Abstract
CYP7B1 is the enzyme responsible for hydroxylation and termination of the estrogenic actions of the androgen metabolite, 5α-androstane-3β, 17β-diol (3βAdiol). 3βAdiol is estrogenic in ERα or ERβ positive cells only if they do not express CYP7B1. In this study we show that female CYP7B1–/– mice experience early onset of growth of the uterus and mammary glands and commence estrus cycles 2 days earlier than their wild-type littermates. Adult mammary glands and uteri appear to be under continuous estrogenic stimulation. We conclude that, by cell-specific regulation of the estrogenicity of 3βAdiol, CYP7B1 performs two major tasks: (i) it allows 3βAdiol to have growth inhibitory effects through ERβ and (ii) it permits estradiol-specific activation of estrogen receptors by protection of certain cells from the estrogenic effects of 3βAdiol. When CYP7B1 is inactivated, 3βAdiol activates estrogen receptors indiscriminately, and the overall effect is prolonged and inappropriate exposure to estrogen.
Keywords: 3βAdiol, estrogen receptor, precocious puberty, cytochrome P-450, mammary gland
Since the discovery that the brain is masculinized by 17β-estradiol (E2) (1) and the more recent discovery of the absolute requirement for estrogen receptors in maintenance of the male skeleton, estrogens are no longer regarded as exclusively female hormones. What is not clear is whether estradiol is the major estrogen in males. Because it cannot be aromatized to estrogen, 5α-dihydrotestosterone (DHT) has traditionally been used when it was necessary to distinguish between the estrogenic and androgenic actions of testosterone in vivo. However, many years ago, it was noted that 5α-androstane-3β, 17β-diol (3βAdiol) is an estrogenic metabolite of DHT (2). In other words, even though aromatase is not involved, DHT is also the precursor of an estrogen. The formation of 3βAdiol is catalyzed by 17β-hydroxysteroid dehydrogenase type 7 (3). 3βAdiol can activate both estrogen receptor (ER) α and ERβ with a Kd of 1.9 × 10–8 M but has no affinity for the androgen receptor (AR) (4).
We have described a unique endocrine pathway in the prostate involving the termination of the estrogenic action of 3βAdiol by the enzyme CYP7B1 (5). At the cellular level, this pathway regulates the balance between ER and AR signaling. We found that 3βAdiol, not E2, is the most abundant ligand of ERβ in ventral prostate, and that the ERβ-3βAdiol complex reduces AR level and decreases proliferation of the prostate epithelium (6). The 3βAdiol-ER pathway is not an endocrine but a paracrine pathway. DHT is synthesized within its target tissue, and its physiological significance is not as a circulating hormone. Therefore, the same is true for 3βAdiol.
The enzyme 3βAdiol hydroxylase was discovered by Isaacs and Coffey (6) in the rat ventral prostate. Its functions in the brain (7, 8) and prostate (9–11) were studied for many years before it was identified as CYP7B1 (12). Analysis of mRNA from various tissues has revealed that, in addition to the brain, prostate, and pituitary, CYP7B1 was also expressed in urogenital organs, such as testis and ovary (12). This pattern of distribution suggests that the CYP7B1 could be playing a more widespread role in regulating ER and AR balance in the body.
In the present study, we compared the effects of administration of 3βAdiol (non-tissue-selective exposure) to the phenotype of female CYP7B1 knockout (CYP7B1–/–) mice (tissue-specific exposure to 3βAdiol). We found that CYP7B1 is essential for maintaining tissue selective estrogenicity of 3βAdiol, and, in its absence, there is precocious development of the mammary gland and uterus.
Materials and Methods
Animals. CYP7B1–/– mice, on a C57BL/6J background, were provided by Richard Lathe (Pieta Research) (12). C57BL/6J WT mice were from our colony, maintained in Huddinge University Hospital Animal Facility. Mice were housed in this facility in a controlled environment on an 12-h light/12-h dark illumination schedule, with food and water provided ad libitum. Mice were fed a standard pellet diet containing soya. The colony was maintained by breeding of heterozygous pairs. Mice were genotyped by PCR with DNA extracted from tail biopsies. All animal experiments were approved by Stockholm's Södra För-söksdjursetiska Nämnd. Animals were decapitated after being asphyxiated with CO2. Tissues were fixed overnight in 4% paraformaldehyde at 4°C for immunohistochemical studies.
Chemicals and Antibodies. 3βAdiol was purchased from Sigma; BrdUrd was from Roche Diagnostics; rabbit polyclonal anti-ERα (MC-20) and rabbit polyclonal anti-AR (N-20) antibodies from Santa Cruz Biotechnology; mouse monoclonal anti-BrdUrd antibody was from Pharmingen. Chicken polyclonal anti-ERβ503 antibody was produced in our laboratory (13). Biotinylated anti-rabbit, anti-mouse, and anti-sheep antibodies raised in goats were from Vector Laboratories (Burlingame, CA).
