Abstract
Background:
Gastrointestinal (GI) malignancies are the most common cancers and account for nearly half of all cancer-related deaths in Iran. There was a strong association between human papillomavirus (HPV) infection and urogenital cancers, in particular the cervix. However, there is no clear causal relationship in all types of cancers, including gastrointestinal cancers. Therefore, the present study as a systematic review and meta-analysis was designed to evaluate the prevalence and relation of HPV in GI cancers.
Methods:
This systematic review and meta-analysis study assess the prevalence of human papillomavirus in GI cancers in Iran. Data were collected by searching electronic databases, including PubMed, Google Scholar, Scopus, SID and Iranmedex by English and Persian key words up to August 2016. Key words included: Human Papillomavirus, HPV, Cancer, Neoplasm, Carcinoma, Esophageal, colorectal, Gastrointestinal and Iran articles were entered in the EndNote software and duplicate papers were excluded. Data were extracted and analyzed by comprehensive meta-analysis software, Version 2 (CMA.V2) and random effects model.
Results:
Finally, we included 17 studies in this meta-analysis. The prevalence of HPV in Iranian patients with GI cancers was 16.4% (CI95%: 10.4-24.9). Considering all HPV types, the odds ratio of GI cancers in positive patients was 3.03 (CI95%: 1.42-6.45) while in patients with HPV-16 was 3.62 (CI: 1.43-4.82).
Conclusion:
The results show a strong relationship between HPV infection especially high-risk HPV type 16 and GI cancers in Iranian population.
Key Words: Gastrointestinal cancers, Human papillomavirus, Systematic review, Meta-analysis
Asian countries such as Iran are encountering increased prevalence and mortality caused by cancer and ranks third as the cause of death after cardiovascular disorders and road traffic injuries (1). Due to demographic changes and increasing life expectancy in Iran, it has been estimated that the incidence of cancer increases from 84,800 cases in 2012 and will rise to 129,700 in 2025 (a 35% increase) (2). This rapid development of different types of cancer has directed the medical scientists and researchers to answer a variety of questions about the sources and reasons of this disease. The increased middle-aged and elderly population, advancements in the new diagnostic tools and screening as a standalone test, cannot be a proper justification for the rising growth of this disease in different societies around the world (3, 4). This issue has remarkably highlighted the other causing factors as the new sources of this disease as lifestyle-related, genetic factors and disease-promoting factors, like microorganisms (bacteria, parasites particularly viruses) (5). It is estimated that 15 to 20% of all cancers may be attributed to viral agents (6-9).
HPV as well as H. pylori, hepatitis B and C viruses are among the well-known that causes cancer infectious agents (10, 11). Primary infection of HPV is acquired through contact with skin lesions, which is usually detected and eliminated by the immune system. In case of persistent infection, virus integrates into host DNA and through increase cell proliferation and disruption, tumor suppressor pathways facilitate the development of cancer (12). HPV develops a range of epithelial hyperplasia and depending on the strength of lesion in the creation of progressive malignancy, two mucosal and cutaneous groups are classified. Mucosal low-risk HPV such as HPV6 and HPV11 create genital warts, while high-risk types like HPV16 and HPV 18 can lead to squamous intraepithelial lesions which can progress to invasive squamous cell carcinoma stage (13).
In 2013, it was stated that of the 12.7 million cancers in the world, 4.8% (about 610,000 cases) may be attributed to HPV. This statistics ranges from 15.5% in India to 1.2% in Australia (14).
HPV is a well-established risk factor for developing urogenital/ oropharynx cancers in particular the cervix (76% in Iranian patients) (15). Nonetheless, there is no clear causal relationship in all types of cancers, including cancers of the digestive (gastrointestinal) system. GI malignancies are the most common cancers and account for almost half of all cancer-related mortality in Iranian population. In the meantime, gastric, esophageal and colorectal cancers are parts of the five most prevalent cancers in Iran (1, 16). Multiple risk factors are involved in the development of these malignancies such as family history, smoking, consumption of alcohol and hot foods, genetic mutations and infections. HPV oncogenic types have been detected in esophageal and colorectal cancers but studies on this topic are conflicting (1).
