Interestingly, other anterior pituitary functions, as determined by levels of TSH, thyroxine, ACTH, or the mouse glucocorticoid analogue corticosterone in plasma, were normal on postnatal day 15 (Fig. 3e through h). These findings show that mice with only one intact gna11 allele in the nervous system suffer from selective somatotroph hypoplasia, leading to strongly reduced plasma GH and IGF-I levels and, consecutively, proportionate dwarfism.
FIG. 3.
Reduced numbers of somatotrophs in pituitaries of mice with only one intact gna11 allele (NestinCre+/− gnaqflox/flox gna11+/−). (a) Immunofluorescence staining for GH in the anterior pituitary of 15-day-old control mice (left) and mice with one remaining gna11 allele (right). (b) Statistical evaluation of immunofluorescence staining (two mice per group, three sections each). (c to h) Levels in plasma, at postnatal day 15, of GH (15 mice per group) (c), IGF-I (6 to 8 mice per group) (d), ACTH (8 mice per group) (e), mouse cortisol analogue corticosterone (8 mice per group) (f), TSH (g), and thyroxine, T4 (h) (6 to 8 mice per group). ***, P < 0.001.
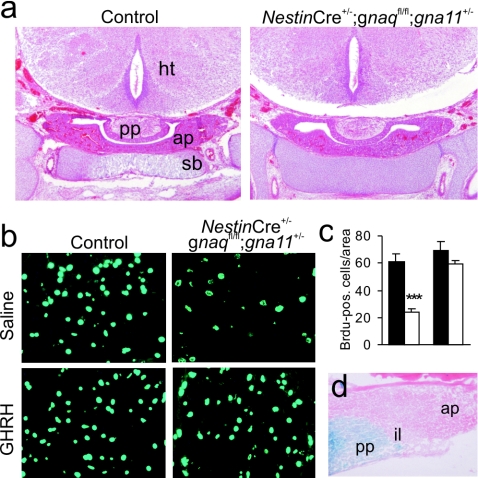
To test whether hypoplasia of the anterior pituitary was due to a defect in prenatal development, we histologically analyzed pituitaries from mice at embryonic day 18.5. At this age, pituitary sizes and development were normal for mice of all genotypes (Fig. 4a), indicating that somatotroph hypoplasia is due to a defect in postnatal proliferation. To test this concept, we performed BrdU labeling of pituitaries in the first postnatal week and found that the number of proliferating cells was reduced in mice with only one intact gna11 allele to 38% of that of the control littermates (Fig. 4b and c). An important stimulator of somatotroph proliferation is GHRH (4), which is produced in neurons of the hypothalamic arcN and which is released into the pituitary portal system at the median eminence. To test whether pituitaries from mice with only one intact gna11 allele would be responsive to exogenously administered GHRH, we treated pups of different genotypes with daily intraperitoneal injections of saline or GHRH from postnatal days 5 through 7. This treatment strongly enhanced anterior pituitary proliferation in mice with only one intact gna11 allele, which almost reached the proliferation levels of saline-treated control littermates (Fig. 4b and c). These findings suggest that somatotroph cells are responsive to GHRH in these mice and that somatotroph hypoplasia is not due to a defect of the pituitary itself but rather to inappropriate GHRH release from the hypothalamus. Consistent with this, β-galactosidase staining of mice carrying both the NestinCre transgene and a Rosa26-lacZ reporter construct (23) did not show any recombination in the anterior pituitary, whereas the posterior pituitary and hypothalamus were recombined (Fig. 4d and data not shown).
FIG. 4.
