Abstract
The increasing frequency of antibiotic resistance in hospital-acquired infections is a major public health concern that has both biological and economic causes. Here we develop conceptual mathematical models that couple the economic incentives and population biology of hospital infection control (HIC). We show that the optimal investment by a hospital for HIC changes with the proportion of patients already colonized with antibiotic-resistant bacteria (ARB) at the time of admission. As that proportion increases, the optimal behavior of a hospital is to increase spending to control ARB with low transmissibility and decrease spending on those with high transmissibility. In some cases, the global optimum investment in HIC can shift discontinuously from one that contains transmission to a do-nothing policy once the proportion already colonized at the time of admission becomes too great. We also show that investments in HIC are determined by a strategic game when several hospitals share patients. Hospitals acting selfishly and rationally will free-ride on the investments of other hospitals, and the level of free-riding should increase with the number of other hospitals in the area. Thus, in areas with many hospitals, the rational strategy for each hospital is to spend less than in areas with few hospitals. Thus, we predict that transmission rates and the prevalence of ARB should be higher in urban hospitals, for instance, compared with rural hospitals. We conclude that regional coordination and planning for HIC is an essential element of public health planning for hospital-acquired infections.
Keywords: game theory, nosocomial infections, infection control, optimal control, transmission dynamics
The emergence and spread of bacteria resistant to multiple antibiotics continues to gain in importance as a major public health concern. One facet of the epidemic is the increasing frequency of hospital-acquired infections resistant to multiple antibiotics, an alarming trend that has continued despite efforts to control transmission through prudent antibiotic use and hospital infection control (HIC) (1, 2). Here we explore the economic incentives for HIC as an underlying cause of the current epidemic and as a hypothesis for the distribution of antibiotic-resistant bacteria (ARB).
The most immediate public health concern is to control transmission of resistant bacteria in hospital intensive-care units, in which most infections occur. More broadly, the public health response to antibiotic resistance must be concerned with those people who are colonized by ARB (i.e., asymptomatic carriers), because these individuals increase colonization pressure and the risk that other patients will become infected (symptomatic) or colonized (asymptomatic) by ARB (3, 4).
In some people, asymptomatic carriage of ARB can be extremely persistent (5). These ARB carriers play an important role in the spread of ARB among hospitals and other institutions in a region. Carriers can move resistance among hospitals, so hospitals with endemic resistance can “infect” other hospitals by discharging colonized patients or hiring from another hospital health-care workers who are carriers (6–9). Moreover, recently hospitalized patients are more likely to be hospitalized again; carriers may be rehospitalized and continue to transmit with important epidemic implications (10–12). Over time, the proportion of people who are already colonized at the time of admission increases, which makes HIC more difficult.
This “spillover effect” that links levels of infection and drug resistance among hospitals has important consequences for incentives faced by hospital administrators to invest in HIC. The benefits of HIC expenditure in any given facility depend on the proportion of patients who are already colonized at the time of admission. Hospitals that are behaving optimally to minimize both costs and levels of ARB within their own facilities will incur additional expenses by admitting ARB carriers who became colonized elsewhere. Similarly, the hospital may ignore the benefits of their HIC programs outside their own walls; hospitals may not benefit from decreasing the overall level of resistance in the catchment population when those patients are admitted later to other hospitals. Instead, hospitals may prefer to free-ride on the HIC investments of other hospitals.
The extent to which a facility will cut back on its expenditures from a level that is socially desirable increases with the probability that the burden of disease created by a hospital's failure to successfully manage ARB within the hospital is borne to a greater extent by other hospitals. Therefore, one would expect to find greater levels of ARB resistance in urban settings, where many hospitals in close proximity share a common pool of carriers and there is a greater likelihood that a discharged patient will be readmitted to a different facility. Conversely, we might expect greater investment in HIC per patient and lower levels of resistance in large hospitals in rural settings, where there are few other hospitals close by.
Variations in incentives to invest in HIC may help explain patterns in the emergence and spread of ARB. A case in point is the emergence and spread of multidrug resistance in hospital-acquired pathogens such as vancomycin-resistant enterococci (VRE) and methicillin-resistant Staphylococcus aureus (MRSA). In both cases, new forms of resistance first emerged in large research hospitals in metropolitan areas, spreading next to other hospitals in the regions and then to other regions (13). Multidrug resistance has been rare or absent in small and isolated communities. In a few notable success stories, large coordinated efforts have managed to reverse epidemics or keep the frequency of resistance low (14–16). Although there are epidemiological reasons why resistance may have arisen more rapidly in urban settings with closer contact between people, the role of HIC within hospitals is important to examine in greater detail. Why did resistance arise more rapidly in urban settings? Did it have anything to do with incentives faced by hospital administrators who have little control over the influx of resistant organisms from other facilities? Also, what types of strategies are likely to work when the control of ARB depends on the actions of multiple institutions, each acting in its own self-interest?