The present range of HPV DNA in colorectal and esophageal cancers in Iran is reported between 0 -34.2% (17, 18) and 0-37.5% (19, 20), respectively. Thus, according to the conflicts and the absence of a comprehensive review about the prevalence and association between HPV infections and GI cancers, we aimed to perform a systematic review and meta-analysis on this issue.
Methods
Literature search strategy: This systematic review and meta-analysis investigated the prevalence and relation between HPV infection and GI cancers in Iran. International electronic databases; PubMed, Scopus, Google Scholar and National databases including Scientific Information Database, Iranmedex, and Magiran were systematically searched up to August 2016. Key words were selected according to medical subheading (MeSH) with all possible meaningful combinations including; Colorectal, Neoplasm, Esophageal, Cancer, Human papilloma virus affiliated to Iran and appropriate equivalents in Farsi.
Inclusion and exclusion criteria: All case-control and cross-sectional studies conducted on HPV infection in GI cancers affiliated to Iran were included in the research. Other article types including case report/series, letter to editor and review were eliminated. Studies that examined HPV seropositivity or detection techniques other than polymerase chain reaction (PCR) were eliminated as well.
Study Selection: Selected articles were assessed by two researchers independently. Initial abstracts and full texts were evaluated. A possible discrepancy between the reviewers was resolved by discussions.
Study Quality Control: Diversified aspects of the methodology including type of survey, measurement and diagnostic methods and statistical analysis were evaluated according to the Strobe checklist.
Data Extraction: According to the prepared checklist, author name, year of study, type of cancer, type of specimen, number of patients, number of healthy controls (in case-control studies), number of positive samples in each group and detected HPV type were extracted.
Data Analysis: Finally, data were analyzed using the comprehensive meta-analysis software version 2.0 and random effects method. Forest diagrams were plotted for the prevalence of HPV in GI cancer and the relationship between HPV and GI cancers. Publication bias examples were evaluated by Funnel plot and were drawn for the relationship between HPV and GI cancers. Heterogeneity was checked by I2 index.
Results
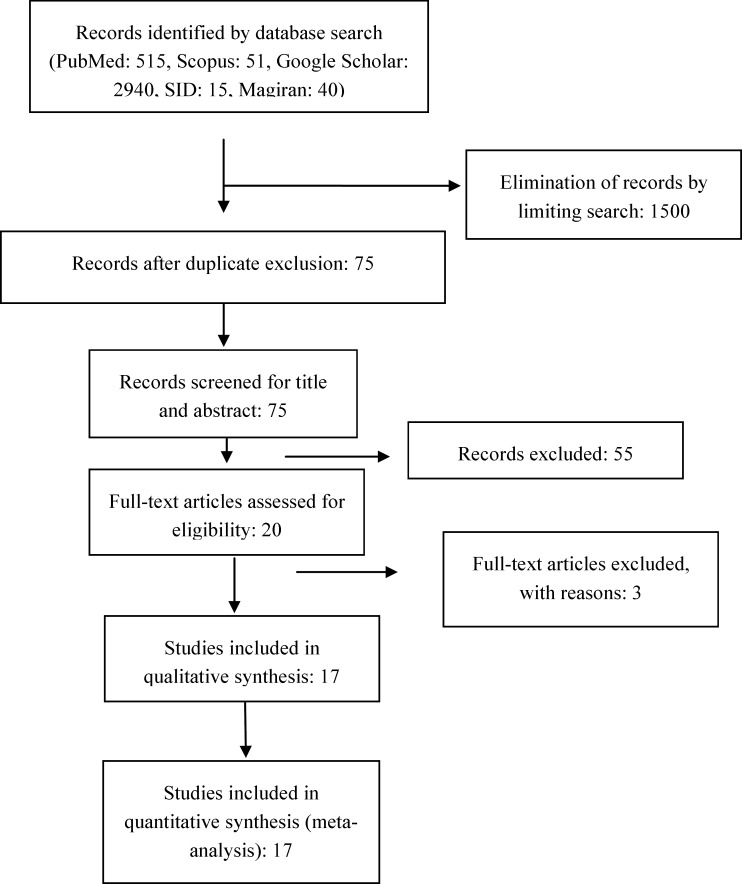
With all possible keyword combinations, our primary search in mentioned databases yielded 3561 articles (figure 1). After removing and eliminating duplicate records by limiting search, two authors (R.A and V.O) independently evaluated the abstracts and full texts of the remaining 75 articles. In this part, papers using diagnostic methods other than PCR (i.e. serology-based), review, case report/series and studies lacking the required qualities were excluded. Finally, we entered 17 studies in this meta-analysis conducted during 2005-2016.