Impaired postnatal proliferation of somatotroph cells can be rescued by administration of GHRH. (a) Hematoxylin-eosin staining of coronal head sections from a control animal (left) and a mouse with only one intact gna11 allele (right) at embryonic day 18.5. (b) BrdU-labeled cells in the anterior pituitary of 7-day-old control animals (left) and mice with only one intact gna11 allele (right) after 3 days of intraperitoneal administration of saline (top) or GHRH (bottom). (c) Statistical analysis of BrdU-positive (pos.) cells (two mice per group, three sections each). Black bars, control mice; white bars, mice with one intact gna11 allele; ***, P < 0.001. (d) β-Galactosidase staining of a pituitary from a mouse carrying both the Rosa26-lacZ reporter construct and the NestinCre transgene. (a and d) ap, anterior pituitary; ht, hypothalamus; il, intermediate lobe; pp, posterior pituitary; sb, sphenoid bone.
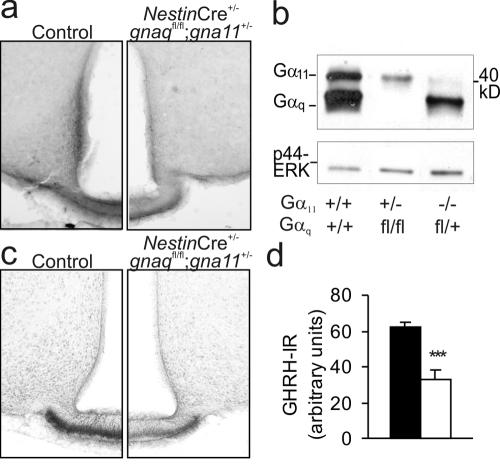
Immunohistochemical analyses of the hypothalamic arcN and median eminence, in which GHRH-producing neurons are located, revealed a considerable immunoreactivity for Gαq/Gα11 in wild-type controls, which was strongly reduced in mice with only one intact gna11 allele (Fig. 5a). To quantify Gαq/Gα11 protein expression in the hypothalami of mice with only one intact Gαq or Gα11 allele, we performed Western blotting experiments using extracts from hypothalami of wild-type or Gαq/Gα11-deficient mice. We found that in wild-type mouse hypothalami, Gαq levels were severalfold higher than Gα11 levels and that the reduction of Gαq/Gα11 immunoreactivity was much stronger in hypothalami from animals with one intact Gα11 allele than in hypothalami from mice with one intact Gαq allele (Fig. 5b).
FIG. 5.
Immunohistochemical changes in the hypothalami of mice with only one gna11 allele. (a) Staining with antibodies against Gαq/11 in the arcN of a control mouse (left) and a mouse with one intact gna11 allele (right). (b) Western blot of extracts from wild-type and mutant mouse hypothalami (10 μg per lane) with antibodies against Gαq/11 and total extracellular signal-regulated kinase (ERK) as the loading control. +, wild-type allele; fl, recombined floxed allele; −, null allele. (c) Staining with antibodies against GHRH in the arcN of a control mouse (left) and a mouse with one intact gna11 allele (right). (d) Statistical evaluation of GHRH immunoreactivity (GHRH-IR) in the median eminence (two mice per group, three sections each). Black bars, control mice; white bars, mice with only one intact gna11 allele. ***, P < 0.001.
Immunohistochemistry using an antibody directed against GHRH revealed that the number of GHRH-immunoreactive terminals was markedly decreased in mice with only one intact Gα11 allele (Fig. 5c and d) but that the total number of arcN neurons, as determined by NeuN immunohistochemistry, was not changed (not shown). These findings suggest that the loss of Gαq/Gα11-mediated signaling in the arcN leads to impaired GHRH production, which in turn causes impaired somatotroph proliferation and, consecutively, dwarfism.