Mathematical models have provided important insights into how ARB evolve and proliferate (6, 11, 12, 17–20) as well as into the relative efficiency of control strategies (21, 22). Integrating a strategic framework that couples the economic incentives faced by institutions with population dynamic models enhances the insights based on models that rely on either one alone. We propose a hospital incentive-based hypothesis for the historical patterns of emergence and spread of ARB and for the distribution of antibiotic resistance and discuss implications for the success or failure of strategies to control the resistance epidemic.
Infection Control When Patients Are Already Colonized at the Time of Admission
We begin by focusing on the optimal investment in HIC made by a single hospital. Let 1/σ denote the average length of stay in a hospital (σ is the per-capita discharge rate), X the proportion of patients that are colonized, and κ the proportion that are admitted colonized.
Let D denote the excess economic costs born by a hospital from each colonized patient, per day; these costs measure the economic burden of resistance generated by extra days of hospitalization and the increased costs incurred from treating resistant infections. To reduce the number of patients who are colonized, we assume hospitals can change their expenditures to reduce transmission through HIC; let c denote the amount of money spent by the institution on HIC per patient per day. Let β(c) denote daily transmission rates within a hospital as a function of money spent. We assume that β(c) is a nonincreasing function of expenditures, β′(c) ≤ 0, where the prime denotes the derivative with respect to c, and we have focused on functions with diminishing marginal reductions in transmission with respect to HIC investments, β″(c) ≥ 0.
Note that extra hospitalization from patients with resistant bacterial infections would also lead to more transmission from these patients. Thus, our assumption of a constant discharge rate, regardless of colonization status, may be slightly biased. On the other hand, longer hospital stays are not expected for colonized patients, and colonization is much more common than infection.
The dynamics are given by
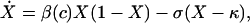 |
[1] |
where the superdot denotes the derivative with respect to time. Let S(c) = β(c)/σ denote the single-stay reproductive number, the number of cases, per case per visit, as a function of expenditures when resistance is absent. S(c) is effectively the basic reproductive, R0, in this model but not in structured population models (see below and ref. 11). The equilibrium prevalence is given by
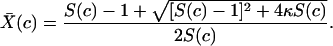 |
[2] |
The turnover of hospital patients is relatively fast (average length of stay is ≈5 days), so hospital prevalence responds rapidly to changes in HIC investments and tracks the equilibrium. Therefore, we ignored the transient dynamic behavior, and the problem of minimizing total costs is reduced to minimizing the quantity c + D X̄(c).
The optimal strategy varies with the proportion colonized on admission, κ, with the excess economic costs associated with resistance, D, with the maximum intrinsic transmissibility of the ARB, S(0), and with the shape of the transmission function, β(c). Local minima, ĉ, if they exist, are solutions to the equation 1 + D X̄′(ĉ) = 0 and subject to the condition X̄″(ĉ) > 0. It is possible that multiple local minima exist or that the global optimum is to spend nothing. For example, a hospital that spends nothing on HIC may see total resistance-related expenses increase for marginal increases in HIC expenditures if -X̄′(0) < 1/D, whereas the global minimum is to invest substantially more. Also note that c* < D, because total costs are at most D dollars per person per day, and it would cost less to let patients become infected and treat them rather than spend on HIC (proof in Appendix). Thus, 0 ≤ c* < D.
For some cost functions, β(c), optimal allocations in HIC can shift dramatically to spending nothing once the proportion colonized at the time of admission exceeds a threshold, effectively amounting to abandoning all infection control (see Fig. 1). For such functions, the optimum strategy is to spend enough to eliminate the pathogen if the proportion already colonized at the time of admission is very low. Once the proportion already colonized at the time of admission reaches a threshold (≈12% in Fig. 1), two minima exist: a local minimum, ĉ, is to spend approximately the same amount it would cost to eliminate the pathogen, but the global minimum c* is to spend nothing and save a few dollars by letting transmission proceed and absorbing the costs. Hospitals investing at the local minimum would see total costs increase if they spent slightly less on infection control, but they would see total costs decrease if they abandon it. Once the proportion already colonized at the time of admission reaches some higher threshold (≈18% in Fig. 1), the local minimum disappears, and total costs decrease smoothly with the proportion already colonized at the time of admission. Thus, it may be optimal to abandon HIC if hospitals admit too many patients who are already colonized by resistant bacteria, because such individuals swamp infection control.
