Abstract
Although chromatin condensation is one of the hallmarks of apoptosis, its relationship with DNA fragmentation has been controversial. We show here that apoptotic chromatin condensation is regulated by nucleoplasmin, a protein that decondenses sperm chromatin during male pronuclear assembly. In Xenopus egg extracts, nucleoplasmin is tyrosine-dephosphorylated during apoptosis. This dephosphorylation inactivates the chromatin decondensation activity of nucleoplasmin and leads to its exclusion from the chromatin. Inhibition of tyrosine dephosphorylation prevents apoptotic chromatin condensation but not DNA fragmentation. Studies with mutant proteins indicate that dephosphorylation of nucleoplasmin at Tyr-124 regulates chromatin condensation through changes in the interaction of nucleoplasmin with chromatin and the loss of its chromatin decondensation activity. These results show that chromatin condensation and DNA fragmentation are independent processes.
Keywords: caspase, nucleoplasmin, protein tyrosine phosphatase
Apoptosis, or programmed cell death, plays a role not only in the development of multicellular organisms and maintenance of tissue homeostasis but also in a number of disease processes ranging from neurodegeneration to cancer (1). Apoptosis-inducing stimuli, such as proapoptotic cytokines, UV irradiation, and DNA-damaging drugs, induce an apoptotic response consisting of characteristic morphological changes in cellular membranes, cytoplasm, and nucleus. The activation of a proteolytic cascade, mediated by a family of cysteine proteases called caspases, is critical to the initiation and progression of apoptosis (2).
Two hallmarks of apoptosis are the condensation and the fragmentation of chromatin. During apoptosis, the inhibitor of caspase-activated DNase/DNA fragmentation factor of 45 kDa (DFF45) is cleaved by activated caspase-3, releasing the coupled caspase-activated DNase/DFF40 to digest chromatin DNA into nucleosomal fragments (3, 4). In the caspase-independent apoptosis pathway, endonuclease G is activated to substitute caspase-activated DNase/DFF40 (5). The mechanism of chromatin condensation is still controversial, because the relationship between DNA fragmentation and chromatin condensation has remained unclear. It has been suggested that DNA fragmentation induces chromatin condensation, because activated DFF40 by itself could induce chromatin condensation when incubated with the nuclei (4), and, in the caspase-independent pathway, apoptosis-inducing factor released from mitochondria could induce large-scale DNA degradation and chromatin condensation (6). Other reports showed, however, that chromatin condensation could occur independent of DNA fragmentation (7, 8).
Nucleoplasmin is an acidic, thermoresistant protein existing in large amounts in the oocytes and unfertilized eggs of Xenopus and other amphibians (9). It is the key decondensation factor that rapidly decondenses sperm chromatin and exchanges sperm nuclear basic proteins in sperm chromatin to histones H2A and H2B in the process of pronuclear formation during fertilization (10–11). Homologs of Xenopus nucleoplasmin are found in other species, such as in human and rat (12–13).
In this study, we show that nucleoplasmin is tyrosine-dephosphorylated and excluded from condensed chromatin during apoptosis. Inhibition of tyrosine dephosphorylation prevents chromatin condensation but does not block DNA fragmentation. A phosphorylation-mimicking mutant, Y124E, delays the occurrence of chromatin condensation during apoptosis in a cell-free system and in intact cells. These results indicate that nucleoplasmin regulates chromatin condensation and that chromatin condensation and DNA fragmentation are two independent processes.
Materials and Methods
Preparation of Xenopus Egg Extracts, Demembraned Xenopus Sperms, and Chicken Erythrocyte Nuclei. Crude egg extract and ultra-speed egg extract (termed extract S-150) were prepared as described in ref. 14. Briefly, eggs were dejellied and rinsed with extraction buffer (50 mM Hepes-KOH, pH 7.4/50 mM KCl/2 mM MgCl2), and centrifuged at 12,000 × g for 20 min for two times, the supernatant was Crude egg extract. S-150 was prepared by centrifuging the crude egg extract at 150,000 × g for 2 h (centrifuge model no. 55P-72, RPS 50-II rotor, Hitachi, Tokyo). Crude extract and extract S-150 were frozen and stored in aliquots in liquid nitrogen. Sperm were released from testes by gently squeezing the dissected testes in nuclear isolation buffer (NIB; 15 mM NaCl/60 mM KCl/15 mM Tris/1 mM DTT/0.5 mM spermine/0.25 M sucrose, pH 7.5) and centrifuged at 100 × g for 1 min at 4°C to remove somatic tissue. Sperm containing supernatant was centrifuged at 1,500 × g for 10 min at 4°C, resuspended in NIB, and incubated at 22°C in the presence of 0.05% lysolecithin for 8–10 min. The reaction was stopped by adding three volumes of cold NIB plus 3% BSA. After being washed three times with NIB, demembraned sperm was stored in aliquots in liquid nitrogen at 108 sperm heads per ml. Chicken blood supplemented with a 1/10 volume of 4% sodium citrate was centrifuged at 1,000 × g for 5 min. The erythrocyte pellet was washed two times with RSB buffer (10 mM NaCl/3 mM MgCl2/10 mM Tris·HCl/0.1 mM PMSF, pH 7.4), then lysed in RSB containing 0.5% Triton X-100. After being centrifuged at 8,000 × g for 10 min and washed three times with RSB, purified erythrocyte nuclei were resuspended in RSB at 105 nuclei per μl and stored in liquid nitrogen.
