Abstract
Objective
To examine associations of perceived stress with cognitive symptoms among adults with systemic lupus erythematosus (SLE).
Methods
Among 777 adult (>18 years) SLE patients, we examined the association of Perceived Stress Scale (PSS) scores with two self-reported cognitive symptoms: forgetfulness [severe/moderate vs. mild/none; from the Systemic Lupus Activity Questionnaire] and difficulty concentrating (all/most vs. some/little/none of the time; from the Lupus Impact Tracker). We used multivariable logistic regression to estimate the odds ratios (ORs) per minimal important difference (MID=0.5*SD) of PSS score and cognitive symptoms.
Results
Forgetfulness and difficulty concentrating were reported by 41.7% and 29.5%, respectively. Women and those with less education and high disease activity had higher PSS scores and were more likely to report cognitive symptoms than their counterparts. With adjustment for age, race, sex, education, and disease activity, each MID increase in PSS score was associated with higher prevalence of forgetfulness (OR=1.43, 95% CI 1.29–1.47) and difficulty concentrating (OR=2.19, 95% CI 1.90–2.52). No substantial differences in this association by age, race, sex, or disease activity were noted.
Conclusions
SLE patients, particularly those with high disease activity, report a high burden of cognitive symptoms, for which stress may be a modifiable risk factor.
Keywords: systemic lupus erythematosus, stress, cognitive function, African-American
Introduction
Signs and symptoms of cognitive dysfunction are common in systemic lupus erythematosus (SLE) patients, with about one-quarter to one-half of patients experiencing some level of cognitive impairment (1–3). Multiple biologic factors, such as immunologic mechanisms and neuropsychiatric involvement in SLE, to explain the so-called “lupus brain fog” have been explored (4–6). However, psychosocial factors such as stress may also act as contributors to this impairment. High levels of perceived stress have been shown to be strongly associated with cognitive impairment in older adults generally (7, 8) and in other chronic, inflammatory diseases as diverse as HIV (9), breast cancer (10), and multiple sclerosis (11). This association may also hold in SLE patients, in whom stress is commonly reported, with nearly half reporting major life stress in the past 6 months (12). Stress represents one of many psychosocial factors that may interact with each other and with disease-related factors to produce symptoms of cognitive impairment in SLE (13), but stress may also exert effects on cognitive function that are independent of the effects of disease-specific SLE activity.
Thus, stress represents a potentially modifiable risk factor for poor cognitive function in SLE patients that would not be addressed by clinical approaches that primarily address the contribution of disease activity to cognitive dysfunction in SLE (14, 15). However, few studies have explicitly examined the independent association of stress with cognitive dysfunction in SLE. While a recent Spanish study found that higher stress was associated with greater cognitive dysfunction in a small sample of SLE patients (16), better understanding of this association in a larger, representative population of SLE patients—particularly, a population that includes African-American patients, who may experience stress and its effects on health differently (17)—will inform clinical care for the broader SLE population. Leveraging cross-sectional data from the ongoing U.S. cohort, Georgians Organized Against Lupus (GOAL), which enrolled large numbers of adult SLE patients who are African-American, we examined whether perceived stress was independently associated with reported cognitive symptoms, including forgetfulness and difficulty concentrating, among SLE patients and whether these associations were independent of SLE-specific disease activity. We also examined whether the association between stress and cognitive symptoms differed among subpopulations of interest.
Methods
Study Design and Population
We used a cross-sectional design to describe the association of perceived stress with cognitive symptoms in baseline data from the ongoing GOAL cohort study. Details of GOAL recruitment and data collection have been published previously (18). Briefly, GOAL study participants were recruited primarily from the existing Georgia Lupus Registry, a population-based registry funded by the Centers for Disease Control and Prevention for the purpose of more accurately estimating the incidence and prevalence of SLE in metropolitan Atlanta (19). The population-based cohort was further enriched with additional patients receiving SLE treatment at Emory University, at Grady Memorial Hospital (the only safety net hospital in Atlanta), or from community rheumatologists in metropolitan Atlanta, who were recruited by mail, by telephone, and in person. Eligible participants were adult patients (aged ≥18 years) with a documented diagnosis of SLE [≥4 revised American College of Rheumatology (ACR) criteria (20), or 3 ACR criteria plus a diagnosis of SLE by the patient’s treating board-certified rheumatologist].