Fig. 1.
As the proportion already colonized at the time of admission increases, the costs and benefits of HIC change. When the proportion of patients already colonized at the time of admission is low, it is cost-effective to invest enough in HIC to eliminate the pathogen. Once that proportion exceeds ≈12%, two minima exist. A local minimum is to continue to invest at levels that would eliminate the pathogen if no patients were colonized at the time of admission, but the globally cost-effective strategy is to abandon HIC and do nothing. Hospitals spending near the no-colonization optimum would see total costs increase if they spent slightly less on infection control, but they would see total costs decrease if they abandon it entirely. Once the proportion colonized on admission reaches 18%, the local minimum disappears, and hospitals would see total costs decrease as they spent less on HIC. The point at which abandoning HIC becomes optimal depends on the particular function that describes the relationship between costs and transmission; here, we use the function S(c) = 4e-0.03c and D = $100 per patient per day.
For some functions, β(c), multiple local minima do not exist, and expenditures change smoothly with changes in κ. Optimal allocation for one such function is illustrated in Fig. 2. When no patients are colonized at the time of admission, the optimal solution is to eliminate the ARB for 1 < S(0) < τ, where τ is a kind of threshold; >τ, it is too expensive to eliminate the ARB, but some investment in HIC reduces total costs. The cutoff for reducing S below 1 changes with the costs, D, and the control function, β(c), but similar patterns were observed for other functions (data not shown).
Fig. 2.
Optimal expenditures on HIC (solid line) vary depending on the transmissibility of the ARB S(0) and the proportion already colonized at the time of admission κ; at the optimum, transmission rates decrease [shown as S(c*), the dotted line]. For κ = 0, the optimum investment is to eliminate the ARB if S(0) < ≈2.2. Otherwise, the optimum reduces transmission but does not eliminate ARB. For κ = 4%, it is not possible to eliminate ARB, so the optimum response curve is not as sharp. For S(0) < ≈1.5, the optimum spent on HIC increases with the proportion already colonized at the time of admission but not for ARB with higher intrinsic transmission rates. The shifts in optimum investment depend on the specific function used; here, S(c) = S(0)(1 + 0.2√c)-1, a function that does not predict sudden shifts in spending.
Total costs associated with resistance increase with the proportion already colonized at the time of admission, κ, but the benefits of HIC differ among bacterial species depending on the intrinsic transmissibility of the ARB, S(0). All else being equal, when S(0) is low, optimal expenditures increase with the proportion already colonized at the time of admission; it is optimal to reduce transmission (Fig. 2). In contrast, the optimal investments in HIC decrease for ARB with high transmissibility, i.e., for S(0) ≫ 1. For a narrow range of intermediate values, optimal expenditures initially increase with κ but later decrease.
Intuitively, lowering the transmission rate costs money but also lowers the burden associated with colonization, D. The increased investment for ARB with low transmissibility limits quasiepidemic transmission (11): secondary transmission from imported cases at levels that are too low to sustain an internal epidemic but that nevertheless lead to substantial increases in prevalence around S = 1. The benefits of controlling transmission are the opposite for ARB with higher transmissibility. When the proportion of admitted patients who are colonized is low, then the marginal benefit of reducing the transmission rate is relatively greater for ARB with higher intrinsic transmissibility, S(0), and it is worth spending more money on HIC. However, when the proportion of admitted patients who are colonized is high, the benefit of reducing the transmission rate is diminished, because newly arriving patients will increase the burden of infection regardless of what one spends on HIC.
Multi-Institutional Epidemics and Infection-Control Strategies
We expand the analysis to consider strategic interactions with other institutions. Let X denote the proportion colonized in a focal hospital. Let n denote the number of hospitals in an area; as n increases, so does the size of the community. Let Y denote the proportion colonized in each one of the n other hospitals, all of which are identical, and let Z denote the proportion colonized in the community from which all of the hospitals draw patients.
To focus on strategic aspects of these interactions, we assume that resource-allocation decisions made by each hospital are time-invariant and that all the hospitals are identical except with respect to the amount of money spent by the focal hospital and other hospitals on infection control. Let c denote the amount of money spent by a focal institution for infection control, and let c̃ denote the investment by the other hospitals in the catchment population. Transmission decreases following the same assumptions and notation as before. Here, hospitals allocate a certain level of resources to infection control depending on the proportion colonized on admission Z; because all hospitals discharge colonized patients into a common catchment population, the decision to allocate is a response to other hospitals in the region.
