Abstract
Cocaine dependence is a difficult-to-treat, chronically relapsing disorder. Multiple scientific disciplines provide distinct perspectives on this disorder; however, connections between disciplines are rare. The competing neurobehavioral decision systems (CNDS) theory posits that choice results from the interaction between two decision systems (impulsive and executive) and that regulatory imbalance between systems can induce pathology, including addiction. Using this view, we integrate a diverse set of observations on cocaine dependence, including bias for immediacy, neural activity and structure, developmental time course, behavioral comorbidities, and the relationship between cocaine dependence and socioeconomic status. From the CNDS perspective, we discuss established and emerging behavioral, pharmacological, and neurological treatments and identify possible targets for future treatments. The ability of the CNDS theory to integrate diverse findings highlights its utility for understanding cocaine dependence and supports that dysregulation between the decision systems contributes to addiction.
Keywords: Cocaine dependence, Competing neurobehavioral decision systems, Impulsivity, Self-control, Executive function, Delay discounting, Dual systems, Transcranial magnetic stimulation
1 Introduction
Cocaine is a powerful psychoactive and addictive substance. Approximately 15% of cocaine users develop dependence within the first decade after initial use, with lifetime incidence of dependence estimated at 20% (Lopez-Quintero et al., 2011; Wagner and Anthony, 2002). In some racial minorities, these estimates are even higher (e.g., 35% lifetime incidence of dependence in African American users) (Lopez-Quintero et al., 2011). Cocaine dependence is difficult to treat and is recognized as a chronically relapsing disorder, in which affected individuals choose continued drug use despite negative consequences, and return to use after periods of abstinence. Understanding the processes that undergird these choices is an important undertaking for the science and treatment of this disorder.
A variety of scientific approaches have tried to understand and explain cocaine dependence. Some have focused on molecular variables, such as pharmacological action (Volkow et al., 1999); others have focused on demographics, including age, race, and socioeconomic status (SES) (Lopez-Quintero et al., 2011; Palamar et al., 2015). These multiple levels of analysis provide distinct perspectives on cocaine dependence, but connections across levels have been rare. A thorough understanding of these multilevel phenomena, in our view, will require a scientific theory or paradigm that not only can integrate observations across levels in a compelling way, but can also suggest novel hypotheses. As Henri Poincaré noted in his classic text, Science and Hypothesis (Poincaré, 1905), “Science is built up of facts, as a house is built of stones; but an accumulation of facts is no more a science than a heap of stones is a house (p. 157).”
The question we should ask is what would we want from such a theory that could set the extant facts in order? At the very least, any such theory should integrate the neuroscience of cocaine's effects on the brain, developmental processes associated with drug use initiation, the relationship of SES to cocaine use, and the high prevalence of certain comorbidities. Such a theory should also have the capacity to suggest novel treatments and perhaps reveal mechanisms underlying established treatments.
We have been involved with formulating a view, referred to as the competing neurobehavioral decision systems (CNDS) theory (Bickel and Yi, 2008; Bickel et al., 2007, 2012a) that has considerable integrative power. This view, consistent with a broad array of dual-systems theories, suggests that choices result from the interaction between the two decision systems and that those who are experiencing addiction suffer from imbalance or dysregulation between these two systems. In this chapter, we will examine cocaine dependence from the perspective of this theory. To accomplish this, we will first give a brief synopsis of this theoretical view and examine the evidence to support the dysregulation between the dual systems in individuals with cocaine dependence. Next, we will examine how this perspective provides insight on the relationship between cocaine dependence and developmental life course, SES, and comorbidities. Finally, we will examine the implications of the CNDS perspective for existing and emerging approaches to the treatment of cocaine dependence.
2 The Competing Neurobehavioral Decision Systems Theory
Dual-systems models of decision-making have been discussed since Descartes and have evolved to many variations and applications (Sanfey and Chang, 2008), particularly in the areas of self-control (Metcalfe and Mischel, 1999) and addiction (Bechara, 2005; Goldstein and Volkow, 2002, 2011; Jentsch and Taylor, 1999). In decision-making research, most models refer to the dual systems as System 1 and System 2. System 1 refers to unconscious and automatic processes, requiring little effort, while System 2 refers to conscious, controlled, and effortful processes (Evans, 2008; Evans and Stanovich, 2013).
The CNDS theory is a dual-systems model that accounts for self-control failure (Bickel et al., 2007, 2011a), has been directly applied to addiction (Bickel et al., 2011a; Sofis et al., 2014) and emphasizes the relative control between impulsive and executive decision systems. The impulsive system, comprised of the limbic and paralimbic brain regions, and executive system, comprised of the prefrontal and parietal cortices, are interdependent and compete for relative control during decision-making (see Bickel et al., 2012a for pictorial representations). Normal functioning results when the systems are in regulatory balance; however, when the two systems are not in regulatory balance, pathology may result (Bickel et al., 2015). Although worthwhile, systematic comparison of the CNDS theory and other dual-systems models is beyond the scope of this chapter, thus we reserve such comparisons for future discussions.
Importantly, delay discounting is a behavioral measure of self-control that designates the relative strength of the competing decision systems (Bickel et al., 2012b; McClure and Bickel, 2014). Delay discounting procedures measure future valuation by asking participants if they would prefer a smaller, immediate amount of a commodity or a larger, delayed amount. The immediate amount is titrated until a point of subjective equality (the indifference point) is determined. A hyperbolic function often best accounts for the fit of the indifference points across delays and is represented by the equation (Mazur, 1987),
where V is the subjective value of the reinforcer, A is the amount of the reinforcer, D is the delay to receipt of the reinforcer, and k is a free parameter that serves as an index of discounting (higher values of k indicate higher rates of discounting). Nicotine- (Bickel et al., 1999), alcohol- (Petry, 2001), cocaine- (Bickel et al., 2011b, 2014a; Heil et al., 2006), and heroin-dependent (Madden et al., 1997) individuals discount future rewards more than controls. Higher rates of discounting, then, reflect hyperactive control by the impulsive decision system, consistent with the bias for immediate reward evident in addiction (Bickel et al., 2011a).
The study of neuroeconomics, which combines psychology, economics, and neu-roscience (Bickel et al., 2011a), has provided confirmatory neural evidence for the actions of the CNDS (described in the following sections). When participants complete delay-discounting procedures in an MRI scanner, relative activity between the executive and impulsive systems varies, dependent upon the choice being made. For example, choices for the immediate and delayed reinforcer result in greater activity in the impulsive and executive systems, respectively (McClure et al., 2004, 2007). Moreover, when the reinforcer is delayed for both choices, the limbic system shows no differential activation. Thus, activation of the impulsive decision system depends on the presence of an immediate reinforcer (McClure et al., 2004).
