Abstract
Dictyostelium discoideum, a social slime mold that forms fruiting bodies with spores, depends on inorganic polyphosphate (poly P) for its cycles of development and for nutritional predation on bacteria. The synthesis of poly P, a polymer of tens or hundreds of phosphate residues linked by high energy, ATP-like bonds, is catalyzed in most bacteria by poly P kinase (PPK1). The eukaryote D. discoideum possesses a homolog of PPK1. We report here that mutants of D. discoideum PPK1 (DdPPK1) have reduced levels of poly P and are deficient in development. Fruiting bodies are smaller and produce fewer spores, which appear to germinate like the wild type (WT). The DdPPK1 mutant formed smaller plaques on bacterial lawns compared with those of the WT. Predation by D. discoideum, assessed by uptake and digestion of Klebsiella aerogenes, showed that fewer bacteria were taken up by the DdPPK1 mutant compared with the WT and were killed less rapidly, indicating a role of poly P and/or DdPPK1 in phagocytosis. On Pseudomonas aeruginosa lawns, cleared plaques were observed with the bacterial PPK1 mutant but not with the WT P. aeruginosa. Thus, poly P is important in predation both for the predator and prey.
Likely an agent in evolution from prebiotic times, inorganic polyphosphate (poly P) in chains of tens to hundreds of phosphate units linked by high-energy phosphoanhydride bonds is now found in volcanic condensates and deep oceanic steam vents and has been conserved in all cells: bacteria, fungi, plants, and animals. Poly P can be formed from Pi simply by dehydration at elevated temperatures. In eukaryotes, it is found in virtually all subcellular organelles, even at levels of 20 percent of the cell dry weight (1). Poly P is required for bacterial responses to a variety of stresses and stringencies and for the virulence of some pathogens (1–6). It is also involved in the proliferation of mammary cells by stimulating the kinase activity of mammalian target of Rapamycin (7). Among many poly P-metabolizing enzymes, polyphosphate kinase (PPK1) reversibly catalyzes the polymerization of the terminal phosphate of ATP to poly P (8). PPK1 homologs have been found in 80 or more prokaryotic species, including 17 pathogens, but in only one eukaryote, the social slime mold Dictyostelium discoideum (9).
The genes used to parasitize protozoa and macrophages are widely conserved. Thus, Legionella pneumophila, with no known animal host, switches on the same genes on entry into either amoebae or macrophages (10). Furthermore, they exit these eukaryotic predators with similar properties of virulence and resistance (11–13). Protozoa feed on bacteria by a phagocytic mechanism, similar to those used by higher eukaryotes, that involves attachment of bacteria to cell-surface receptors, influenced by relative surface hydrophobicity (14). Engulfment of the bacteria depends on actin polymerization, membrane exocytosis, and formation of phagolysosomes. The regulation of protozoal cell motility, membrane trafficking, and internalization events resembles that of neutrophils and macrophages (15–17). That coevolution of bacteria and protozoa may have led to animal pathogens (18–21) has promoted the use of protozoa as convenient models for virulence pathways.
D. discoideum has been used to study host–pathogen interactions, in particular for Legionella pneumophilia, Mycobacterium avium, and Pseudomonas aeruginosa (18, 22–25). With a small haploid genome, D. discoideum is a genetically tractable host and undergoes a developmental cycle. When nutrients are available, either in the form of bacteria or an axenic medium, the cells grow vegetatively as amoebae. When starved, amoebae use a cAMP signal relay to stream into aggregates of up to 105 cells and finally form a fruiting body that contains stalk cells and spores that can germinate to complete the cycle (15). From amoebae to spores, poly P is found in every stage of development of D. discoideum (26), mostly in vacuoles called acidocalcisomes (27). Sims and Katz first observed that resistance of D. discoideum mutants to polyene antibiotics mapped in a genetic locus homologous to E. coli PPK1 and observed that the mutant is abnormal in development (M. Sims and E. Katz, personal communication). We have confirmed that the D. discoideum homolog is indeed a PPK1 (D. discoideum PPK1, DdPPK1), and we found that null mutants are defective in development, sporulation, and predation.