We assume that no transmission occurs in the community, and therefore those who are colonized by ARB have been hospitalized. Because colonization status is a marker for previous hospitalization, the average waiting time to hospitalization for colonized individuals, denoted 1/r, may be much higher than for a typical person in the population. We assume that the average persistence time for ARB is 1/λ regardless of an individual's location.
The dynamics of ARB in multi-institutional epidemics are governed by the following equations:
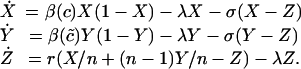 |
[3] |
The dynamics of multi-institutional epidemics have been explored by Smith et al. (11) and Cooper et al. (12). We are interested in understanding how the strategy changes with actions undertaken by other institutions and how these strategies affect the infection dynamics.
As before, we assume that each colonized patient costs the hospital D dollars per patient per day. For the hospital, the total economic costs of resistance at any point in time is given by c + DX(t, c; n, c̃). Greater spending on HIC, c, both reduces prevalence in the focal institution in the current period and the proportion of patients discharged colonized who may remain colonized if and when the patient is later rehospitalized to one of the hospitals at random.
The focal institution decides how much to invest in HIC by minimizing the net present value of discounted costs of HIC and hospitalization, given by the formula 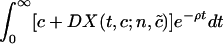 , where ρ is the economic discounting rate.
, where ρ is the economic discounting rate.
In these equations, colonization originates in hospitals, and after discharge, each patient can generate additional cases, if they remain colonized when rehospitalized. The probability that a newly colonized patient is discharged colonized and remains colonized on readmission is given by the formula p = σr/(σ + λ)(r + λ). Typically, hospital visits are short (1/σ ≈ 5 days), so most people remain colonized while hospitalized. The probability of remaining colonized on next admission is higher if persistence times are long and the intervals between hospital visits are short.
The economic incentives to invest in HIC change depending on the number of other hospitals in a region. The proportion already colonized at the time of admission depends on the probability that a patient remains colonized on readmission, p, as well as the spending on HIC by all the hospitals in an area. In multi-institutional epidemics, each hospital allocates resources to decrease the number of cases generated by each case in its own hospital during a single hospital visit, either S(c) = β(c)/(λ + σ) or S(c̃) = β(c̃)/(λ + σ). The total number of cases generated is found by summing over all visits; a patient remains colonized for p/(1 - p) hospital visits. The expected number of secondary cases generated by each colonized patient, summed over the duration of colonization, after being discharged from a hospital is p/(1 - p)[S(c)/n + (n - 1)S(c̃)/n)].
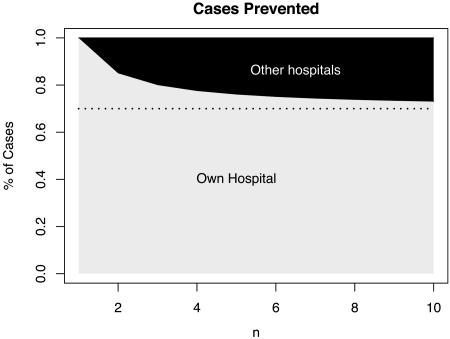
As the number of other hospitals in the area increases, a focal hospital's allocation decision has less influence on the proportion colonized in a catchment population because of the decisions taken by the remaining n - 1 hospitals. In this model, patients enter another hospital, chosen at random. By preventing transmission through HIC, a focal hospital prevents [1 + np/(1 - p)]S(c) new cases in its own hospital and (n - 1)p/[n(p - 1)]S(c̃) in other hospitals. Thus, as the size of the catchment population increases, the benefits of HIC decrease as realized by the focal hospital, and they increasingly depend on the expenditures of other hospitals (Fig. 3).
Fig. 3.
A fraction of the total secondary cases prevented are expected to occur during the initial visit (below the dotted line), but some occur on subsequent visits. As the number of other hospitals that share a population increases (n), the benefits of reducing transmission are increasingly shared among hospitals (dark gray) as the number of other hospitals in a region increases. Thus, infection control is an economic game. The fraction of benefits that accrue to other hospitals increases when the interval between hospitalization is short and persistence times are long. We have illustrated the relationship for p = 0.3, equal expenditures (i.e., c = c̃) and values of n that range from a rural hospital to a hospital in a city of several million people.
