Abstract
Background and Objective:
Human papilloma virus (HPV) 16 and HPV18 have been detected in head and neck squamous cell carcinomas (HNSCC) and there is evidence that detection of HPVs would have better prognostic value than patients with HNSCC negative for HPVs. Thus, this study was conducted to evaluate frequency of HPV 16 and HPV 18 genotypes in patients with laryngeal carcinoma.
Materials and methods:
Fifty formalin-fixed, paraffin-embedded (FFPE) tissue blocks of laryngeal cancers were collected. Sections were prepared at 5 µm and DNA was extracted from each sample and subjected to the polymerase chain reaction (PCR) to detect HPV-16/18 DNA s.
Results:
All samples were squamous cell carcinomas (SCCs). Overall 14/50 (28%) were positive for HPVs, 8 (18%) with HPV-16 and 6 (12%) with HPV-18. Additionally, 2 (4%) mixed infections of HPV 16 and 18 genotypes were observed among these cases.
Conclusions:
Overall, 28% of HNSCC samples proved positive for HPV16 and HPV18 genotypes, two high-risk HPV types. It is important to further assess whether such viral infection, could be a risk factor in HNSCC progression.
Keywords: Laryngeal carcinoma, HPV16, HPV18, PCR
Introduction
Head and neck squamous cell carcinoma (HNSCC) comprises cancers of the nasal cavity, sinuses, lips, mouth, salivary glands, throat, and larynx (Kristy, 2015). Laryngeal cancer account for 3% of all the cancers, and sixth common prevalent cancer worldwide (Siegel et al., 2011; Xu et al., 2014). Almost all laryngeal cancers (90-95%) are squamous cell carcinomas (SCC) and males are more prone to infection than females (Hobbs et al., 2004; Güvenç et al., 2008). The survival rate varies between 20% and more than 90%, depending on tumor stage and localization of the primary tumor (Claus et al., 2012). Smoking is a known carcinogenic that can result in changes in the cellular DNA and lead to the enhanced risk for several types of cancers. Many studies exhibit that smoking status can cause the persistence of HPV infection (Haukioja et al., 2014; Kero et al., 2014b). There is possibly an association with poor oral hygiene that results in an increased risk of developing HPV-positive HNSCC (Bui et al, 2013; Tezal et al., 2012). PVs contribute to a wide spectrum of tumors range from benign to malignancy (Hobbs et al., 2004; Torrente et al., 2005). PVs are members of the Papillomaviridae, which can infect humans as well as a variety of animals. More than 150 HPV genotypes cause a variety of epithelial benign and malignancies (Bernard, 2010). PVs genotypes are categorized as low or high risk. Low-risk types, such as HPV-6 and HPV-11, can cause benign papilloma (wart).
However, high-risk types such as HPV-16 and HPV-18 can lead to chronic infection, cellular ab-normalities, and then cancer. HPV-16 is linked to HNSCC of the oropharynx (American Cancer So¬ciety, 2015; Sannigrahi et al., 2016; Ryan et al., 2016). Approximately 90% of HPV-related oropharyngeal cancers are positive for the HPV-16 subtype (Blitzer et al., 2014; Gan et al., 2014; Morshed et al., 2010). The frequency of HPV18 in a lesser extend have been reported among the patients with HNSCC (Luciano et al., 2012; Mohamadian et al.,2014; Nema et al., 2014).
The transforming activity of HPV relies upon E5, E6 and E7 ocnoproteins which can interact with different cellular molecules. The E5 protein is able to interact with cellular epidermal growth factor receptor (EGFR) and result in transforming cells, whereas E6 and E7 proteins can form complexes with cellular tumor suppressor proteins (Rb, p53) and change normal cells to the malignant transformation (Hernandez et al., 2014). Various molecular methods have been applied to determine the potential viral oncoprotein role in laryngeal carcinoma, these techniques are, mainly southern blotting, in situ hybridization, polymerase chain reaction (PCR) and real time PCR (Ru et al., 2015). Laryngeal cancer mostly exhibit with squamous-cell carcinoma (SCC) or squamous-cell cancer (SqCC), which is a cancer of epithelial cell. The knowledge of molecular epidemiology of the HPV genotypes among the patients with laryngeal carcinoma is very limited in Iran, although some studies on HPV16 and HPV18 have been reported among the patients with HNSCC in some regions of Iran (Mahnaz et al., 2009; Jalal et al., 2011). Thus, this study was conducted to determine the frequency of HPV genotypes 16 and 18 in patients with larynx carcinoma in Ahvaz city, Iran. Ahvaz city is the capital city of Khuzestan province with two million population located in the south west region of Iran.