Baseline (8/2011–7/2012) data were available for 800 patients. For analyses, patients were excluded if they were missing data on perceived stress (n=1) or cognitive symptoms (n=7). Because of small sample sizes, we were unable to stratify by race other than African-American or white; thus, patients were further excluded if their race was not African-American or white (n=15), leaving 777 SLE patients in the analytic sample. The Emory University Institutional Review Board, Grady Health System Research Oversight Committee, and Georgia Department of Public Health Institutional Review Board approved the GOAL study protocol. All GOAL participants provided informed consent.
Study Variables
Perceived stress
Perceived stress was estimated using the 10-item Perceived Stress Scale (PSS) score (21), which measures the degree to which the subject found life situations stressful during the prior month. PSS scores range from 0 to 40, with higher scores indicating greater perceived stress; mean scores in a probability-based U.S. sample were 12.1 and 13.7 in males and females, respectively (22).
Cognitive symptoms
Self-reported cognitive symptoms were taken from items included in instruments measuring disease activity and lupus impact at baseline.
Forgetfulness
Forgetfulness was assessed by the item “In the past 3 months, how bad has your forgetfulness been?” (with possible responses of “no problem,” “mild,” “moderate,” or “severe”) from the Systemic Lupus Activity Questionnaire (SLAQ) (23). We dichotomized forgetfulness as severe/moderate vs. mild/none.
Difficulty concentrating
Difficulty concentrating was assessed by the item “During the past 4 weeks, I experienced difficulty concentrating” (with possible responses of “none of the time,” “a little of the time,” “some of the time,” “most of the time,” and “all of the time”), from the Lupus Impact Tracker (LIT) (24). We dichotomized difficulty concentrating as all/most vs. some/little/none of the time.
Other variables
Sociodemographics, including age, sex, race, years of education completed, employment status, income, and marital status, were self-reported at baseline. Age was grouped into categories of 20–39, 40–59, and >60 years for stratified analyses. Current disease activity was assessed via SLAQ (23) (range, 0–44, with higher scores indicating greater SLE-related disease activity). Strata for high vs. low disease activity were defined by the median SLAQ score, excluding the forgetfulness item. SLE duration was calculated as the number of years between age at SLE onset and age at survey. SLE-related organ damage was assessed via the self-administered Brief Index of Lupus Damage (BILD; range, 0–30, with higher scores indicating greater levels of damage) (25, 26).
Statistical Analysis
Sociodemographic and clinical patient characteristics were summarized overall, and PSS scores and cognitive symptoms were summarized overall and by select characteristics. Odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between dichotomized cognitive symptoms and PSS score were estimated with multivariable logistic regression models. PSS score was transformed such that resulting ORs representing relative change in odds per minimal important difference (MID), estimated as 0.5*SD (27), since, to our knowledge, there is no published minimal clinically important difference for this non-clinical assessment, particularly in SLE (25). Adjustment for a priori confounders age, sex, race, education, and disease activity was performed. In sensitivity analyses, we examined the effects of additional adjustment for employment, income, marital status, and current steroid use. Stata v. 14 (StataCorp, College Station, TX) was used for all analyses and the threshold for statistical significance was set at α =0.05.
Results
Patient Characteristics
The mean age of patients at baseline was 46.4 years; 6.4% were male and 80.3% were African-American (Table 1). Most had at least 12 years of education, but only 35% of patients were employed (with 67% of those who were not working being disabled) and nearly half earned <$20,000 annually (Table 1). The mean duration of SLE at baseline was 14 years, and the median disease activity (SLAQ) score, excluding the forgetfulness symptom, was 15 (Table 1).
Table 1.
Baseline characteristics of a cohort of systemic lupus erythematosus patients, August 2011–July 2012
| Characteristic | N | Overall |
|---|---|---|
| Sociodemographic | ||
| Mean (SD) age at survey | 777 | 46.4 (13.3) |
| Male, n (%) | 777 | 50 (6.4) |
| African-American race, n (%) | 777 | 624 (80.3) |
| Years of education, n (%) | 774 | |
| <12 | 71 (9.2) | |
| 12–15 | 456 (58.9) | |
| 16+ | 247 (31.9) | |
| Employment, n (%) | 776 | |
| Employed | 272 (35.1) | |
| Not employed* | 166 (21.4) | |
| Disabled | 338 (43.6) | |
| Income, n (%) | 754 | |
| <$20,000 | 368 (48.8) | |
| $20–49,999 | 200 (26.5) | |
| ≥$50,000 | 186 (24.7) | |
| Marital status, n (%) | 777 | |
| Never married | 276 (35.5) | |
| Married | 271 (34.9) | |
| Separated/divorced/widowed | 230 (29.6) | |
| Clinical | ||
| Mean (SD) duration of SLE, years | 775 | 13.6 (9.3) |
| Mean (SD) BMI | 774 | 29.0 (7.6) |
| On steroids, n (%) | 746 | 425 (57.0%) |
| Median (IQR) SLAQ score** | 777 | 15 (9–22) |
| Median (IQR) BILD score | 777 | 2 (0–3) |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; BILD, Brief Index of Lupus Damage; SLAQ, Systemic Lupus Activity Questionnaire.