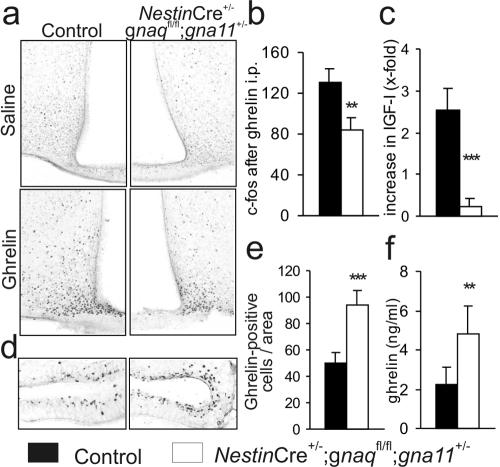
The peptide hormone ghrelin, in addition to having a regulatory function in food uptake and energy homeostasis, is a potent inducer of hypothalamic GHRH release, and the ghrelin receptor GHS-R was reported to couple to Gq/11 family G proteins (12, 22). We therefore tested whether impaired GHS-R signaling might contribute to reduced GHRH release and weight loss in mice with only one intact gna11 allele. Since ghrelin administration is known to cause c-fos expression in the arcN of rodents (8, 30, 33), we performed c-fos immunohistochemistry after intraperitoneal ghrelin application and found that the number of activated neurons in these mice was reduced to 65% of the number in controls (Fig. 6a and b). In contrast to its effect on wild-type animals, ghrelin administration did not increase plasma IGF-I levels in mice with only one gna11 allele (Fig. 6c), indicating that ghrelin-induced GH release is inhibited in these mice. We then tested whether impaired GHS-R signaling led to a compensatory up-regulation of ghrelin production and found both the number of ghrelin-immunoreactive cells in the stomach (Fig. 6d and e) and the plasma ghrelin levels (Fig. 6f) to be increased. These findings suggest that defective signaling via the ghrelin receptor GHS-R contributes to deregulation of somatic growth and food uptake in mice with only one intact gna11 allele.
FIG. 6.
Impaired ghrelin actions in mice with only one intact gna11 allele (NestinCre+/− gnaqflox/flox gna11+/−). (a) c-fos immunohistochemistry of the arcN in control mice (left) or mice with one intact gna11 allele (right) after the i.p. administration of saline (top) or ghrelin (bottom). (b) Statistical evaluation of c-fos immunohistochemistry of mouse arcN (two mice per group, three sections each). (c) Relative increase in plasma IGF-I levels after i.p. administration of ghrelin (six mice per group). (d) Immunohistochemistry for ghrelin-positive cells in the stomachs of control mice (left) and mice with only one intact gna11 allele (right). (e) Statistical evaluation of immunochemistry for ghrelin-positive cells (two mice per group, three sections each). (f) Plasma ghrelin levels on postnatal day 20 (eight mice per group). (b, c, e, and f) **, P < 0.005; ***, P < 0.001.
DISCUSSION
We show in this study that conditional inactivation of Gq/11-mediated signaling in the nervous system leads to different degrees of impairment, depending on the number of intact gnaq/gna11 alleles. Newborn mice which lacked all four alleles did not show any gross morphological abnormalities but did not take up rhythmic breathing activity. This outcome suggests that Gαq/Gα11-mediated signaling is crucial for the postnatal function of the nervous system but not for its prenatal development. Pups carrying one intact gna11 allele survived the early postnatal period but died as underweight dwarfs between the third and sixth postnatal weeks. In contrast, mice which inherited one intact gnaq allele were viable and fertile. gnaq and gna11 are obviously equally able to guarantee vital functions in neonates, but normal postnatal development and weight gain depend on the presence of an intact gnaq allele. Two factors might account for these differences between Gαq and Gα11: functional differences and differential expression patterns of the two proteins. Potential functional differences between Gαq and Gα11 have been intensively studied, but up to now, neither differential receptor coupling (34, 38) nor differences with regard to effector preferences (7, 29, 38) have been described. Our data show that total Gαq/Gα11 immunoreactivity is much lower in hypothalami from mice with only one intact Gα11 allele than in hypothalami from mice with one intact Gαq allele. Though both Gαq and Gα11 are basically ubiquitously expressed in the central nervous system (26), differences in expression levels have also been described for other brain regions, e.g., in the cerebellum or the hippocampus, in which expression levels of Gα11 are also lower than those of Gαq (6, 11, 15). Thus, a single gna11 allele, but not a single gnaq allele, might be unable to compensate for the loss of the other alleles. This finding is in line with observations for constitutively gnaq- and/or gna11-deficient mice, in which the presence of either two gna11 alleles or one gnaq allele ensures normal cardiomyocyte development (17), craniofacial development (10, 17), and melanoblast migration and proliferation (32).
