Abstract
Objective
This study was conducted to locate quantitative trait loci (QTL) influencing fatty acid (FA) composition in a large F2 intercross between Landrace and Korean native pigs.
Methods
Eighteen FA composition traits were measured in more than 960 F2 progeny. All experimental animals were genotyped with 165 microsatellite markers located throughout the pig autosomes.
Results
We detected 112 QTLs for the FA composition; Forty seven QTLs reached the genome-wide significant threshold. In particular, we identified a cluster of highly significant QTLs for FA composition on SSC12. QTL for polyunsaturated fatty acid on pig chromosome 12 (F-value = 97.2 under additive and dominance model, nominal p-value 3.6×10−39) accounted for 16.9% of phenotypic variance. In addition, four more QTLs for C18:1, C18:2, C20:4, and monounsaturated fatty acids on the similar position explained more than 10% of phenotypic variance.
Conclusion
Our findings of a major QTL for FA composition presented here could provide helpful information to locate causative variants to improve meat quality traits in pigs.
Keywords: Fatty Acid, Quantitative Trait Locus, Genome-wide Linkage Analysis, Landrace, Korean Native Pigs
INTRODUCTION
Fatty acids (FAs) composition plays a crucial role in pork quality. For example, high linoleic acid (C18:2) contents in meat, which is deposited in muscle phospholipid at a high level, is associated with low juiciness and lower consumer acceptance [1]. In addition, FA composition regulates the oxidative stability of pork, which in turn influences muscle color and meat flavor [2]. Ingested FAs could enhance the susceptibility to cardiovascular disease in humans. Thus, pork may be considered as unhealthy food. However, several of FAs such as oleic, linoleic, and linolenic FAs are associated with a hypocholesterolemic condition associated with a lower risk of suffering cardiovascular disease [3,4]. Therefore, FA composition is relevant to human health.
Two types of native pigs exist in Korea: i) the Jeju native pigs raised on the Jeju Island, and ii) native pigs raised on the Korean Peninsula. The Jeju native pig has unique genetic characteristics that differ from those of the native pig in the Korean Peninsula since it has been isolated from the main Korean Peninsula for more than 1,000 years (hereafter we will refer to Jeju native pig as ‘KNP’ in this paper). The KNP has a uniformly black coat color and its overall growth performance is low, as for most indigenous breeds. However, it has excellent meat quality such as a white colored fat, solid fat structure, good marbling, and red meat color [5]. Although Cho et al [6] recently reported several quantitative trait loci (QTLs) influencing meat quality related traits, studies on the genetic factors that influence the FA profile of KNP are still limited. Hence, this study presents the identification of novel and previously reported QTL influencing intramuscular FA composition in longissimus dorsi muscle in a large F2 intercross between Landrace and KNP pigs.
MATERIALS AND METHODS
Experimental animals
A three-generation resource population was generated and managed as described by Cho et al [7]. In brief, 19 purebred KNPs were crossed to 17 purebred Landrace. A total of 91 F1 progeny and 1,105 F2 progeny (568 males and 537 females) from 79 full-sib families were generated. Whole experimental procedures were performed according to national and institutional guidelines and approved by the Ethical Committee of the National Institute of Animal Science.
Genotypes and phenotypes
All experimental animals were genotyped with 165 microsatellite markers located throughout the pig autosomes and linkage map construction was also according to Cho et al [7]. Eighteen of FA compositions were measured in F2 progeny. A full description of phenotypic analysis can be found in our previous publication [8].
Statistical and quantitative trait loci analyses
We used a web-based GridQTL program to conduct QTL analysis (http://www.gridqtl.org.uk/). An interval mapping model based on least squares regression was used for QTL analysis, including the cofactors of sex, batch, and carcass weight. The F-ratios were computed at 1-cM intervals across the 18 autosomes. At the peak QTL location, the additive and dominance coefficients of each F2 pig were extracted to obtain significance of each variable. After evaluating the nominal significance of the additive and dominance components, only the significant additive component was included for subsequent analysis. If only the dominance effect was significant, the dominance variable was included together with the additive variable, regardless of the significance of the additive coefficient. To address the multiple testing issue in QTL analysis, genome-wide empirical significant thresholds of the test statistic were obtained by 1,000 permutations of data [9]. Genome-wide thresholds for highly significant (α = 0.01) and significant linkage (α = 0.05) were employed. Suggestive linkage was employed using a 5% chromosome-wide threshold. The 1.5-logarithm of odds (LOD) drop method was used to estimate support intervals for identified QTL at the suggestive and significant levels of significance [10]. The verifications of novel QTL were done by comparing the flanking markers of previously reported QTL in the pig QTLdb (http://www.animalgenome.org/QTLdb/pig) with those of newly detected QTL in this study.