Whole-Mount Analysis of Mammary Glands. Excised abdominal mammary glands were spread on glass slides and fixed in a mixture of ethanol, chloroform, and glacial acetic acid (6:3:1 vol/vol) for 4 h at room temperature. Glands were then processed as follows: 70% ethanol for 15 min followed by rinsing in distilled water for 5 min and staining overnight at 4°C in carmine alum solution (1 g of carmine red, 2.5 g of aluminum potassium sulfate in 500 ml of water). Stained glands were dehydrated in graded ethanol (70%, 95%, and 100% for 15 min at each step) and xylene for 1 h before mounting. Whole mounts were photographed by using Leica dissecting microscope and video system.
Immunohistochemical Staining of Mammary Glands and Uterus. Representative blocks of paraffin-embedded tissues were cut at 4-μm thickness, dewaxed, and rehydrated. Antigens were retrieved by microwaving in 10 mM citrate buffer (pH 7.0) for 15 min. Sections were incubated in 0.5% H2O2 in PBS for 30 min at room temperature to quench endogenous peroxidase, and then incubated in 0.5% Triton X-100 in PBS for 15 min. For BrdUrd staining, before Triton X-100 incubation, sections were incubated in 2M hydrochloric acid for 10 min, followed by incubation for 15 min at room temperature in a solution of 0.05 M NaCl and 0.05 M sodium tetraborate (pH 8.5) in 0.2 M boric acid. To block nonspecific binding, sections were incubated in 10% normal serum prepared from the host of secondary antibodies for 1 h at 4°C. Sections were incubated with the following antibodies: anti-ERα (1:150), anti-AR (1:350), anti-ERβ (1:100), and anti-BrdUrd (1:100) in 3% BSA in PBS overnight at 4°C. After washing, sections were incubated with the corresponding secondary antibodies (all in 1:200 dilution) for 1 h at room temperature. The Vectastain ABC kit (Vector Laboratories) was used for ABC method following the manufacturer's instructions. Peroxidase activity was visualized with 3,3′-diaminobenzidine (Dako). The sections were lightly counterstained with hematoxylin. Negative controls were incubated without primary antibody.
Counting of Proliferating Cells. For proliferation studies, BrdUrd was injected s.c. (100 mg/kg of body weight) 2 h before death. Data were obtained by counting labeled cells and the total number of cell nuclei from three to four individual fields taken by ×400 magnification and are expressed as percentage of proliferating cells. A total of >1,000 cells were counted.
Estrus Cycles of CYP7B1–/– Mice. A total of four CYP7B1–/– and nine WT mice were examined to date the starting of estrus cycles. Vaginal smears were taken every morning from 28 days after birth and stained with Giemsa solution. The age at first estrus was taken as the day when vaginal smears contained primary leukocytes.
To check the regularity and duration of estrus cycles, eight CYP7B1–/– and six WT mice at 4 months of age and 10 CYP7B1–/– and five WT 15-month-old mice were used. Vaginal smears were taken every morning for 40 days and stained with Giemsa solution. The stage of the estrus cycle (proestrus, estrus, metestrus, and diestrus) was determined by microscopic examination of the slides. In normal mice, the duration of the cycle was 4–5 days. If the mouse had consecutive and regular estrus cycles more than twice, this animal was considered as having “normal cycles.” The length of one cycle was calculated by division of whole observed period by frequency of estrus.
3βAdiol Exposure Study. C57BL/6J WT mice were injected s.c. with 1 μlof3βAdiol in DMSO (6 mg/kg) or vehicle every second day from 2 days after birth until 4 weeks of age. These mice were killed either immediately after the last hormone treatment when they were 4 weeks of age, or 1 week later. There were two to three mice in each group.
Results
Body Weight Examination. At 2 weeks of age, CYP7B1–/– mice (n = 32) had significantly lower body weight (7.20 ± 0.45 g) than WT littermates (n = 22; 7.77 ± 0.75 g; P = 0.027). After puberty, although CYP7B1–/– mice tended to be leaner than WT littermates, the weight differences were not significant.