We showed that growth retardation in mice with only one intact gna11 allele is due to a massive hypoplasia of anterior pituitary somatotroph cells, resulting in GH deficiency. The anterior pituitary is a derivative of the oral ectoderm and is not of neuronal origin; hence, it does not undergo NestinCre-mediated recombination (Fig. 4d) (31). The defect leading to somatotroph hypoplasia is therefore unlikely to reside in the pituitary but rather involves brain regions which regulate somatotroph proliferation, e.g., the hypothalamus. In rats, somatotroph cells are first detected at embryonic day 18.5 and rapidly increase in number during the first 10 days after birth (27). An important stimulator of somatotroph proliferation is GHRH, and the overexpression of GHRH leads to somatotroph hyperplasia, increased growth, and tumorigenesis, whereas inactivating mutations cause somatotroph hypoplasia and dwarfism (4). Our data show that impaired somatotroph proliferation in mice with only one gna11 allele is accompanied by decreased production of GHRH in the hypothalamus and that exogenous application of GHRH can restore normal proliferation.
The synthesis and release of GHRH from arcN neurons are modulated by a variety of neuropeptides and neurotransmitters, e.g., somatostatin, galanin, neuropeptide Y, and the peptide hormone ghrelin (1). Ghrelin enhances GHRH release via the activation of the GHS-R, which was shown to activate protein kinase C and to release calcium from intracellular stores via the pathway mediated by phospholipase C-β and IP3 (12). However, several other effectors seem to contribute to GHS-R function, like adenylyl cyclase-mediated cyclic AMP production or L-type Ca2+ channels (12, 22). We showed that impaired hypothalamic Gq/11 signaling led to a significant reduction of ghrelin-mediated c-fos activation in arcN neurons, suggesting that Gq/11 proteins are indeed crucial for the signal transduction of the GHS-R. This notion is corroborated by the finding that ghrelin-induced IGF-I surges are inhibited in mice with only one intact gna11 allele. The latter finding also suggests that the pituitary GHS-R (5, 9), which should signal normally because gnaq is not inactivated in the anterior pituitary of these mice, does not play a major role in ghrelin-mediated GH release. This finding is in line with the observation that GHRH antiserum inhibits the GH response to ghrelin in rats (28) and that patients with hypothalamopituitary disconnection do not respond with GH release to synthetic GHS-R ligands (19).
Impaired proliferation of the somatotroph cell line explains very well the dwarf phenotype but not the premature deaths of these mice. Dwarfism itself is not expected to be lethal, but it might handicap the pup in competing for the dam's milk or in reaching the food tray. However, even in very small litters and when food and water were put directly into the cage, the survival of dwarf mice was not improved. In addition to being of short stature, these mice are also impaired with regard to motor development, i.e., they develop an ataxia resembling the one seen in constitutively Gαq-deficient mice (15). However, constitutively Gαq-deficient mice have, except for an increased perinatal mortality due to defective primary hemostasis (16), a normal life expectancy (15), and therefore, ataxia cannot account for the premature deaths of mice with only one intact gna11 allele. We hypothesized that additional defects must exist in these mice, but neither levels of other pituitary hormones in plasma (Fig. 3) nor histological analyses of internal organs revealed any abnormalities. However, stomach weight, size of retrorenal fat deposits, and food uptake were reduced in mice with only one intact gna11 allele (Fig. 2), suggesting that they suffer from a deregulation of energy homeostasis and/or appetite control. Ghrelin, besides having a role as a growth-hormone secretagogue, is a well-known regulator of food intake and energy expenditure (12), and impaired hypothalamic ghrelin signaling is expected to result in dwarfism and reduced food intake. Surprisingly, genetic inactivation of the ghrelin gene did not affect growth or food intake in mice (25, 37). On the other hand, a small interfering RNA-based knockdown of the GHS-R in rats impaired somatic growth, weight gain, and food uptake (21). These studies suggest that the GHS-R, but not ghrelin itself, is indispensable for normal regulation of growth and energy homeostasis. It is quite possible that parallel hormonal systems, known or unknown, are involved in the regulation of these vital processes, and our findings strongly suggest that they converge on Gαq/Gα11-coupled receptors, including the GHS-R.