RESULTS AND DISCUSSION
Basic statistics on the phenotypic measurements of the FA traits have been described in our previous report [8]. Oleic acids (C18:1, 40.9%) were the most abundant, followed by palmitic acids (C16:0, 25.0%), and stearic acids (C18:0, 13.7%) in longissimus dorsi muscle. The results of QTL analysis are summarized in Table 1 and Supplementary Table 1. A total of 112 QTLs were revealed across all pig autosomes. Forty seven QTLs reached the genome-wide significant threshold. In particular, we identified a cluster of highly significant QTLs for FA compositions on pig chromosome (SSC) 12 (Figure 1). Among these, the QTL for total polyunsaturated fatty acid (PUFA) was identified with an F-value of 97.2 (nominal p-value 3.6×10−39) and highest proportion of phenotypic variance was explained by this QTL (16.9%). This QTL hotspot on SSC 12 harbored additional extremely significant QTLs which explained more than 10% of phenotypic variances: the QTL for C18:2 was found with an F-value of 90.4 (nominal p-value 1.1×10−36) and accounted for 15.9% of phenotypic variance, while the QTL for C20:4 was found with an F-value of 159.7 (nominal p-value 5.7× 10−34) and explained 14.3% of phenotypic variance. Two more QTLs explained more than 10% of phenotypic variance: the QTL for total monounsaturated fatty acid (MUFA) was found with an F-value of 138.1 and explained 12.6% of phenotypic variance whereas the QTL for C18:1 was found with an F-value of 78.3 and explained 14.1% of phenotypic variance. At the similar position, eight QTLs for the percentage of C10:0, C12:0, C16:0, C17:0, C17:1, C18:0, C18:3, C20:0, and C20:1 were identified with highly significant threshold. We also mapped highly QTLs affecting total saturated fatty acid, and total unsaturated fatty acid on the similar region on SSC12. These QTLs accounted for up to 6.3% of phenotypic variance. With the exception of QTL for C20:1, detected significant QTLs on SSC12 were identified as novel. The clustering of highly significant FA composition QTL indicates that this chromosomal region would be expected to contain causative genes that impact on the fundamental biology of FA composition. Interestingly, this QTL region was co-localized with QTLs influencing meat quality traits (e.g., crude fat, meat color, etc.) with highly significance levels in our previous study [6].
Table 1.
Summary of identified significant QTLs for fatty acid composition
| SSC | Trait | Position | F-ratio1) | Inheritance of mode2) | %Var3) | Support interval4) | Reference5) |
|---|---|---|---|---|---|---|---|
| 1 | C18:0 | 123 | 16.2* | A | 1.7 | SW962-SW1301 | |
| C18:1 | 0 | 12.9* | A | 1.3 | SW1514-SW1515 | ||
| SFA | 123 | 9.3* | AD | 1.9 | SW803-SW1957 | ||
| UFA | 123 | 10.2** | AD | 2.1 | SW803-SW1957 | ||
| 2 | C16:0 | 22 | 13.7* | A | 1.4 | SW2623-SW776 | Ramayo-Caldas et al [15] |
| 3 | C20:1 | 1 | 16.0* | A | 1.6 | APR22-SE47329 | |
| 4 | C10:0 | 94 | 13.8* | A | 1.4 | SW1364-MP77 | |
| 6 | C20:4 | 79 | 13.6* | A | 1.4 | APR8-S0059 | |
| 7 | C17:0 | 63 | 21.7** | A | 2.2 | SW1369-SW147 | |