2.1 The Impulsive Decision System
The impulsive decision system, comparable to System 1, is embodied in the limbic (e.g., midbrain, amygdala, habenular commissure, and striatum) and paralimbic (e.g., insula and nucleus accumbens) brain regions (Bickel et al., 2007). Habit formation, emotional responding, and the acquisition of primary reinforcers to satisfy biological needs (Bickel et al., 2013) are controlled by the impulsive decision system.
As discussed above, imaging studies have confirmed that the impulsive decision system is involved in the choice for immediate reinforcers in delay discounting. Choice for immediate reinforcers (McClure et al., 2004) selectively activate the paralimbic cortex and parts of the limbic system, including ventral striatum, medial orbitofrontal cortex, medial prefrontal cortex, posterior cingulate cortex, and left posterior hippocampus (McClure et al., 2004, 2007).
2.2 The Executive Decision System
The second decision system of the CNDS, comparable to System 2, is embodied in the parietal lobes and portions of the prefrontal cortex, including the dorsolateral pre-frontal cortex (Bickel et al., 2007). Some overlap of function in the decisions systems exists for several areas of the prefrontal cortex, including the orbitofrontal cortex. The cortical pathways of the executive decision system are responsible for planning, memory, attention, and future valuation (Bickel et al., 2013). Neuroeconomic evidence has demonstrated activation of the lateral prefrontal cortex and parietal lobe during decision-making in delay discounting for monetary and primary reinforcers (i.e., juice) regardless of delay, indicating the executive system is involved in all decisions (McClure et al., 2004, 2007). Moreover, greater activation in the executive system structures occurs during the more difficult choices requiring greater executive function.
2.3 The Competing Neurobehavioral Decision Systems Theory in Health and Addiction
When regulatory balance is achieved between the impulsive and executive decision systems, an individual is considered self-controlled and is likely to have no dysfunction (Bickel et al., 2015). Conversely, hyperactive control by either the impulsive or executive decision system can lead to pathological behavior. Many combinations of relative strength of each system are possible (Bickel et al., 2013). Consider Fig. 1 that shows a continuum from low to high control by the impulsive decision system on the y-axis and on the x-axis, low to high executive control. The diagonal line represents regulatory balance between the two decision systems. Shaded regions represent high risk for engaging in negative health behaviors. The pathological decision-making strategies associated with these behaviors emerge when control by the impulsive decision system overpowers control by the executive decision system. For example, high impulsive system control coupled with low or medium executive control results in greater relative control by the impulsive decision system and can result in pathological decision-making (e.g., bias toward smaller, immediate over larger, delayed consequences) (Bickel et al., 2011a, 2013).
Figure 1.
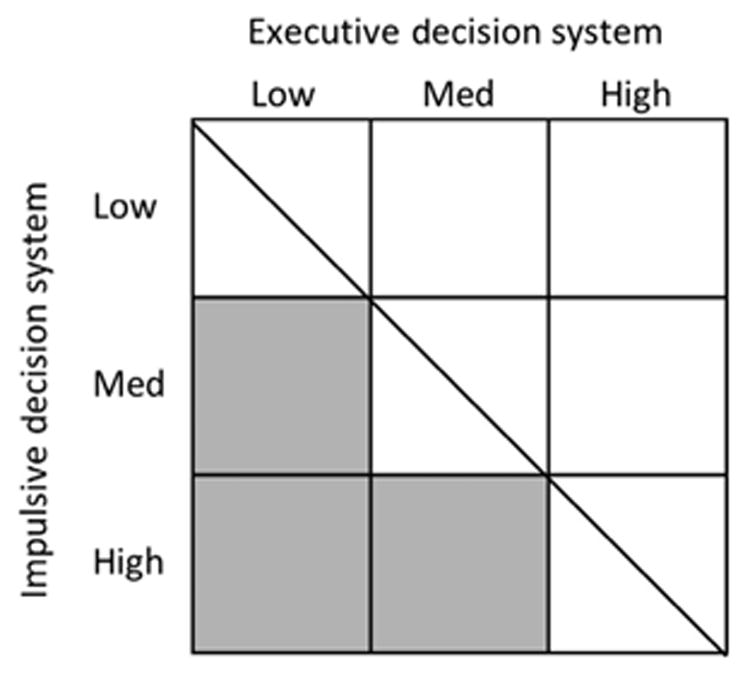
The relative control of the impulsive and executive decision systems, represented graphically. The diagonal line represents regulatory balance between the systems. The shaded regions indicate an imbalance between the two systems producing a bias for immediate over delayed rewards.
Imbalance of the CNDS is evident in many disease states where individuals have bias for immediate consequences over delayed, healthier choices. Hyperactive control of the impulsive decision system results in patterns of behavior consistent with obesity, legal and illicit substance use, and gambling problems. Regulatory imbalance can also be a result of hyperactive control by the executive system.
3 The Competing Neurobehavioral Decisions Systems Theory and Cocaine
3.1 Neural Evidence of the Imbalance of Decision Systems
Imbalance of the CNDS contributes to excessive discounting and addiction behaviors (Bickel et al., 2012b). Addiction occurs when the executive system is weak and the hyperactive impulsive decision system drives choice (Bechara, 2005). Cocaine use, via neuronal plasticity, promotes a transition in regulation from the prefrontal cortices to the striatum leading to compulsive and habitual drug seeking (Everitt et al., 2008). Advances in imaging have provided us with tools to examine the neural evidence of this imbalance in cocaine addiction.
3.1.1 Hyperactivation of the impulsive system
The impulsive decision system is comprised of regions of the limbic system and related areas (McClure et al., 2004). One of these regions, the orbitofrontal cortex, is associated with: (1) the reinforcing aspects of cocaine, (2) immediate choice preferences, and (3) craving and cocaine salience (Lucantonio et al., 2012; McClure et al., 2004; Steinberg, 2007). Compared to healthy controls, cocaine addicts show increased activation in limbic regions (i.e., the amygdala, anterior cingulate cortex, and striatum) following exposure to cocaine cues (Childress et al., 1999; Garavan et al., 2000). These increases in activation suggest regions responsible for drug craving and hyperactivation in craving states. Hyperactivation of the impulsive system in cocaine users is also consistent with findings of acute withdrawal circuits becoming hypermetabolic during spontaneous craving (Kalivas and Volkow, 2005; Lucantonio et al., 2012). Moreover, hyperactivation of the medial orbitofrontal cortex and anterior cingulate cortex (structures with impulsive functions) occurs following acute methylphenidate administration in cocaine addicts (Wilcox et al., 2011), suggestive of system over-activation following repeated stimulant administration (akin to sensitization observed in animals) (Robinson and Berridge, 1993).