Induction of Apoptosis in Chicken Erythrocyte Nuclei and Assembled Nuclei. Chicken erythrocyte nuclei (1 × 105) were added into 50 μl of extract S-150 with a 1 μM final concentration of cytochrome c (from horse heart) (Sigma) and incubated at 23°C for the indicated times in Figs. 2A and 3A. Phosphatase inhibitors were added before incubation to examine their effect of on apoptosis. Demembranated Xenopus sperm nuclei were mixed with crude egg extracts and an ATP-regenerating system (2 mM ATP/20 mM phosphocreatine/50 mg/ml creatine phosphokinase). After incubation at 22°C for 1 h, the assembled nuclei were round; then a 1 μM final concentration of cytochrome c was added to induce apoptosis. To assemble nuclei containing nucleoplasmin or its mutants, a 2 μM final concentration of purified prokaryotic expressed proteins were added with sperm nuclei before incubation. The chromatin change of nuclei was observed under a fluorescence microscope (Olympus IX71) by DAPI staining, and images were taken with a cooled charge-coupled device camera and processed with spot and photoshop (Adobe Systems, San Jose, CA) software. For DNA fragmentation examination, 10 volumes of Buffer D (100 mM Tris·HCl, pH 8.0/5 mM EDTA/0.2 M NaCl/0.4% SDS/0.2 mg/ml proteinase K) was added into each reaction and incubated at 37°C overnight. DNA was prepared and loaded onto a 1.5% agarose gel for electrophoresis.
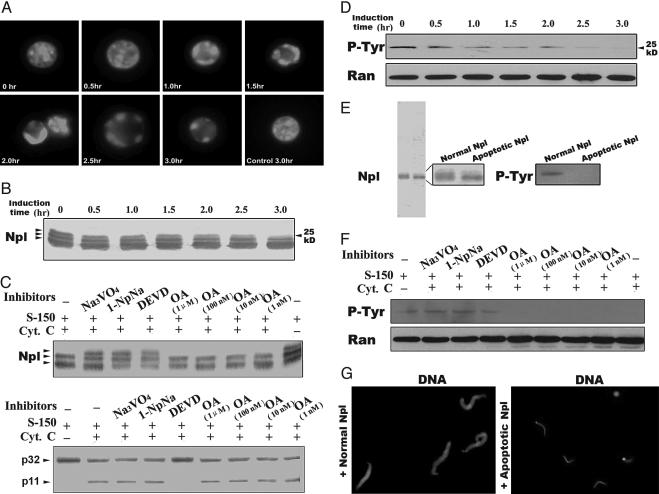
Fig. 2.
Nucleoplasmin (Npl) was tyrosine-dephosphorylated and lost its chromatin decondensation activity. (A) Xenopus egg extracts were induced to apoptosis by adding cytochrome c with chicken erythrocyte nuclei. Samples were taken out at the times indicated and stained with DAPI to observe chromatin condensation. (B) At the same times as in A, samples were taken out for Western blot with anti-nucleoplasmin antibody. (C) Different phosphatase inhibitors were added with cytochrome c (Cyt. C) to Xenopus egg extracts, and samples were taken out at 3 h for Western blot analysis with anti-nucleoplasmin and anti-caspase-3 antibodies. (D) Apoptotic egg extracts of the time course were taken for Western blot analysis with anti-p-tyrosine monoclonal antibody (p-Tyr), with ant-Ran as a loading control (Ran). (E) Immunopurified nucleoplasmin from normal and apoptotic extracts were taken for electrophoresis (12% SDS/PAGE) and for anti-p-tyrosine Western blot. (F) Samples from D were taken for Western blot analysis with anti-p-tyrosine antibody, with anti-Ran as a loading control. (G) Sperm chromatin was incubated with normal or apoptotic purified nucleoplasmin (at final concentration of 700 ng/μl) at 23°C for 10 min and then stained with DAPI without fixation.