Figure 1.
PRISMA diagram of study identification and selection
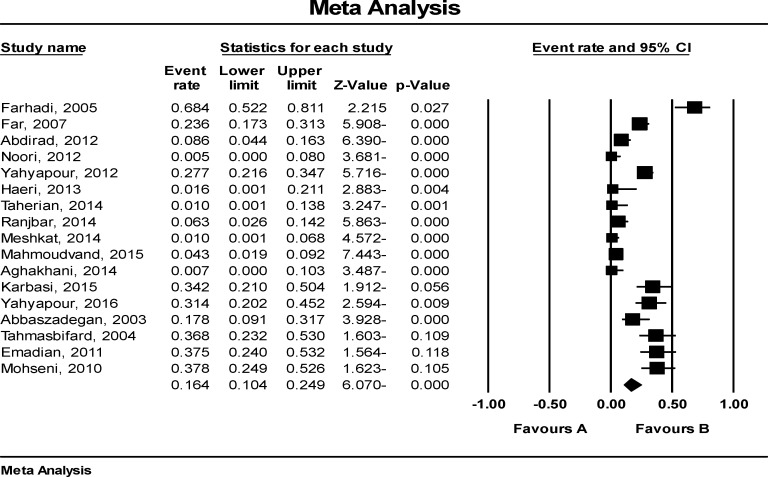
A total of 1337 patients and 561 controls were investigated to assess the prevalence and the relationship between HPV and GI cancer. Features of study participants are shown in table 1. Studies were carried out in five different provinces in Iran: Tehran (21), Mazandaran (12), Mashhad (20), Shiraz (2) and Gilan (1). All of the specimens were paraffin-embedded tissues with the exception of one study (fresh frozen). As shown in figure 2, the prevalence of HPV in Iranian patients with GI cancer was 16.4% (CI 95%: 10.4-24.9).
Table 1.
Characteristics of studies enrolled in the systematic review and meta-analysis investigating the prevalence and association between HPV infection and GI cancers
| Author | Province | Year of publication | Cancer type | Specimen | No. case | No. positive | No. ctrl | No. positive | Detection method | HPV-Type detected |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbaszadegan et al. (20) | Mash’had | 2003 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 45 | 8 | - | - | HPV16/18: E6/E7 | HPV16 |
| Tahmasebi Fard et al.(21) | Tehran | 2004 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 38 | 14 | 38 | 5 | L1 gene: MY09/MY11 HPV16/18: E6/E7 |
HPV16,18 |
| Farhadi et al. (22) | Tehran | 2005 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 38 | 14 | 38 | 5 | L1 gene: MY09/MY11 HPV16/18: E6/E7 |
HPV18,16 |
| Far et al. (23) | Tehran | 2007 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 140 | 33 | 140 | 12 | L1 gene: GP5+/GP6+ sequencing |
HPV16,18,33 and 31 |
| Mohseni et al. (24) | Gilan | 2010 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 45 | 17 | - | - | L1 gene: GP5+/GP6+ genotyping |
HPV16,18, 31,33,51,52,58 |
| Emadian et al. (19) | Mazandaran | 2011 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 40 | 15 | 40 | 5 | PCR, genotyping by Kit | HPV16,45 |
| Abdirad et al. (25) | Tehran | 2012 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 93 | 8 | - | - | L1 gene: SPF10 sequencing |
