Significance
Specific gene functions have been successfully suppressed by gene silencing or editing in many organisms. However, genetic manipulation to suppress the function of a target tissue has not been achieved using cytotoxin genes. We established transgenic silkworms with posterior silk glands (PSGs) that express the enzymatic domain of the cytotoxin pierisin-1A (P1A). The larvae with the modified PSGs produced the sericin cocoons with potential utilities in tissue engineering. The targeted P1A expression was found to cause site-specific repression of certain protein synthesis that appeared to have no impact on the developmental stages of individuals. Thus, the new approach through targeted P1A expression could be applicable to the development of biologically useful model organisms with tissue-specific dysfunction.
Keywords: transgenic silkworm, cytotoxin, silk proteins, hydrogel
Abstract
Genetically manipulated organisms with dysfunction of specific tissues are crucial for the study of various biological applications and mechanisms. However, the bioengineering of model organisms with tissue-specific dysfunction has not progressed because the challenges of expression of proteins, such as cytotoxins, in living cells of individual organisms need to be overcome first. Here, we report the establishment of a transgenic silkworm (Bombyx mori) with posterior silk glands (PSGs) that was designed to express the cabbage butterfly (Pieris rapae) cytotoxin pierisin-1A (P1A). P1A, a homolog of the apoptosis inducer pierisin-1, had relatively lower DNA ADP ribosyltransferase activity than pierisin-1; it also induced the repression of certain protein synthesis when expressed in B. mori-derived cultured cells. The transgene-derived P1A domain harboring enzymatic activity was successfully expressed in the transgenic silkworm PSGs. The glands showed no apoptosis-related morphological changes; however, an abnormal appearance was evident. The introduced truncated P1A resulted in the dysfunction of PSGs in that they failed to produce the silk protein fibroin. Cocoons generated by the silkworms solely consisted of the glue-like glycoprotein sericin, from which soluble sericin could be prepared to form hydrogels. Embryonic stem cells could be maintained on the hydrogels in an undifferentiated state and proliferated through stimulation by the cytokines introduced into the hydrogels. Thus, bioengineering with targeted P1A expression successfully produced silkworms with a biologically useful trait that has significant application potential.
Pierisin-1 is a 98-kDa cytotoxic protein that is produced by the cabbage butterfly Pieris rapae (1–4). Previous studies have shown that the addition of purified pierisin-1 to culture media can induce apoptosis of various human cancer cell lines (1–4). The N-terminal domain of pierisin-1 features an ADP ribosyltransferase that transfers the ADP ribose moiety of nicotinamide-adenine dinucleotide (NAD) to the 2′-deoxyguanosine residues of DNA (2–4), whereas the C-terminal region carries a domain that mediates binding to receptors on cell membranes and uptake by target cells (4).
To date, the suppression of a specific gene function by gene silencing or editing has been successfully performed in many organisms (5, 6). In addition genetically manipulated model organisms with cytotoxic protein-induced dysfunction of specific tissues would be crucial for the study of various biological applications and mechanisms. Based on its function, the ectopic expression of pierisin-1 could be used to induce cell or tissue dysfunction, and to develop model organisms with modified traits. However, no studies have examined whether cells that are genetically engineered to express pierisin-1 in vivo locally and intracellularly undergo apoptosis or have other physiological alterations because the expression of pierisin-1 in any kind of living cell has been unsuccessful due to its strong cytotoxicity. Several proteins homologous with pierisin-1 have been found in P. rapae (7). A pierisin-1 homolog named pierisin-1A (P1A) was recently identified that has relatively lower DNA ADP ribosylating activity than pierisin-1. Consequently, the successful expression of P1A in living cells of individual organisms is considered possible due to its lower enzymatic activity.