Materials and Methods
Cells and Growth Conditions. The D. discoideum cell lines include wild type (WT) (AX2) and mutant AX2M1 [AX2 ΔDdppk1::Bsr (Blasticidin resistance)] (see below). All strains were grown at 21°C in HL5 (28) medium. Cells were also grown in association with Klebsiella aerogenes on SM5 agar plates (29). P. aeruginosa WT PAO1 and mutant PAOM5 [PAO1 Δppk1::tet (Tcr)] (2) were grown in LB at 37°C. Antibiotics were Blasticidin, 5 μg/ml (13); G418, 10 μg/ml (30); and tetracycline, 15 μg/ml.
Mutant Construction. Two segments of Ddppk1 (GenBank accession no. AF176830) were amplified from AX2 genomic DNA by PCR. Primer 5U with a XhoI site (in bold) (CCGCTCGAGATTGCATTGTATTTTCAGACTA) and 5L with a HindIII site (in bold) (CCCAAGCTTGTTTCAAAAGGACCGCTATGTT) were used for the 5′ segment. Primer 3U with a XbaI site (in bold) (GCTCTAGATTGGCAAATTTGGATACACTC) and 3L with an EcoRI site (in bold) (GCGAATTCGTCTTTACCTTCTCTGGCGTTC) were used for the 3′ segment. The knockout plasmid pSP72-Ddppk1-Bsr was obtained by inserting the 5′ segment of Ddppk1 into pSP72-Bsr (31) between the XhoI and HindIII sites at the 3′ end of the bsr (blasticidin resistance) gene, and the 3′ segment of Ddppk1 into XbaI and EcoRI sites at the 5′ end of bsr. pSP72-Ddppk1-Bsr was digested by XhoI and EcoRI, the 4.3-kb fragment containing Ddppk1 segments on both ends of bsr was recovered and transformed into AX2 by electroporation. Transformed cells were selected by resistance to 5 μg/ml blasticidin. Individual clones were screened by PCR with primers 5U, 3L, and several other primer sets for the correct deletion-insertion alleles of Ddppk1; PPK1 activities were measured. One of the clones, AX2M1 (AX2 ΔDdppk1::Bsr), was used for further study.
Complementation Constructions. A two-step PCR procedure was followed to create a fragment containing Ddppk1 under the act15 promoter. A 0.35-kb actin 15 promoter region was amplified from pTX-gfp (30) by using primers P1 (GGGCGAATTGGAGCTGG) and P2 (TGAGTTAGTTATCATTTTTTAAGCTTGG); a 3.2-kb Ddppk1 fragment was amplified from AX2 genomic DNA by primer P3 (CAAGCTTAAAAAATGATAACTCAAAAATGG) and DK1-Xba-L4 (ATCTAGATTTGTTTATTTTGACCAA). The two PCR products were purified and mixed as templates for the second-round PCR. Because the 5′ end of P2 and P3 could anneal to each other, P1 and DK1-Xba-L4 were used to amplify the 3.55-kb fragment containing act15/Ddppk1. This fragment was then digested by SalI and XbaI and inserted into SalI- and XbaI-digested pTX-gfp to obtain pTX-Pa-Ddppk1, which was then transferred into AX2M1 by electroporation and selected by G418 (10 μl/ml) and blasticidin(5 μl/ml). It was designated AX2M1 (act15/Ddppk1).
Biochemical Assays. Poly P was extracted and determined by both radioactive and nonradioactive methods (3, 32). The PPK1 assay for D. discoideum was performed as described in ref. 8 with modified reaction conditions. Cells were lysed by freeze-thawing. After centrifugation at 13,000 × g at 4°C for 10 min, the supernatant (crude lysate) was used for the PPK1 reaction. The reaction mixture (25 μl) contained 50 mM Hepes (pH 7.2), 80 mM (NH4)2SO4, 4 mM MgCl2, 0.5 mM poly P (Sigma type 75, in phosphate residues), 1 mM ATP, 1 mM creatine phosphate, and 20 μg/ml creatine kinase.