HPV6,16 and 18 |
| Noori et al. (18) | Shiraz | 2012 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 92 | 0 | - | - | L1 gene: GP5+/GP6+ | - |
| Yahyapour et al. (26) | Mazandaran | 2012 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 177 | 49 | - | - | L1 gene: MY09/MY11 | - |
| Haeri et al. (27) | Tehran | 2013 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 30 | 0 | 30 | 0 | L1 gene: GP5+/GP6+ | - |
| Yahyapour et al. (28) | Mazandaran | 2016 | esophageal squamous cell carcinoma (ESCC) | paraffin blocks | 51 | 16 | 45 | 20 | L1 gene: MY09/MY11 genotyping |
HPV11,16, 33,35,39,45, 52,56 58 and 59 |
| Taherian et al. (29) | Tehran | 2014 | Colorectal cancer | paraffin blocks | 50 | 0 | 50 | 0 | L1 gene: MY09/MY11 | - |
| Ranjbar et al. (30) | Tehran | 2014 | colorectal cancer | paraffin blocks | 80 | 5 | 80 | 1 | L1 gene: MY09/MY11, GP5+/GP6+ sequencing |
HPV18 |
| Meshkat et al. (31) |
Mashhad | 2014 | colorectal cancer | paraffin blocks | 100 | 1 | - | - | L1 gene: GP5+/GP6+ | |
| Aghakhani et al. (16) | Tehran | 2014 | Colon adenocarcinoma colon adenoma | 70 70 |
0 0 |
30 | 0 | L1 gene: MY09/MY11, GP5+/GP6+ | - | |
| Mahmoudvand et al. (32) | Shiraz | 2015 | colorectal adenocarcinoma colorectal adenomatous polyps | paraffin blocks | 70 70 |
2 4 |
70 | 0 | L1 gene: MY09/MY11 HPV16/18: E6 |
HPV16,18 |
| Karbasi et al. (17) | Tehran | 2015 | colorectal cancer | fresh frozen tissue | 38 | 13 | - | - | HPV16,18 |
Figure 2.
Forest plot of HPV prevalence in Iranian patients with GI cancers
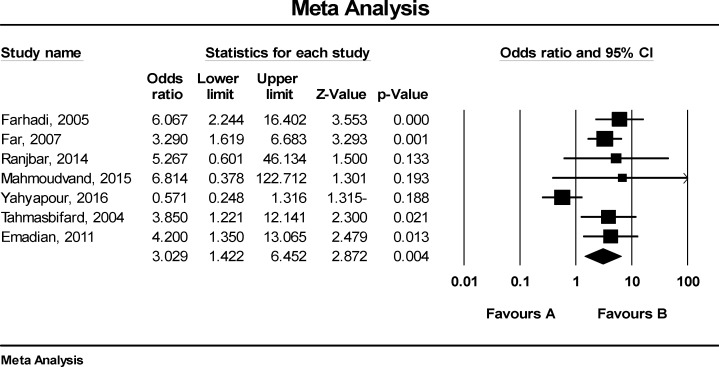
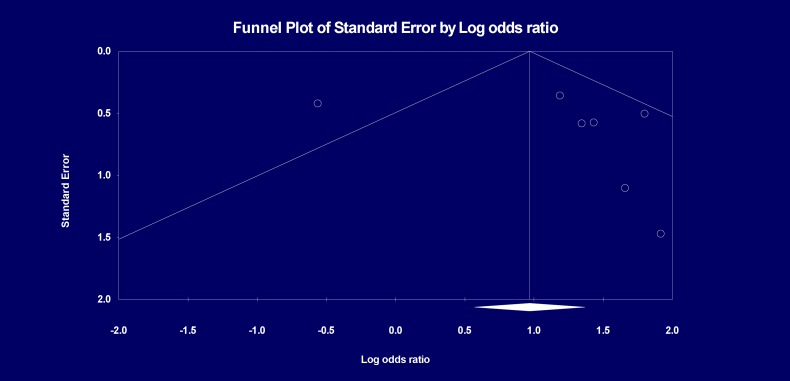
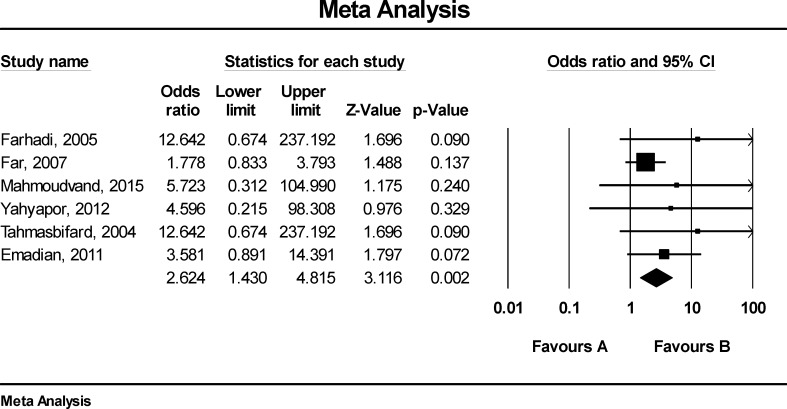
Figure 3 shows that the odds ratio (OR) for GI cancers in HPV positive patient was 3.03 (CI95%: 1.42-6.45). According to figure 4, there was publication bias in the reviewed studies between HPV with GI cancer in Iranian patients. Figure 5 shows that the OR for GI cancers in patients with HPV16 was 3.62 (CI: 1.43-4.82).
Figure 3.
The relationship between HPV and GI cancers in Iranian patients
Figure 4.
Funnel plot of the relationship between HPV and GI cancers in Iranian patients