Taken together, our data show that the complete loss of Gαq/Gα11-mediated signaling in the developing nervous system does not cause obvious developmental abnormalities but leads to functional defects incompatible with extrauterine life. The presence of a single gnaq allele or a single gna11 allele is sufficient to maintain vital functions after birth, but only the presence of a gnaq allele, not of a gna11 allele, allows normal postnatal proliferation of the somatotroph cell line. In addition to showing somatotroph hypoplasia and consecutive dwarfism, mice with only one intact gna11 allele show abnormal eating behavior, and our findings suggest that both defects involve impaired signaling via the GHS-R.
Acknowledgments
We thank Rüdiger Klein for providing the NestinCre line and Anke Rogatzki and Marianne Hillesheim for expert technical assistance.
This work was funded by Collaborative Research Center 488 of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Bluet-Pajot, M. T., J. Epelbaum, D. Gourdji, C. Hammond, and C. Kordon. 1998. Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol. Neurobiol. 18:101-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Exton, J. H. 1996. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 36:481-509. [DOI] [PubMed] [Google Scholar]
- 3.Freeman, M. E., B. Kanyicska, A. Lerant, and G. Nagy. 2000. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 80:1523-1631. [DOI] [PubMed] [Google Scholar]
- 4.Frohman, L. A., and R. D. Kineman. 2002. Growth hormone-releasing hormone and pituitary development, hyperplasia and tumorigenesis. Trends Endocrinol. Metab. 13:299-303. [DOI] [PubMed] [Google Scholar]
- 5.Gnanapavan, S., B. Kola, S. A. Bustin, D. G. Morris, P. McGee, P. Fairclough, S. Bhattacharya, R. Carpenter, A. B. Grossman, and M. Korbonits. 2002. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 87:2988. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann, J., R. Blum, Y. Kovalchuk, H. Adelsberger, R. Kuner, G. M. Durand, M. Miyata, M. Kano, S. Offermanns, and A. Konnerth. 2004. Distinct roles of Galpha(q) and Galpha11 for Purkinje cell signaling and motor behavior. J. Neurosci. 24:5119-5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hepler, J. R., T. Kozasa, A. V. Smrcka, M. I. Simon, S. G. Rhee, P. C. Sternweis, and A. G. Gilman. 1993. Purification from Sf9 cells and characterization of recombinant Gq alpha and G11 alpha. Activation of purified phospholipase C isozymes by G alpha subunits. J. Biol. Chem. 268:14367-14375. [PubMed] [Google Scholar]
- 8.Hewson, A. K., and S. L. Dickson. 2000. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J. Neuroendocrinol. 12:1047-1049. [DOI] [PubMed] [Google Scholar]
- 9.Howard, A. D., S. D. Feighner, D. F. Cully, J. P. Arena, P. A. Liberator, C. I. Rosenblum, M. Hamelin, D. L. Hreniuk, O. C. Palyha, J. Anderson, P. S. Paress, C. Diaz, M. Chou, K. K. Liu, K. K. McKee, S. S. Pong, L. Y. Chaung, A. Elbrecht, M. Dashkevicz, R. Heavens, M. Rigby, D. J. Sirinathsinghji, D. C. Dean, D. G. Melillo, L. H. Van der Ploeg, et al. 1996. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974-977. [DOI] [PubMed] [Google Scholar]
- 10.Ivey, K., B. Tyson, P. Ukidwe, D. G. McFadden, G. Levi, E. N. Olson, D. Srivastava, and T. M. Wilkie. 2003. Galphaq and Galpha11 proteins mediate endothelin-1 signaling in neural crest-derived pharyngeal arch mesenchyme. Dev. Biol. 255:230-237. [DOI] [PubMed] [Google Scholar]