Materials and Methods
Study specimens
For the present retrospective study, 50 blocks of formalin-fixed, paraffin-embedded tissues from patients suffering from laryngeal cancers were collected from the referral Imam Khomeini Hospital, Ahvaz city, Iran during a 10- year period (2004-2014). The diagnosis of laryngeal cancer and tumor grade was approved by a pathologist. The histological grading was performed according to the World Health Organization (WHO) criteria, which divides the tumor into: well differentiated (G1), moderately well differentiated (G2), and poorly differentiated (G3) types (Mohamadian et al., 2014).
DNA extraction from formalin-fixed and paraffin- embedded tissues
The sections of 5 µm were prepared from each block of formalin-fixed, paraffin-embedded tissues of laryngeal cancer and transferred into a 1.5 ml DNase free Microtube. Then, 1000 μl xylen (Merck Company, Germany) was added into each tube, incubated at 45 °C for 15 minutes and centrifuged at 14000rpm. The supernatant was discarded, fresh xylene was added and centrifuged, the pellet was washed two times with 98% ethanol to remove xylen. Ethanol was removed and evaporated completely, the pellet was collected and utilized for DNA extraction. DNA was extracted using high pure PCR template preparation kit (Roche, Germany. The extracted DNA was stored at -70°C until use.
PCR
All the extracted DNA samples were initially subjected to PCR with consensus primers PCO3/PCO4 (β-globin) to confirm the quality of the extracted DNA (used as an internal control). The following primers (PCO3: 5´ ACA CAA CTG TGT TCA CTA GC /PCO4: 5´ CAA CTTC AT CCACGT TCA CC with PCR product of 110 bp (Aguayo et al., 2011; LUCIANO et al., 2012; Shahab et al., 2015).
Polymerase chain reaction (PCR) Amplification
The following primers were used for detection of HPV 16 and 18 DNA (Table 1) 25 µl PCR reaction mixture containing 10 μl DNA template, 2.5μl PCR buffer 10X (Roche), 1.5 mM MgCl2 (Merck Company, Germany), 0.5 mM, dNTP (Roche, Germany), 20 pmol of each primer, 1 U Taq polymerase (Roche, Germany) D/W up to 25 µl. The PCR reaction mixture with positive and negative controls were subjected to thermocycler (TC-512, Techne, UK) and programmed: Initial denaturation, at 95°C for 5 minutes, then 35 cycles of: denaturation, 95°C for 40 seconds, annealing 50°C for 1 minutes, and extension were 72°C for 45 seconds. The final extension cycle was 72°C for 5 minutes. Six μl of PCR products were loaded on the 2% agarose gel and safe stain, electrophoresed for about 1 hour at 100 volts. The results were visualized on UV Transiluminater (Figures 1, 2).
Table 1.
The primer used for HPV16 and HPV18
| Primer Sequence (5-3) | Target | Amplimer length (bp) | Reference |
|---|---|---|---|
| E6F TCAAAAGCCACTGTGTCCTG E6R CGTGTTCTTGATGATCTGCA |
HPV-16 | 120 | Ding, et al., 2010 |
| HPV-18F ACCTTAATGAAAAACGACGA HPV-18R CGTCGTTGGAGTCGTTCCTG |
HPV-18 | 100 | Luciano et al., 2012 |
Figure 1.

The Amplification of E6 Gene Human Papillomavirus (HPV) 16 by PCR. Lane M: DNA Marker (100bp), Lane1: HPV 16-Positive Control (PC), Lanes 3, 4 and 5 Positive HPV 16 Positive Cases, Lane 6: Negative Control (NC).
Figure 2.
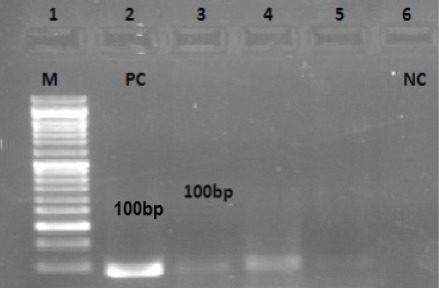
Detection of HPV 18 DNA in Patients with Larynx Carcinoma by PCR. Lane 1:M DNA Marker (100bp), Lane2: HPV 18-Positive Control (PC) (Hella Cell), Lanes 3, 4 and 5 Positive HPV 18 Cases, Lane 6: Negative Control (NP)
Statistical analysis
In addition to demographic data, pathological and molecular results were recorded and analyzed using SPSS V22.0. Chi-square and Fisher’s exact tests were employed to perform statistical comparisons. P-values< 0.05 were considered as statistically significant. Further more logistic regression was used to determine relationship between histological grading and HPV genotypes, and the association value was reported through OR.