Figure 5.
The relationship between HPV-16 and GI cancers in Iranian patients
Discussion
The aim of this systematic review and meta-analysis was to determine the prevalence of HPV in gastrointestinal cancers and its relation as a risk factor for these cancers. Result of the present study shows that the prevalence of HPV in Iranian patients with GI cancer was 16.4% and it seems that this rate is higher than the expected value in Bucchi’s review study which reported that the exposure to HPV is very common and the majority of HPV infections are asymptomatic. Nevertheless, there is a 10% chance that individuals can develop a persistent infection and may have an increased risk of developing a cancer (33).
In the present study, the OR of getting cancer in Iranian patients with HPV positive was 3 times more than HPV negative. This rate for HPV16 was 3.6, in other words, there was a strong association between gastrointestinal cancer and infections with HPV particularly HPV16 in Iranian population. A meta-analysis to assess HPV-16/18 in esophageal cancer was performed by Yong et al. Of the 5755 cases, 11.67% and 1.82% harbored HPV-16 and 18, respectively. The OR reported for HPV-16 and risk of cancer (3.55) is quite similar to ours (34). Zhang et al. investigated the association between HPV-16 and esophageal cancer in Chinese population and reported that being infected with HPV-16 increases the chance of esophageal squamous cell carcinoma more than six folds (35). About one-third of samples they analyzed were fresh frozen biopsies which increase the chance of HPV detection. Also, higher OR can be attributed to differences in target populations (36). Li et al. in 2014 carried out a similar meta-analysis on HPV infection and esophageal squamous cell carcinoma and adenocarcinoma worldwide. Overall, the prevalence of HPV was 22.2% in SCC and 35% in adenocarcinoma. In line with our results, the analysis association for HPV-16 and SCC showed an OR of 3.52. This incidence in Asia was approximately two times more than America and Europe (37).