| C18:1 | 64 | 20.3** | A | 2.1 | 207G8-4-SW252 | Sanchez et al [16]; Guo et al [17] | |
| C18:2 | 63 | 13.4* | A | 1.4 | SW1369-SW2108 | Sanchez et al [16]; Guo et al [17] | |
| MUFA | 64 | 17.6** | A | 1.8 | 207G8-4-SW632 | Guo et al [17] | |
| 8 | C14:0 | 77 | 21.9** | A | 2.2 | SW933-S0086 | |
| C16:0 | 117 | 32.3** | A | 3.3 | S0069-SW790 | Estellé et al [18]; Ramayo-Caldas et al [15]; Zhang et al [19] | |
| C16:1 | 110 | 64.4** | A | 6.3 | S0069-SW790 | Estellé et al [18]; Ramayo-Caldas et al [15]; Zhang et al [19] | |
| C18:0 | 96 | 20.5** | A | 2.1 | SW444-S0144 | Guo et al [17]; Uemoto et al [20] | |
| C18:3 | 0 | 14.6* | A | 1.5 | SW2410-SW1345 | ||
| C20:0 | 141 | 13.7* | A | 1.4 | S0144-KS188 | ||
| C20:1 | 119 | 16.2* | A | 1.7 | S0086-SW790 | ||
| 9 | C16:0 | 145 | 13.2** | AD | 2.7 | SW2093-SW749 | |
| C18:0 | 39 | 17.9** | A | 1.9 | SW911-SW1434 | Nii et al [21]; Uemoto et al [20] | |
| C20:0 | 145 | 13.1** | AD | 2.7 | SW2093-SW749 | ||
| SFA | 145 | 14.2** | AD | 2.9 | SW2093-SW749 | ||
| UFA | 145 | 13.9** | AD | 2.8 | SW2093-SW749 | ||
| 12 | C10:0 | 102 | 64.8** | A | 6.3 | S0106-SWR1021 | |
| C12:0 | 109 | 21.7** | A | 2.2 | SW1962-SWR1021 | ||
| C16:0 | 111 | 49.7** | A | 5.0 | S0106-SWR1021 | ||
| C17:0 | 110 | 57.9** | A | 5.7 | S0106-SWR1021 | ||
| C17:1 | 112 | 32.6** | A | 3.3 | S0106-SWR1021 | ||
| C18:0 | 99 | 18.4** | A | 1.9 | SW1962-SWR1021 | ||
| C18:1 | 101 | 78.3** | AD | 14.1 | S0106-SWR1021 | ||
| C18:2 | 101 | 90.4** | AD | 15.9 | S0106-SWR1021 | ||
| C18:3 | 100 | 24.0** | AD | 4.8 | SW1962-SWR1021 | ||
| C20:0 | 108 | 41.5** | A | 4.2 | S0106-SWR1021 | ||
| C20:1 | 71 | 44.6** | A | 4.4 | SW957-SW1962 | Muñoz et al [22] | |
| C20:4 | 101 | 159.7** | A | 14.3 | S0106-SWR1021 | ||
| UFA | 110 | 33.5** | A | 3.4 | SW1962-SWR1021 | ||
| MUFA | 101 | 138.0** | A | 12.6 | S0106-SWR1021 | ||
| PUFA | 101 | 97.2** | AD | 16.9 | S0106-SWR1021 | Zhang et al [19] | |
| 14 | C16:1 | 69 | 50.2** | A | 5.0 | SW2519-S0116 | Sanchez et al [16]; Zhang et al [19] |
| C18:0 | 70 | 39.8** | A | 4.1 | SW2519-S0116 | Sanchez et al [16]; Uemoto et al [20] | |
| C20:1 | 55 | 13.0 | A | 1.3 | SW1975-SW886 | ||
| SFA | 73 | 14.4* | A | 1.5 | SW2519-SW2515 | ||
| UFA | 72 | 16.0* | A | 1.7 | SW2519-SW2515 | ||
| 16 | C14:0 | 42 | 8.5* | AD | 1.7 | SW419-SW2517 | Uemoto et al [23]; Quintanilla et al [24] |
| C20:0 | 49 | 23.6** | A | 2.4 | SW419-SW2517 | Guo et al [17]; Zhang et al [19] |
QTL, quantitative trait loci; SFA, saturated fatty acid; UFA, unsaturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; LOD, logarithm of odds.
Test statistic and level of significance: ** genome-wide 1%; * genome-wide 5%.
A represents additive effect; AD represents additive and dominance effects.
%Var is the reduction in residual variance of the F2 population obtained by inclusion of a QTL at the given position.
SI represents support interval of identified QTL estimated by the 1.5-LOD drop method.
Papers reporting QTL at similar positions in Pig QTLdb compared with QTL from this study.
Figure 1.