Results
Out of 50 patients, 38 patients (76%) were male and 12 patients (24%) were female. The patients’ age was between 23-88 years with mean age of 52±13 years. All laryngeal cancers were diagnosed as a squamous cell carcinomas (SCCs) with grade tumor G1-G3 (table 2). Out of 50 patients, 14 patients (28%) of samples shown to be positive for HPV among the 8 (16%) HPV 16 and 6 (12%) HPV 18, respectively, among them 2 (4%) patients were positive for mixed HPV-16 and HPV-18 genotypes (Figures 1 and 2). Totally, of 50, 11 (22%) males and 3 (6%) females were shown to be positive for HPV infection (Odds ratio = 1.2, 95% CI = 0.3 - 5.4, P= 0.8). Table 2 shows the distribution of HPV16 and HPV18 genotypes diagnosed in squamous cell carcinomas (SCCs) with gender, age, location and histological grade.
Table 2.
Distribution of HPV-16 and HPV-18 Based on Demographic and Clinical Characteristics
| Frequency of HPV t ypes 16 & 18 | Total | Positive | sample (%) | Negative sample (%) | p value | OR | |||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HPV16 | HPV18 | HPV16 & 18 | Total of positive samples | 0.791 | 95% CI | |||
| Gender | Male | 38 | 6 (15.78%) | 5 (13.15%) | 2 (5.26%) | 11 (28.94 %) | 27 (71.0%) | 0.8 (.19, 3.6) | |
| Female | 12 | 2 (16.66%) | 1 (8.33%) | 0 | 3 (25%) | 9 (75.0%) | |||
| Age | ≥50 | 39 | 7 (17.94%) | 4 (10.25%) | 2 (5.12%) | 11 (28.2%) | 28 (71.8%) | 0.951 | 1.0 (.2, 4.7) |
| <50 | 11 | 1 (12.5%) | 2 (25%) | 0 | 3 (21%) | 8 (22.0%) | |||
| Localization | Supraglottic | 8 (16%) | 2 (4%) | 3 (6%) | 1 (2%) | 5 (10%) | 3 (6.0%) | ||
| Glottis | 36 (72%) | 5(10%) | 2 (4%) | 1(2%) | 8 (16%) | 28 (56.0%) | 0.272 | 0.4m(.13, 1.4) | |
| Subglottic | 6 (12%) | 1(2%) | 1 (2%) | 0 | 2(4%) | 4 (8.0%) | |||
| Histologic grade | G1 | 11 (22%) | 2(4%) | 1 (2%) | 0 | 3 (6%) | 8 (16.0%) | 0.982 | |
| G2 | 23 (46%) | 4 (8%) | 2 (4%) | 1(2%) | 7 (14%) | 16 (32.0%) | 1.0 | ||
| G3 | 16 (32%) | 2 (4%) | 3 (6%) | 1(2%) | 5 (10%) | 11 (22.0%) | (0.4, 2.3) | ||
| Sample total | All tissues | 50 | 8 (16%) | 6 (12%) | 2(4%) | 14 (28%) | 36 (72.0%) |
Table 2 shows the relation between the distribution of HPV-16 /18 with male and female (p= 0.791), age(p=0.951), localization (p= 0.272) and histology grade (p=0.983); the last column reveals the OR and 95% CI correspondingly; among the HPV and gende, age; localization and histological grading. Result indicates no association is found among the HPV and the mentioned variables significantly.
Table 2 shows the relation between the distribution of HPV-16 /18 with male and female (p= 0.791), age(p=0.951), localization (p= 0.272) and histology grade (p=0.983). the last column reveals the OR and 95% CI correspondingly, among the HPV and gende, age, localization and histological grading. Result indicates no association is found among the HPV and the mentioned variables significantly.