In terms of colorectal cancer in the study of 2630 cases of colon adenocarcinoma, the total prevalence of HPV was 11.2% and the obtained OR of case-control studies was about 6 (38). In contrast to our findings, the most common HPV type found by Damin et al. in Asia was HPV18. As there were fewer case-control reports positive for HPV-18, we did the type specific analysis for HPV-16 only. In addition, the overall incidence and OR in mentioned studies was considerably higher than our results (31.9% and 10.04% respectively). This conflicting result can be affected by the higher incidence of infections in South America (34). Although it has been more than 30 years since the introduction of HPV infection in GI cancers, no strong evidence has been proven with regard to the role of this virus in the mentioned cancers. The pattern of HPV infection is globally diverse and ranges from more than 80% in China to 0% in Brazil (39). The studies enrolled in the present meta-analysis had contrary reports, too. Noori and Haeri with regard to esophageal cancer reported no HPV infection, similar to Taherian and Aghalshai regarding colorectal. High HPV-positive sample was seen in Mohseni et al;s study in the North of Iran considered as a high risk area for gastric cancer (table 1). The differences in diagnostic methods may be responsible for diverse reports of HPV infection; consequently, we included published studies with a similar technique. Accordingly, it is possible that geographic differences in HPV distribution patterns and the existence of other risk factors contributing to HPV-associated cancers be involved in this issue. Hence, it is necessary that future studies in Iran consider the risk factors for GI cancers more precisely.
Another point that should be kept in mind is the existence of HPV DNA which does not indicate an active infection. Most HPV infections are spontaneously eliminated by immune response within one year, and only a small proportion of them established transformed infections. As a result, research targeting viral oncogenes (E6 and E7) through ribonucleic acid (RNA) and protein level can provide more accurate information (40, 41). A (other) meta-analysis done investigating HPV in breast and cervical cancers in Iran has similar outcomes (42, 43) It appears that HPV increased the risk of certain cancers in Iranian population.
As limitations of the current study, all of the ORs extracted from primary study had crude rate and there were no adjusted rates in all studies. Moreover, the strength of the present study as far as we know, is its being the first systematic review and meta-analysis that aimed to investigate the prevalence and relation between HPV and GI cancers in Iranian population. In conclusion, the results of our study indicate that there is a strong relationship between gastrointestinal cancer and HPV infection particularly the high risk HPV type 16 in Iran. HPV screening and preventive vaccine-based policies in high-risk groups need further investigations that should be considered.
Acknowledgments
We are thankful to Mazandaran University of Medical Sciences for their cooperation.
Funding:
Financial support provided.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kolahdoozan S, Sadjadi A, Radmard AR, Khademi H. Five common cancers in Iran. Arch Iran Med. 2010;13:143–6. [PubMed] [Google Scholar]
- 2.Abachizadeh K, Keramatinia A. Anticipating Cancer Rates of Iran in 2025. Community Health. 2016;3:66–73. [Google Scholar]
- 3.Belpomme D, Irigaray P, Sasco AJ, Newby JA, Howard V, Clapp R, et al. The growing incidence of cancer: role of lifestyle and screening detection (Review) Int J Oncol. 2007;30:1037–49. doi: 10.3892/ijo.30.5.1037. [DOI] [PubMed] [Google Scholar]