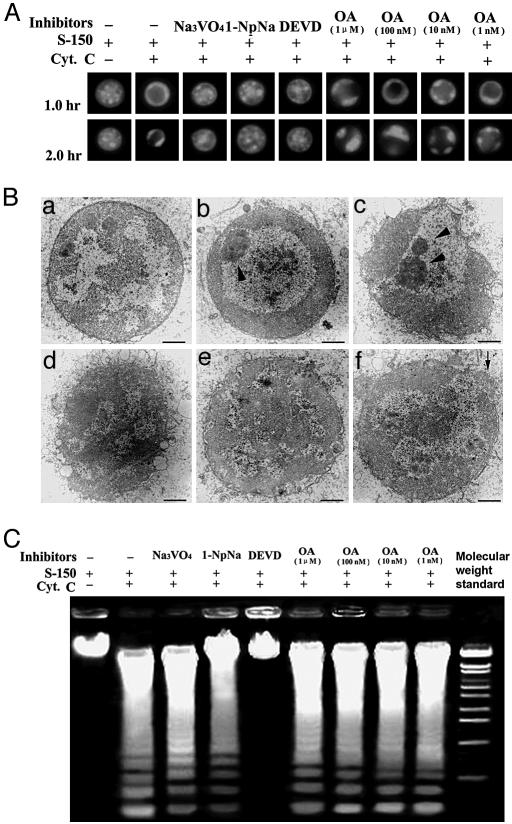
Fig. 3.
Effects of phosphatase inhibitors on chromatin condensation and DNA fragmentation during apoptosis. (A) Different phosphatase inhibitors were added with cytochrome c (Cyt. C) to Xenopus egg extracts supplemented with 105 chicken erythrocyte nuclei. After incubation at 23°C at the times indicated, samples were taken out for chromatin condensation observation. (B) Transmission electron microscopy of chicken erythrocyte nuclei in apoptotic Xenopus egg extracts with or without phosphatase inhibitors. (a) Nuclei in normal egg extracts (2 h). (b) Cytochrome-c-induced apoptotic nuclei (1 h). (c) Cytochrome-c-induced apoptotic nuclei (2 h). (d) Added tyrosine phosphatase inhibitor Na3VO4 with cytochrome c (2 h). (e) Added broad phosphatase inhibitor 1-NpNa with cytochrome c (2 h). (f) Added caspase-3-specific inhibitor DEVD with cytochrome c (2 h). Arrowheads indicate condensed chromatin; the arrow indicates NE disruption. (Bars, 1 μm.) (C) Different phosphatase inhibitors were added with cytochrome c to Xenopus egg extracts supplemented with 105 chicken erythrocyte nuclei. After incubation at 23°C for 2 h, the samples were taken out for DNA fragmentation assay.
Molecular Cloning, Protein Expression and Purification, Antibody Raising, and Expression Vector Transfection. The coding region of nucleoplasmin was amplified from the cDNA library of Xenopus oocytes (Clontech), and the GeneEdit kit (Promega) was used to create point mutations of nucleoplasmin. After digestion by EcoRI/SalI, nucleoplasmin or its mutants were constructed into pEGFP-C2 (Clontech) and pET28a (Invitrogen), respectively. For prokaryotic expression of GFP fusion proteins, a GFP fragment was amplified from pEGFP-C2 and cloned into pET28a-nucleoplasmin between NdeI and BamHI. For expression, pET28a-GFP-nucleoplasmin or pET28a-nucleoplasmin (or mutants) was transfected into BL21 (pLys), and clones were picked and then grown in liquid LB containing 50 μg/ml kanamycin. Protein expression was induced at an OD600 of 0.5 by adding isopropyl β-d-thiogalactoside to the final concentration of 1 mM and by continuous shaking at 30°C for an additional 5 h. Expressed proteins were purified with His6X Talon-spin columns (Clontech) according to the manual provided. Proteins were desalted by using a G-25 column to KHM buffer (78 mM KCl/50 mM Hepes, pH 7.0/4 mM MgCl2/2 mM EGTA/1 mM DTT) and then concentrated to 1 mg/ml by using a 10-K cutoff Centricon (Millipore). Antibody against nucleoplasmin was raised by immune rabbit with purified prokaryotic expressed 6His-tagged nucleoplasmin and characterized by Western blot (Fig. 5, which is published as supporting information on the PNAS web site). Eukaryotic expression vectors containing nucleoplasmin or its mutants were transfected into Xenopus XTC cells by Lipofectin (Invitrogen).