In a region with one hospital, that hospital readmits its own patients and realizes the full benefit of HIC investment. In areas with many hospitals, a patient who was previously hospitalized is more likely to have been hospitalized elsewhere, so hospitals are affected by the decisions of other hospitals to invest in HIC. Because the proportion already colonized at the time of admission is increasingly out of its control, the economics become increasingly like a model with a constant proportion of patients already colonized at the time of admission (Eq. 1).
Thus, in multi-institutional epidemics, the optimal allocation in HIC for a focal hospital changes in response to other hospitals. The decisions are represented as response curves (Fig. 4). When the initial conditions are the same for the focal hospital and other hospitals, the optimum for cooperating hospitals is the same as the optimal strategy for a single hospital. In contrast, a strategic optimum is the point at which a focal hospital's optimal expenditures in response to its neighbors matches the expenditures of the neighbors. It is important to note that the strategic optimum decreases with the number of hospitals that share a catchment population, a surrogate for the population density in a region (Fig. 4a). If other hospitals invest in HIC, a focal hospital can decide to spend less and “free-ride” on other hospital's investments. As a consequence of free-riding, hospitals in metropolitan areas will tend to follow the game-theoretic optimum and spend less on HIC, leading to epidemics that develop earlier and faster (Fig 4b).
Fig. 4.
The strategic responses and corresponding dynamics change with the number of hospitals that interact. (a) The response curves (solid) for increasing n. The strategic optimum, the points at which a focal hospital's optimal investment matches the investment of other hospitals, decreases with n (the dashed line shows equal investments). Note also that the coordinated optimum for many hospitals is the same as the optimum investment for a single hospital, n = 1. (b) These decisions affect the rate at which resistance increases. For n = 10, an epidemic occurs if hospitals allocate at the strategic optimum, whereas the coordinated optimum prevents emergence for more than a decade. Hence, the epidemic will be delayed and less severe in isolated areas. The transmission function here is the same one used for Fig. 2, with 1/λ = 2,000 days and 1/r ≈ 1,500 days.
Discussion
Two examples of successful HIC efforts have been the Dutch public health response to MRSA and the Siouxland response to VRE. In The Netherlands, the frequency of MRSA infections is <0.5% after an intensive “search-and-destroy” campaign, compared with 50% in some areas (14). In Siouxland, an epidemic of VRE was reversed (15). Economic studies have shown that the high level of expenditure on HIC in The Netherlands (a total of 2.8 million Euros between 1991 and 2000) was justified on the basis of averted MRSA infections, vancomycin intermediate-susceptible S. aureus, and VRE (23).
In both examples, an important but overlooked fact is that the public health response was coordinated among institutions. From an economic perspective, the hospitals sought a cooperative optimum that would not have been in the interest of any single institution to seek on its own. In the absence of coordination, an intensive HIC effort by any single hospital may have resulted in other hospitals cutting back on their HIC expenditures if they were behaving selfishly. Similar arguments apply to isolated hospitals: because they admit their own patients, they will tend to make optimal decisions and adopt more aggressive HIC responses. In contrast, hospitals in metropolitan areas will tend to free-ride and invest less than the coordinated optimum.
In the language of economics, investment in HIC by a single hospital produces positive externalities, or benefits that do not accrue to the individual hospital. Because the hospital will typically ignore these benefits unless it is altruistic toward other facilities in the region, it will invest in a lower level of HIC that is socially desirable. Therefore, the failure to invest adequately in controlling ARB within the hospital that we currently observe may not necessarily be a consequence of failure of hospital administrators and HIC committees to respond adequately to problems in their facilities but may actually be the most optimal response from the hospital's perspective. This kind of behavior is observed in other settings. Individuals frequently ignore the consequences of their driving on overall traffic congestion, as do factories that pollute without regard to the adverse effect of that pollution on others. Although taxes on hospitals that have high levels of transmission is a theoretical (although impractical) way to deal with the problem, a coordinated strategy may be in the interest of all hospitals.
From a game-theory perspective, the spirit of the problem of noncooperative investment in HIC is captured as the well known prisoner's dilemma. The two (or more) players (hospitals) in the game can make one of two decisions: either “cooperate” (fight ARB aggressively) or “defect” (invest little in HIC). Each hospital gains when both cooperate, but if only one of them cooperates, the other one (who defects) will gain more because they can free-ride on the efforts of the other hospital(s). If both choose to not cooperate, both lose (or gain very little) but not as much as the “cheated” hospital, the cooperation of which in controlling ARB is not reciprocated by other hospitals. The Nash equilibrium, of course, is for both hospitals to defect. Needless to say, it may be in the interest of any single hospital to free-ride on the HIC efforts of other facilities and lower its own resource allocation to HIC. Hence, it may be in the public interest to establish a transparent system that permits hospitals to observe transmission levels and HIC expenditures in other hospitals. Third-party verification of the efforts of each hospital also may be a possible solution.