Developmental Assay. Multicellular development was examined on K. aerogenes lawns or on nitrocellulose (NC) filters. For development on K. aerogenes lawns, D. discoideum was grown to mid-log phase in HL5 medium; 106 or 103 cells were mixed with 0.2 ml of overnight culture of K. aerogenes and plated on SM5 plates. Pictures of plaques and fruiting body formation were taken at various times. After the fruiting bodies were fully developed, the plates were held bottom-up and banged down on the bench; spores that fell to the cover of the Petri dish were collected and counted with a hemocytometer. For development on NC filters, mid-log phase cells were washed in Sorensen C buffer (16.7 mM Na2H/KH2PO4/50 μM CaCl2, pH 6.0). Cells (1 × 107) were plated on 25-mm-diameter (0.45-μm pore size) NC filters (Millipore) resting on Whatman no. 3 paper soaked with 20 mM KCl/5 mM MgCl2/9mMK2HPO4/13 mM KH2PO4, pH 6.4 (33).
For germination, spores were washed three times with water and inoculated at a final density of 2 × 106 spores per ml in HL5 medium and shaken at 21°C. The proportion of nascent amoebae was determined by phase-contrast microscopy (34, 35).
Plate Killing and Gentamicin Protection Assays. Both assays were as described in ref. 24 with small modifications. For the plate-killing assay, an overnight P. aeruginosa culture was collected, washed once, and resuspended in Sorensen C buffer to OD600 of 5.5. Mid-log D. discoideum cells were collected, diluted in Sorensen C buffer, and added to bacterial suspensions at a final concentration of 200 cells per ml; 0.4 ml of this mixture was plated on SM5 plates, incubated for 3–5 days, and examined for plaque formation. For the gentamicin protection assay, mid-log D. discoideum cells were collected, washed, and resuspended in SM5 liquid medium at a concentration of 1 × 106 cells per ml. Aliquots of 3 ml were added to six-well tissue culture dishes (Falcon); subsequent treatments were as described in ref. 24.
Results
Ddppk1, the Gene and Mutant. D. discoideum contains a homolog of Escherichia coli PPK1, designated DdPPK1. Ddppk1 has 3,153 base pairs without an intron, the only bacterial ppk1 homolog yet found in eukaryotic cells. The deduced amino acid sequence shares 30% identity and 51% homology with E. coli PPK1. However, E. coli PPK1 contains only 688 amino acids compared with 1,050 amino acids in DdPPK1; about one-third of the N-terminal sequence has no homology with E. coli PPK1. Moreover, the deduced protein has an asparagine (N818) in place of one of the essential histidines (H460) of E. coli PPK1.
Low PPK1 activity (200 units/mg protein) in extracts of the WT under standard reaction conditions was increased 15-fold by the addition of poly P and optimizing pH and salt concentrations. At the levels of 3,600 units/mg protein, the optimized activity exceeds that of the PPK1 value of 2,000 in an E. coli lysate. When grown in HL5 medium, the DdPPK1 activity peaked at mid-log phase and declined when the cells entered stationary phase.
A mutant of Ddppk1 that disrupts the single copy of Ddppk1 in the haploid D. discoideum genome was constructed (see Materials and Methods). The coding sequence from 1591 to 1705 was replaced by a bsr gene. The correct deletion-insertion mutation allele of Ddppk1 was confirmed by PCR with at least four sets of primers. PPK1 activity in the mutant was only 5% that of the WT in all six individual mutant clones tested; one of these clones, AX2M1, was used in these studies.
Poly P levels in vegetative cells were highest in the stationary phase but far lower than in spores (Table 1). The chain lengths of poly P were from a few to 500 residues in cells in the stationary phase of vegetative growth; of these ≈30% were short chains (collected in a sodium perclorate-soluble fraction with an estimated chain length of <20). The poly P level in the mutant was reduced to 20–50% of the WT level, but the short-chain poly P was only ≈10% of the WT level.
Table 1. Poly P content of D. discoideum cells at different stages.
| Poly P content,* nmol/mg protein
|
||
|---|---|---|
| WT | Mutant | |
| Vegetative growth stages | ||
| Logarithmic | 4.9 | 1.9 |
| Early stationary | 11.3 | 3.9 |
| Stationary | 15 | 3.2 |
| Spore | 59 | 15 |
Poly P content is calculated in phosphate residues. Numbers were the average of several experiments.