Purification of Nucleoplasmin from Xenopus Egg Extracts and Decondensation Assay of Nucleoplasmin. Anti-nucleoplasmin polyclonal antibodies were loaded into CNBr-activated Sepharose (Amersham Pharmacia) according to the manual provided. Normal or cytochrome-c-induced apoptotic egg extract was subjected to an anti-nucleoplasmin Sepharose column. After being washed with three volumes of binding buffer (20 mM sodium phosphate, pH 7.0), nucleoplasmin proteins were eluted by elution buffer (0.1 M glycine-HCl, pH 3.0) and collected into 0.1 volume of Tris buffer (1 mol/liter, pH 9.0). The collected proteins were desalted by G-25 and changed to buffer N (15 mM Tris·HCl, pH 7.8/60 mM KCl/15 mM NaCl/10 mM mercaptoethanol/0.5 mM spermine/0.15 mM spermidine), then concentrated to 1 mg/ml by 10-K cutoff Centricon (Millipore). Decondensation activity of nucleoplasmin was assayed as described in refs. 10 and 11. Demembraned sperm chromatin (500 per μl) was added to a final concentration of 700 ng/μl nucleoplasmin. Buffer N was set for control. After incubation at 23°C for 10 min, sperms were taken out and stained with DAPI without fixation for immediate observation.
Western Blot. Samples were electrophoresed in 12% SDS/PAGE and transferred onto nitrocellulose membranes. After blocking with 5% milk in TTBS (20 mM Tris, pH 7.5/0.5 M NaCl/0.5% Tween 20) at 37°C for 1 h, the membranes were probed with rabbit anti-nucleoplasmin or mouse monoclonal anti-p-Tyr (Sc-7020, Santa Cruz Biotechnology) or mouse monoclonal anti-Ran (R32620, Transduction Laboratories, Lexington, KY) antibodies overnight at 4°C. After being washed with TTBS and blocked with 5% milk, the membranes were reprobed with horseradish peroxidase-conjunct correlative secondary antibodies (Bio-Rad) and detected by Luminol/peroxide solution (Pierce).
Preparation and Observation of Samples for Transmission Electron Microscopy. The samples were taken after incubation at 23°C for the indicated time in Fig. 3B and then fixed at 4°C for 1 h in a final concentration of 2.0% (vol/vol) glutaraldehyde. The sediments were collected at 500 × g for 3 min and then fixed at 4°C for 1 h in a final concentration of 1% OsO4. After dehydration in a graded series of ethanol and acetone (15 min each) and embedding in Epon 812, the samples were cut into the silver-gray or white sections by using a Leica ultramicrotome Ultracut R. After being stained with uranyl acetate and lead citrate, the sections were observed and photographed under a transmission electron microscope (JEOL-1010).
Results and Discussion
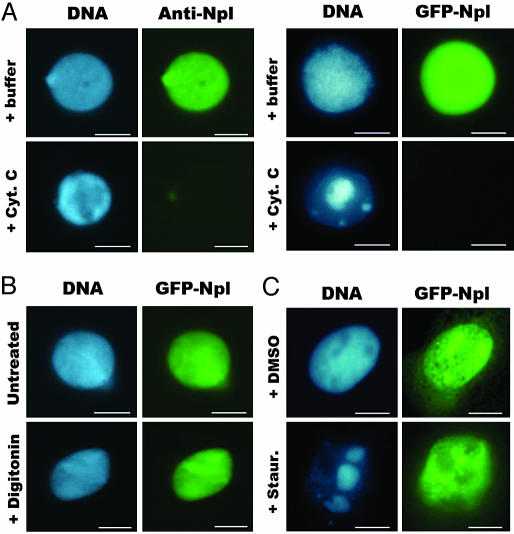
Nucleoplasmin Is Excluded from Condensed Chromatin During Apoptosis. To study whether nucleoplasmin is involved in chromatin condensation during apoptosis, we assessed the localization of nucleoplasmin during apoptosis in the nuclei. During nuclear assembly around the sperm chromatin in the crude Xenopus egg extract, endogenous nucleoplasmin entered the nuclei and co-localized with chromatin. After the nuclear assembly, we added cytochrome c (at a 1 μM final concentration) to induce apoptosis. We found that nucleoplasmin was totally excluded from the apoptotic chromatin, probed by anti-nucleoplasmin indirect immunofluorescence microscopy (Fig. 1A). We also assembled nuclei with the recombinant GFP–nucleoplasmin fusion protein and found that this fusion protein also left the chromatin during the cytochrome c-induced apoptosis (Fig. 1 A). To address whether the exclusion of nucleoplasmin from condensing chromatin was due to the nuclear envelope (NE) leaking or the failure of nuclear import of this protein rather than the loss of its chromatin-binding activity, we treated the assembled nuclei containing GFP–nucleoplasmin with digitonin to permeabilize the NE and found that GFP–nucleoplasmin was still present in nuclei (Fig. 1B).
Fig. 1.
Location changes of nucleoplasmin during apoptosis. (A) Demembraned sperm heads were added to Xenopus egg crude extracts without or with purified GFP–nucleoplasmin protein to assemble nuclei containing endogenous (Anti-Npl) or GFP-labeled nucleoplasmin (GFP-Npl), and then cytochrome c (Cyt. C) was added to examine the distribution change of nucleoplasmin. (B) Demembraned sperm heads were added to Xenopus egg crude extracts to assemble nuclei containing GFP–nucleoplasmin, treated with digitonin, and fixed by ethylene glycol-bis. (C) pEGFP-C2-nucleoplasmin-transfected Xenopus XTC cells were treated with staurosporine (Staur.) for 16 h to induce apoptosis to show the distribution of nucleoplasmin during apoptosis. (Bar, 10 μm.)