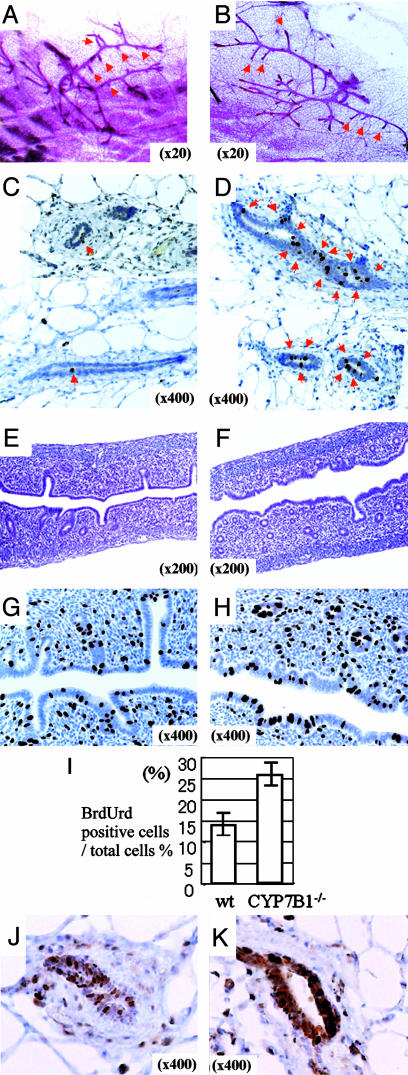
Morphological Phenotype of 2-Week-Old Mice. At 2 weeks of age, the first ovulation had not occurred in either WT (n = 3) or CYP7B1–/– (n = 3) mice and there were no corpora lutea in the ovaries. Whole mounts of mammary glands from these mice showed that there was much more ductal growth in the CYP7B1–/– (n = 4; Fig. 1B) than in the WT mice (n = 5; Fig. 1 A). Two hours after administration of BrdUrd, many epithelial cells of mammary ducts were BrdUrd-labeled in CYP7B1–/– mice (n = 3; Fig. 1D), but it was difficult to find a single labeled cell in WT mice (n = 3; Fig. 1C).
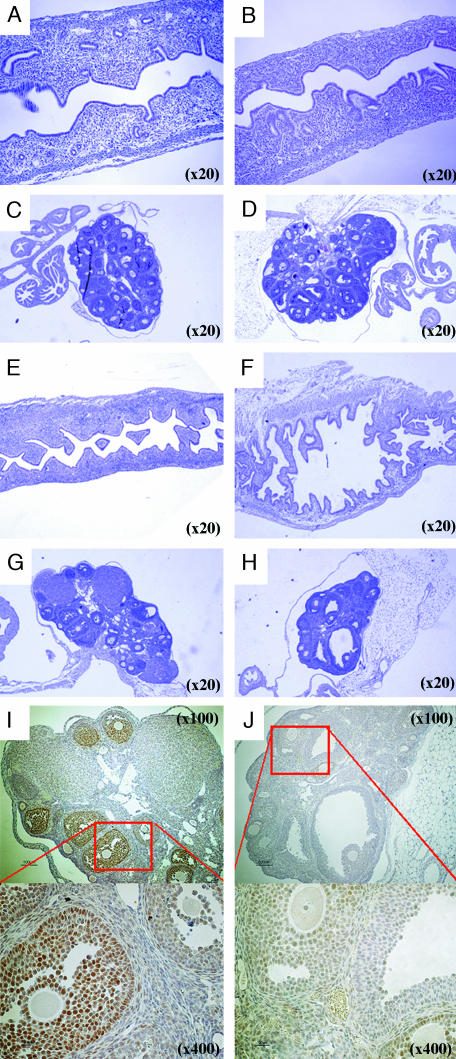
Fig. 1.
Morphological phenotype of 2-week-old mice. (A and B) Whole-mount staining of mammary glands. In 2-week-old WT mice (A), there was no elongated side branching of ducts (red arrows), as is seen in CYP7B1–/– mice (B). (C and D) BrdUrd staining of mammary glands after 2-h BrdUrd treatment. BrdUrd-labeled DNA was visualized with an anti-BrdUrd antibody. In WT mice, very little BrdUrd incorporation could be detected (C), but in CYP7B1–/– mice, there were many BrdUrd-positive nuclei (D), suggesting that mammary gland growth in CYP7B1–/– mice started earlier than in WT mice. The red arrow signifies a BrdUrd labeled cell. (E and F) Hematoxylin and eosin staining of uterus from WT (E) and CYP7B1–/– (F) mice. There are more glands in the uteri of CYP7B1–/– mice than in WT mice. (G–I) BrdUrd staining of uterus after 2-h BrdUrd treatment. There was more incorporation of BrdUrd into DNA in the uteri of CYP7B1–/– mice (H) than there was in WT mice (G). The percentage of total epithelial cells, which were BrdUrd stained in WT and CYP7B1–/– mice is shown in I.(J and K) ERα staining of mammary glands. There are more ERα-positive epithelial cells and staining is stronger in CYP7B1–/– mice (K) than WT mice (J).
In the uterus, glands were much more abundant in CYP7B1–/– (n = 3; Fig. 1F) than WT (n = 3; Fig. 1E) mice. Two hours after BrdUrd treatment, twice as many epithelial cells were labeled in CYP7B1–/– than in WT mice (Fig. 1 G–I), These data suggest that even though the ovary had not yet begun to secrete E2, there was a precocious start of development of mammary glands and uteri in CYP7B1–/– mice.