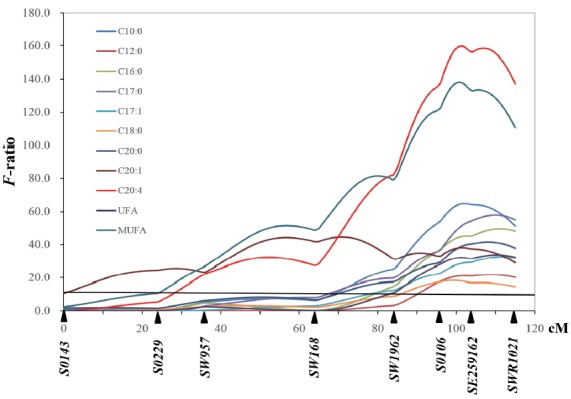
Quantitative trait loci (QTL) profiles for fatty acid composition traits on SSC12. The y-axis represents the F-value testing the hypothesis of a single QTL under additive model on a given position on the chromosome. The marker map with genetic distance between microsatellite (MS) markers in Kosambi cM is given on the x-axis. The horizontal line indicates the 5% genome-wide significant threshold.
Despite the large confidence interval for the detected QTLs, we would like to point out some apparent positional candidate genes. The QTL for C17:1, C18:1, C18:2, C20:4, MUFA, and PUFA maps to a region containing the fatty acid binding protein 3 (FABP3) gene on SSC6 [11].
The QTL regions on SSC8 (for 16:0 and C20:1) and 16 (for C12:0, C14:0, C17:1, C20:0, C20:0, C20:4) harbor the ELOVL fatty acid elongase 6 (ELOVL6) gene and the ELOVL fatty acid elongase 7 (ELOVL7) gene, respectively [12,13]. In the QTL region on SSC14, we found the stearoyl-CoA desaturase (SCD) gene. We previously reported that the SCD gene has a substantial effect on FA composition in this study population [8]. Additionally, acyl-CoA dehydrogenase, very long chain, which is involved in a fatty acid β-oxidation pathway, was found in the QTL interval on SSC12 [14].
In conclusion, the findings enhance our understanding of the genetic structure of FA composition in longissimus dorsi muscle in pig and contribute to further high-resolution QTL analyses to delineate genes affecting muscle FA profile.
Supplementary Data
ACKNOWLEDGMENTS
This study was supported by grants from Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01010502 & PJ01262701) and Postdoctoral Fellowship Program of National Institute of Animal Science (2016), Rural Development Administration, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Lawrence TLJ, Fowler VR. Growth of farm animals. 2nd ed. Wallingford, UK: CABI Publishing; 2002. pp. 1–347. [Google Scholar]
- 2.Wood JD, Enser M, Fisher AV, et al. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008;78:343–58. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Kurushima H, Hayashi K, Toyota Y, Kambe M, Kajiyama G. Comparison of hypocholesterolemic effects induced by dietary linoleic acid and oleic acid in hamsters. Atherosclerosis. 1995;114:213–21. doi: 10.1016/0021-9150(94)05486-3. [DOI] [PubMed] [Google Scholar]
- 4.Saha SS, Chakraborty A, Ghosh S, Ghosh M. Comparative study of hypocholesterolemic and hypolipidemic effects of conjugated linolenic acid isomers against induced biochemical perturbations and aberration in erythrocyte membrane fluidity. Eur J Nutr. 2012;51:483–95. doi: 10.1007/s00394-011-0233-0. [DOI] [PubMed] [Google Scholar]
- 5.Park JC, Kim YH, Jung HJ, et al. Comparison of meat quality and physicochemical characteristics of pork between Korean native black pigs, (KNBP) and Landrace by market weight. J Anim Sci Technol. 2005;47:91–8. [Google Scholar]
- 6.Cho IC, Yoo CK, Lee JB, et al. Genome-wide QTL analysis of meat quality-related traits in a large F2 intercross between Landrace and Korean native pigs. Genet Sel Evol. 2015;47:7. doi: 10.1186/s12711-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho IC, Park HB, Yoo CK, et al. QTL analysis of white blood cell, platelet and red blood cell-related traits in an F2 intercross between Landrace and Korean native pigs. Anim Genet. 2011;42:621–6. doi: 10.1111/j.1365-2052.2011.02204.x. [DOI] [PubMed] [Google Scholar]
- 8.Maharani D, Park HB, Lee JB, et al. Association of the gene encoding stearoyl-CoA desaturase (SCD) with fatty acid composition in an intercross population between Landrace and Korean native pigs. Mol Biol Rep. 2013;40:73–80. doi: 10.1007/s11033-012-2014-0. [DOI] [PubMed] [Google Scholar]