Includes retired, student, and homemaker.
Excluding the forgetfulness item.
Perceived Stress
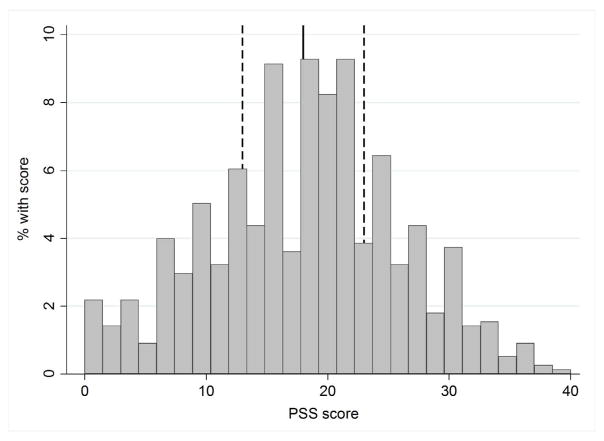
The overall mean PSS score was 18, with the entire range of possible scores (0–40) represented in the cohort (Figure 1); 16.1% and 6.1% reported scores <10 and >30, respectively. PSS scores were, on average, higher in younger patients, patients with fewer years of education and with lower income, and patients who were not married (Table 2). African-American and female participants reported higher stress scores than white and male participants, respectively, but no statistically significant differences by race or sex were noted. On average, PSS scores were 1.5-fold higher in participants reporting higher vs. lower disease activity (mean scores of 22 vs. 14; Table 1).
Figure 1.
Distribution of perceived stress scores among a cohort of systemic lupus erythematosus patients, August 2011–July 2012. Solid line, mean/median (=18); dashed lines, interquartile range (=13–23). IQR, interquartile range; PSS, Perceived Stress Scale.
Table 2.
Perceived stress and cognitive symptoms among a cohort of systemic lupus erythematosus patients, August 2011–July 2012, overall and by patient characteristics
| Patient characteristic | N | PSS score,* Mean (SD) | Reported cognitive symptom,**
n (%)
|
|
|---|---|---|---|---|
| Forgetfulness | Difficulty concentrating | |||
| Overall | 777 | 18.0 (8.1) | 324 (41.7%) | 229 (29.5%) |
| Age | ||||
| 18–39 | 252 | 19.2 (8.1) | 110 (43.7%) | 67 (26.6%) |
| 40–59 | 398 | 17.9 (8.0) | 169 (42.5%) | 127 (31.9%) |
| 60+ | 127 | 15.8 (7.8) | 45 (35.4%) | 35 (27.6%) |
| P*** | <0.001 | 0.28 | 0.31 | |
| Race | ||||
| African-American | 624 | 18.2 (8.1) | 263 (42.2%) | 184 (29.5%) |
| White | 153 | 17.3 (8.0) | 61 (39.9%) | 45 (29.4%) |
| P*** | 0.23 | 0.61 | >0.9 | |
| Sex | ||||
| Male | 50 | 16.9 (7.6) | 11 (22.0%) | 10 (20.0%) |
| Female | 727 | 18.1 (8.1) | 313 (43.1%) | 219 (30.1%) |
| P*** | 0.33 | 0.003 | <0.001 | |
| Years of education | ||||
| <12 | 71 | 19.8 (6.7) | 33 (46.5%) | 24 (33.8%) |
| 12–15 | 456 | 18.8 (7.9) | 203 (44.5%) | 150 (32.9%) |
| 16+ | 247 | 16.1 (8.4) | 87 (35.2%) | 55 (22.3%) |
| P*** | <0.001 | 0.04 | 0.009 | |
| Employment | ||||
| Employed | 272 | 16.0 (8.3) | 88 (32.4%) | 49 (18.0%) |
| Not employed | 166 | 16.7 (7.8) | 61 (36.8%) | 42 (25.3%) |
| Disabled | 338 | 20.3 (7.4) | 175 (51.8%) | 138 (40.8%) |
| P*** | <0.001 | <0.001 | <0.001 | |
| Income | ||||
| <$20,000 | 368 | 19.8 (7.4) | 174 (47.3%) | 131 (35.6%) |
| $20–49,999 | 200 | 17.8 (8.0) | 90 (45.0%) | 59 (29.5%) |
| ≥$50,000 | 186 | 15.0 (6.8) | 53 (28.5%) | 36 (19.4%) |
| P*** | <0.001 | <0.001 | <0.001 | |
| Marital status | ||||
| Never married | 176 | 18.5 (7.9) | 117 (42.4%) | 78 (28.3%) |
| Married | 271 | 16.8 (8.2) | 103 (38.0%) | 69 (25.5%) |
| Separated/divorced/widowed | 230 | 18.9 (7.9) | 104 (45.2%) | 82 (35.7%) |
| P*** | 0.006 | 0.25 | 0.04 | |
| Disease activity**** | ||||
| High | 412 | 21.5 (7.0) | 242 (58.7%) | 187 (45.4%) |
| Low | 365 | 14.0 (7.3) | 82 (22.5%) | 42 (11.5%) |
| P*** | <0.001 | <0.001 | <0.001 | |
PSS, Perceived Stress Scale. Score range, 0–40.