- 11.Kleppisch, T., V. Voigt, R. Allmann, and S. Offermanns. 2001. G(alpha)q-deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J. Neurosci. 21:4943-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korbonits, M., A. P. Goldstone, M. Gueorguiev, and A. B. Grossman. 2004. Ghrelin—a hormone with multiple functions. Front. Neuroendocrinol. 25:27-68. [DOI] [PubMed] [Google Scholar]
- 13.Naor, Z., S. Shacham, D. Harris, R. Seger, and N. Reiss. 1995. Signal transduction of the gonadotropin releasing hormone (GnRH) receptor: cross-talk of calcium, protein kinase C (PKC), and arachidonic acid. Cell Mol. Neurobiol. 15:527-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro, V. M., J. M. Castellano, R. Fernandez-Fernandez, M. L. Barreiro, J. Roa, J. E. Sanchez-Criado, E. Aguilar, C. Dieguez, L. Pinilla, and M. Tena-Sempere. 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565-4574. [DOI] [PubMed] [Google Scholar]
- 15.Offermanns, S., K. Hashimoto, M. Watanabe, W. Sun, H. Kurihara, R. F. Thompson, Y. Inoue, M. Kano, and M. I. Simon. 1997. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Galphaq. Proc. Natl. Acad. Sci. USA 94:14089-14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Offermanns, S., C. F. Toombs, Y. H. Hu, and M. I. Simon. 1997. Defective platelet activation in G alpha(q)-deficient mice. Nature 389:183-186. [DOI] [PubMed] [Google Scholar]
- 17.Offermanns, S., L. P. Zhao, A. Gohla, I. Sarosi, M. I. Simon, and T. M. Wilkie. 1998. Embryonic cardiomyocyte hypoplasia and craniofacial defects in G alpha q/G alpha 11-mutant mice. EMBO J. 17:4304-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orban, P. C., D. Chui, and J. D. Marth. 1992. Tissue- and site-specific DNA recombination in transgenic mice. Proc. Natl. Acad. Sci. USA 89:6861-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popovic, V., S. Damjanovic, D. Micic, M. Djurovic, C. Dieguez, and F. F. Casanueva. 1995. Blocked growth hormone-releasing peptide (GHRP-6)-induced GH secretion and absence of the synergic action of GHRP-6 plus GH-releasing hormone in patients with hypothalamopituitary disconnection: evidence that GHRP-6 main action is exerted at the hypothalamic level. J. Clin. Endocrinol. Metab. 80:942-947. [DOI] [PubMed] [Google Scholar]
- 20.Seminara, S. B., S. Messager, E. E. Chatzidaki, R. R. Thresher, J. S. Acierno, Jr., J. K. Shagoury, Y. Bo-Abbas, W. Kuohung, K. M. Schwinof, A. G. Hendrick, D. Zahn, J. Dixon, U. B. Kaiser, S. A. Slaugenhaupt, J. F. Gusella, S. O'Rahilly, M. B. Carlton, W. F. Crowley, Jr., S. A. Aparicio, and W. H. Colledge. 2003. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 349:1614-1627. [DOI] [PubMed] [Google Scholar]
- 21.Shuto, Y., T. Shibasaki, A. Otagiri, H. Kuriyama, H. Ohata, H. Tamura, J. Kamegai, H. Sugihara, S. Oikawa, and I. Wakabayashi. 2002. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J. Clin. Investig. 109:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith, R. G., L. H. Van der Ploeg, A. D. Howard, S. D. Feighner, K. Cheng, G. J. Hickey, M. J. Wyvratt, Jr., M. H. Fisher, R. P. Nargund, and A. A. Patchett. 1997. Peptidomimetic regulation of growth hormone secretion. Endocr. Rev. 18:621-645. [DOI] [PubMed] [Google Scholar]