- 9.Churchill GA, Doerge RW. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–71. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupuis J, Siegmund D. Statistical methods for mapping quantitative trait loci from a dense set of markers. Genetics. 1999;151:373–86. doi: 10.1093/genetics/151.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmerman AW, Veerkamp JH. New insights into the structure and function of fatty acid-binding proteins. Cell Mol Life Sci. 2002;59:1096–116. doi: 10.1007/s00018-002-8490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naganuma T, Sato Y, Sassa T, Ohno Y, Kihara A. Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Lett. 2011;585:3337–41. doi: 10.1016/j.febslet.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Corominas J, Ramayo-Caldas Y, Puig-Oliveras A, et al. Polymorphism in the ELOVL6 gene is associated with a major QTL effect on fatty acid composition in pigs. PLoS One. 2013;8:e53687. doi: 10.1371/journal.pone.0053687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregersen N, Andresen BS, Corydon MJ, et al. Mutation analysis in mitochondrial fatty acid oxidation defects: exemplified by acyl-CoA dehydrogenase deficiencies, with special focus on genotype-phenotype relationship. Hum Mutat. 2001;18:169–89. doi: 10.1002/humu.1174. [DOI] [PubMed] [Google Scholar]
- 15.Ramayo-Caldas Y, Mercadé A, Castelló A, et al. Genome-wide association study for intramuscular fatty acid composition in an Iberian × Landrace cross. J Anim Sci. 2012;90:2883–93. doi: 10.2527/jas.2011-4900. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez MP, Iannuccelli N, Basso B, et al. Identification of QTL with effects on intramuscular fat content and fatty acid composition in a Duroc × Large White cross. BMC Genet. 2007;8:55. doi: 10.1186/1471-2156-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T, Ren J, Yang K, et al. Quantitative trait loci for fatty acid composition in longissimus dorsi and abdominal fat: results from a White Duroc × Erhualian intercross F2 population. Anim Genet. 2009;40:185–91. doi: 10.1111/j.1365-2052.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 18.Estellé J, Fernández AI, Pérez-Enciso M, et al. A non-synonymous mutation in a conserved site of the MTTP gene is strongly associated with protein activity and fatty acid profile in pigs. Anim Genet. 2009;40:813–20. doi: 10.1111/j.1365-2052.2009.01922.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Zhang J, Cui L, et al. Genetic architecture of fatty acid composition in the longissimus dorsi muscle revealed by genome-wide association studies on diverse pig populations. Genet Sel Evol. 2016;48:5. doi: 10.1186/s12711-016-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemoto Y, Soma Y, Sato S, et al. Genome-wide mapping for fatty acid composition and melting point of fat in a purebred Duroc pig population. Anim Genet. 2012;43:27–34. doi: 10.1111/j.1365-2052.2011.02218.x. [DOI] [PubMed] [Google Scholar]
- 21.Nii M, Hayashi T, Tani F, et al. Quantitative trait loci mapping for fatty acid composition traits in perirenal and back fat using a Japanese wild boar × Large White intercross. Anim Genet. 2006;37:342–47. doi: 10.1111/j.1365-2052.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz G, Alves E, Fernández A, et al. QTL detection on porcine chromosome 12 for fatty-acid composition and association analyses of the fatty acid synthase, gastric inhibitory polypeptide and acetyl-coenzyme A carboxylase alpha genes. Anim Genet. 2007;38:639–46. doi: 10.1111/j.1365-2052.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 23.Uemoto Y, Sato S, Ohnishi C, et al. The effects of single and epistatic quantitative trait loci for fatty acid composition in a Meishan × Duroc crossbred population. J Anim Sci. 2009;87:3470–6. doi: 10.2527/jas.2009-1917. [DOI] [PubMed] [Google Scholar]
- 24.Quintanilla R, Pena RN, Gallardo D, et al. Porcine intramuscular fat content and composition are regulated by QTLs with muscle-specific effects. J Anim Sci. 2011;89:2963–71. doi: 10.2527/jas.2011-3974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