Forgetfulness, severe/moderate vs. mild/none; difficulty concentrating, all/most vs. some/little/none of the time.
By t test, ANOVA, or chi-square test, as appropriate.
Systemic Lupus Activity Questionnaire score (excluding forgetfulness item) ≥15 (high) or <15 (low).
Association of Perceived Stress with Reported Cognitive Symptoms in a Cohort of SLE Patients
Forgetfulness
Overall, 42% of patients reported severe or moderate forgetfulness (Table 2); 34% of these patients (14% of all patients) reported severe forgetfulness. Female patients were nearly twice as likely as male patients to report severe or moderate forgetfulness (43% vs. 22%, P=0.003), but no differences by age or race were seen (Table 2). Patients with the most years of education and highest income reported were less likely, whereas patients who were not working due to disability were more likely, to report forgetfulness, relative to other patients (Table 2). Forgetfulness was reported nearly 3-fold more often with high vs. low disease activity (59% vs. 22%, P<0.001; Table 2).
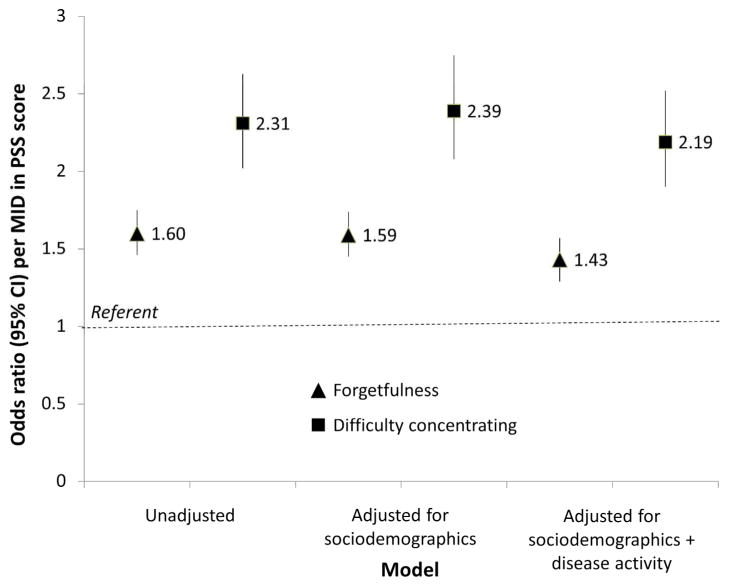
Overall, each MID increase in PSS score was associated with 60% higher reported forgetfulness (OR=1.60, 95% CI 1.46–1.74); adjustment for sociodemographics did not change the association (Figure 2). Further adjustment for disease activity resulted in only a slightly attenuated association (OR=1.43, 95% CI 1.29–1.57), despite high vs. low disease activity being associated with nearly 3-fold greater prevalence of reported forgetfulness in the same model (OR=2.92, 95% CI 2.05–4.15). Additional adjustment for employment, income, marital status, and steroid use did not change the results substantially (data not shown). Finally, stratified analyses showed no statistically significantly differences in association of PSS score with forgetfulness by age, sex, race, or disease activity (Table 3), although the magnitude of the associations were stronger in males vs. females and in those with low vs. high disease activity.
Figure 2.
Association of cognitive symptoms with perceived stress among a cohort of systemic lupus erythematosus patients, August 2011–July 2012. Triangles, forgetfulness (severe/moderate vs. mild/none); squares, difficulty concentrating (all/most vs. some/little/none of the time). MID, minimal important difference (=4.03 points); PSS, perceived stress score. Sociodemographics include age group, sex, race, and education; disease activity includes the Systemic Lupus Activity Questionnaire score (excluding the forgetfulness item).