Proliferation in response to exogenous estrogen is first seen when mice are 3 weeks of age (14); before this time, the mammary gland is reported to be unresponsive to the proliferative effects of estradiol (14). We examined the glands of 2-week-old mice for expression of ERα. In CYP7B1–/– mice (Fig. 1K), levels of ERα were much higher those in WT mice (Fig. 1J) and, unlike the WT glands where <50% of the epithelial cells were positive, all of the epithelial cells were stained in the CYP7B1–/– mice.
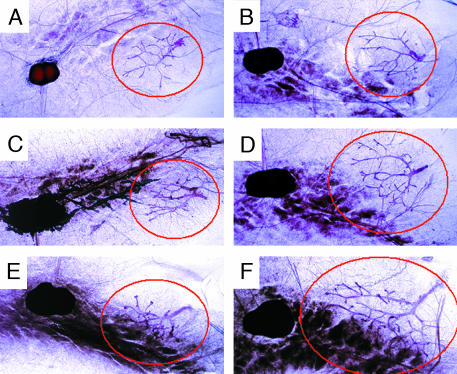
Morphological Change of Mammary Glands from 2- to 4-Week-Old Mice. Ductal elongation in the mammary glands normally begins when mice are 3 weeks old and is completed by 7 weeks of age. We examined changes in ductal morphology in mice at 2, 3, and 4 weeks of age. Four mice (two WT and two CYP7B1–/– mice from two litters) were compared. Comparison of whole mounts of mammary glands from CYP7B1–/– and WT mice during development showed that, at every age, in the absence of CYP7B1 there was more ductal elongation and branching than in WT mice (Fig. 2).
Fig. 2.
Whole-mount examination of mammary glands from 2- to 4-week-old mice. Two-week-old WT (A) and CYP7B1–/– (B) mice, 3-week-old WT (C) and CYP7B1–/– (D) mice, and 4-week-old WT (E) and CYP7B1–/– (F) mice are shown. CYP7B1–/– mice show more ductal elongation and branching than WT mice at every age. Mammary ducts are encircled in red.
Estrus Cycle of CYP7B1–/– Mice. Because mammary glands and uteri of CYP7B1–/– mice appeared to be under chronic estrogenic stimulation, the question was whether the ovary was precociously secreting estrogen. The first estrus cycle started on day 38 ± 1 in CYP7B1–/– and on day 40 ± 1 in WT mice (P = 0.01), suggesting a precocious puberty in CYP7B1–/– mice. Both CYP7B1–/– (n = 7) and WT (n = 6) mice at 4 months of age had normal cycles and the duration of each cycle (4 or 5 days) was not significantly different between the two groups. However, at 15 months of age, when three of five WT mice had normal cycles, none of the 10 CYP7B1–/– mice examined was cycling. These data suggest premature exhaustion of ovarian function in CYP7B1–/– mice.
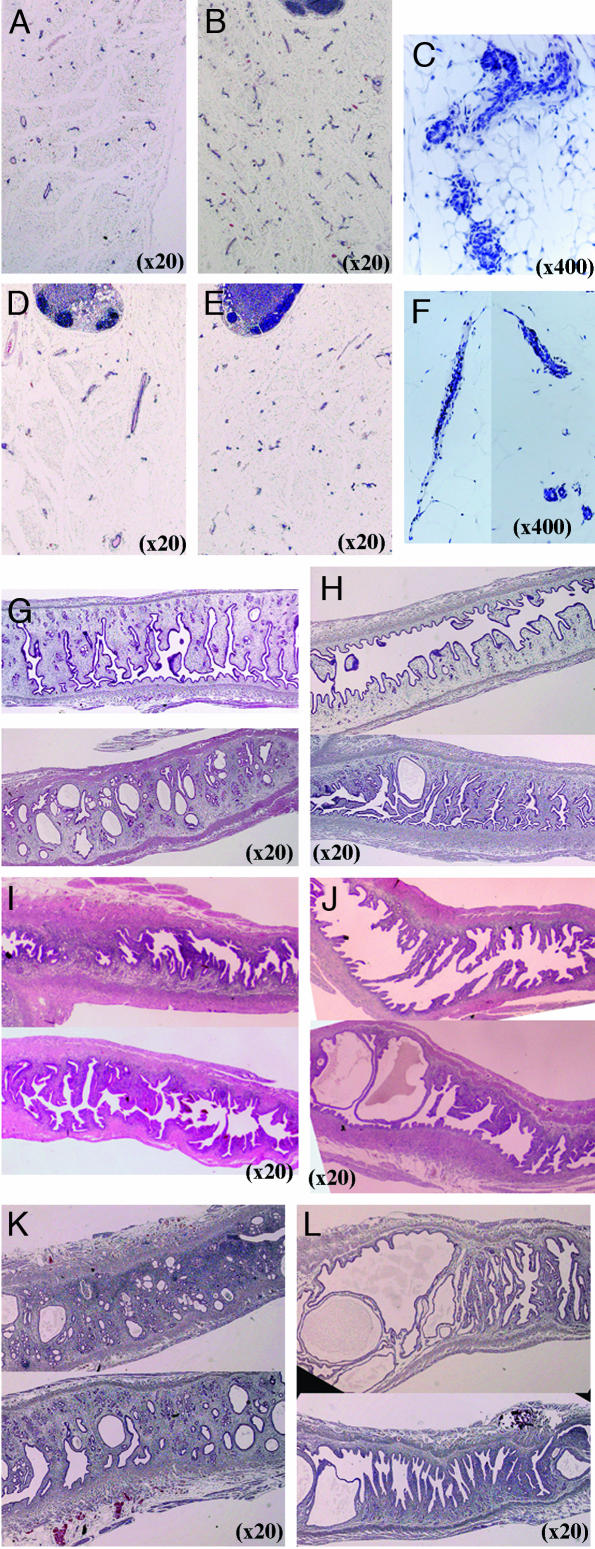