We have focused on the role of hospitals in spreading resistance, but the use of antibiotics in agriculture for prophylaxis and growth promotion have also been implicated in generating a reservoir resistance in the community (24–26). The interplay between heterospecific transmission of ARB due to agricultural antibiotic use and horizontal transmission of ARB in hospitals has been explored (20, 27–29). By increasing the reservoir of ARB in the community, agricultural antibiotic use increases the difficulty and expense of HIC, but it can also undermine a hospital's incentives to invest in HIC to control resistance in rapidly transmitted ARB (30).
We have provided an economic hypothesis for the distribution of ARB, but other epidemiological mechanisms can explain the same patterns. For example, the prevalence of resistance could be higher in cities because transmission correlates with population density, or in other words because community transmission is density-dependent (31). One way to test these competing hypotheses would be to examine community prevalence in urban and rural settings: higher community transmission in large cities would weaken the association between previous hospitalization and colonization status. Thus, a comparison of the strength of association between previous hospitalization and colonization status in urban and rural areas would provide a crucial test of the theory.
Another competing hypothesis is that ARB are introduced into city hospitals earlier because of patient sharing and that prevalence is higher in urban hospitals because the epidemic is more advanced. One possible test would be to compare the prevalence of ARB in hospitals that draw from the same patient population but that readmit different proportions of their own patients. Another test would be to look for correlations between prevalence and the date when ARB was first detected in an area. None of these alternative hypotheses explain the success of HIC in limiting the prevalence of MRSA and VRE in Dutch hospitals or in reversing the VRE epidemic in the Siouxland region.
The results we present here are based on several simplifying assumptions. We assumed that hospitals are identical with respect to their incentives to invest in HIC, but hospitals can differ from one another in important ways. For example, patients do not flow randomly among hospitals; rehospitalized patients are often transferred or readmitted to tertiary care facilities. Such hospitals often perform organ transplants and other procedures that require long hospital stays and heavy use of antimicrobial drugs. Moreover, tertiary care facilities, including large teaching hospitals, are often located in large urban areas. Such hospitals could play an important role by infecting other hospitals or by acting as major sources for colonization (6, 11, 18). The implications of differences among hospitals could be explored in models of this sort.
Another caveat is that hospitals adjust their allocation decisions over time depending on their situation. Although our assumption of time-invariant HIC expenditures is admittedly restrictive, it is largely true that hospitals do not make dynamically optimal resource allocations and are more likely to be responsive to problems with ARB as they arise. It is also possible that early investments in HIC can have long-term benefits by selecting for ARB that have lower transmissibility; an additional benefit of the Dutch campaign was to reduce the prevalence of those strains of S. aureus with the highest intrinsic transmission rates, making future HIC efforts easier (14). In our models, we ignored selection for lower transmissibility.
We have shown also that admitting patients who are already colonized can seriously undermine HIC. For example, it may be optimal for hospitals to abandon HIC once the proportion already colonized at the time of admission exceeds a threshold. Sudden decreases in spending would lead to sudden and dramatic increases in transmission. Similar dramatic increases in transmission may occur as the proportion already colonized at the time of admission increases if hospitals exceed their limited capacity to isolate colonized patients (12). Large, sudden decreases in the amount invested in HIC at the optimum that amount to abandoning HIC are not a universal property of models such as this one. For functions with more mundane responses, optimal allocation decisions change with the proportion already colonized at the time of admission, but the direction of the change depends on the intrinsic rate of increase of the ARB. For ARB with low transmissibility, including many ARB that can sustain transmission within a hospital, coupled epidemiological and economic models predict that hospitals should increase their expenditures in HIC as the proportion colonized increases. For ARB with high transmissibility, expenditures decrease as the proportion already colonized at the time of admission increases. Thus, hospitals may adopt different strategies for different ARB. On the other hand, the same control strategies generally affect multiple ARB, increasing the benefits of every dollar spent on HIC (32). These insights become increasingly complicated in metapopulations in which long persistence guarantees that some discharged patients will continue to generate new cases in other hospitals.