Several methods have been established for generating transgenic silkworms (Bombyx mori) (8–11). Accordingly, transgenic silkworms have been studied as tools for large-scale production of foreign recombinant proteins because silkworm silk glands have a high capacity for protein biosynthesis (12–14). Silk proteins from silkworm cocoons, which are mainly composed of fibroin and sericin, are candidate biomaterials with numerous biomedical applications (15, 16). Genetic manipulations that alter the properties of silk glands would help expand the biomedical utilities of silk proteins. One study described a silkworm with ablation in several tissues as a result of forced expression of the mouse Bcl-2–associated X protein; however, it is unclear whether the manipulation altered the biological attributes of its cocoon (17). To explore a strategy for genetic manipulation to induce tissue-specific dysfunction that would modify the individual traits, we established a transgenic silkworm with forced expression of the putative ADP ribosyltransferase domain from P1A in the posterior silk gland (PSG).
Results
Expression and Physiological Effects of Truncated P1A in Silkworm PSGs.
Cabbage butterfly-derived P1A has ∼75% amino acid identity with the apoptosis-inducing humoral protein pierisin-1 (1–4) (SI Appendix, Fig. S1). In vitro-translated P1A protein exhibited ADP ribosylation activity, which was manifested as the transfer of the ADP ribose moiety of substrate [32P]NAD to DNA (SI Appendix, Fig. S2A); however, the HPLC analyses of the reaction products revealed that P1A had ∼5% of DNA ADP ribosylating activity compared with pierisin-1 (P < 0.001; SI Appendix, Fig. S2B). Transfection experiments with vectors (SI Appendix, Fig. S3) followed by immunoblotting confirmed the transient expression of the P1A N-terminal portion containing the DNA ADP ribosyltransferase domain (P1A269) in insect cultured cell lines, B. mori-derived BM-N and Spodoptera frugiperda-derived Sf21 (Fig. 1A and SI Appendix, SI Results and Fig. S7). The ectopic gene expression of P1A269 and full-length P1A caused unique nonapoptotic effects on BM-N cells that were characterized by an increase in the number of flattened cells and the repression of reporter protein synthesis (18) (SI Appendix, SI Results and Figs. S4 and S5).
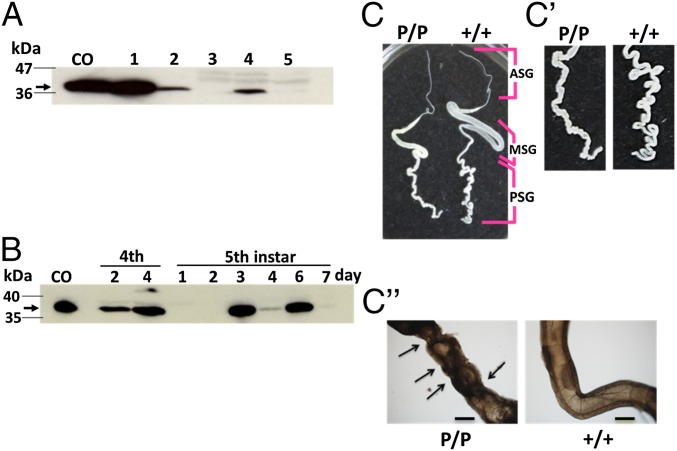
Fig. 1.
Expression of the truncated pierisin-1 protein (P1A269) in PSGs from w1-pndP1A269/P1A269 silkworms (B. mori). (A) P1A269 protein detection in PSGs by immunoblotting with a FLAG-tag antibody: lane CO, cypovirus polyhedron-encapsulated P1A269; lanes 1 and 2, P1A269-expressing Sf21 and BM-N cells, respectively (SI Appendix, Figs. S4 and S5); lane 3, PSGs from w1-pnd+/+ on day 7 of the fifth instar; and lanes 4 and 5, PSGs from w1-pndP1A269/P1A269 on days 6 and 7 of the fifth instar, respectively. A protein size marker is shown in the left-hand lane, and the arrow indicates the P1A269 protein. (B) P1A269 protein expression in PSGs from w1-pndP1A269/P1A269 larvae during the fourth and fifth instars. The arrow indicates the P1A269 protein. Normal and abnormal silk glands from w1-pnd+/+ (+/+) and w1-pndP1A269/P1A269 (P/P) larvae, respectively, on day 7 of the fifth instar (C), showing enlarged images (C′) and microscopic observations (4× objective) (C′′) of PSGs. The anterior silk gland (ASG), middle silk gland (MSG), and PSG of w1-pnd+/+ larvae are indicated. Arrows indicate the observed dents. (Scale bars: C and C′, 10 mm; C′′, 500 μm.) Full gel images for the immunoblotting and SDS/PAGE for the same series of samples shown in A and B are presented in SI Appendix, Figs. S7 and S8, respectively.