We also localized nucleoplasmin in Xenopus XTC cells that were undergoing apoptosis. pEGFP-C2-nucleoplasmin was transfected into XTC cells, and nucleoplasmin was found to colocalize with chromatin (Fig. 1C). We then treated the cells with staurosporin to induce apoptosis. Apoptotic chromatin condensation occurred after 16 h, and the GFP–nucleoplasmin was again found to be excluded from the apoptotic condensed chromatin (Fig. 1C). Different from the situation in the cell-free system (Fig. 1 A and B), GFP–nucleoplasmin in XTC cells remained in the nuclei (Fig. 1C). These differences may be due in part to the difference between the two systems. In the cell-free system, the assembled nuclei were directly exposed to apoptotic extracts, so when the NE was destroyed, the detached nucleoplasmin could easily exit from the nuclei. The situation in intact cells is more complex, and the rate of apoptosis is relatively slower. Although the GFP–nucleoplasmin was detached from the chromatin, it might stay in nucleus for a longer period. Even so, it seems common that the detachment of nucleoplasmin from the apoptotic condensed chromatin occurs in the cell-free and intact cell systems.
Nucleoplasmin Is Tyrosine-Dephosphorylated and Loses Its Decondensation Activity During Apoptosis. The function of nucleoplasmin depends highly on its phosphorylation state (15). To assess the phosphorylation state of nucleoplasmin during apoptosis, Xenopus egg extracts S-150 were treated with cytochrome c to induce the apoptotic reactions (Fig. 2A), Western blot analysis with anti-nucleoplasmin showed that nucleoplasmin in normal S-150 existed as triple bands, which was not changed when incubated at 23°C without cytochrome c (Fig. 6A, which is published as supporting information on the PNAS web site). When cytochrome c was added to induce apoptosis, the upper band started to disappear as early as 30 min after apoptosis induction (Fig. 2B), consistent with the kinetics of chromatin condensation (Fig. 2 A).
The disappearance of the upper band of nucleoplasmin might be due to dephosphorylation. To prove this possibility, we added a number of phosphatase inhibitors, together with cytochrome c, to egg extract S-150 and examined the state of nucleoplasmin. Serine/threonine phosphatases PP1A and PP2A regulate many processes in the cell, and okadaic acid (OA) inhibits their activities. Our results indicated that OA could not inhibit the disappearance of the upper band of nucleoplasmin (Fig. 2C), suggesting that PP1A and PP2A do not participate in nucleoplasmin dephosphorylation. On the other hand, 1-naphthyl acid phosphate sodium [1-NpNa (a broad phosphatase inhibitor)] and [Na3VO4 an inhibitor of protein tyrosine phosphatases (PTPs)] inhibited this phenomenon (Fig. 2C), indicating that one type of PTPs might be involved. DEVD, a caspase-3 inhibitor, inhibited not only caspase-3 activity but also the upper-band shift-down of nucleoplasmin (Fig. 2C). Control experiments showed that the phosphatase inhibitors did not affect the activation of caspase-3, because Western blot with anti-caspase-3 antibodies showed that caspase-3 was activated normally (Fig. 2C).
We further confirmed nucleoplasmin was tyrosine-dephosphorylated during apoptosis by Western blots with an anti-phosphorylated-tyrosine monoclonal antibody. The antibody could recognize one band corresponding to the upper band of nucleoplasmin, and the band gradually reduced during apoptosis, compared with the band of Ran as a loading control, which was not degraded during apoptosis (16) (Fig. 2D). The control without cytochrome c showed no reduction of this p-tyrosine-specific band (Fig. 6B). For further verification, we used purified nucleoplasmin from normal or apoptotic S-150 for Western blot and found that apoptotic nucleoplasmin was indeed tyrosine-dephosphorylated (Fig. 2E). To confirm the effect of phosphatase inhibitors on the dephosphorylation of nucleoplasmin, we also performed Western blots with the anti-phosphorylated-tyrosine antibody for those samples in Fig. 2C and found that nucleoplasmin dephosphorylation was inhibited by 1-NpNa, Na3VO4, and DEVD but not by OA (Fig. 2F). These results indicate there is at least one apoptosis-specific PTP, which is possibly activated by caspase-3 directly or indirectly, that dephosphorylates nucleoplasmin during apoptosis.