Discussion
Several factors, including ethnicity, dietary habits, smoking, alcohol, poor nutrition, genetic predisposition and geographic origin may involve in head and neck Squamus cell carcinoma (HNSCC) (Mohamadian et al., 2014). The implication of HPV in laryngeal squamous cell carcinoma is controversial and varies from zero to 100%. The zero rate of HPV among patients with head and neck squamous cell carcinoma (HNSCC) is reported in Brasília (Rivero et al., 2006; Mohamadian et al., 2014), while in Japan, 100% of HNSCC were found to be positive for HPV (Koyama et al., 2007).
In our study, HPV was detected in 14 of 50 (28%) in HNSCC, among them, 8 of 14 (16%) HPV 16, 6 of 14 (12%) HPV-18 and 2 (4%) had mixed HPV-16 and HPV-18 infection. In Lucknow, India, Vineeta et al. have detected HPV in 23 of 250 (9.2%) patients with SCC, which among them, 30.4% were with HPV 16, 17.4% were with HPV 18 and 26.1% had co-infections HPV 16 and HPV18 (Vineeta et al., 2015). In USA, Stephen et al. (2012) have detected HPV16 among 21 of 77 (27%) patients with laryngeal squamous cell cancer (LSCC).
In 2005, Kreimer et al. (2005) have detected HPV in 24% of patients with laryngeal (24%), which is in accordance with our finding. Eleni et al. (2014) have described that males are more prone to HPV than female. Our results have shown HPV infection was associated with 11 of 38 (28.94%) males and 3 of 12 (25%) female (p=0.791).
Morshed K et al. (2005) have detected HPV DNA in 6 of 40 (15%) patients with laryngeal squamous cell carcinoma, among them 5 of 6 (83.4%) samples were with histology grade G2 (moderately differentiated), 1 (16.6%) histology grade G3 (poorly differentiated), and no HPV associated with histology grade G1 (well-differentiated) tumor. In addition, 4 of 6 (66.6%) of patients showed tumor location in supraglottic region, 1 of 6 (16.6%) in glottis, and 1 of 6 (16.6%) in subglottic. Our finding showed that association of positive HPV in the tumor site was glottis (57%), supraglottic (28%) and subglottic (14%). In Italy (2001), Almodori et al. (2001) reported that the association of positive HPV in the tumor site in supraglottis was 9 (43%), Glottis 9 (43%), and Subgottis) 0 (0%) (23).
Almadori et al. (2001) have detected HPV DNA in 9 of 45 (20%) of the patients with laryngeal squamus cell carcinoma, of which 55% and 33% samples were histology grade G2 and G3, respectively. In our study, HPV DNA was detected in SCC histology grade G1(3 of 11(21%), G2 (8 of 23 (34.4%) and G3 (5 of 16 (31.2%) which are in agreement with Almodori et al.’s and Morshed et al.’s (2005) finding. While there is no specific treatment available for HPV infection, detection of PVs is of important prognostic value for patients with HNSCC. According to the reports, HPV-positive HNSCCs have been shown to have a better clinical outcome than HPV-negative cases (Syrjanen., 2010; Josena et al., 2013).
It was reported that individuals with HPV-positive SCC had a lower risk of dying and of recurrence, especially when restricted to HPV-positive oropharyngeal cancers (Ang et al., 2010; Ragin et al., 2007). It was also observed that patients with HPV-positive SCC respond better to chemotherapy and radiotherapy than HPV-negative individuals (Dayyani et al., 2010). Additionally, the prognostic value of tumor stage was shown to be significant only among HPV-positive tonsillar cancers (Hong et al., 2013). However, not all studies show consistent results and the prognostic value of HPV remains uncertain especially among OSCC. It was observed that HPV-16- positive individuals with advanced OSCC had a poor survival rate compared to HPV-16-negative cases (Lee et al., 2012). However, more research is warranted before establishing the use of HPV status to guide treatment and to predict the outcome of HNSCC.
Prophylactic HPV vaccines are available. Clinical trials have revealed that the high efficacy of the vaccine could prevent anal, cervical, vaginal, and vulvar cancer development among individuals have not been exposed to HPV (Villa., 2011). Vaccination induces not only an active immune response but also B-cell immune memory response will persist for years. Determination of vaccine efficacy against head and neck HPV-16 infection and tumor development is still necessary, because there are no published data on this topic (Syrjanen S, 2010).
Overall, 28% of HNSCC samples showed positive for 16% HPV16 and 12% HPV18 genotypes. Additionally, 2 (4%) co-infection of HPV16 and HPV18 were found among these cases. Both HPV16 and HPV18 are high-risk HPV. It is important to point up that viral infection, especially HPV-16, and HPV-18 could be a risk factor in HNSCC progression.