Table 3.
Association of cognitive symptoms with perceived stress among a cohort of systemic lupus erythematosus patients, August 2011–July 2012, by patient characteristics
| Patient characteristic | Odds ratio (95% CI),per minimal important difference in PSS score (=4.03 points)
|
||
|---|---|---|---|
| Unadjusted | Adjusted* for sociodemographics | Adjusted* for sociodemographics + disease activity | |
| Forgetfulness (severe/moderate vs. mild/none) | |||
|
| |||
| Age | |||
| <40 | 1.74 (1.47–2.07) | 1.77 (1.47–2.12) | 1.59 (1.32–1.93) |
| 40–59 | 1.53 (1.35–1.72) | 1.55 (1.36–1.75) | 1.38 (1.20–1.58) |
| 60+ | 1.58 (1.25–1.99) | 1.59 (1.25–2.02) | 1.47 (1.13–1.91) |
| P** | 0.37 | 0.40 | 0.33 |
| Sex | |||
| Male | 2.99 (1.43–6.22) | 8.58 (1.74–42.3) | 9.37 (1.41–62.4) |
| Female | 1.57 (1.43–1.72) | 1.56 (1.42–1.72) | 1.40 (1.27–1.55) |
| P** | 0.09 | 0.09 | 0.14 |
| Race | |||
| African-American | 1.57 (1.29–1.92) | 1.67 (1.33–2.09) | 1.43 (1.28–1.60) |
| White | 1.60 (1.45–1.77) | 1.59 (1.43–1.76) | 1.49 (1.17–1.89) |
| P** | 0.87 | 0.76 | 0.75 |
| Disease activity*** | |||
| High | 1.33 (1.18–1.51) | 1.33 (1.17–1.50) | 1.33 (1.17–1.50) |
| Low | 1.59 (1.35–1.87) | 1.61 (1.36–1.90) | 1.61 (1.36–1.90) |
| P** | 0.09 | 0.10 | 0.10 |
|
| |||
| Difficulty concentrating (all/most vs. some/little/none of the time) | |||
|
| |||
| Age | |||
| <40 | 2.66 (2.02–3.51) | 2.62 (1.98–3.46) | 2.40 (1.79–3.21) |
| 40–59 | 2.18 (1.83–2.60) | 2.18 (1.83–2.60) | 1.99 (1.66–2.38) |
| 60+ | 2.93 (1.97–4.37) | 3.14 (2.04–4.84) | 3.23 (2.03–5.13) |
| P** | >0.9 | 0.88 | >0.9 |
| Sex | |||
| Male | 2.44 (1.27–4.70) | 2.61 (1.19–5.72) | 1.69 (0.73–3.93) |
| Female | 2.30 (2.01–2.63) | 2.40 (2.08–2.77) | 2.22 (1.91–2.57) |
| P** | 0.86 | 0.88 | 0.87 |
| Race | |||
| African-American | 2.36 (2.03–2.75) | 2.45 (2.09–2.87) | 2.26 (1.92–2.66) |
| White | 2.15 (1.63–2.83) | 2.22 (1.65–2.99) | 1.97 (1.45–2.70) |
| P** | 0.56 | 0.53 | 0.52 |
| Disease activity*** | |||
| High | 2.12 (1.79–2.50) | 2.16 (1.82–2.56) | 2.16 (1.82–2.56) |
| Low | 2.09 (1.64–2.65) | 2.33 (1.77–3.07) | 2.33 (1.77–3.07) |
| P** | 0.24 | >0.9 | >0.9 |
PSS, Perceived Stress Scale.
Adjusted for: sociodemographics (age group, sex, race, and education) and disease activity (Systemic Lupus Activity Questionnaire score, excluding the forgetfulness item).
P for interaction of characteristic with perceived stress score.
Systemic Lupus Activity Questionnaire score (excluding forgetfulness item) ≥15 (high) or <15 (low).
Difficulty concentrating
Nearly one-third (30%) of patients reported difficulty concentrating all or most of the time (Table 2); 35% of these patients (10% of all patients) reported difficulty concentrating all of the time. Female patients were 1.5-fold more likely than male patients to report difficulty concentrating all or most of the time (30% vs. 20%, P<0.001), but there were no differences by age or race (Table 2). As with forgetfulness, patients with the most years of education and highest income reported were less likely, and patients who were not working due to disability were more likely, to report difficulty concentrating, relative to other patients (Table 2). Those who were separated, divorced, or widowed were also more likely than married or never-married patients to report difficulty concentrating. Difficulty concentrating was reported nearly 4-fold more often with high vs. low disease activity (45% vs. 12%, P<0.001; Table 2).