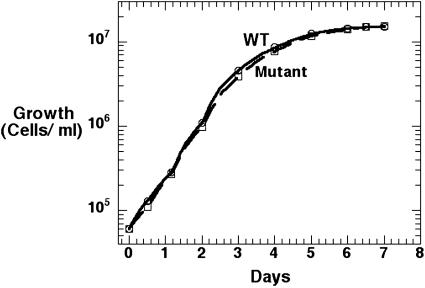
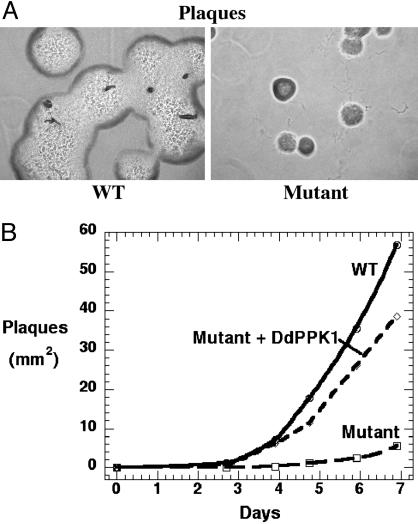
Growth Defect of Mutant on Bacterial Lawns. The mutant was severely deficient in growth on bacterial lawns measured by the size of plaque formed by single cells on K. aerogenes, although growth appeared the same for the WT and mutant in HL5 medium (Fig. 1). When mid-log phase cells were diluted into an overnight K. aerogenes culture and plated on SM5 agar plates, the average size of a plaque after 7 days was only about 1/10th the size for the mutant compared with the WT (Fig. 2); complementation of the mutant restored growth to the WT level (Fig. 2). Similar results were obtained on lawns of the P. aeruginosa PPK1 mutant PAOM5 (data not shown).
Fig. 1.
Growth of D. discoideum in HL5 medium.
Fig. 2.
Growth of D. discoideum on a K. aerogenes lawn. (A) D. discoideum cells (1 × 103) were mixed with K. aerogenes and plated on SM5 plate. Plaques were formed by WT (Left) and mutant (Right) cells on lawns after 2 days. (B) D. discoideum cells (20–50) were mixed with K. aerogenes and plated on SM5 agar. Plaque sizes were measured over a 7-day period.
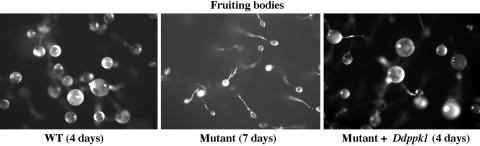
Defects of Mutant in Development. The DdPPK1 mutant was delayed in fruiting body formation when examined on a K. aerogenes lawn (Fig. 3) because of slower growth. WT cells were fully developed into fruiting bodies in 3–4 days, but those of the mutant were only about one-fifth the size of the WT even after 7 days (Fig. 3). The spore number produced by the mutant was only ≈20% that of WT (Table 2). Growth of the complemented mutant was similar to that of the WT, it formed a normal-sized fruiting body (Fig. 3), and produced as many spores (Table 2). With development on filters, the mutant was slightly delayed and formed smaller fruiting bodies as compared with the WT and could also be complemented as above (data not shown).
Fig. 3.
Development of D. discoideum on K. aerogenes lawns. D. discoideum cells (1 × 103) were mixed with K. aerogenes and plated on a SM5 plate. Fruiting bodies formed on lawns after 4 days for WT (Left) and mutant + Ddppk1 (Right) and 7 days for the mutant (Center).
Table 2. D. discoideum Ddppk1 mutant shows reduced sporulation.
| Strain | Sporulation,* % |
|---|---|
| WT | 100 |
| Mutant | 20 |
| Mutant + Ddppk1 | 135 |
D. discoideum cells (1 × 106) were mixed with K. aerogenes and plated on SM5 agar plates. Spores were collected on the fourth day for AX2 (WT) and AX2M1 (act15/Ddppk1) (mutant + Ddppk1) and on the seventh day for AX2M1 (mutant). Spore numbers were counted in a hemocytometer.
Sporulation refers to the number of spores produced by a strain as a percentage of the number of spores produced by the WT.
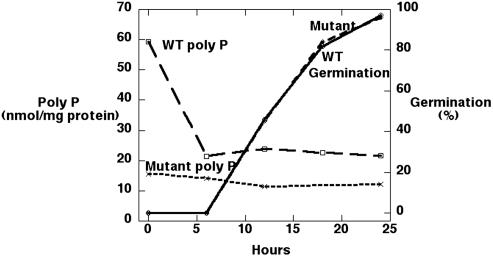
During germination, the poly P level in the WT dropped dramatically before amoebae emerged and remained constant thereafter (Fig. 4), whereas the mutant, which had not accumulated poly P, showed only a small decrease. Yet, the levels of poly P did not appear to affect the germination as measured in HL5 medium (Fig. 4).