Interestingly, while hyperactivation and hypermetabolism of the limbic system occurs under certain conditions, cocaine-dependent participants show an overall reduction in aspects of the impulsive decision system compared to healthy controls. These reductions include decreased activation of the orbitofrontal cortex and cingulate gyrus (Volkow et al., 1993) and decreased gray matter volume of the amygdala (Makris et al., 2004) and the ventromedial, orbitofrontal, anterior cingulate, and anteroventral insular cortices (Franklin et al., 2002). Although these findings may seem counterintuitive from the viewpoint of CNDS (i.e., reduced function and structure of the impulsive decision system in cocaine addicts), cocaine may prime the limbic regions associated with cue salience and motivation, consistent with hyperactivation of the impulsive decision system following drug administration and contributes to increased craving and compulsive intake (Volkow et al., 2005). Consistently, acute methylphenidate administration may normalize limbic activation by working similarly to cocaine but with slower pharmacokinetics. That is, in cocaine addicts, acute methylphenidate increases activation in the anterior cingulate cortex during a cue-reactivity task (Goldstein et al., 2010), restores response levels to normal after fatigue in a Stroop task (Moeller et al., 2012), and increases resting-state functional connectivity in limbic regions, including the anterior cingulate cortex (Konova et al., 2013). Thus, hyperactivation of the impulsive decision system, as a consequence of cocaine priming the system, weakens relative control of the executive system and decreases self-control (Noel et al., 2013).
3.1.2 Hypoactivation of the executive system
In addition to hyperactivity of the impulsive system, drugs of abuse cause an interruption of the top-down processes required for self-control (Dalley et al., 2011). Neural evidence suggests that cocaine induces executive dysfunction. Although hyperactivation may occur in some instances, as mentioned above, overall reductions in signaling, glucose metabolism (Kalivas and Volkow, 2005), and structural volume (Franklin et al., 2002) in both the impulsive and executive systems are observed after cocaine use. Moreover, the degree of cocaine use is associated with both structural and functional deficits in the executive system (Beveridge et al., 2008).
3.2 Developmental Processes and Cocaine Addiction
3.2.1 Differential development
Evidence of differential development between the CNDS explains impaired self-control in adolescents, as the two decision systems appear to differentially mature. During the first half of adolescence (i.e., ages 10–15), dopaminergic activity increases dramatically in brain areas associated with the impulsive decision system (Sisk and Zehr, 2005), including a dramatic dopamine and dendritic synaptic over-expression in the striatum (Andersen et al., 2000). Related to this overexpression, the nucleus accumbens, an area of the impulsive decision system responsible for the rewarding properties of stimuli, and orbitofrontal cortex display hyperactivation in children and adolescents compared to adults in resting state (Galvan et al., 2006) and when completing a monetary reward task (Ernst et al., 2005). Moreover, differential myelination between limbic and nonlimbic regions enhances activation in the impulsive decision system (Galvan et al., 2006). As adolescents mature, the overexpression and hyperactivation of the impulsive system begins to prune to model an inverted U-shaped function over time (Sisk and Zehr, 2005; Teicher et al., 1995). That is, after the overexpression peaks, extra connectivity begins to decline while the slower to mature executive decision system continues to develop.
Development of the executive decision system includes increases in parietal gray matter volume (Sisk and Zehr, 2005) along with dramatic dopamine and dendritic synaptic overexpression in the prefrontal cortex (Andersen et al., 2000). Gray matter density development and myelination in the frontal and parietal cortices continues into adulthood (Sowell et al., 2003), thus increasing relative control of the executive over the impulsive decision systems with age.
3.2.2 Related behaviors
The differential development of the two systems and inverted U-shaped curve of impulsive decision system development is evident in self-control. Paralleling the over-expression of dopamine and activation of the impulsive decision system, a drastic increase in risky behavior is present in adolescence. Specifically, self-reported sensation-seeking and risky sexual behavior increases drastically, peaks in early adolescence, and declines as self-regulatory behavior begins to mature (Baams et al., 2015; Steinberg, 2007; Steinberg et al., 2008).
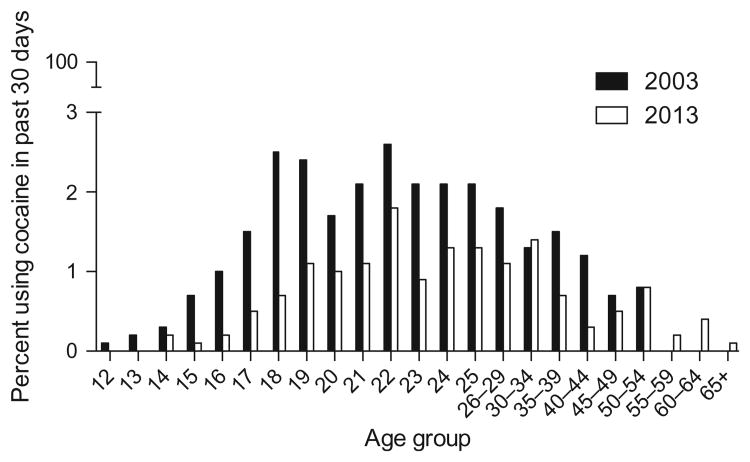
Importantly, a longitudinal study modeled the imbalance of the CNDS and reported that high rates of delay discounting and poor working memory (both measures of weak executive control) predicted greater subsequent initiation of drug use (i.e., alcohol, marijuana, and tobacco) (Khurana et al., 2015). Using data from two large national surveys (Substance Abuse and Mental Health Services Administration, 2004, 2013), Fig. 2 highlights this increased vulnerability in adolescents by illustrating the percentage of adolescents who used cocaine in the last 30 days by age group. Note, the percentage of use rapidly peaks in adolescence and declines with increasing age.
Figure 2.
Percentage of cocaine use in the past month, by age group. The results from the 2003 and 2013 National Survey on Drug Use and Health Surveys are presented. Percentage of use increases with age, which then slowly dissipates over time.