Overall, each MID increase in PSS score was associated with 2.3-fold higher prevalence of reported difficulty concentrating (OR=2.31, 95% CI 1.90–2.52); adjustment for sociodemographics did not change the association (Figure 2). Further adjustment for disease activity resulted in only a slightly attenuated association (OR=2.19, 95% CI 1.90–2.52), although high vs. low disease activity was associated with 2.7-fold greater prevalence of difficulty concentrating (OR=2.70, 95% CI 1.75–4.19). Additional adjustment for employment, income, marital status, and steroid use did not change the results substantially (data not shown). Finally, there were no differences in association of PSS score with difficulty concentrating by age, sex, race, or disease activity (Table 3).
Discussion
In this large, predominantly African-American U.S. cohort of SLE patients, we found perceived stress levels are high, with only about one-quarter of the population having scores below the comparable mean score in the general U.S. population (22). Furthermore, >40% of these patients reported moderate-to-severe problems with forgetfulness, and nearly 30% reported difficulty concentrating all or most of the time. Both stress scores and prevalence of these cognitive symptoms were positively associated with younger age, less education, lower income, and high disease activity. However, even with adjustment for these and other potentially confounding factors, higher stress scores remained strongly associated with higher prevalence of these cognitive symptoms.
These results suggest that, in addition to managing disease activity, an approach that also includes stress reduction may be necessary to manage cognitive symptoms in patients with SLE. For example, self-management programs may work to decrease stress among SLE patients (29). Cognitive behavioral therapy has also been shown to decrease perceived stress in the SLE population (30). In another randomized study of SLE patients (32), a stress reduction program consisting of biofeedback-assisted cognitive behavioral therapy reduced pain perception and improved psychological and physical functioning. Although not currently available on a routine basis, these types of interventions could have multiple patient-centered benefits, in addition to the potential for decreasing perceived stress and improving cognitive symptoms.
One limitation of our study is the lack of measured cognitive performance. Peralta-Ramirez et al. (16) looked at the association between stress and measured cognitive domains (e.g., visual memory, fluency attention) in 21 Spanish patients and found a similar association between higher stress and poorer cognitive function. However, while the self-reported cognitive symptoms that were used as outcomes in this study may be only perceived and may not reflect underlying changes in cognitive processes, these symptoms remain associated with concomitant stress. Furthermore, SLE patients see their perceived cognitive impact of lupus (including impaired ability to think clearly, concentration problems, and memory problems) as a primary component of their overall health-related quality of life, which further affects other important aspects of their quality of life, particularly employment and social functioning (32).
We also have the advantage of being able to examine many more patients who are more representative of the diverse U.S. population with SLE than in previous studies such as Peralta-Ramirez et al. (16). We were able to leverage this population to examine stress, cognitive symptoms, and their association across several subgroups of interest. Interestingly, older age (≥60 years) was not associated with higher prevalence of cognitive symptoms, as might be expected for indicators of cognitive dysfunction; in fact, the younger patients reported higher prevalence of these symptoms, as well as higher stress levels. While fewer reported cognitive symptoms among older patients may reflect changing expectations and better coping strategies with increasing age, it is still important to note that younger patients, who are more likely to be in school or to be employed or seeking employment, are reporting high prevalence of these potentially devastating cognitive symptoms. Male SLE patients were far less likely than female SLE patients to report cognitive symptoms, but our results suggest that the effect of stress on cognitive symptoms may be stronger in male vs. female SLE patients. However, the latter results did not reach statistical significance, which may be due to lack of power, given the small sample of male SLE patients. Notably, there were no differences by race in reported stress, cognitive symptoms, or their association, despite generally poorer SLE outcomes in African-American vs. white SLE patients. Other psychosocial factors, including fewer years of education, disabled employment status, lower income, and unmarried status, were all associated with higher reported stress and higher prevalence of cognitive symptoms. Disease activity was a stronger predictor of stress and cognitive symptoms than any individual psychosocial factor. However, disease activity and psychosocial factors combined still did not explain the strong association between higher stress and higher prevalence of reported cognitive symptoms. Even additional adjustment for other factors such as use of steroids, which have been shown to be associated with reduced cognitive function (33), did not change the association.