- 4.Irigaray P, Newby JA, Clapp R, et al. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed Pharmacother. 2007;61:640–58. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Vedham V, Verma M, Mahabir S. Early-life exposures to infectious agents and later cancer development. Cancer Med. 2015;4:1908–22. doi: 10.1002/cam4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano JS, Blaser M, Buendia MA, et al. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14:453–71. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Tokudome S, Hosono A, Suzuki S, et al. Helicobacter pylori infection as an essential factor for stomach cancer. Asian Pac J Cancer Prev. 2006;7:163. [PubMed] [Google Scholar]
- 8.Tokudome S, Ghadimi R, Suzuki S, et al. Helicobacter pylori infection appears the prime risk factor for stomach cancer. Int J Cancer. 2006;119:2991. doi: 10.1002/ijc.22223. [DOI] [PubMed] [Google Scholar]
- 9.Sotuneh N, Hosseini SR, Shokri-Shirvani J, Bijani A, Ghadimi R. Helicobacter pylori infection and metabolic parameters: is there an association in elderly population? Int J Prev Med. 2014;5:1537–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 11.Feller L, Khammissa RA, Wood NH, Lemmer J. Epithelial maturation and molecular biology of oral HPV. Infect Agents Cancer. 2009;4:16. doi: 10.1186/1750-9378-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahyapour Y, Shamsi-Shahrabadi M, Mahmoudi M, et al. High-Risk and Low-Risk Human papillomavirus in esophageal squamous cell carcinoma at Mazandaran, Northern Iran. Pathol Oncol Res. 2013;19:385–91. doi: 10.1007/s12253-012-9590-0. [DOI] [PubMed] [Google Scholar]
- 13.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Khorasanizadeh F, Hassanloo J, Khaksar N, et al. Epidemiology of cervical cancer and human papilloma virus infection among Iranian women - analyses of national data and systematic review of the literature. Gynecol Oncol. 2013;128:277–81. doi: 10.1016/j.ygyno.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Pourhoseingholi MA, Fazeli Z, Ashtari S, Bavand-Pour FS. Mortality trends of gastrointestinal cancers in Iranian population. Gastroenterol Hepatol Bed Bench. 2013;6:S52–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Aghakhani A, Hamkar R, Ramezani A, et al. Lack of human papillomavirus DNA in colon adenocarcinama and adenoma. J Cancer Res Ther. 2014;10:531–4. doi: 10.4103/0973-1482.137674. [DOI] [PubMed] [Google Scholar]
- 17.Karbasi A, Borhani N, Daliri K, Kazemi B, Manoochehri M. Downregulation of external death receptor genes FAS and DR5 in colorectal cancer samples positive for human papillomavirus infection. Pathol Res Pract. 2015;211:444–8. doi: 10.1016/j.prp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Noori S, Monabati A, Ghaderi A. The prevalence of human papilloma virus in esophageal squamous cell carcinoma. Iran J Med Sci. 2012;37:126–33. [PMC free article] [PubMed] [Google Scholar]
- 19.Emadian O, Naghshvar F, Rafiei A, et al. Correlation of human papillomavirus infection with esophageal squamous cell carcinoma. J Babol Univ Med Sci. 2011;13:54–9. [in Persian] [Google Scholar]
- 20.Abbaszadegan MR, Omidi A, Niyazi A, et al. Prevalence of human papillomavirus type 16 and 18 and p53 mutant protein expression in esophageal squamous cell carcinomas. Iran J Basic Med Sci. 2003;6:38–42. [Google Scholar]
- 21.Tahmasebi Fard Z, Farhadi Langroody M, Malekzadeh R, et al. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. Govaresh. 2004;9:21–6. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farhadi M, Tahmasebi Z, Merat S, et al. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200–3. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Far AE, Aghakhani A, Hamkar R, et al. Frequency of human papillomavirus infection in oesophageal squamous cell carcinoma in Iranian patients. Scand J Infect Dis. 2007;39:58–62. doi: 10.1080/00365540600740496. [DOI] [PubMed] [Google Scholar]
- 24.Mohseni Mehran SM. Detection of human papilloma virus in esophageal squamous cell carcinoma in Guilan province. J Clin Diagn Res. 2010;4:2373–7. [Google Scholar]