To explore the functional consequences of intracellularly expressed P1A269 in specific tissues, we generated the transgenic silkworm line w1-pndP1A269/P1A269 (SI Appendix, SI Materials and Methods), which carries a homogeneous transgene for P1A269 expression under the control of the fibroin heavy-chain (FibH) promoter (11) (SI Appendix, Fig. S6), which is particularly active in the PSGs. The effects of P1A269 were expected to be limited to the PSGs because it was designed to lack both the signal sequence needed for P1A secretion from PSG cells and the C-terminal receptor-binding domain needed for its invasion into target cells. Inverse PCR and DNA database analysis showed that the transgene was inserted into chromosome 16 (SI Appendix, SI Materials and Methods and Table S1). Through the use of immunoblotting and SDS/PAGE, the expressed P1A269 protein could be detected in larval PSGs on day 6 of the fifth instar (Fig. 1A and SI Appendix, Fig. S7), which was similar to control samples isolated from cypovirus polyhedron-encapsulated P1A269 (SI Appendix, SI Materials and Methods) and P1A269-expressing Sf21 and BM-N cells. During the larval stage, P1A269 protein expression increased on day 4 of the fourth instar and on days 3 and 6 of the fifth instar, but it decreased on day 7 of the fifth instar (Fig. 1B and SI Appendix, Fig. S8). Quantitative RT-PCR also showed that P1A269 mRNA expression increased on days 3 and 6 of the fifth instar (SI Appendix, Fig. S9). The PSGs dissected from the w1-pndP1A269/P1A269 larvae were not ablated by apoptosis, but instead exhibited morphological abnormalities, such as a dented appearance compared with PSGs from nontransformed w1-pnd+/+ larvae (Fig. 1 C–C′′). In the present study, fifth-instar larvae of w1-pndP1A269/P1A269 started to spin on day 6 of the fifth instar and continued to spin vigorously following a gut purge on day 7. Previous studies have shown that FibH promoter activity in the PSGs increases in response to a pulse of ecdysteroid secreted by larvae on day 3 of the fifth instar and at later stages during the fifth instar (12). On day 4 of the fourth instar, larvae in the present study were considered to be in the molting stage, when an ecdysteroid pulse influences the expression of certain genes. Thus, P1A269 expression on day 4 of the fourth instar and on days 3 and 6 of the fifth instar was likely to be controlled by ecdysteroid secretion, suggesting that ecdysteroid pulses during larval development repeatedly induced the P1A269 expression that was responsible for the abnormal appearance of the PSGs.
Genetic Traits of w1-pndP1A269/P1A269 Silkworms.