Acknowlegement
This study was done as a research project and carried out by Seyed Zinab Hosseini MSc student with registration number 93117 in Health Research Institute, Infectious and Tropical Disease Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
References
- Aguayo F, Khan N, Koriyama C, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agent Cancer. 2011;6:7. doi: 10.1186/1750-9378-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almadori G, Cadoni G, Cattani P, et al. Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin cancer Res. 2001;7:3988–93. [PubMed] [Google Scholar]
- American Cancer Society. Oral cavity and oropharyngeal cancer. 2015. Retrieved from http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-key-statistics .
- Ang KK, Harris J, Wheeler R, et al. Weber R, Rosenthal DI, Nguyen-Tan PF, et al., editors. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer GC, Smith MA, Harris SL, et al. Review of the clinical and biologic aspects of human papillomavirus positive squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phy. 2014;88:761–70. doi: 10.1016/j.ijrobp.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard HU, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virol J. 2010;401:70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TC, Markham CM, Ross MW, Mullen PD. Examining the association between oral health and oral HPV infection ?Cancer Prev Res (Philadel-phia) 2013;6:917–24. doi: 10.1158/1940-6207.CAPR-13-0081. [DOI] [PubMed] [Google Scholar]
- Claus W, Steffen W, Christina SM, et al. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:09. doi: 10.3205/cto000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayyani F, Etzel CJ, Liu M, et al. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC) Head Neck Oncol. 2010;2:15. doi: 10.1186/1758-3284-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding GC, Ren JL, Chang FB, et al. Human papillomavirus DNA and P16INK4A expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol. 2010;16:5901–6. doi: 10.3748/wjg.v16.i46.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleni B, Ryan Li David E, et al. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014;50:565–74. doi: 10.1016/j.oraloncology.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan LL, Zhang H, Guo J H, et al. Prevalence of human papillomavirus infection in oral squamous cell carcinoma:A case-control study in Wuhan, China. Asian Pac J Cancer Prev. 2014;15:5861–65. doi: 10.7314/apjcp.2014.15.14.5861. [DOI] [PubMed] [Google Scholar]
- Güvenç MG, Midilli K, Ozdoğan A, et al. Detection of HHV-8 and HPV in laryngeal carcinoma. Auris Nasus Larynx. 2008;35:357–62. doi: 10.1016/j.anl.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hong AM, Martin A, Armstrong BK. Human papillomavirus modifies the prognostic significance of T stage and possibly N stage in tonsillar cancer. Ann Oncol. 2013;24:215–19. doi: 10.1093/annonc/mds205. [DOI] [PubMed] [Google Scholar]
- Hobbs CGL, Birchall MA. Human papillomavirus infection in the etiology of laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2004;12:88–92. doi: 10.1097/00020840-200404000-00006. [DOI] [PubMed] [Google Scholar]
- Hernandez BY, Goodman MT, Lynch CF, et al. Human Papillomavirus Prevalence in Invasive Laryngeal Cancer in the United States. PLoS ONE. 2014;9:e115931. doi: 10.1371/journal.pone.0115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukioja A, Asunta M, Söderling E, Syrjänen S. Persistent oral human papillomavirus infection is associ¬ated with smoking and elevated salivary immunoglobu¬lin G concentration. J Clin Virol. 2014;61:101–6. doi: 10.1016/j.jcv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Jalal MS, Nasrin Y, Zahra OA, et al. TP53 gene expression in HPV-positive oral tongue SCC and its correlation with nodal metastasis. Pathology -Research and Practice. 2011;207:758–61. doi: 10.1016/j.prp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Josena KS, George D, Kang MC, et al. Significance of p16 in site-specific HPV positive and HPV negative head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;2:51–61. doi: 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kero K, Rautava J, Syrjänen K, et al. Smoking increases oral HPV persistence among men:7-year follow-up study. Eur J Clin Microbiol Infect Dis. 2014b;33:123–33. doi: 10.1007/s10096-013-1938-1. [DOI] [PubMed] [Google Scholar]