Fig. 4.
A comparison of spore germination and poly P levels.
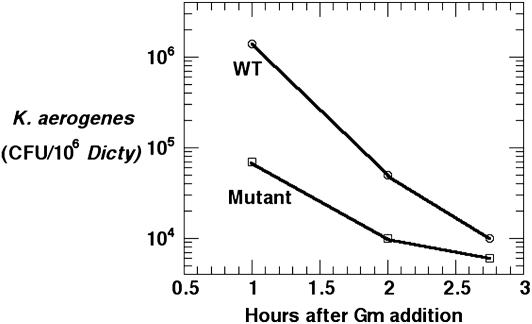
Role of DdPPK1 in Phagocytosis and Digestion. The reduced ability of the mutant DdPPK1 to feed on bacterial lawns could be due to defects in phagocytosis, in endosome processing, or in motility. To appraise phagocytosis, a modified gentamicin-protection assay was used to measure the uptake and digestion rate by K. aerogenes. The WT and mutant were each incubated with K. aerogenes for 30 min; bacteria that was not taken up by the amoebae were killed by the addition of gentamicin. One hour after the addition of gentamicin, 1.4 × 106 K. aerogenes cells were detected inside the WT, but only 5% as many (8 × 104 cells) were inside the mutant (Fig. 5), indicating reduced phagocytosis by the Dictyostelium mutant. After 2 hours more, digestion of bacteria by the WT left only 1% of the phagocytosed bacteria viable, whereas 15% of the bacteria were still detected in the mutant D. discoideum. Thus, during the 2-hour interval, the WT digested >10 times as many bacteria as did the mutant, suggestive of a role of DdPPK1 in the endocytic pathway of D. discoideum.
Fig. 5.
Uptake and digestion of K. aerogenes by D. discoideum cells. WT and mutant cells (1 × 106 per ml) were placed in tissue culture wells and infected with K. aerogenes at a multiplicity of ≈100:1. Cultures were incubated at 22°C for 30 min, at which time gentamicin (Gm) was added to kill extracellular K. aerogenes. D. discoideum were collected at indicated time points, lysed, and plated on nutrient agar plates to determine the colony-forming units (cfu)/ml D. discoideum cell lysates.
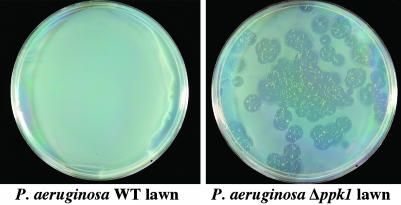
Further Role of Poly P and/or PPK1 in Predator–Prey Relations. The role of poly P and/or PPK in the contest between predator and prey can be observed in the outcome of interactions between P. aeruginosa and D. discoideum. When P. aeruginosa WT (PAO1) and Δppk1 (PAOM5) were used in the plaque formation assay with the WT D. discoideum, the P. aeruginosa WT was taken up by the amoebal form of D. discoideum and killed them, leaving an intact bacterial lawn. However, on a lawn of Δppk1, the WT D. discoideum proved to be an effective predator as observed by the plaques formed (Fig. 6). The mutant D. discoideum also forms plaques on the Δppk1 lawn, but they are smaller than the WT. Thus, poly P and/or PPK1 are crucial in the balance between predator and prey.
Fig. 6.
Plaque formation of D. discoideum cells on P. aeruginosa lawns. WT cells were mixed with P. aeruginosa WT (Left) and mutant (Right) and plated on SM5 agar and incubated at 22° for 5 days.
Discussion
The enzyme responsible for the synthesis of poly P from ATP in a wide range of bacterial species is a poly P kinase, designated as PPK1. In this article, we describe an instance in which a PPK1 homologue has been found in a eukaryote, the social slime mold D. discoideum. Mutants lacking the enzyme (DdPPK1) are defective in development, sporulation, and predation. Poly P levels in the DdPPK1 mutant are reduced both in the vegetative cells and spores (Table 1). Development of fruiting bodies is delayed in the mutant (Fig. 3), and sporulation is diminished (Table 2); these developmental deficiencies can be overcome by complementation with the Ddppk1 gene.
