Abstract
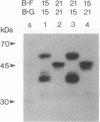
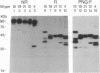
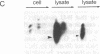
B-G antigens are cell-surface molecules encoded by a highly polymorphic multigene family located in the chicken major histocompatibility complex (MHC). Rabbit antisera to B-G molecules immunoprecipitate 3-6 bands from iodinated erythrocytes by sodium dodecyl sulfate (SDS) gels under reducing conditions. These are all B-G molecules because they all map to the B-G region of the chicken MHC in congenic and recombinant chickens, most are directly recognized by the antisera, most form disulfide-linked dimers, and none bear N-linked carbohydrate. Both apparent homodimers and heterodimers are found, which bear intrachain disulfide bonds. All 3-6 bands have different mobilities in SDS gels between different haplotypes, ranging from 30 to 55 kDa. This size polymorphism is not affected by glycosidase treatment or addition of protease inhibitors. Partial proteolysis of cell surface-iodinated B-G molecules generates extremely similar patterns of spots, both within and between haplotypes. These surface-iodinated peptides bear either interchain or intrachain disulfide bonds. Additional peptides are generated by proteolysis of B-G molecules iodinated after isolation. Thus, it appears that the extracellular regions of these molecules are very similar and that the length polymorphism is due to variations in the cytoplasmic regions. Inspection of the cDNA-derived protein sequence in this region shows many heptad repeats, which may allow variation in length by step deletion and alternative splicing. The repeats indicate an alpha-helical coiled-coil structure, which could form an interaction between subunits of the dimer or with the cytoskeleton or both.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto R., Miyada C. G., Young S., Wallace R. B., Abplanalp H., Bloom S. E., Briles W. E., Miller M. M. Isolation of a cDNA clone from the B-G subregion of the chicken histocompatibility (B) complex. Immunogenetics. 1988;27(2):102–109. doi: 10.1007/BF00351083. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Kaufman J. F., Skjoedt K., Auffray C. The major histocompatibility complex in the chicken. Trends Genet. 1989 Sep;5(9):300–304. doi: 10.1016/0168-9525(89)90112-1. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Hála K., Plachý J., Schulmannová J. Role of the B-G-region antigen in the humoral immune response to the B-F-region antigen of chicken MHC. Immunogenetics. 1981;14(5):393–401. doi: 10.1007/BF00373319. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Salomonsen J., Skjødt K. B-G cDNA clones have multiple small repeats and hybridize to both chicken MHC regions. Immunogenetics. 1989;30(6):440–451. doi: 10.1007/BF02421176. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Skjoedt K., Salomonsen J., Simonsen M., Du Pasquier L., Parisot R., Riegert P. MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive xenoantisera. J Immunol. 1990 Mar 15;144(6):2258–2272. [PubMed] [Google Scholar]
- Kaufman J., Skjoedt K., Salomonsen J. The MHC molecules of nonmammalian vertebrates. Immunol Rev. 1990 Feb;113:83–117. doi: 10.1111/j.1600-065x.1990.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Longenecker B. M., Mosmann T. R. "Natural" antibodies to chicken MHC antigens are present in mice, rats, humans, alligators and allogeneic chickens. Immunogenetics. 1980;11(3):293–302. doi: 10.1007/BF01567795. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Abplanalp H. Analysis of the B-G antigens of the chicken MHC by two-dimensional gel electrophoresis. Immunogenetics. 1984;20(4):373–385. doi: 10.1007/BF00345612. [DOI] [PubMed] [Google Scholar]
- Miller M. M., Goto R., Briles W. E. Biochemical confirmation of recombination within the B-G subregion of the chicken major histocompatibility complex. Immunogenetics. 1988;27(2):127–132. doi: 10.1007/BF00351086. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Salomonsen J., Skjødt K., Crone M., Simonsen M. The chicken erythrocyte-specific MHC antigen. Characterization and purification of the B-G antigen by monoclonal antibodies. Immunogenetics. 1987;25(6):373–382. doi: 10.1007/BF00396103. [DOI] [PubMed] [Google Scholar]
- Schierman L. W., McBride R. A. Adjuvant activity of erythrocyte isoantigens. Science. 1967 May 5;156(3775):658–659. doi: 10.1126/science.156.3775.658. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]