- 23.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 24.Strathmann, M., and M. I. Simon. 1990. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc. Natl. Acad. Sci. USA 87:9113-9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, Y., S. Ahmed, and R. G. Smith. 2003. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 23:7973-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka, J., S. Nakagawa, E. Kushiya, M. Yamasaki, M. Fukaya, T. Iwanaga, M. I. Simon, K. Sakimura, M. Kano, and M. Watanabe. 2000. Gq protein alpha subunits Galphaq and Galpha11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur. J. Neurosci. 12:781-792. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi, Y., S. Yasutaka, R. Kominami, and H. Shinohara. 2001. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anat. Embryol. (Berlin) 204:469-475. [DOI] [PubMed] [Google Scholar]
- 28.Tannenbaum, G. S., J. Epelbaum, and C. Y. Bowers. 2003. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology 144:967-974. [DOI] [PubMed] [Google Scholar]
- 29.Taylor, S. J., and J. H. Exton. 1991. Two alpha subunits of the Gq class of G proteins stimulate phosphoinositide phospholipase C-beta 1 activity. FEBS Lett. 286:214-216. [DOI] [PubMed] [Google Scholar]
- 30.Traebert, M., T. Riediger, S. Whitebread, E. Scharrer, and H. A. Schmid. 2002. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J. Neuroendocrinol. 14:580-586. [DOI] [PubMed] [Google Scholar]
- 31.Tronche, F., C. Kellendonk, O. Kretz, P. Gass, K. Anlag, P. C. Orban, R. Bock, R. Klein, and G. Schutz. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23:99-103. [DOI] [PubMed] [Google Scholar]
- 32.Van Raamsdonk, C. D., K. R. Fitch, H. Fuchs, M. H. de Angelis, and G. S. Barsh. 2004. Effects of G-protein mutations on skin color. Nat. Genet. 36:961-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, L., D. H. Saint-Pierre, and Y. Tache. 2002. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 325:47-51. [DOI] [PubMed] [Google Scholar]
- 34.Wange, R. L., A. V. Smrcka, P. C. Sternweis, and J. H. Exton. 1991. Photoaffinity labeling of two rat liver plasma membrane proteins with [32P]gamma-azidoanilido GTP in response to vasopressin. Immunologic identification as alpha subunits of the Gq class of G proteins. J. Biol. Chem. 266:11409-11412. [PubMed] [Google Scholar]
- 35.Wettschureck, N., A. Moers, T. Hamalainen, T. Lemberger, G. Schütz, and S. Offermanns. 2004. Heterotrimeric G proteins of the Gq/11 family are crucial for the induction of maternal behavior in mice. Mol. Cell. Biol. 24:8048-8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wettschureck, N., H. Rutten, A. Zywietz, D. Gehring, T. M. Wilkie, J. Chen, K. R. Chien, and S. Offermanns. 2001. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat. Med. 7:1236-1240. [DOI] [PubMed] [Google Scholar]
- 37.Wortley, K. E., K. D. Anderson, K. Garcia, J. D. Murray, L. Malinova, R. Liu, M. Moncrieffe, K. Thabet, H. J. Cox, G. D. Yancopoulos, S. J. Wiegand, and M. W. Sleeman. 2004. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA 101:8227-8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, D., A. Katz, C. H. Lee, and M. I. Simon. 1992. Activation of phospholipase C by alpha 1-adrenergic receptors is mediated by the alpha subunits of Gq family. J. Biol. Chem. 267:25798-25802. [PubMed] [Google Scholar]
- 39.Yu, R., and P. M. Hinkle. 1999. Signal transduction and hormone-dependent internalization of the thyrotropin-releasing hormone receptor in cells lacking Gq and G11. J. Biol. Chem. 274:15745-15750. [DOI] [PubMed] [Google Scholar]
- 40.Zimmerman, L., B. Parr, U. Lendahl, M. Cunningham, R. McKay, B. Gavin, J. Mann, G. Vassileva, and A. McMahon. 1994. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12:11-24. [DOI] [PubMed] [Google Scholar]