3.3 Socioeconomic Status and Cocaine Addiction
A widely demonstrated negative linear relationship exists between SES and illicit drug use, health problems, and mortality. This monotonic gradient, describing the relationship between SES and health status, extends from the lowest to the highest ends of the socioeconomic spectrum. As a result, this relationship cannot be entirely accounted for by poverty-induced deprivation or healthcare access (Adler and Stewart, 2010). This gradient represents the health disparity in prevalence of negative health behaviors (e.g., drug use, risky sexual behavior, and obesity) such that a lower prevalence of disease states is observed in high-SES individuals and a higher prevalence is observed in lower SES individuals. The greater the income inequality within a society, the larger the health disparity (Banks et al., 2006). This trend is apparent within the United States and is representative of the general trend showing larger health disparities in countries with more income inequality (Wilkinson and Pickett, 2011). Rates of mental illness, obesity, and substance use are disease states strongly associated with SES inequalities (Pampel et al., 2010).
One measure included in SES, education level, contributes to the prevalence of past year cocaine use and exemplifies this general trend in health disparities. In 2012, 2.4% of people who did not graduate from high school, while only 1.1% of college graduates, used cocaine in the past year. This relationship between SES and cocaine use began in the 1990s when risk perception of using cocaine increased and therefore became less culturally acceptable. As a result, high-SES individuals were more likely to discontinue cocaine use while low SES individuals continued use (Miech, 2008). The increased prevalence of cocaine use among lower income individuals demonstrates the negative socioeconomic gradient present across a wide variety of negative health behaviors, including cigarette smoking (Hiscock et al., 2012), illicit drug use (Buka, 2002), and obesity (Baum and Ruhm, 2009).
The CNDS theory can be used as a conceptual framework to explain the discrepancy between the prevalence of negative health behaviors among individuals with varying SES (Bickel et al., 2014b). The experiences associated with low SES, including increased allostatic load and lack of resources (Haushofer and Fehr, 2014; Mani et al., 2013), disrupt the development and regulatory balance between the impulsive and executive decision systems (Bickel et al., 2014b; Noble et al., 2012). Exposure to these environmental circumstances facilitates a biased decision-making process favoring immediate over delayed, healthier consequences. This executive dysfunction results from hyperactivation of the impulsive decision system and results in continued choice for immediate rewards, which perpetuates the disparity in negative health behaviors, including cocaine use.
3.4 Comorbidities with other Substance Use and Risky Sexual Behavior
The CNDS theory also provides a framework for understanding the relationship between comorbid disease states and cocaine use. Regulatory imbalance of the systems resulting from hyperactivity of the impulsive decision system may explain the presence of decision-making favoring immediate rewards (e.g., cocaine use and risky sexual behavior) (Chesson et al., 2006; Johnson and Bruner, 2012) over delayed, more healthy consequences. These decision-making processes are central to many disease states, which contributes to the incidence of comorbidity (Bickel and Mueller, 2009). Comorbid substance use, including tobacco (Budney et al., 1993; Burling et al., 1996), alcohol (Bierut et al., 2008), marijuana (Narvaez et al., 2014), and opiate use (Bierut et al., 2008), is common in cocaine users although few treatments intended for cocaine dependence take these comorbidities into account (Yoon et al., 2013).
4 The CNDS and Cocaine Treatment
The CNDS theory has been used previously to understand and categorize the effects of various delay-discounting manipulations (Koffarnus et al., 2013). Here, we apply a similar analysis to current and emerging treatments for cocaine dependence.
4.1 Conventional Treatment for Cocaine Dependence
A number of therapies have been successfully used to treat cocaine dependence, among which cognitive behavioral therapy (CBT) has the largest evidence base (Carroll and Onken, 2005; Carroll et al., 2008; Maude-Griffin et al., 1998). However, behavioral measures of executive dysfunction (e.g., poor Stroop performance) consistently predict poor response to these treatments (Aharonovich et al., 2006; Bleiberg et al., 1994; Moeller et al., 2001; Simpson et al., 1999; Streeter et al., 2008; Worhunsky et al., 2013; Xu et al., 2010). Likewise, functional and structural neuroimaging data, such as diminished prefrontal cortex activation and white matter integrity, further implicate executive dysfunction in poor treatment response (Brewer et al., 2008; Moeller et al., 2005; Worhunsky et al., 2013; Xu et al., 2010).
Many of these conventional treatments, including CBT, require a complex repertoire of executive skills (e.g., coping strategies or the ability to recognize dynamic relapse cues and modify behavior accordingly), which are likely compromised in individuals demonstrating regulatory imbalance between decision systems. From the viewpoint of the CNDS, a more promising approach would be to precisely target areas of dysfunction to produce more uniformly efficacious treatment outcomes compared to conventional treatment strategies (Bickel et al., 2012b). In the sections that follow, we consider a number of treatments that may accomplish this goal.
4.2 Treatments to Decrease Control of the Impulsive Decision System
4.2.1 Contingency management
One of the most reliable treatments for cocaine and other substance dependence in recent decades has been contingency management, a behavioral approach that arranges immediate delivery of monetary or other tangible reinforcers contingent on physiologically verified drug abstinence (Higgins et al., 1991, 1994) (for review and meta-analysis, see Lussier et al., 2006; Prendergast et al., 2006). This approach rapidly reduces cocaine use (Robles et al., 2000) and maintains abstinence over long periods of time (Poling et al., 2006; Rawson et al., 2002), even in the absence of continued treatment (Epstein et al., 2003; Higgins et al., 1995; Petry and Martin, 2002). Moreover, contingency management for cocaine use may be implemented successfully at relatively low cost (Petry and Martin, 2002; Petry et al., 2004) and may be paired with adjunctive therapies (e.g., CBT) (Epstein et al., 2003) to further improve treatment outcomes.
As discussed previously, substance use may be viewed as an intertemporal choice between immediate drug reinforcement and the temporally diffuse and distant outcomes associated with drug abstinence (e.g., sustained physical and mental health and attainment of occupational goals). Regulatory imbalance between decision systems may predispose individuals toward cocaine use by disproportionately weighting the value of immediate drug reinforcement. With this in mind, the provision of extrinsic, relatively immediate reinforcement for abstinence in contingency management therapies may supplant the naturalistic, delayed outcomes of abstinence (e.g., improved health and social function) that are otherwise insufficient to impact behavior in those suffering from regulatory imbalance. In addition, cessation of cocaine use during contingency management likely facilitates initial contact with these naturalistic outcomes, perhaps contributing to continued abstinence following treatment (Epstein et al., 2003; Higgins et al., 1995; Petry and Martin, 2002). Consistent with these mechanisms, a recent study examining contingency management for opioid abuse allowed participants to either redeem these earnings immediately at each laboratory visit or accumulate their earnings in an account over the course of the study (Bickel et al., 2010). Participants with the highest baseline rates of delay discounting more frequently redeemed their earnings immediately than participants with lower rates of delay discounting, demonstrating the selective importance of immediate outcomes for participants with regulatory imbalance. Future studies should be designed to determine whether a similar finding would be observed with contingency management for cocaine dependence.