It has been reported that only the hyperphophorylated form of nucleoplasmin in Xenopus egg extracts can decondense sperm chromatin (17). It remained unclear, however, whether dephosphorylated nucleoplasmin in apoptotic extracts loses its decondensation activity. An anti-nucleoplasmin immunoaffinity column was prepared to purify nucleoplasmin from normal and apoptotic egg extract S-150, and the sperm decondensation activities of these purified proteins were examined (Fig. 2 E and G). Results showed that only nucleoplasmin from normal S-150, but not apoptotic S-150, could decondense sperm chromatin (Fig. 2G). These findings indicated that dephosphorylated nucleoplasmin had lost its decondensation activity.
Inhibition of Nucleoplasmin Dephosphorylation Prevents Chromatin Condensation but Not DNA Fragmentation. To explore the relationship between nucleoplasmin dephosphorylation and chromatin condensation during apoptosis, we examined the chromatin states of exogenous chicken erythrocyte nuclei in apoptotic egg extract S-150 supplemented with various phosphatase inhibitors as well as DEVD (Fig. 3A). The results showed that 1-NpNa, Na3VO4, and DEVD, which inhibited nucleoplasmin dephosphorylation (Fig. 2C), did inhibit the apoptotic chromatin condensation and that OA, which did not inhibit nucleoplasmin dephosphorylation, had no affect on the apoptotic chromatin condensation (Fig. 3A). To confirm these results, we prepared all of the samples (except those treated with OA) for transmission electron microscopy. The results showed that chromatin condensation did not occur in samples treated with these nucleoplasmin dephosphorylation inhibitors (Fig. 3B). In samples treated with Na3VO4, the nuclear membrane was completely disrupted, and in samples treated with 1-NpNa, the degree of nuclear membrane disruption was slightly decreased, probably because of the inhibition of other phosphatases. In samples treated with caspase-3 inhibitor DEVD, chromatin condensation did not occur as expected, and nuclear membrane was relatively intact, although there was still some breakage (Fig. 3B); we could not rule out the possibility that this was caused by other caspases, such as caspase-9, which was activated upstream of caspase-3 and was not inhibited by DEVD. These results suggested that the inhibition of nucleoplasmin dephosphorylation inhibited chromatin condensation during apoptosis and that these inhibitors did not affect other apoptosis process, such as NE disruption.
Because it has been reported that apoptotic chromatin condensation is the direct result of DNA fragmentation, it might be possible that, in the above experiments, inhibition of DNA fragmentation caused the inhibition of apoptotic chromatin condensation. To test this possibility, we performed DNA fragmentation assays. Our results indicated, unexpectedly, that DNA was fragmented normally in all of the samples that were treated with phosphatases inhibitors as well as those not treated. In DEVD-treated samples, DNA fragmentation was inhibited as expected (Fig. 3C). These results suggested that apoptosis-specific DNA nuclease, DFF40/caspase-activated DNase, and caspase-3, were activated normally in phosphatase inhibitor-treated samples, and inhibition of chromatin condensation was not caused by the inhibition of DNA fragmentation but was caused by the inhibition of dephosphorylation. Our findings also suggested that, at least in our experimental system, DNA fragmentation is not the cause of apoptotic chromatin condensation.
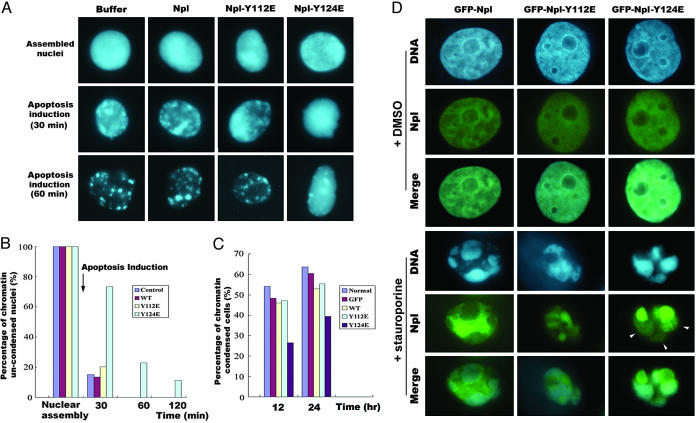
A Phosphorylation-Mimicking Nucleoplasmin Mutant, Y124E, Delays Apoptotic Chromatin Condensation. We next tested whether nucleoplasmin was involved in PTP-mediated chromatin condensation. Because there were only two tyrosine residues in the amino acid sequence of nucleoplasmin, we mutated these two sites to glutamine separately to mimic phosphorylation. By using purified bacterial recombinant wild-type and mutated nucleoplasmin, we explored the functions of these proteins on chromatin condensation. We added the purified proteins to Xenopus egg extracts with sperm heads to assembly nuclei containing the purified proteins. Cytochrome c (at a 1 μM final concentration) was then added to induce apoptosis, and samples were taken every 30 min for chromatin condensation observation. The results showed no obvious difference between control samples and samples supplemented with wide-type nucleoplasmin or mutant nucleoplasmin Y112E. However, in samples supplemented with Y124E, apoptotic chromatin condensation was largely inhibited (Fig. 4A). At 30 min, most nuclei in the samples, except those supplemented with Y124E, underwent apoptotic chromatin condensation. At 60 min, most of nuclei in the control sample and samples supplemented with nucleoplasmin or Y112E were highly condensed, but Y124E-supplemented samples still