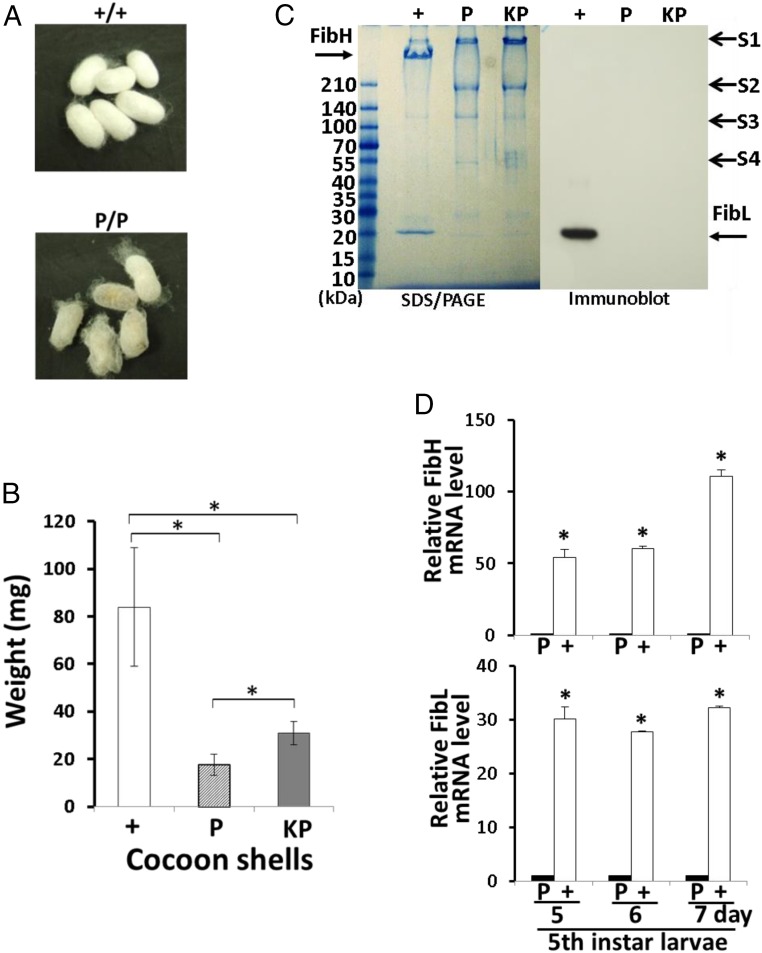
The w1-pndP1A269/P1A269 larvae produced thin-layered cocoon shells (Fig. 2A) that weighed 79% less than the cocoon shells produced by w1-pnd+/+ larvae (P < 0.001; Fig. 2B). Wild-type silkworm cocoon shells are composed of ∼70% fibroin protein [FibH and fibroin light-chain (FibL)], 25% sericin protein, and other materials. The fibroin is a raw silk component specifically produced by the PSGs, whereas the sericin is a glue-like protein produced mainly in the middle silk glands that promotes cocoon cohesion by surrounding and gluing fibroin threads together (15). Hence, the reduced weight of the cocoon shells made by w1-pndP1A269/P1A269 larvae was considered most likely due to reduced fibroin content. SDS/PAGE in a gradient gel (5–20%) followed by immunoblotting with a FibL-specific antibody (13) showed that FibL and FibH proteins were present in the cocoon shells of w1-pnd+/+ larvae but not in the cocoons shells of w1-pndP1A269/P1A269 larvae, in which only nonfibroin proteins, mainly sericins (15, 19–22), were detectable (sericin 1–4; Fig. 2C). Quantitative RT-PCR analysis showed that FibH and FibL mRNA levels in the PSGs of w1-pndP1A269/P1A269 larvae were strongly decreased on days 5, 6, and 7 of the fifth instar relative to w1-pnd+/+ larvae on the corresponding days (Fig. 2D). Indeed, on day 7 of the fifth instar, FibH and FibL mRNA levels of w1-pndP1A269/P1A269 larvae were decreased to 3.1% and 0.9%, respectively, of FibH and FibL mRNA levels of w1-pnd+/+ larvae (P < 0.001; Fig. 2D). The mRNA level of a housekeeping gene, cytoplasmic actin A3 in the PSGs of w1-pndP1A269/P1A269 larvae on the same days did not appear to be decreased relative to the level of cytoplasmic action A3 of w1-pnd+/+ larvae (23) (SI Appendix, Fig. S10). These results revealed that intracellularly expressed P1A269 represses FibH and FibL protein synthesis in the PSGs of the transgenic silkworms.
Fig. 2.