4.2.2 Medications
Currently no approved medication exists for stimulant addiction (Brackins et al., 2011) and replacement therapies with stimulants for cocaine and methamphetamine addiction have produced equivocal results (Moeller et al., 2008). However, the possibility remains that replacement agonist therapy may be a viable avenue to decrease or buffer the hyperactivation of the impulsive decision system during or to prevent crave states.
For example, dexamphetamine and methylphenidate are long-acting stimulants, with similar mechanisms of action to cocaine (i.e., increases in extracellular dopamine) and have shown positive results in reducing behaviors related to cocaine addiction. In intravenous cocaine users, dexamphetamine reduced positive urine samples for cocaine, self-reported use, craving, and criminal activity (Shearer et al., 2003). Dexamphetamine maintenance also reduces choice preferences for immediate cocaine over money (Rush et al., 2009). Likewise, methylphenidate reduces reaction to cocaine cues and attenuates anterior cingulate cortex activation in cocaine-dependent individuals (see review Mariani and Levin, 2012) without impairing inhibitory control in a go/no-go task (Vansickel et al., 2008), offering a stimulant agonist medication without over activating the impulsive decision system. Moreover, replacement therapies such as methylphenidate, dexamphetamine, and atomoxetine are pharmacologically safe for maintenance therapy (Grabowski et al., 1997; Rush et al., 2009; Stoops et al., 2008). Thus, the benefit of longer acting agonist medications for use as partial agonist therapies offers a potential avenue to buffer hyperactivation of the impulsive decision system in cocaine-dependent individuals.
4.2.3 Neurotherapeutic stimulation
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation tool which enables us to selectively activate or inhibit populations of neurons by altering the frequency and placement of cortical stimulation. When stimulation is delivered repetitively, at frequencies known to induce long-term potentiation (LTP) or depression (LTD) of cortical activity, this technique is known as repetitive TMS (rTMS) (Fitzgerald et al., 2006; Hoogendam et al., 2010; Thickbroom, 2007; Ziemann et al., 2008). LTP of both behavioral and neural activity is possible by applying either a single high frequency (e.g., 10 Hz) or an intermittent theta burst frequency to the cortex. In contrast, transient LTD of behavioral and neural activity is possible by applying either a single low-frequency (e.g., 1 Hz) or continuous theta burst frequency to the cortex. rTMS is an FDA-approved treatment for depression and is the only noninvasive brain stimulation tool available for humans.
A growing body of substance dependence literature suggests that we may be able to directly dampen limbic circuitry or amplify executive control circuitry in substance-dependent individuals through rTMS. Consequently, rTMS has garnered significant attention as an innovative tool for treating substance dependence from both the National Institutes of Health and in the literature (Barr et al., 2011; Bellamoli et al., 2014; Gorelick et al., 2014; Wing et al., 2013). In context with the CNDS, several strategies could be used to develop treatments for substance dependence, including altering the relative control of the impulsive and executive decision systems.
The vulnerability to drug-related cues in treatment-seeking cocaine users is likely sustained by high functional activity in the impulsive decision system (Ersche et al., 2012; Moeller et al., 2010; Moreno-Lopez et al., 2012). Consequently, application of low-frequency TMS, for example, applying LTD-like stimulation to the limbic system may reduce sensitivity to cocaine and other substance cues. Given that the nucleus accumbens is one of the primary brain regions involved in craving (Robinson and Berridge, 1993) and the medial prefrontal cortex is that structure's primary cortical input, targeting the medial prefrontal cortex would be a method to modulate nucleus accumbens activity among substance-dependent populations. Recent work by Cho et al. (2015) demonstrated that LTP-like rTMS (i.e., 10 Hz) to the medial prefrontal cortex in a group of healthy, nondrug-using individuals was associated with a significant decrease in dopamine binding potential in the dorsal striatum, reflecting a release of dopamine in these areas. Although they did not find a significant change in dopamine binding in the nucleus accumbens, LTP-like stimulation of the medial prefrontal–striatal circuit increased delay discounting (a behavioral marker of executive dysfunction). This finding suggests that an LTD-like rTMS strategy over the medial prefrontal cortex would attenuate activity in this neural circuit and may reduce drug craving and impulsive decision system control. Prior data from our laboratory demonstrate that in cocaine users, continuous theta burst stimulation to the frontal lobe selectively decreases activation in the medial prefrontal cortex and nucleus accumbens (Hanlon et al., 2015). Given that craving for cocaine is associated with an increase in striatal dopamine, decreasing the sensitivity of this circuit through rTMS may be a valuable treatment strategy. Future research is required to determine whether stimulating this location is tolerable in substance-dependent populations because medial prefrontal cortex stimulation has not been widely pursued and is subjectively more painful than dorsolateral prefrontal cortex rTMS.
4.3 Treatments to Increase Control of the Executive Decision System
4.3.1 Neurocognitive training
Executive function deficits in chronic cocaine users are well established (Bolla et al., 2000). Specifically, compared to healthy controls, cocaine-dependent individuals demonstrate significant impairments of multiple measures of attention, visual and spatial memory, language and sensory perception functions (Jovanovski et al., 2005). This executive dysfunction is related to retention rates for relapse prevention therapy in cocaine users (Aharonovich et al., 2003, 2006). Because functional and regional overlap exists between executive function areas, including those involved in making delay-discounting decisions for the delayed reinforcer (Bickel et al., 2011c; Wesley and Bickel, 2014), training specific executive functions, such as working memory, may increase executive decision system control leading to program retention and a rebalance of the CNDS.
4.3.1.1 Working memory training
Of the impaired executive systems in cocaine addicts, working memory is an executive function mediated by the prefrontal cortex and is involved in goal-directed behavior (Miller and Cohen, 2001). Interestingly, following working memory training, healthy participants demonstrate increases in prefrontal and parietal region activation (Olesen et al., 2004). Consistent with the CNDS theory, more activation in these areas indicate increases in executive decision system functionality and is important because greater frontoparietal activity occurs when participants choose larger delayed rewards (McClure et al., 2004). In fact, we have demonstrated decreased delay discounting of monetary rewards following working memory training in cocaine addicts (Bickel et al., 2011c), thus providing support for this potential approach to increase executive system functionality. In addition to working memory training, a second potential treatment, episodic future thinking, shows beneficial executive neurocognitive improvement capabilities.