contained ≈23% intact nuclei with uncondensed chromatin. Even at 2 h, nuclei in all other samples were disrupted absolutely; there was still 11% uncondensed nuclei in Y124E-supplemented samples (Fig. 4B). Consistent with these results, overexpression of Y124E in XTC cells significantly delayed the apoptotic chromatin condensation (Fig. 4C). We also examined the location of nucleoplasmin in the nucleoplasmin-overexpressing cells with apparent apoptotic chromatin condensation during apoptosis. Although Y112E and wild-type nucleoplasmin detached from the condensed chromatin, Y124E was still bound to the condensed chromatin, although there was much of this protein aggregated and left out (Fig. 4D). These results indicated that nucleoplasmin was involved with regulating chromatin condensation during apoptosis and that Tyr-124 was the key residue that was regulated by its upstream PTP(s). It was also apparent, not like the inhibition effects of 1-NpNa and Na3VO4, that Y124E mutant only delayed the occurrence of apoptotic chromatin condensation. This fact might imply that other substrates, which function together with nucleoplasmin to regulate the apoptotic chromatin condensation process, existed at the downstream of apoptosis-specific PTP(s) or that, through an additional mechanism, these inhibitors inhibit the apoptotic chromatin condensation. Taken together, dephosphorylation of nucleoplasmin on Y124 is required for a timely apoptotic chromatin condensation.
Fig. 4.
Nucleoplasmin-Y124E delayed the occurrence of apoptotic chromatin condensation. (A) Purified wild-type nucleoplasmin (Npl) and mutant Y112E or Y124E proteins were added to Xenopus egg extracts with demembraned sperm nuclei to assemble nuclei containing added nucleoplasmin proteins; then cytochrome c was added to induce apoptosis. Samples were taken out at the indicated times to observe chromatin condensation. (B) The percentages of the nuclei with uncondensed chromatin. In each sample, at least 100 nuclei were counted. (C) GFP-fused wild-type nucleoplasmin or mutant Y112E- or Y124E-overexpressing XTC cells were induced to apoptosis with a final concentration of 0.5 μmol/liter staurosporine or DMSO as a normal control. Cells at 16 h were fixed with methyl alcohol/acetone (1:1) for 10 min on ice, and the percentage of the chromatin-condensed cells was counted. In each sample, at least 100 cells were counted. (D) At the same time as for C, the location of nucleoplasmin or its mutants was observed in the highly chromatin-condensed apoptotic cells. It could be seen that GFP-Npl-Y124E was still on the condensed chromatin (arrowheads).
Based on our current and available data, we propose that PTP-S2/TC45-PTP is one highly likely candidate for an apoptosis-specific PTP. PTP-S2/TC45-PTP locates in nuclei by binding to chromosomal DNA during interphase (18), just like nucleoplasmin (Fig. 1). Overexpressing PTP-S2 in MCF-7 and A549 cells can lead to p53-dependent apoptosis (19), and the expression and activation of caspase-1 (20), indicating PTP-S2/TC45-PTP is an apoptosis-related factor. In a stressed environment, PTP-S2/TC45-PTP translocates from nuclei to the cytoplasm (21). During mitosis, PTP-S2/TC45-PTP is excluded from condensed mitotic chromatin (22), similar to nucleoplasmin translocation during apoptosis (Fig. 1). At the same time, the important Y124 residue of nucleoplasmin is in the (E/D/Y)Z (where Z is phosphotyrosine) motif, which accords with the substrate-recognizing motif of TC-PTP (23). These results imply that PTP-S2/TC45-PTP may play a role in nucleoplasmin dephosphorylation during apoptosis.
In conclusion, our data suggest a mechanism of apoptotic chromatin condensation that is independent of DNA fragmentation. In this scheme, caspase-3 is activated during apoptosis. This caspase then activates the apoptosis-specific PTP(s) by direct or indirect means. The apoptosis-specific PTP(s) then dephosphorylates nucleoplasmin and other possible substrates. And finally, nucleoplasmin and the other possible substrates regulate the apoptotic chromatin condensation.
Supplementary Material
Acknowledgments
We thank Prof. Tung-Tien Sun (New York University Medical School, New York) for revision and discussion and Dr. Qing Jiang and other members in our laboratory for valuable comments and discussion. This work was supported by National Natural Science Foundation of China Grants for Creative Research Groups 30225016 and 30330200, State Key Basic Research and Development Plan Grants G 1999053908 and 2004CB720003, Peking University Special Fund 985, and by the Scientific Research Foundation for the Returned Overseas Chinese Scholars of the State Education Ministry.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 1-NpNa, 1-naphthyl acid phosphate sodium; PTP, protein tyrosine phosphatase; OA, okadaic acid; NE, nuclear envelope; DFF, DNA fragmentation factor.