There are several important limitations to this work not discussed above. In this cross-sectional study, causal inference is limited. Further, the cross-sectional nature of the study does not allow for longitudinal examinations of cognitive symptoms or chronic stress (e.g., monthly stress inventories implemented by Peralta-Ramirez et al. (16)) or for the ability to distinguish effects of perceived stress on subsequent disease activity vs. effects of disease activity on subsequent perceived stress. However, regardless of the direction of this association, our results still suggest that a clinical approach that addresses both disease activity and stress reduction is likely to be more effective than an approach that primarily addresses disease activity. Another important limitation is that we did not have a comparison group; thus, we cannot know whether the prevalence of cognitive symptoms is higher than that in a similar or even matched population. Despite this, knowledge of the association between stress and cognitive symptoms among SLE patients still informs their clinical care. Finally, as in any observational study, there is the possibility of residual confounding. For example, unmeasured factors such as social support and coping strategies (12, 34, 35) might at least partially explain our results. Our study also had important strengths, including relative large sample sizes allowing subgroup analyses, a population-based sample of SLE patients, and adequate representation of African-American SLE patients.
SLE patients of all ages may be highly susceptible to cognitive symptoms, which are often considered to be geriatric issues in the general population. Further, these cognitive symptoms are strongly associated with higher perceived stress, regardless of disease activity or other patient characteristics. Stress is a modifiable risk factor that could be incorporated into a more patient-centered approach to cognitive symptoms in SLE.
Acknowledgments
We thank the participants of the GOAL study.
Funding
This study was supported in part by PHS Grant UL1TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Advancing Translational Sciences. The GOAL study was supported by Human Genome Science Inc. and GlaxoSmithKline (GHO-11-3366). S.S.L. and C.D. are supported by the NIH (R01AR065493) and the CDC (U01DP005119).
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.Monastero R, Bettini P, Del Zotto E, Cottini E, Tincani A, Balestrieri G, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184:33–39. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 2.Olazaran J, Lopez-Longo J, Cruz I, Bittini A, Carreno L. Cognitive dysfunction in systemic lupus erythematosus: prevalence and correlates. Eur Neurol. 2009;62:49–55. doi: 10.1159/000215879. [DOI] [PubMed] [Google Scholar]
- 3.Utset TO, Fink J, Doninger NA. Prevalence of neurocognitive dysfunction and other clinical manifestations in disabled patients with systemic lupus erythematosus. J Rheumatol. 2006;33:531–538. [PubMed] [Google Scholar]
- 4.Mackay M. Lupus brain fog: a biologic perspective on cognitive impairment, depression, and fatigue in systemic lupus erythematosus. Immunol Res. 2015;63:26–37. doi: 10.1007/s12026-015-8716-3. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y, Lau EY, Wan JH, Lau CS, Mok MY. Systemic lupus erythematosus patients with past neuropsychiatric involvement are associated with worse cognitive impairment: a longitudinal study. Lupus. 2016;25:637–644. doi: 10.1177/0961203315624022. [DOI] [PubMed] [Google Scholar]
- 6.Tay SH, Mak A. Anti-NR2A/B antibodies and other major molecular mechanisms in the pathogenesis of cognitive dysfunction in systemic lupus erythematosus. Int J Mol Sci. 2015;16:10281–102300. doi: 10.3390/ijms160510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz MJ, Derby CA, Wang C, Sliwinski MJ, Ezzati A, Zimmerman ME, et al. Influence of perceived stress on incident amnestic mild cognitive impairment: results from the Einstein Aging Study. Alzheimer Dis Assoc Disorder. 2016;30:93–98. doi: 10.1097/WAD.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz E, Sliwinski MJ, Scott SB, Hofer S. Global perceived stress predicts cognitive change among older adults. Psychol Aging. 2015;30:487–499. doi: 10.1037/pag0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, et al. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol. 2015;21:422–432. doi: 10.1007/s13365-015-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips SM, Lloyd GR, Awick EA, McAuley E. Relationship between self-reported and objectively measured physical activity and subjective memory impairment in breast cancer survivors: role of self-efficacy, fatigue and distress. Psycho-oncology. doi: 10.1002/pon.4156. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beier M, Amtmann D, Ehde DM. Beyond depression: Predictors of self-reported cognitive function in adults living with MS. Rehab Psychol. 2015;60:254–262. doi: 10.1037/rep0000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozora E, Ellison MC, Waxmonsky JA, Wamboldt FS, Patterson TL. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus. 2005;14:363–372. doi: 10.1191/0961203305lu2094oa. [DOI] [PubMed] [Google Scholar]