4.3.1.2 Episodic future thinking
Episodic future thinking is a form of prospection which involves mental simulation of future events (Atance and O'Neill, 2001). Neural evidence demonstrates that future thinking tasks activate frontal cortices (Okuda et al., 2003) associated with the executive decision system. Moreover, goal-directed simulations activate the prefrontal cortex and associated regions (Gerlach et al., 2011). Behaviorally, episodic future thinking decreases delay discounting, which is predicted by anterior cingulate cortex activation (Daniel et al., 2013; Peters and Buchel, 2010). Thus, given that poor performance of future thinking is associated with poor executive function (de Vito et al., 2012), repetition of either working memory training or episodic future thinking may increase control of the executive decision system, improve valuation of future rewards, and provide a valuable adjunct to cocaine cessation therapy.
4.3.2 Medications
Modafinil acts on several neurotransmitter systems including glutamate, GABA, and dopamine. Similar to the previously proposed agonist therapies to decrease control of the impulsive decision system, modafinil produces a similar mechanism of action to cocaine (i.e., increases in dopamine) and produces protracted mild stimulant properties to promote wakefulness. Modafinil reduces activity in the ventral tegmental area, an impulsive decision system brain region, and reduces self-reported craving in response to cocaine cues (Goudriaan et al., 2013), indicative of an attenuation of craving. Though modafinil has been investigated as an agonist replacement therapy (i.e., to buffer hyperactivation of the impulsive decision system) with mixed results (Dackis et al., 2012; Hart et al., 2008), modafinil's actions may be most beneficial by activating the executive decision system. Modafinil promotes enhanced activation of the frontoparietal regions and reduced activation of the ventro-medial prefrontal cortex (Schmaal et al., 2014), both regions associated with the valuation of rewards. Behaviorally, modafinil increases several measures of working memory and attention in cocaine users (Kalechstein et al., 2013). Modafinil reduces delay discounting in alcohol-dependent participants compared to controls (Schmaal et al., 2014), and importantly, modafinil does not impair inhibitory control in a go/no-go task in cocaine-dependent individuals (Vansickel et al., 2008) offering another stimulant medication that increases executive function without overactivating the impulsive system.
Modafinil, alongside other medications, has been classified as a nootropic, or a cognitive enhancer. Nootropics are reported to increase working and visual memory, decision-making, and planning (Turner et al., 2004), indicating that pharmacological interventions can improve executive decision system function and regulatory balance of the CNDS. Interestingly, improving deficits in neurotransmitter systems with nicotine agonists, norepinephrine transporter inhibitors, or alpha-2 adrenergic agonists, coincide with some improved attention, response inhibition, and working memory (Sofuoglu, 2010). Evidence that these other systems modulate executive function warrants further investigation into nootropics enhancing the executive decision system to improve treatment outcomes. Moreover, the benefits of pharmacological treatments can provide a valuable adjunct therapy to behavioral interventions such as contingency management or working memory training, allowing for synergistic treatment.
4.3.3 Neurotherapeutic stimulation
Vulnerability to drug-related cues may be due to low functional activity in the executive decision system of substance-dependent individuals (Goldstein et al., 2004; Kubler et al., 2005; Moeller et al., 2010) suggesting that an LTP-like rTMS stimulation of the executive decision system (e.g., dorsolateral prefrontal cortex) might enable better resistance against drug cues. To date, the vast majority of rTMS studies in addiction have targeted the dorsolateral prefrontal cortex (Amiaz et al., 2009; Camprodon et al., 2007; Eichhammer et al., 2003; Herremans et al., 2012, 2013; Hoppner et al., 2011; Li et al., 2013; Mishra et al., 2010; Politi et al., 2008; Pripfl et al., 2014). While many of these studies demonstrated that LTP-like rTMS stimulation to the dorsolateral prefrontal cortex can result in a significant reduction of craving, the neurobiological mechanism is unclear. For example, in a comprehensive review on the efficacy of rTMS for smoking cessation, Wing et al. (2013) reported beneficial effects on tobacco craving following LTP-like rTMS on the dorsolateral prefrontal cortex.
Neurotherapeutic stimulation is a developing area of research for treatment of drug dependence. Future research needs to resolve two questions, which cortical location should be targeted in order to maximally affect the circuitry associated with regulatory balance between decision systems and what stimulation frequency should be used. Identification of a single “optimal” protocol for all individuals or all drug classes is not likely. For example, some individuals may benefit the most from a treatment strategy that amplifies the executive decision system (e.g., 10 Hz dorsolateral prefrontal cortex stimulation) while others may benefit most from a strategy that attenuates the impulsive decision system (e.g., 1 Hz medial prefrontal stimulation). Before moving forward with expensive and slow multisite clinical trials investigating the efficacy of rTMS as a viable treatment tool for addiction, exploration of these combinations of frequencies and cortical targets to maximize potential impact should be considered. TMS may provide a powerful new tool to use as an adjunct to behavioral and pharmacotherapeutic addiction treatment. Given that no FDA-approved pharmacotherapy for cocaine dependence exists, brain stimulation may be a particularly useful therapeutic technique.
5 Conclusion and Future Directions
Integration of findings from multiple scientific disciplines and levels of analysis into a robust conceptual system will permit and suggest experiments, and perhaps lead to novel treatments for cocaine dependence. Scientific paradigms in the field of addiction have continuously evolved and have had at least four major paradigm shifts in the last hundred years (Bickel et al., 2013). The CNDS theory constitutes the most recent paradigm shift and is a valuable perspective for addiction research in two ways. First, it stipulates that a fundamental contributor to the addiction process is a dysregulation between the impulsive and executive decision systems. Second, it identifies those two decision systems as targets for interventions.
In this chapter, we have shown that numerous observations could be integrated when viewed from the perspective of the CNDS. Armed with that view, we connect observations regarding the immediacy bias evident in addiction, neural activity and structure, the developmental pattern associated with cocaine and other drug use vulnerabilities, the SES gradient of cocaine and other drug dependencies, and the presence of comorbidities. Such integration supports use of the CNDS theory to guide treatment strategies.
For treatment of cocaine dependence, our view is that treatments or interventions should be supported by a theoretical conceptualization. If the conceptualization of a disorder changes, that change should force a reevaluation of the treatment efficacy. The CNDS is a relatively new conceptualization and permits understanding of the efficacy of existing treatments (e.g., CBT), but also suggests novel approaches (e.g., rTMS) to either decrease activity in the impulsive decision system or increase activity in the executive decision system. Efficacy of these novel approaches will, in part, continue to test the CNDS and indicate the range of its relevance.