A striking effect of the lack of PPK1 and the reduction in poly P level is crucial in the predator–prey relationships of the mutant. The WT Dictyostelium consumes a P. aeruginosa mutant that lacks PPK1 but becomes the prey of the WT bacterium that possess it. DdPPK1 mutants are less effective in feeding on bacteria, as judged by a reduced level of phagocytosis and endosomal digestion of engulfed bacteria. Thus, poly P plays an important role in tipping the balance in the contest between predator and prey.
A comparison of the PPK1 sequences of Dictyostelium and bacteria identifies two clear differences. One is the length of DdPPK1, which is extended by 369 amino acids at the NH2 terminus with no homology to any sequence in the databases. The other distinction is the substitution of an asparagine (N818) for H460, which along with H441 are the two highly conserved histidine residues in bacterial PPK1, both located in the ATP-binding pocket (Y. Zhu and W. Xu, personal communication) and essential for PPK activity (36).
A fortuitous outcome of the study of DdPPK1 was the revelation that mutants lacking the enzyme still had significant levels of poly P and PPK activity. The residual PPK activity has been located in a vacuole, the acidocalcisome, that contains poly P and Ca2+ and is responsible for the flux of Ca2+ into the cytosol in D. discoideum, related protozoa (e.g., Trypanosoma, Leishmania, and Toxoplasma), and the alga Chlamydomonas (27, 37–39). The PPK activity when separated from its membrane location and purified to homogeneity has proven to be an enzyme (DdPPK2) that assembles from a globular state to an actin-like filament concurrent with its synthesis of poly P (40). This remarkable confluence of two biopolymers, poly P and actin, has invited comments (41) with relevance to the role of poly P in the origin and survival of species (42).
Acknowledgments
We thank Dr. James Spudich (Stanford University) and his group for advice and D. discoideum strains and plasmids. This work was assisted in part by the Ellison Medical Foundation for supporting a sabbatical visit by M.R.W.B. and by grants from the National Institutes of Health.
Abbreviations: poly P, inorganic polyphosphate; PPK, polyphosphate kinase; DdPPK, Dictyostelium discoideum PPK.
References
- 1.Kornberg, A., Rao, N. N. & Ault-Riché, D. (1999) Annu. Rev. Biochem. 68, 89–125. [DOI] [PubMed] [Google Scholar]
- 2.Rashid, M. H. & Kornberg, A. (2000) Proc. Natl. Acad. Sci. USA 97, 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao, N. N. & Kornberg, A. (1996) J. Bacteriol. 178, 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashid, M. H., Rumbaugh, K., Passador, L., Davies, D. G., Hamood, A. N., Iglewski, B. H. & Kornberg, A. (2000) Proc. Natl. Acad. Sci. USA 97, 9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rashid, M. H., Rao, N. N. & Kornberg, A. (2000) J. Bacteriol. 182, 225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao, N. N., Liu, S. & Kornberg, A. (1998) J. Bacteriol. 180, 2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, L., Fraley, C. D., Faridi, J., Kornberg, A. & Roth, R. A. (2003) Proc. Natl. Acad. Sci. USA 100, 11249–11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn, K. & Kornberg, A. (1990) J. Biol. Chem. 265, 11734–11739. [PubMed] [Google Scholar]
- 9.Zhang, H., Ishige, K. & Kornberg, A. (2002) Proc. Natl. Acad. Sci. USA 99, 16678–16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal. G. & Shuman, H. A. (1999) Infect. Immun. 67, 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker, J., Brown, M. R. W., Collier, P. J., Farrell, I. & Gilbert, P. (1992) Appl. Environ. Microbiol. 58, 2420–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker, J., Scaife, H. & Brown, M. R. W. (1995) Antimicrob. Agents Chemother. 39, 2684–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirillo, J. D., Cirillo, S. L. G., Yan, L., Bermudez, L. E., Falkow, S. & Tompkins, L. S. (1999) Infect. Immun. 67, 4427–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Oss, C. J. (1978) Annu. Rev. Microbiol. 32, 19–39. [DOI] [PubMed] [Google Scholar]
- 15.Kessin, R. H. (2001) Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity (Cambridge Univ. Press, Cambridge, UK).