Cocoon weight and fibroin content of P1A transgenic silkworms. (A) Cocoons of w1-pnd+/+ (+/+) and w1-pndP1A269/P1A269 (P/P) silkworms. (B) Weights of the cocoon shells from w1-pnd+/+ (+; n = 57), w1-pndP1A269/P1A269 (P; n = 71), and KWP1A269/P1A269 (KP; n = 60; SI Appendix, SI Materials and Methods) silkworms measured after 30 min of lyophilization are shown. Data are means ± SD. *P < 0.001. (C) SDS/PAGE (Left) and FibL-specific immunoblot detection (Right) of proteins from the cocoon shells produced by w1-pnd+/+, w1-pndP1A269/P1A269, and KWP1A269/P1A269 larvae. Proteins were run on a 5–20% gradient gel; arrows indicate bands for FibH, FibL, sericin 1 (S1), sericin 2 (S2), sericin 3 (S3), and sericin 4 (S4) proteins. Size markers are indicated on the left-hand side. (D) Relative FibL and FibH mRNA levels in the PSGs from w1-pnd+/+ and w1-pndP1A269/P1A269 larvae on days 5, 6, and 7 of the fifth instar as analyzed by quantitative RT-PCR. The values of expression levels for FibH and FibL mRNAs were normalized to the value of expression levels of 18S rRNA. Data are means ± SD of relative values obtained by comparing the value on each day of the fifth instar with w1-pndP1A269/P1A269 larvae (set as 1) (n = 3 independent samples). *P < 0.001 versus w1-pndP1A269/P1A269 larvae on each corresponding day.
The average weights of male and female w1-pndP1A269/P1A269 pupae on the third day after pupation were 1.5- and 1.3-fold higher, respectively, than the average weights of male and female w1-pnd+/+ pupae (P < 0.001; SI Appendix, Fig. S11). This additional weight may be due to the retention of nutritional resources that would have otherwise been devoted to protein production, particularly fibroin, which is needed for cocoon spinning. Indeed, in classical experiments, the surgical excision of silk glands from fourth- and fifth-instar larvae caused an accumulation of excess humoral amino acids, such as Gly, Thr, Ser, and Tyr, before pupation, whereas larvae from which the entire silk gland was removed failed to pupate likely due to the presence of excess amino acids (19). The w1-pndP1A269/P1A269 pupae with excess weight showed no noticeable developmental defects after pupation; to date, these transgenic silkworms can mature to adulthood and produce healthy offspring that can successfully reproduce. These results suggest that the retention of nutritional resources for fibroin synthesis does not appear to have negative effects on the transgenic silkworms.
Noncocoon silk threads that are spun during the first to fourth ecdysis are known to fix the larval prolegs to surfaces, a behavior that is crucial for supporting larval ecdysis. The larvae that spun fibroin-free, noncocoon threads in this study did not fail to shed their skins. This outcome is consistent with evidence shown by Takasu et al. (20) that the noncocoon threads of wild-type silkworms that support larval ecdysis contain little fibroin, but are instead mainly composed of sericin components, particularly sericin-2.
Application of the P1A269 Allele to Silkworm Breeding.
We next established the KWP1A269/P1A269 line (SI Appendix, SI Materials and Methods) by mating a commercial silkworm strain (Kinshu x Showa) used in the sericulture industry with the w1-pndP1A269/P1A269 line, screening for individuals that were positive for the genetic marker (EGFP-positive eyes) and then conducting successive sib matings. The commercial silkworm strain produces heavier cocoons than w1-pnd+/+. The resulting KWP1A269/P1A269 larvae produced sericin cocoon shells in which FibL and FibH proteins were undetectable by SDS/PAGE and FibL-specific immunoblotting (Fig. 2C). Moreover, the KWP1A269/P1A269 shells were 1.7-fold heavier than w1-pndP1A269/P1A269 cocoon shells (P < 0.001; Fig. 2B). This result suggests that the simple mating of the w1-pndP1A269/P1A269 line with preserved lines described in the Silkworm Genetic Resource Database (shigen.nig.ac.jp/silkwormbase/top.jsp) that are known to produce heavy cocoons could be used to produce fibroin-free, heavy sericin cocoons.
Sericin Cocoons Can Be Used to Prepare Hydrogels.