Morphological Phenotype of Adult Mice After Puberty. At 6 months of age, when mice were examined at diestrus or proestrus, both the mammary glands and the uteri of CYP7B1–/– mice had the appearance of E2-treated mice. There was much more extensive ductal formation in the mammary glands (Fig. 3 B and C) and the lumina of the uteri were larger (Fig. 3H) than in WT mice (Fig. 3 A and G). Enlarged lumina of the uteri were still evident at 8 months (Fig. 3J) and 15 months of age (Fig. 3L). However, by 15 months of age, the uterine glands were sclerotic and atrophic (Fig. 3L) and the mammary glands of CYP7B1–/– mice also showed signs of atrophy (Fig. 3 E and F). Age-matched WT mice had normal mammary glands (Fig. 3D) and uteri (Fig. 3 I and K).
Fig. 3.
Morphological phenotype of adult CYP7B1–/– mice. Comparison of mammary glands from 6-month-old WT (A) and CYP7B1–/– (B and C) mice showed a much more extensively branched ductal tree in CYP7B1–/– mice. (C) Higher magnification of the ducts in B. There was no difference between the two genotypes in lobular formation. The mammary glands from 15-month-old mice (D–F) show that, in CYP7B1–/– mice (E and F), there are signs of involution (F, higher magnification of the ducts in E), whereas ducts appear still to be active in WT mice (D). (G–L) Uteri at the ages of 6 (G and H),8(I and J), and 15 (K and L) months. Uteri of 6-month-old CYP7B1–/– mice (H) had larger lumina than did WT mice (G). Enlarged lumina of the uteri were evident in 8-month-old CYP7B1–/– mice (J) but not in WT mice (I). By 15 months of age, the lumina of the CYP7B1–/– mice (L) were extremely enlarged with sclerotic and atrophic glands, whereas the uterine morphology in the age matched WT mice (K) was essentially similar to that of 6-month-old WT mice (G).
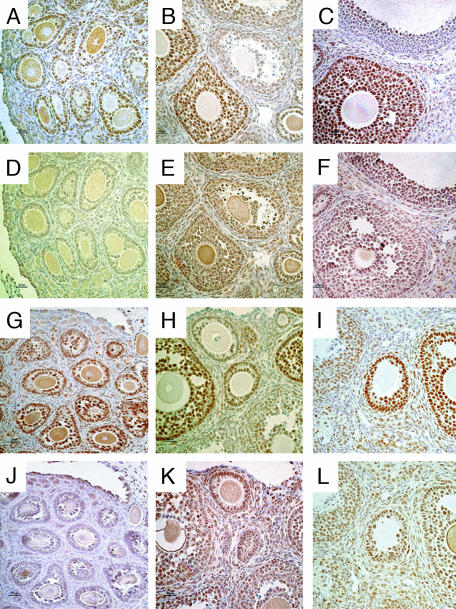
AR and ERβ Staining in CYP7B1–/– Mouse Ovary. At 2, 4, and 24 weeks of age, ovaries of CYP7B1–/– and their WT littermates were examined for expression of AR and ERβ. At 2 weeks of age, no ERβ was detectable in the ovary (Fig. 4 D and J), and AR staining in granulosa cells in developing follicles was strong (Fig. 4 A and G). At 4 (Fig. 4 E and K) and 24 (Fig. 4 F and L) weeks, there was abundant ERβ staining in follicles, and AR staining was high in developing follicles and down regulated in large antral follicles as expected (Fig. 4 B, C, H, and I). No differences in AR or ERβ staining were seen between CYP7B1–/– mice and their WT littermates.
Fig. 4.