The CNDS, like many paradigmatic approaches, is an approximation of a more complete paradigm. The examination and use of the CNDS in the treatment of cocaine and other drug dependence disorders are not based on the ultimate value of the theory, but rather its proximal utility in making new discoveries and assisting those trapped by cocaine dependence. Whether the CNDS continues to provide new research insights that contribute to treatment or will instead give way to an even more robust perspective will await subsequent investigation. In either case, the continued exploration and elaboration of this integrated view contributes to the science of addiction, in general, and cocaine dependence in particular.
Acknowledgments
The following grants contributed to the support of the authors during the development of this work: NIH grants U19CA157345, R01DA034755, R01AA021529, and R01DA036617.
References
- Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Atance CM, O'Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5(12):533. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Baams L, Dubas JS, Overbeek G, van Aken MAG. Transitions in body and behavior: a meta-analytic study on the relationship between pubertal development and adolescent sexual behavior. J Adolesc Health. 2015;56(6):586–598. doi: 10.1016/j.jadohealth.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int. Rev. Psychiatry. 2011;23(5):454–466. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Baum CL, II, Ruhm CJ. Age, socioeconomic status and obesity growth. J Health Econ. 2009;28:635–648. doi: 10.1016/j.jhealeco.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G. rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol. 2014;2014:815215. doi: 10.1155/2014/815215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond Ser B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Mueller ET. Toward the study of trans-disease processes: a novel approach with special reference to the study of co-morbidity. J Dual Diagn. 2009;5:131–138. doi: 10.1080/15504260902869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R. Temporal discounting as a measure of executive function: Insights from the competing neuro-behavioral decision system hypothesis of addiction. In: Houser D, McCabe K, editors. Neuroeconomics: Advances in Health Services Research. Vol. 20. Emerald Group Publishing; Bingley, UK: 2008. pp. 289–309. [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90S:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jones BA, Landes RD, Christensen DR, Jackson L, Mancino M. Hypothetical intertemporal choice and real economic behavior: delay discounting predicts voucher redemptions during contingency-management procedures. Exp Clin Psycho-pharmacol. 2010;18(6):546–552. doi: 10.1037/a0021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM. The behavioral economics and neuroeconomics of reinforcer pathologies: implications for etiology and treatment of addiction. Curr Psychiatry Rep. 2011a;13:406–415. doi: 10.1007/s11920-011-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, et al. Single- and cross-commodity discounting among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology (Berl) 2011b;217(2):177–187. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol. Psychiatry. 2011c;69(3):260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012a;221(3):361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012b;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Mueller ET, Jarmolowicz DP. What is addiction? In: McCrady B, Epstein E, editors. Addictions: A Comprehensive Guidebook. second. Oxford University Press; New York: 2013. pp. 3–16. [Google Scholar]
- Bickel WK, Landes RD, Kurth-Nelson Z, Redish AD. A quantitative signature of self-control repair: rate-dependent effects of successful addiction treatment. Clin Psychol Sci. 2014a;2:685–695. doi: 10.1177/2167702614528162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody L, Quisenberry AJ, Ramey CT, Sheffer CE. A competing neurobehavioral decision systems model of SES-related health and behavioral disparities. Prev Med. 2014b;68:37–43. doi: 10.1016/j.ypmed.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Moody L, Wilson AG. Therapeutic opportunities for self-control repair in addiction and related disorders: change and the limits of change in trans-disease processes. Clin Psychol Sci. 2015;3:140–153. doi: 10.1177/2167702614541260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Strickland JR, Thompson JR, Afful SE, Cottler LB. Drug use and dependence in cocaine dependent subjects, community-based individuals, and their siblings. Drug Alcohol Depend. 2008;95:14–22. doi: 10.1016/j.drugalcdep.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiberg JL, Devlin P, Croan J, Briscoe R. Relationship between treatment length and outcome in a therapeutic community. Int J Addict. 1994;29:729–740. doi: 10.3109/10826089409047906. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology. 2000;54(12):2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24:541–550. doi: 10.1177/0897190011426557. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abus Treat. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Buka SL. Disparities in health status and substance use: ethnicity and socioeconomic factors. Public Health Rep. 2002;117:S118–S125. [PMC free article] [PubMed] [Google Scholar]
- Burling TA, Salvio MA, Seidner AL, Ramsey TG. Cigarette smoking in alcohol and cocaine abus. J Subst Abus. 1996;8:445–452. doi: 10.1016/s0899-3289(96)90005-x. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rtms) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162(8):1452–1460. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Martino S, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165(7):881–888. doi: 10.1176/appi.ajp.2008.07111835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson HW, Leichliter JS, Zimet GD, Rosenthal SL, Bernstein DI, Fife KH. Discount rates and risky sexual behavior among teenagers and young adults. J Risk Uncertain. 2006;32:217–230. [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatr. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SS, Koshimori Y, Aminian K, et al. Investing in the future: stimulation of the medial prefrontal cortex reduces discounting of delayed rewards. Neuropsychopharmacology. 2015;40(3):546–553. doi: 10.1038/npp.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abus Treat. 2012;43:303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, Epstein LH. The future is now: reducing impulsivity and energy intake using episodic future thinking. Psychol Sci. 2013;24:2339–2342. doi: 10.1177/0956797613488780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vito S, Gamboz N, Brandimonte MA, Barone P, Amboni M, Della Sala S. Future thinking in Parkinson's disease: an executive function? Neuropsychologia. 2012;50(7):1494–1501. doi: 10.1016/j.neuropsychologia.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J. Clin. Psychiatry. 2003;64(8):951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in response to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Muller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169(9):926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Evans JS, Stanovich KE. Dual-process theories of higher cognition-advancing the debate. Perspect Psychol Sci. 2013;8:223–241. doi: 10.1177/1745691612460685. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc B. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol. Psychiatry. 2002;51(2):134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroan-atomical specificity for drug users and drug stimuli. Am J Psychiatr. 2000 Nov 1;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. 2000. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage. 2011;55(4):1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatr. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327:79–93. doi: 10.1111/nyas.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav. 2013;38:1509–1517. doi: 10.1016/j.addbeh.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol. 1997;17:485–488. doi: 10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Austelle CW, et al. What goes up, can come down: novel brain stimulation paradigms may attenuate craving and craving-related neural circuitry in substance dependent individuals. Brain Res. 2015 doi: 10.1016/j.brainres.2015.02.053. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33(4):761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Haushofer J, Fehr E. On the psychology of poverty. Science. 2014;344(6186):862–867. doi: 10.1126/science.1232491. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, et al. No influence of one right-sided prefrontal hf-rtms session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120:1–3. 209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C. Reduced intraindividual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. 2013;48:552–557. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatr. 1991;148:1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Badger G, Foerg F, Ogden D. Outpatient behavioral treatment for cocaine dependence: one-year outcome. Exp Clin Psychopharmacol. 1995;3:205–212. [Google Scholar]