Commercial sericin prepared by degumming the raw silk from regular cocoons was unable to form hydrogels (SI Appendix, Fig. S12 A and A′) because the high temperature and alkaline pH conditions that are involved in degumming result in protein decomposition to the point where sericin is no longer detectable by SDS/PAGE (SI Appendix, Fig. S12B). However, intact, soluble, fibroin-free sericin at concentrations that were detectable by SDS/PAGE (Fig. 2C and SI Appendix, Fig. S12B) could be readily prepared from w1-pndP1A269/P1A269 sericin cocoons using 6 M LiBr (Materials and Methods). Hydrogel formation of the intact, soluble sericin could then be induced following a 1-h incubation with 10% ethanol (15, 21) (SI Appendix, Fig. S12 A and A′). It was not possible to use the same chemical method to obtain a fibroin-free, intact sericin solution from the wild-type silkworm cocoons due to the high level of fibroin contamination in the extracted low-concentration sericin solution. The intact, soluble sericin has moisture retention properties that are similar to collagen and can gelate in solutions of up to 96% water. The solution of intact sericin autonomously forms hydrogels after long-term storage for 2–3 wk at 4 °C. Slight modifications to the sericin protein structure following long-term storage at 4 °C appear to be sufficient to promote gelation; thus, the addition of ethanol probably accelerated this process.
Sericin Hydrogels Can Be Used for Cell Culture Matrices.
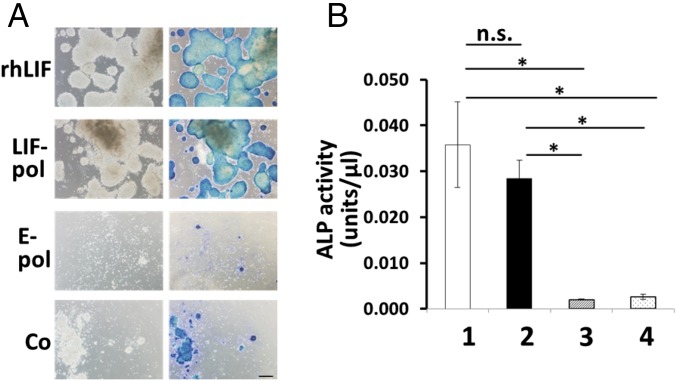
We previously showed that the activity of cytokines encapsulated in polyhedra, which are protein microcrystals of cypovirus, can be stably maintained over long periods in both ex vivo and in vivo environments, and can be slowly released to stimulate the growth and differentiation of many cell types continually (14, 24–29). Therefore, to determine whether sericin hydrogels can act as scaffolds to support cell growth and differentiation, we cultivated the mouse embryonic stem (ES) cell line EB5 on sericin hydrogels that were overlaid with a medium containing canonical recombinant human leukemia inhibitory factor (rhLIF) or that incorporated polyhedron-encapsulated LIF (24) (SI Appendix, SI Materials and Methods). EB5 cells that can be kept undifferentiated in the presence of LIF can proliferate, form characteristic dome-shaped colonies, and express alkaline phosphatase (ALP) (30). Differentiated EB5 cells are unable to proliferate in the medium containing blasticidin, which was used for all cultivations in this study (24, 30). EB5 cells did not proliferate and form ALP-staining positive colonies in control cultures that were grown on sericin hydrogel lacking polyhedra or that incorporated empty polyhedra (Fig. 3A and SI Appendix, SI Materials and Methods). The ALP activity extracted from cells grown on control hydrogels did not increase after cultivation (Fig. 3B). By contrast, EB5 cells grown on hydrogels with polyhedron-encapsulated LIF and on hydrogels overlaid with medium containing rhLIF formed dome-shaped, ALP-positive colonies (Fig. 3A) that had 14- and 18-fold greater activities of extracted ALP, respectively, than cells grown on control hydrogels incorporating empty polyhedra in a medium lacking rhLIF (P < 0.005; Fig. 3B); however, there was no significant difference between these two experimental groups (P > 0.05; Fig. 3B). These findings suggest that sericin hydrogels can serve as a scaffold that supports EB5 cell growth provided that LIF is available.
Fig. 3.