- Koyama K, Uobe K, Tanaka A. Highly sensitive detection of HPV-DNA in paraffin sections of human oral carcinomas. J Oral Pathol Med. 2007;36:18–24. doi: 10.1111/j.1600-0714.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide:a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- Kristy LB. Significance of human papillomavirus in head and neck cancers. J Adv Pract Oncol. 2015;6:256–62. doi: 10.6004/jadpro.2015.6.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LA, Huang CG, Liao CT, et al. Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One. 2012;7:e40767. doi: 10.1371/journal.pone.0040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano MS, Lucyana CF, Carlos AD, et al. HPV-16/18 detection does not affect the prognosis of head and neck squamous cell carcinoma in younger and older patients. Oncol Lett. 2012;3:945–49. doi: 10.3892/ol.2012.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnaz S, Marzieh B, Saeed-Reza G, et al. Human papillomavirus in saliva of patients with oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2009;1(14):525–8. doi: 10.4317/medoral.14.e525. [DOI] [PubMed] [Google Scholar]
- Mohamadian Roshan N, Jafarian A, Ayatollahi H, et al. Correlation of laryngeal squamous cell carcinoma and infections with either HHV-8 or HPV-16/18. Pathol Res Pract. 2014;210:v205–9. doi: 10.1016/j.prp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Morshed K, Korobowicz E, Szymański M, et al. Immunohistochemical demonstration of multiple HPV types in laryngeal squamous cell carcinoma. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2005;262:917–20. doi: 10.1007/s00405-005-0925-2. [DOI] [PubMed] [Google Scholar]
- Morshed K. Association between human papillomavirus infection and laryngeal squamous cell carcinoma. J Med Virol. 2010;82:1017–23. doi: 10.1002/jmv.21749. [DOI] [PubMed] [Google Scholar]
- Nema MR, Amirhossein J, Hossein A, et al. Correlation of laryngeal squamous cell carcinoma and infections with either HHV-8 or HPV-16/18. Pathol Res Pract. 2014;210:205–9. doi: 10.1016/j.prp.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection:review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- Rivero ER, Nunes FD. HPV in oral squamous cell carcinomas of a Brazilian population:amplification by PCR. Braz Oral Res. 2006;20:21–4. doi: 10.1590/s1806-83242006000100005. [DOI] [PubMed] [Google Scholar]
- Ru J, Oleksandr E, Tara MM, et al. Association between HPV/EBV co-infection and oral carcinogenesis. J Oral Pathol Med. 2015;44:28–36. [Google Scholar]
- Ryan CC, Yenkai L, Ian HF, et al. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16INK4a expression in head and neck squamous cell carcinoma patients. BMC Cancer. 2016;16:178. doi: 10.1186/s12885-016-2217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannigrahi MK, Singh V, Sharma R, et al. Detection of active human papilloma virus-16 in head and neck cancers of Asian North Indian patients. Oral Diseases. 2016;22:62–8. doi: 10.1111/odi.12382. [DOI] [PubMed] [Google Scholar]
- Syrjanen S. The role of human papillomavirus infection in head and neck cancers. Ann Oncol. 2010;21:243–45. doi: 10.1093/annonc/mdq454. [DOI] [PubMed] [Google Scholar]
- Shahab M, Akbar S, Nasrollah E, et al. Presence of human Papillomavirus DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Asian Pac J Cancer Prev. 2015;16:7883–87. doi: 10.7314/apjcp.2015.16.17.7883. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, et al. Cancer statistics. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Stephen JK, Chen KM, Shah V, et al. Human papillomavirus outcomes in an access-to-care laryngeal cancer cohort. Otolaryngol Head Neck Surg. 2012;146:730–38. doi: 10.1177/0194599811434482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezal M, Scannapieco FA, Wactawski-Wende J, et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg. 2012;138:669–75. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa LL. HPV prophylactic vaccination:The first years and what to expect from now. Cancer Lett. 2011;305:106–12. doi: 10.1016/j.canlet.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Vineeta S, Nuzhat H, Naseem A, et al. Do human papilloma viruses play any role in oral squamous cell carcinoma in north Indians? Asian Pac J Cancer Prev. 2015;16:7077–84. doi: 10.7314/apjcp.2015.16.16.7077. [DOI] [PubMed] [Google Scholar]
- Torrente MC, Ampuero S, Abud M, et al. Molecular detection and typing of human papillomavirus in laryngeal carcinoma specimens. Acta Otolaryngol. 2005;125:888–93. doi: 10.1080/00016480510038220. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu S, Yi H, et al. Human papillomavirus infection in 674 Chinese patients with laryngeal squamous cell carcinoma. PLoS One. 2014;9:e115914. doi: 10.1371/journal.pone.0115914. [DOI] [PMC free article] [PubMed] [Google Scholar]