These principles also apply to other bioeconomic problems such as conservation biology in a metapopulation, in which local populations are demographically unstable, but the population at a larger scale is stabilized by migration from other populations nearby. In such cases, persistence in local populations naturally free-rides on migration from healthy populations nearby. Local economic decisions may have a marginal effect on global persistence. Thus, coordinated control may lead to persistence, with which uncoordinated control would lead to a game-theoretic optimum at which none of the local populations are sustained and the populations deterministically go extinct.
Key messages can be summarized as follows. The level of expenditure of a hospital on infection control may be influenced by the number of other hospitals in an area. Therefore, a single hospital in a rural setting is more likely to invest in HIC than a large hospital in an urban setting. In fact, it may actually be in the best interests of a hospital to engage in a lower level of infection control than is desirable from a societal standpoint, which may result in a failure to control ARB in settings in which a number of institutions are in close geographical proximity and share a common catchment population of patients. Search-and-destroy strategies of the kind observed from the Netherlands and Siouxland experiences are successful not only because they engaged in a massive investment in HIC but also because a number of hospitals acted in a coordinated manner. Organizing regional HIC committees that share information on ARB prevalence within their facilities and act in a coordinated manner to manage ARB within the region may be a useful first step.
Acknowledgments
We thank F. Ellis McKenzie and Gardner Brown for reading and commenting on earlier drafts.
Appendix: Proof That c* < D
Because X is the proportion colonized, X < 1. Suppose the optimum is c* and c* > D. Total costs are c* + DX(c*). By spending nothing, total costs would be DX(0), but DX(0) < D < c* < c* + DX(c*), which is a contradiction because we assumed that total costs were minimized at c* + DX(c*).
Abbreviations: HIC, hospital infection control; ARB, antibiotic-resistant bacteria; VRE, vancomycin-resistant enterococci; MRSA, methicillin-resistant Staphylococcus aureus.
See Commentary on page 2683.
References
- 1.Hospital Infection Control Practices Advisory Committee (1995) MMWR Recomm. Rep. 44 (RR-12), 1-13. [PubMed] [Google Scholar]
- 2.National Nosocomial Infections Surveillance (2001) Am. J. Infect. Control 29, 404-421. [DOI] [PubMed] [Google Scholar]
- 3.Mest, D. R., Wong, D. H., Shimoda, K. J., Mulligan, M. E. & Wilson, S. E. (1994) Anesth. Analg. 78, 644-650. [DOI] [PubMed] [Google Scholar]
- 4.Bonten, M. J., Slaughter, S, Ambergen, A. W., Hayden, M. K., van Voorhis, J., Nathan, C. & Weinstein, R. A. (1998) Arch. Intern. Med. 158, 1127-1132. [DOI] [PubMed] [Google Scholar]
- 5.Bonten, M. J., Hayden, M. K., Nathan, C., Rice, T. W. & Weinstein, R. A. (1998) J. Infect. Dis. 177, 378-382. [DOI] [PubMed] [Google Scholar]
- 6.Austin, D. J. & Anderson, R. M. (1999) Philos. Trans. R. Soc. London B 354, 721-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trick, W. E., Kuehnert, M. J., Quirk, S. B., Arduino, M. J., Aguero, S. M., Carson, L. A., Hill, B. C., Banerjee, S. N. & Jarvis, W. R. (1999) J. Infect. Dis. 180, 391-396. [DOI] [PubMed] [Google Scholar]
- 8.Trick, W. E., Weinstein, R. A., DeMarais, P. L., Kuehnert, M. J., Tomaska, W, Nathan, C., Rice, T. W., McAllister, S. K., Carson, L. A. & Jarvis, W. R. (2001) J. Am. Geriatr. Soc. 49, 270-276. [DOI] [PubMed] [Google Scholar]
- 9.Tansel, O., Kuloglu, F., Mutlu, B., Anthony, R. M., Uyar, A., Vahaboglu, H. & French, G. L. (2003) New Microbiol. 26, 175-180. [PubMed] [Google Scholar]