AR and ERβ staining in CYP7B1–/– and WT ovaries. AR staining in 2-week-old (A), 4-week-old (B), and 24-week-old (C) WT mice; ERβ staining in 2-(D), 4-(E), and 24-week-old (F) WT mice; AR staining in 2-week-old (G), 4-week-old (H), and 24-week-old (I) CYP7B1–/– mice; and ERβ staining in 2-week-old (J), 4-week-old (K), and 24-week-old (L) CYP7B1–/– mice. ERβ staining was not detectable in 2-week-old mouse ovaries (D and J) but the granulosa cells in developing follicles were positively stained for AR (A and G). At 4 and 24 weeks of age, ERβ was abundant in follicles (E, F, K, and L). AR was highly expressed in developing follicles, but levels were reduced in large antral follicles (B, C, H, and I). All pictures were taken at ×400 magnification.
The Effect of 3βAdiol Exposure in Postnatal WT Mice. When 3βAdiol was administered to mice, it had estrogenic effects on the uterus but it also affected pituitary function. 3βAdiol was injected s.c. 6 mg/kg every second day from 2 days after birth until 4 weeks of age. At 4 weeks of age there was no obvious difference in the uterus between treated (Fig. 5B) and untreated mice (Fig. 5A), but at 5 weeks of age, the uteri of the treated mice (Fig. 5F), but not of untreated mice (Fig. 5E), had the appearance of E2-treated mice. The treated uteri were large and filled with fluid, and had distended lumina (Fig. 5F). In the ovaries at 4 weeks of age, there were developing follicles in both of groups (Fig. 5 C and D). At 5 weeks, no AR was detectable in the ovaries of treated mice (Fig. 5J), but there was strong staining in granulosa cells in control mice (Fig. 5I). Whole ovaries were completely sectioned. There were corpora lutea and healthy follicles in untreated ovaries (Fig. 5G), but in 3βAdiol-treated mice, there were no corpora lutea and there were many more atretic follicles than in the ovaries of WT mice (Fig. 5H).
Fig. 5.
3βAdiol exposure in postnatal mice: WT mice were treated with 6 mg/kg 3βAdiol or vehicle per day from day 2 of birth until 4 weeks of age. (A–D) Uterus and ovary from 4-week-old mice. (E–J) Uterus and ovary from mice killed at 5 weeks of age. At 4 weeks of age, there was no obvious difference in the uterus between treated (B) and untreated (A) mice, but at 5 weeks of age, the uteri of the treated mice (F) had the appearance of estrogen-treated mice. Uteri of estrogen-treated mice were large, filled with fluid, and had distended lumina, but not those of control mice (E). In the ovaries at 4 weeks of age, there were developing follicles in both of groups (C and D); by 5 weeks, corpora lutea were present in untreated ovary (G), whereas atretic follicles and no corpora lutea were seen in treated ovaries (H). AR staining in 5-week-old ovary showed very weak staining in treated mice (J) but strong staining in granulosa cells in control mice (I).
Discussion
Here, we show that 3βAdiol is estrogenic in female reproductive organs and that CYP7B1 is the main enzymatic route for the inactivation and excretion of this estrogen.
We have shown previously that the prostates, brains and pituitaries of the CYP7B1–/– mice have lost the capacity to metabolize 3βAdiol to triols (12). We found that the uteri and mammary glands of CYP7B1–/– female mice have characteristics of tissues chronically exposed to estrogen. Normally, sexual maturation begins in mice ≈3 weeks of age, and mammary glands and uteri grow dramatically at puberty (15). The prepubertal uterus does not express ERα, but does express ERβ (16), whereas the prepubertal mammary gland expresses both ERα and ERβ. In CYP7B1–/– mice, ductal elongation in the mammary gland and proliferation in the uterus started before the first ovulation (Figs. 1 and 2).
In the WT mouse mammary gland, E2 does not elicit epithelial proliferation before 3 weeks of age (14). We have shown previously that during late pregnancy and lactation, when ERα and ERβ are coexpressed in the same cells, the mammary gland does not proliferate in response to estradiol (13). In the present study, there was no proliferation in the 2-week-old WT mouse mammary gland in response to 3βAdiol. Surprisingly, however, in the CYP7B1–/– mouse, there was a strong proliferative response caused by high exposure to 3βAdiol. The most likely explanation for this proliferation is the higher expression level of ERα in the mammary epithelium of the CYP7B1–/– mice. Because ERα signals proliferation and ERβ signals inhibition of proliferation, the overall response of a cell to estrogen depends on the ratio of ERα to ERβ. Quiescence in response to estrogen in the 2-week-old WT mice suggests that ERα and ERβ signals neutralize each other. The increase in ERα expression in the CYP7B1–/– mice is probably sufficient for ERα signaling to dominate and this results in proliferation.