- 16.Cardelli, J. (2001) Traffic 2, 311–320. [DOI] [PubMed] [Google Scholar]
- 17.May, R. C. & Machesky, L. M. (2001) J. Cell Sci. 114, 1061–1077. [DOI] [PubMed] [Google Scholar]
- 18.Chen, J., De Felipe, K. S., Clark, M., Lu, H., Anderson, O. G., Segal, G. & Shuman, H. A. (2004) Science 303, 1358–1361. [DOI] [PubMed] [Google Scholar]
- 19.Greub, G. & Raoult, D. (2004) Clin. Microbiol. Rev. 17, 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker, J. & Brown, M. R. W. (1994) Microbiology 140, 1253–1259. [DOI] [PubMed] [Google Scholar]
- 21.Harb, O. S., Gao, L.-Y. & Abu Kwaik, Y. (2000) Environ. Microbiol. 2, 251–265. [DOI] [PubMed] [Google Scholar]
- 22.Solomon, J. M., Rupper, A., Cardelli, J. A. & Isberg, R. R. (2000) Infect. Immun. 68, 2939–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skriwan, C., Fajardo, M., Hägele, S., Horn, M., Wagner, M., Michel, R., Krohne, G., Schleicher, M., Hacker, J. & Steinert, M. (2002) Int. J. Med. Microbiol. 291, 615–624. [DOI] [PubMed] [Google Scholar]
- 24.Pukatzki, S., Kessin, R. H. & Mekalanos, J. J. (2002) Proc. Natl. Acad. Sci. USA 99, 3159–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosson, P., Zulianello, L., Lambert, O. J., Faursson, F., Gebbie, L., Benghezal, M., Delden, C. V., Curty, L. K. & Köhler, T. (2002) J. Bacteriol. 184, 3027–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gezelius, K. (1974) Arch. Microbiol. 98, 311–329. [DOI] [PubMed] [Google Scholar]
- 27.Marchesini, N., Ruiz, F. A., Vieira, M. & Docampo, R. (2001) J. Biol. Chem. 277, 8146–8153. [DOI] [PubMed] [Google Scholar]
- 28.Spudich, J. A. (1982) Methods Cell Biol. 25, 359–364. [DOI] [PubMed] [Google Scholar]
- 29.Loomis, W. F. (1975) Dictyostelium discoideum. A Developmental System (Academic, New York).
- 30.Levi, S., Polyakov, M. & Egelhoff, T. T. (2000) Plasmid 44, 231–238. [DOI] [PubMed] [Google Scholar]
- 31.Wang, N., Wu, W. & De Lozanne, A. (2002) J. Cell. Biochem. 86, 561–570. [DOI] [PubMed] [Google Scholar]
- 32.Ault-Riché, D., Fraley, C. D., Tzeng, C. M. & Kornberg, A. (1998) J. Bacteriol. 180, 1841–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sussman, M. (1987) Methods Cell Biol. 28, 9–29. [DOI] [PubMed] [Google Scholar]
- 34.Lydan, M. A. & Cotter, D. A. (1995) J. Cell Sci. 108, 1921–1930. [DOI] [PubMed] [Google Scholar]
- 35.Van Dijken, P. & Van Haastert, P. J. M. (2001) BMC Cell Biol. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumble, K. D., Ahn, K. & Kornberg, A. (1996) Proc. Natl. Acad. Sci. USA 93, 14391–14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz, F. A., Rodrigues, C. O. & Docampo, R. (2001) J. Biol. Chem. 276, 26114–26121. [DOI] [PubMed] [Google Scholar]
- 38.Seufferheld, M., Vieira, M. C., Ruiz, F. A., Rodrigues, C. O., Moreno, S. N. & Docampo, R. (2003) J. Biol. Chem. 278, 29971–29978. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz, F. A., Marchesini, N., Seufferheld, M., Govindjee & Docampo, R. (2001) J. Biol. Chem. 276, 46196–46203. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-García, M. R. & Kornberg, A. (2004) Proc. Natl. Acad. Sci. USA 101, 15876–15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spudich, J. A. (2004) Proc. Natl. Acad. Sci. USA 101, 15825–15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown, M. R. W. & Kornberg, A. (2004) Proc. Natl. Acad. Sci. USA 101, 16085–16087. [DOI] [PMC free article] [PubMed] [Google Scholar]