- Hiscock R, Bauld L, Amos A, Fidler JA, Munafo M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107–123. doi: 10.1111/j.1749-6632.2011.06202.x. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Hoppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J. Biol. Psychiatry. 2011;12(Suppl. 1):57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. The sexual discounting task: HIV risk behavior and the discounting of delayed sexual rewards in cocaine dependence. Drug Alcohol Depend. 2012;123:1–3. 15–21. doi: 10.1016/j.drugalcdep.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in longterm, high-dose cocaine users. Neuropharmacology. 2013;64:472–478. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Khurana A, Romer D, Betancourt LM, Brodsky L, Giannetta JM, Hurt H. Experimentation versus progression in adolescent drug use: a test of an emerging neurobehavioral imbalance model. Dev Psychopathol. 2015;27:901–913. doi: 10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. 2013;70(8):857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73(8):714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the national epidemiologic survey on alcohol and related conditions (NESARC) Drug Alcohol Depend. 2011;115:1–2. 120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci. 2012;15:358–366. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Makris N, Gasic GP, Seidman LJ, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44(4):729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. 2012;35:425–439. doi: 10.1016/j.psc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel DJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: main and matching effects. J Consult Clin Psychol. 1998;66:832–837. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative Analyses of Behavior. Vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- McClure SM, Bickel WK. A dual-systems perspective on addiction: contributions from neuroimaging and cognitive training. Ann N Y Acad Sci. 2014;1327:62–78. doi: 10.1111/nyas.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004 Oct 15;306(5695):503–507. doi: 10.1126/science.1100907. 2004. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Mischel W. A hot/cold-system analysis of delay of gratification: dynamics of willpower. Psychol Rev. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Miech R. The formation of a socioeconomic health disparity: the case of cocaine use during the 1980s and 1990s. J Health Soc Behav. 2008;49:352–366. doi: 10.1177/002214650804900308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105(1):49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abus Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Herin D, Kjome KL. Use of stimulants to treat cocaine and methamphetamine abuse. Curr Psychiatry Rep. 2008;10:385–391. doi: 10.1007/s11920-008-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, et al. Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 2010;181:174–182. doi: 10.1016/j.pscychresns.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl. Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125:208–214. doi: 10.1016/j.drugalcdep.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Narvaez JC, Jansen K, Pinheiro RT, et al. Psychiatric and substance-use comorbidities associated with lifetime crack cocaine use in young adults in the general population. Compr. Psychiatry. 2014;55(6):1369–1376. doi: 10.1016/j.comppsych.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–638. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, et al. Thinking of the future and the past: the roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19(4):1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, Davies S, Ompad DC, Cleland CM, Weitzman M. Powder cocaine and crack use in the United States: an examination of risk for arrest and socioeconomic disparities in use. Drug Alcohol Depend. 2015;149:108–116. doi: 10.1016/j.drugalcdep.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349–370. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99(3):349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poincaré H. Science and Hypothesis. The Walter Scott Publishing Company, Ltd; New York: 1905. [Google Scholar]
- Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch. Gen. Psychiatry. 2006;63(2):219–228. doi: 10.1001/archpsyc.63.2.219. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17:345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robles E, Silverman K, Preston KL, et al. The brief abstinence test: voucher-based reinforcement of cocaine abstinence. Drug Alcohol Depend. 2000;58:1–2. 205–212. doi: 10.1016/s0376-8716(99)00090-3. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR. Cocaine effects during D-amphetamine maintenance: a human laboratory analysis of safety, tolerability and efficacy. Drug Alcohol Depend. 2009;99:1–3. 261–271. doi: 10.1016/j.drugalcdep.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Chang LJ. Multiple systems in decision making. Ann N Y Acad Sci. 2008;1128:53–62. doi: 10.1196/annals.1399.007. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, Joos L, et al. Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychol Med. 2014;44:2787–2798. doi: 10.1017/S0033291714000312. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98(8):1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 1999;56(6):507–514. doi: 10.1001/archpsyc.56.6.507. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:3–4. 163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sofis MJ, Jarmolowicz DP, Martin LE. Competing neurobehavioral decision systems and the neuroeconomics of craving in opioid addiction. Neurosci Neuroecon. 2014;3:87–89. [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105(1):38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16:55–59. [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44:1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Blackburn JW, Hudson DA, Hays LR, Rush CR. Safety, tolerability and subject-rated effects of acute intranasal cocaine administration during atomoxetine maintenance. Drug Alcohol Depend. 2008;92:1–3. 282–285. doi: 10.1016/j.drugalcdep.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, et al. Performance on the stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology. 2008;33(4):827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2003 National Survey on Drug Use and Health: National Findings. 04. Office of Applied Studies; Rockville, MD: 2004. p. 3964. SMA. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Office of Applied Studies; Rockville, MD: 2013. pp. 14–4863. SMA. [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–593. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ. Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2004;55(10):1031–1040. doi: 10.1016/j.biopsych.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Fillmorex MT, Hays LR, Rush CR. Effects of potential agonist-replacement therapies for stimulant dependence on inhibitory control in cocaine abusers. Am J Drug Alcohol Abuse. 2008;34(3):293–305. doi: 10.1080/00952990802013565. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156(9):1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Bickel WK. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol Psychiatry. 2014;75(6):435–448. doi: 10.1016/j.biopsych.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling JY, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;1115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R, Pickett K. Greater equality: the hidden key to better health and higher scores. Am Educ. 2011;35:5–9. [Google Scholar]
- Wing VC, Barr MS, Wass CE, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Stevens MC, Carroll KM, et al. Functional brain networks associated with cognitive control, cocaine dependence, and treatment outcome. Psychol Addict Behav. 2013;27:477–488. doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35(7):1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Newton TF, Haile CN, et al. Effects of d-cycloserine on cue-induced craving and cigarette smoking among concurrent cocaine- and nicotine-dependent volunteers. Addict Behav. 2013;38:1518–1526. doi: 10.1016/j.addbeh.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Nitsche MA, et al. Consensus: motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]