- 25.Abdirad A, Eram N, Behzadi AH, et al. Human papillomavirus detected in esophageal squamous cell carcinoma in Iran. Eur J Intern Med. 2012;23:e59–62. doi: 10.1016/j.ejim.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Yahyapour Y, Shamsi-Shahrabadi M, Mahmoudi M, et al. Evaluation of human papilloma virus infection in patients with esophageal squamous cell carcinoma from the Caspian Sea area, north of Iran. Asian Pacific J Cancer Prev. 2012;13:1261–6. doi: 10.7314/apjcp.2012.13.4.1261. [DOI] [PubMed] [Google Scholar]
- 27.Haeri H, Mardany O, Asadi-Amoli F, Shahsiah R. Human papilloma virus and esophageal squamous cell carcinoma. Acta Med Iranica. 2013;51:242–5. [PubMed] [Google Scholar]
- 28.Yahyapour Y, Sadeghi F, Alizadeh A, Rajabnia R, Siadati S. Detection of merkel cell polyomavirus and human papillomavirus in esophageal squamous cell carcinomas and non-cancerous esophageal samples in northern Iran. Pathol Oncol Res. 2016;22:667–72. doi: 10.1007/s12253-016-0048-7. [DOI] [PubMed] [Google Scholar]
- 29.Taherian H, Tafvizi F, Fard ZT, Abdirad A. Lack of association between human papillomavirus infection and colorectal cancer. Przeglad Gastroenterol. 2014;9:280–4. doi: 10.5114/pg.2014.46163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranjbar R, Saberfar E, Shamsaie A, Ghasemian E. The aetiological role of human papillomavirus in colorectal carcinoma: an Iranian population- based case control study. Asian Pac J Cancer Prev. 2014;15:1521–5. doi: 10.7314/apjcp.2014.15.4.1521. [DOI] [PubMed] [Google Scholar]
- 31.Meshkat M, Tayyebi Meibodi N, Sepahi S, et al. The frequency of human papillomaviruses in colorectal cancer samples in Mashhad, northeastern Iran. Turk J Med Sci. 2014;44:501–3. doi: 10.3906/sag-1303-81. [DOI] [PubMed] [Google Scholar]
- 32.Mahmoudvand S, Safaei A, Erfani N, Sarvari J. Presence of human papillomavirus DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Asian Pac J Cancer Prev. 2015;16:7883–7. doi: 10.7314/apjcp.2015.16.17.7883. [DOI] [PubMed] [Google Scholar]
- 33.Bucchi D, Stracci F, Buonora N, Masanotti G. Human papillomavirus and gastrointestinal cancer: A review. World J Gastroenterol. 2016;22:7415–30. doi: 10.3748/wjg.v22.i33.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol. 2013;23:726–34. doi: 10.1016/j.annepidem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SK, Guo LW, Chen Q, et al. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta-analysis. BMC Cancer. 2015;15:1096. doi: 10.1186/s12885-015-1096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo LW, Zhang SK, Liu SZ, et al. Human papillomavirus type-18 prevalence in oesophageal cancer in the Chinese population: a meta-analysis. Epidemiol Infect. 2016;144:469–77. doi: 10.1017/S0950268815001703. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Gao C, Yang Y, Zhou F, et al. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39:270–81. doi: 10.1111/apt.12574. [DOI] [PubMed] [Google Scholar]
- 38.Baandrup L, Thomsen LT, Olesen TB, et al. The prevalence of human papillomavirus in colorectal adenomas and adenocarcinomas: a systematic review and meta-analysis. Eur J Cancer. 2014;50:1446–61. doi: 10.1016/j.ejca.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk: a meta-analysis. Colorectal Dis. 2013;15:e420–e8. doi: 10.1111/codi.12257. [DOI] [PubMed] [Google Scholar]
- 40.Cattani P, Siddu A, D'Onghia S, et al. RNA (E6 and E7) assays versus DNA (E6 and E7) assays for risk evaluation for women infected with human papillomavirus. J Clin Microbiol. 2009;47:2136–41. doi: 10.1128/JCM.01733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plummer M, Schiffman M, Castle PE, et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195:1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 42.Haghshenas MR, Mousavi T, Moosazadeh M, Afshari M. Human papillomavirus and breast cancer in Iran: a meta- analysis. Iran J Basic Med Sci. 2016;19:231–7. [PMC free article] [PubMed] [Google Scholar]
- 43.Jalilvand S, Shoja Z, Nourijelyani K, Tohidi HR, Hamkar R. Meta-analysis of type-specific human papillomavirus prevalence in Iranian women with normal cytology, precancerous cervical lesions and invasive cervical cancer: Implications for screening and vaccination. J Med Virol. 2015;87:287–95. doi: 10.1002/jmv.24053. [DOI] [PubMed] [Google Scholar]