References
- 1.Marsden, V. S. & Strasser, A. (2003) Annu. Rev. Immunol. 21, 71–105. [DOI] [PubMed] [Google Scholar]
- 2.Budihardjo, I., Oliver, H., Lutter, M., Luo, X. & Wang, X. (1999) Dev. Biol. 15, 269–290. [DOI] [PubMed] [Google Scholar]
- 3.Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A. & Nagata, S. (1998) Nature 391, 43–50. [DOI] [PubMed] [Google Scholar]
- 4.Liu, X., Li, P., Widlak, P., Zou, H., Luo, X., Garrard, W. T. & Wang, X. (1998) Proc. Natl. Acad. Sci. USA 95, 8461–8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, L. Y., Luo, X. & Wang, X. (2001) Nature 412, 95–99. [DOI] [PubMed] [Google Scholar]
- 6.Susin, S. A., Lorenzo, H. K., Zamzami, N., Marzo, I., Snow, B. E., Brothers, G. M., Mangion, J., Jacotot, E., Costantini, P., Loeffler, M., et al. (1999) Nature 397, 441–446. [DOI] [PubMed] [Google Scholar]
- 7.Bezvenyuk, Z., Miettinen, R. & Solovyan, V. (2003) Mol. Brain Res. 110, 140–146. [DOI] [PubMed] [Google Scholar]
- 8.Sakahira, H., Enari, M., Ohsawa, Y., Uchiyama, Y. & Nagata, S. (1999) Curr. Biol. 9, 543–546. [DOI] [PubMed] [Google Scholar]
- 9.Earnshaw, W. C., Honda, B. M., Laskey, R. A. & Thomas, J. O. (1980) Cell 21, 373–383. [DOI] [PubMed] [Google Scholar]
- 10.Philpott, A. & Leno, G. H. (1992) Cell 69, 759–767. [DOI] [PubMed] [Google Scholar]
- 11.Philpott, A., Leno, G. H. & Laskey, R. A. (1991) Cell 65, 569–578. [DOI] [PubMed] [Google Scholar]
- 12.Colombo, E., Marine, J. C., Danovi, D., Falini, B. & Pelicci, P. G. (2002) Nat. Cell Biol. 4, 529–533. [DOI] [PubMed] [Google Scholar]
- 13.Dalenc, F., Drouet, J., Ader, I., Delmas, C., Rochaix, P., Favre, G., Cohen-Jonathan, E. & Toulas, C. (2002) Int. J. Cancer 100, 662–668. [DOI] [PubMed] [Google Scholar]
- 14.Lu, Z. G., Zhang, C. M. & Zhai, Z. H., (2004) Cell Res. 14, 134–140. [DOI] [PubMed] [Google Scholar]
- 15.Hierro, A., Arizmendi, J. M., De Las Rivas, J., Urbaneja, M. A., Prado, A. & Muga, A. (2001) Eur. J. Biochem. 268, 1739–1748. [PubMed] [Google Scholar]
- 16.Ferrando-May, E., Cordes, V., Biller-Ckovric, I., Mirkovic, J., Gorlich, D. & Nicotera, P. (2001) Cell Death Differ. 8, 495–505. [DOI] [PubMed] [Google Scholar]
- 17.Leno, G. H., Mills, A. D., Philpott, A. & Laskey, R. A. (1996) J. Biol. Chem. 271, 7253–7256. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzen, J. A., Dadabay, C. Y. & Fischer, E. H. (1995) J. Cell Biol. 131, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radha, V., Sudhakar, C. & Swarup, G. (1999) FEBS Lett. 453, 308–312. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, S., Radha, V., Sudhakar, C. & Swarup, G. (2002) FEBS Lett. 532, 61–66. [DOI] [PubMed] [Google Scholar]
- 21.Lam, M. H. C., Michell, B. J., Fodero-Tavoletti, M. T., Kemp, B. E., Tonks, N. K. & Tiganis, T. (2001) J. Biol. Chem. 276, 37700–37707. [DOI] [PubMed] [Google Scholar]
- 22.Nambirajan, S., Radha, V., Kamatkar, S. & Swarup, G. (2000) J. Biosci. 25, 33–40. [DOI] [PubMed] [Google Scholar]
- 23.Asante-Appiah, E., Ball, K., Bateman, K., Korey, K., Friesen, R., Desponts, C., Payette, P., Bayly, C., Zamboni, R., Scapin, G., et al. (2001) J. Biol. Chem. 276, 26036–26043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.