Puberty is not a single event, but the result of a coordinated multiorgan, multistage program involving the brain and the reproductive system. It is still not clear what initiates puberty. Local accumulation of 3βAdiol in the mammary gland and uterus is unlikely to trigger ovarian cycling. Therefore, it remains possible that the early initiation of cycling, which we have observed here, results from estrogenic activity in the brain.
The ovary does not synthesize E2 before puberty, but it does make testosterone, DHT, and 3βAdiol. Eckstein (2) showed that one of the major steroids secreted by the prepubertal ovary is 3βAdiol, and suggested that this steroid had a role in the onset of puberty (17). The significance of this steroid remained obscure for many years. After the discovery of ERβ in the prostate, the question arose as to what was the endogenous ligand of this receptor. We found that 3βAdiol is quantitatively the major estrogenic steroid in the prostate (5) and is the most likely ligand for ERβ in this tissue. This finding means that 3βAdiol, by means of ERβ, regulates AR expression and epithelial cell proliferation in adult prostate (5) and that CYP7B1, by regulating tissue levels of 3βAdiol, is also important in cellular homeostasis in the prostate. If the prepubertal ovary secretes 3βAdiol and this steroid interacts with ERβ, cellular proliferation would be inhibited. If in addition, ERα-containing cells express CYP7B1 and are therefore unresponsive to 3βAdiol, the overall effect would be to keep the uterus and mammary gland quiescent.
In the absence of CYP7B1, there is early proliferation in both the mammary glands and uterus. After puberty, when the ovary begins estrus cycles, the growth and development of the mammary gland and uterus are regulated by a combination of ovarian estrogen and progesterone. Because the mammary glands of adult CYP7B1–/– mice had many more ductal branches, it appears that the continuous exposure to 3βAdiol had effects even though estrogen was being secreted from the ovary in a cyclical fashion. Because only ductal and not lobular growth was observed, the excessive stimulation of the mammary glands in CYP7B1–/– mice is caused by estrogen, not progesterone. The uteri of adult CYP7B1–/– mice, with their extremely enlarged lumen and abundance of glands, also had the appearance of a tissue under continuous estrogen exposure.
We have previously shown that ERβ reduces expression of AR in the ovary (18). Before 2 weeks of age, ERβ is not detectable in the ovary and expression of AR is high in both WT and CYP7B1–/– mice. By 5 weeks of age when ERβ is abundant in the ovary, AR expression is high in developing follicles and is down-regulated in large antral follicles. Loss of CYP7B1 did not alter this regulation. This finding is not surprising because the mature ovary does not produce DHT or 3βAdiol and thus should not be affected by loss of CYP7B1. However, when 3βAdiol is administered to mice lacking CYP7B1, this exogenous 3βAdiol was sufficient to down-regulate AR in the ovary.
If 3βAdiol is estrogenic, the lack of effect of 3βAdiol on the pituitary has to be explained. Estrogenic 3βAdiol would have been expected to cause feedback inhibition on gonadotropin secretion from the pituitary and consequently, shut down of estrogen synthesis in the ovary. This feedback clearly did not occur. The lack of estrogen repression in the pituitary in CYP7B1–/– mice is due to the fact that the 3βAdiol-CYP7B1 system is a paracrine and not an endocrine system. If a tissue does not express significant levels of 5α-reductase, it will not synthesize 3βAdiol and will remain unaffected when CYP7B1 is inactivated. The pituitary normally expresses very low levels of 5α-reductase (19). The pituitary is sensitive to administered 3βAdiol (20). In the present study, 3βAdiol treatment of mice resulted in complete loss of AR from the uterus and a block in ovulation, but the uteri of these mice had the appearance of fully estrogenized mice. It appears that, when 3βAdiol is administered to mice, all tissue selectivity is lost. Under these conditions, which are completely unphysiological, 3βAdiol is acting as an endocrine, not a paracrine, hormone.
Another question arising from our data is why 3βAdiol does not fulfill the role of estrogen in the body after menopause. Because 3βAdiol is an effective estrogen before puberty, it should be equally so after menopause. The answer to this question is that, after menopause, the major steroid secreted from the ovary is androstenedione, not DHT or 3βAdiol (21). In fact, in the mature ovary, 5α-reductase levels are extremely low (22) and the activity of 5α-reductase after the menopause may be too low to maintain sufficient concentrations of 3βAdiol.
Although CYP7B1 has been studied by several groups, the focus has been on its possible role in oxysterol metabolism (23–26). Our data show that this enzyme may have a far more important role in the body, i.e., regulating the cellular specificity of estrogen signaling and influencing the balance between ER and AR signaling.
Acknowledgments
We thank Patricia Humire and AnnMarie Witte for excellent technical support. This study was supported by grants from the Wenner-Gren foundation, the Swedish Cancer Fund, and KaroBio AB.
Abbreviations: E2, 17β-estradiol; DHT, 5α-dihydrotestosterone; 3βAdiol, 5α-androstane-3β, 17β-diol; ER, estrogen receptor; AR, androgen receptor.