- 10.Lai, K. K., Fontecchio, S. A., Kelley, A. L., Baker, S. & Melvin, Z. S. (2003) Infect. Control Hosp. Epidemiol. 24, 264-268. [DOI] [PubMed] [Google Scholar]
- 11.Smith, D. L., Dushoff, J., Perencevich, E. N., Harris, A. D. & Levin, S. A. (2004) Proc. Natl. Acad. Sci. USA 101, 3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper, B. S., Medley, G. F., Stone, S. P., Kibbler, C. C., Cookson, B. D., Roberts, J. A., Duckworth, G., Lai, R. & Ebrahim, S. (2004) Proc. Natl. Acad. Sci. USA 101, 10223-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman, R. S., Smith, J., Walker, M., Byrne, S., Ramotar, K., Dyck, B., Kabani, A. & Nicolle, L. E. (1997) Clin. Infect. Dis. 25, 698-705. [DOI] [PubMed] [Google Scholar]
- 14.Verhoef, J., Beaujean, D., Blok, H., Baars, A., Meyler, A., van der Werken, C. & Weersink, A. (1999) Eur. J. Clin. Microbiol. Infect. Dis. 18, 461-466. [DOI] [PubMed] [Google Scholar]
- 15.Ostrowsky, B. E., Trick, W. E., Sohn, A. H., Quirk, S. B., Holt, S, Carson, L. A., Hill, B. C., Arduino, M. J., Kuehnert, M. J. & Jarvis, W. R. (2001) N. Engl. J. Med. 344, 1427-1433. [DOI] [PubMed] [Google Scholar]
- 16.Sohn, A. H., Ostrowsky, B. E., Sinkowitz-Cochran, R. L., Quirk, S. B. & Jarvis, W. R. (2001) Am. J. Infect. Control 29, 53-57. [DOI] [PubMed] [Google Scholar]
- 17.Austin, D. J., Kakehashi, M & Anderson, R. M. (1997) Proc. R. Soc. London Ser. B 264, 1629-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin, D. J. & Anderson, R. M. (1999) J. Infect. Dis. 179, 883-891. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitch, M., Bergstrom, C. T. & Levin, B. R. (2000) Proc. Natl. Acad. Sci. USA 97, 1938-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, D. L., Harris, A. D., Johnson, J. A., Silbergeld, E. K. & Morris, J. G., Jr. (2002) Proc. Natl. Acad. Sci. USA 99, 6434-6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonhoeffer, S., Lipsitch, M. & Levin, B. R. (1997) Proc. Natl. Acad. Sci. USA 94, 12106-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perencevich, E. N., Fisman, D. N., Lipsitch, M., Harris, A. D., Morris, J. G., Jr., & Smith, D. L. (2004) Clin. Infect. Dis. 38, 1108-1115. [DOI] [PubMed] [Google Scholar]
- 23.Vriens, M., Blok, H., Fluit, A., Troelstra, A., van der Werken, C. & Verhoef, J. (2002) Eur. J. Clin. Microbiol. Infect. Dis. 21, 782-786. [DOI] [PubMed] [Google Scholar]
- 24.Wegener, H. C., Aarestrup, F. M., Jensen, L. B., Mammerum, A. M. & Bager, F. (1999) Emerg. Infect. Dis. 5, 329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonten, M. J., Willems, R. & Weinstein, R. A. (2001) Lancet Infect. Dis. 1, 314-325. [DOI] [PubMed] [Google Scholar]
- 26.Aarestrup, F. M., Seyfarth, A. M., Emborg, H. D., Pedersen, K., Hendriksen, R. S. & Bager, F. (2001) Antimicrob. Agents Chemother. 45, 2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith, D. L., Johnson, J. A., Harris, A. D., Furuno, J. P., Perencevich, E. N. & Morris, J. G., Jr. (2003) Lancet Infect. Dis. 3, 241-249. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, L., Smith, D. L., Snary, E. L., Johnson, J. A., Harris, A. D., Wooldridge, M. & Morris, J. G., Jr. (2004) Int. J. Antimicrob. Agents 24, 205-212. [DOI] [PubMed] [Google Scholar]
- 29.Smith, D. L., Dushoff, J. & Morris, J. G., Jr. (2005) PLoS Med., in press. [DOI] [PMC free article] [PubMed]
- 30.Ridwan, B., Mascini, E., van Der Reijden, N., Verhoef, J. & Bonten, M. (2002) Br. Med. J. 324, 666-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruinsma, N., Hutchinson, J. M., van den Bogaard, A. E., Giamarellou, H., Degener, J. & Stobberingh, E. E. (2003) J. Antimicrob Chemother. 51, 385-390. [DOI] [PubMed] [Google Scholar]
- 32.Harris, A. D., Nemoy, L., Johnson, J. A., Martin-Carnahan, A., Smith, D. L., Standiford, H. & Perencevich, E. N. (2004) Infect. Control Hosp. Epidemiol. 25, 105-108. [DOI] [PubMed] [Google Scholar]