- 13.Aberer E. Epidemiologic, socioeconomic and psychosocial aspects in lupus erythematosus. Lupus. 2010;19:1118–1124. doi: 10.1177/0961203310370348. [DOI] [PubMed] [Google Scholar]
- 14.Wallace DJ, Hahn BH. Dubois’ Lupus Erythematosus and Related Syndromes. Elsevier-Saunders; 2013. [Google Scholar]
- 15.Bertsias GK, Ioannidis JP, Aringer M, Bollen E, Bombardieri S, Bruce IN, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69:2074–2082. doi: 10.1136/ard.2010.130476. [DOI] [PubMed] [Google Scholar]
- 16.Peralta-Ramirez MI, Coin-Mejias MA, Jimenez-Alonso J, Ortego-Centeno N, Callejas-Rubio JL, Caracuel-Romero A, et al. Stress as a predictor of cognitive functioning in lupus. Lupus. 2006;15:858–864. doi: 10.1177/0961203306071404. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SA, Gonzalez-Prendes AA. Powerlessness, anger, and stress in African American women: implications for physical and emotional health. Health Care Women Int. 2009;30:93–113. doi: 10.1080/07399330802523709. [DOI] [PubMed] [Google Scholar]
- 18.Drenkard C, Rask KJ, Easley KA, Bao G, Lim SS. Primary preventive services in patients with systemic lupus erythematosus: study from a population-based sample in Southeast U.S. Semin Arthritis Rheum. 2013;43:209–216. doi: 10.1016/j.semarthrit.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthr Rheumatol. 2014;66:357–368. doi: 10.1002/art.38239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 22.Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. SAGE; 1988. pp. 31–67. [Google Scholar]
- 23.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–286. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 24.Jolly M, Garris CP, Mikolaitis RA, Jhingran PM, Dennis G, Wallace DJ, et al. Development and validation of the Lupus Impact Tracker: a patient-completed tool for clinical practice to assess and monitor the impact of systemic lupus erythematosus. Arthritis Care Res. 2014;66:1542–1550. doi: 10.1002/acr.22349. [DOI] [PubMed] [Google Scholar]
- 25.Yazdany J, Trupin L, Gansky SA, Dall’era M, Yelin EH, Criswell LA, et al. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res. 2011;63:1170–1177. doi: 10.1002/acr.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drenkard C, Yazdany J, Trupin L, Katz PP, Dunlop-Thomas C, Bao G, et al. Validity of a self-administered version of the brief index of lupus damage in a predominantly african american systemic lupus erythematosus cohort. Arthritis Care Res. 2014;66:888–896. doi: 10.1002/acr.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 28.Rai SK, Yazdany J, Fortin PR, Avina-Zubieta JA. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthr Res Ther. 2015;17:143. doi: 10.1186/s13075-015-0658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams EM, Penfield M, Kamen D, Oates JC. An intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: the Balancing Lupus Experiences with Stress Strategies Study. Open J Prevent Med. 2014;4:22–31. doi: 10.4236/ojpm.2014.41005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarrete-Navarrete N, Peralta-Ramirez MI, Sabio JM, Martinez-Egea I, Santos-Ruiz A, Jimenez-Alonso J. Quality-of-life predictor factors in patients with SLE and their modification after cognitive behavioural therapy. Lupus. 2010;19:1632–1639. doi: 10.1177/0961203310378413. [DOI] [PubMed] [Google Scholar]
- 31.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum. 2004;51:625–634. doi: 10.1002/art.20533. [DOI] [PubMed] [Google Scholar]
- 32.Gallop K, Nixon A, Swinburn P, Sterling KL, Naegeli AN, Silk ME. Development of a conceptual model of health-related quality of life for systemic lupus erythematosus from the patient’s perspective. Lupus. 2012;21:934–943. doi: 10.1177/0961203312441980. [DOI] [PubMed] [Google Scholar]
- 33.Montero-Lopez E, Santos-Ruiz A, Navarrete-Navarrete N, Ortego-Centeno N, Perez-Garcia M, Peralta-Ramirez MI. The effects of corticosteroids on cognitive flexibility and decision-making in women with lupus. Lupus. doi: 10.1177/0961203316642313. (in press) [DOI] [PubMed] [Google Scholar]
- 34.Mazzoni D, Cicognani E. Positive and problematic support, stress and quality of life in patients with systemic lupus erythematosus. Anxiety Stress Coping. 2016;29:542–551. doi: 10.1080/10615806.2015.1134785. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi S, Ghisi M, Iaccarino L, Zampieri S, Ghirardello A, Sarzi-Puttini P, et al. Influence of coping skills on health-related quality of life in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;55:427–433. doi: 10.1002/art.21993. [DOI] [PubMed] [Google Scholar]