References
- 1.Roselli, C. E. & Klosterman, S. A. (1998) Endocrinology 139, 3193–3201. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein, B. & Ravid, R. (1974) Endocrinology 94, 224–229. [DOI] [PubMed] [Google Scholar]
- 3.Luu-The, V. (2001) J. Steroid Biochem. Mol. Biol. 76, 143–151. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper, G. G., Carlsson, B., Grandien, K., Enmark, E., Haggblad, J., Nilsson, S. & Gustafsson, J.-Å. (1997) Endocrinology 138, 863–870. [DOI] [PubMed] [Google Scholar]
- 5.Weihua, Z., Lathe, R., Warner, M. & Gustafsson, J.-Å. (2002) Proc. Natl. Acad. Sci. USA 99, 13589–13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaacs, J. T., McDermott, I. R. & Coffey, D. S. (1979) Steroids 33, 639–657. [DOI] [PubMed] [Google Scholar]
- 7.Stromstedt, M., Warner, M., Banner, C. D., MacDonald, P. C. & Gustafsson, J.-Å. (1993) Mol. Pharmacol. 44, 1077–1083. [PubMed] [Google Scholar]
- 8.Warner, M., Stromstedt, M., Moller, L. & Gustafsson, J.-Å. (1989) Endocrinology 124, 2699–2706. [DOI] [PubMed] [Google Scholar]
- 9.Sundin, M., Warner, M., Haaparanta, T. & Gustafsson, J.-Å. (1987) J. Biol. Chem. 262, 12293–12297. [PubMed] [Google Scholar]
- 10.Isaacs, J. T., McDermott, I. R. & Coffey, D. S. (1980) Steroids 35, 139–156. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs, J. T., McDermott, I. R. & Coffey, D. S. (1979) Steroids 33, 675–692. [DOI] [PubMed] [Google Scholar]
- 12.Rose, K., Allan, A., Gauldie, S., Stapleton, G., Dobbie, L., Dott, K., Martin, C., Wang, L., Hedlund, E., Seckl, J. R., et al. (2001) J. Biol. Chem. 276, 23937–23944. [DOI] [PubMed] [Google Scholar]
- 13.Saji, S., Jensen, E. V., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodward, T. L., Xie, J. W. & Haslam, S. Z. (1998) J. Mammary Gland Biol. Neoplasia 3, 117–131. [DOI] [PubMed] [Google Scholar]
- 15.Richert, M. M., Schwertfeger, K. L., Ryder, J. W. & Anderson, S. M. (2000) J. Mammary Gland Biol. Neoplasia 5, 227–241. [DOI] [PubMed] [Google Scholar]
- 16.Weihua, Z., Saji, S., Makinen, S., Cheng, G., Jensen, E. V., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 5936–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein, B. (1975) J. Steroid Biochem. 6, 873–878. [DOI] [PubMed] [Google Scholar]
- 18.Cheng, G., Weihua, Z., Makinen, S., Makela, S., Saji, S., Warner, M., Gustafsson, J.-Å. & Hovatta, O. (2002) Biol. Reprod. 66, 77–84. [DOI] [PubMed] [Google Scholar]
- 19.Bandivdekar, A. H., Karp, R., Sundaram, K. & Kumar, N. (2000) J. Androl. 21, 268–275. [PubMed] [Google Scholar]
- 20.Lund, T. D., Munson, D. J., Haldy, M. E. & Handa, R. J. (2004) Neurosci. Lett. 365, 43–47. [DOI] [PubMed] [Google Scholar]
- 21.Noyan, V., Yucel, A. & Sagsoz, N. (2004) Acta Obstet. Gynecol. Scand. 83, 487–490. [DOI] [PubMed] [Google Scholar]
- 22.Haning, R. V., Jr., Tantravahi, U., Zhao, Q., Hackett, R. J. & Canick, J. A. (1996) J. Steroid Biochem. Mol. Biol. 59, 199–204. [DOI] [PubMed] [Google Scholar]
- 23.Wu, Z. & Chiang, J. Y. (2001) Gene 272, 191–197. [DOI] [PubMed] [Google Scholar]
- 24.Toll, A., Wikvall, K., Sudjana-Sugiaman, E., Kondo, K. H. & Bjorkhem, I. (1994) Eur. J. Biochem. 224, 309–316. [DOI] [PubMed] [Google Scholar]
- 25.Li-Hawkins, J., Lund, E. G., Turley, S. D. & Russell, D. W. (2000) J. Biol. Chem. 275, 16536–16542. [DOI] [PubMed] [Google Scholar]
- 26.Li-Hawkins, J., Lund, E. G., Bronson, A. D. & Russell, D. W. (2000) J. Biol. Chem. 275, 16543–16549. [DOI] [PubMed] [Google Scholar]