Culture of mouse ES cells (EB5) on sericin hydrogels. (A, Left) EB5 colonies formed on sericin hydrogels that were overlaid with culture medium containing blasticidin plus rhLIF or colonies were formed on sericin hydrogels incorporating polyhedron-encapsulated LIF (LIF-pol) and empty polyhedra (E-pol) or no polyhedra (Co) that were overlaid with culture medium containing blasticidin after cultivation for 7 d. (A, Right) ALP-staining positive cells are also presented. (Scale bar: 200 μm.) (B) ALP activities of EB5 on the sericin hydrogels incorporating no polyhedra after cultivation in medium containing blasticidin and rhLIF (column 1) or ALP activities of EB5 cells on sericin hydrogels incorporating polyhedron-encapsulated LIF (column 2), empty polyhedra (column 3), or no polyhedra (column 4) after cultivation for 7 d in medium containing blasticidin. Data are means ± SD (n = 3 independent samples). *P < 0.005. n.s., not significant (P > 0.05).
Discussion
The present study generated the transgenic silkworm w1-pndP1A269/P1A269, in which the PSGs express the DNA ADP ribosyltransferase domain of P1A named P1A269. The successful expression of P1A in the silkworm cells may be due to its lower enzymatic activity (Fig. 1 and SI Appendix, Fig. S2) relative to the strongly cytotoxic pierisin-1, which cannot be expressed in any kind of living cell. Intracellularly expressed P1A269 induced the repression of reporter luciferase synthesis in BM-N cultured cells (SI Appendix, Figs. S4 and S5). An increase in the number of cells with a characteristic flattened morphology was seen in the BM-N cells that transiently express P1A269 (SI Appendix, Fig. S4). Thus, it appears that responses of BM-N cells to P1A269 are related to cell cycle arrest and involve entry into the quiescent phase, which is associated with impaired protein synthesis and activation of apoptotic pathways that respond to DNA damage (31). Also, P1A269 induced the decrease of FibH and FibL mRNA levels in the PSG cells, which are nonproliferative but grow in size during the larval stages (Figs. 1 and 2), suggesting the P1A269 function that represses the transcription of FibH and FibL genes. Cytoplasmic actin A3 mRNA level was not decreased by the function of P1A269 (SI Appendix, Fig. S10), suggesting that P1A269 repressed the transcription of some genes, including fibroin genes that are initiated concurrently with the expression of introduced P1A269. Thus, it appears that P1A269-induced repression of some genes may be responsible for the growth of PSG cells and cause the observed morphological abnormalities (Figs. 1 and 2). P1A269 function superficially appeared to induce an increase in the level of cytoplasmic actin A3 mRNA (SI Appendix, Fig. S10). However, because P1A269 function is estimated to repress the substantial increase in the rate of transcription of rRNAs in PSGs, which has been reported to occur concurrently with the fibroin synthesis (32), it would be better to consider the result an impractical, numerical event emerging from the normalization of data to P1A269-altered 18S rRNA levels.
To date, no cellular responses other than apoptosis have been observed following the treatment of mammalian cells with extracellular pierisin-1, which can enter target cells (4). Accordingly, only intracellularly expressed P1A proteins would be expected to induce characteristic cellular responses, including the repression of cellular protein synthesis, which suggests that an intracellularly expressed P1A N-terminal domain could cause site-specific, nonapoptotic effects that neither expand to neighboring tissues nor have an impact on the developmental stages of the individual. P1A could also be effective in any cells where it induces protein synthesis repression. As such, intracellularly expressed P1A encoding only the N-terminal enzymatic activity region could be used to generate model organisms with tissue-specific dysfunction that would be applicable for a variety of genetic manipulations, including the induction of quiescence in cancerous cells in mammals and the development of animal models with impaired islet function for diabetes mellitus studies or with compromised immune system function. However, for future applications of intracellularly expressed partial P1A in other organisms, we must first understand how the P1A fragment induces PSG dysfunction by repressing protein synthesis rather than inducing apoptosis. One possibility is that a certain level of DNA ADP ribosylation in cells is required to repress protein synthesis but that beyond this level, it induces apoptosis. This hypothesis is supported by the observation that P1A269 is more strongly expressed in Sf21 cells, where it stimulates their apoptotic cell death, than in BM-N cells (Fig. 1 and SI Appendix, SI Results and Figs. S4 and S5). Thus, future studies need to investigate whether the protein synthesis repression that occurred in the PSGs depends on the intensity of the expressed DNA ADP ribosylating activity as well as how P1A activity can be modulated either by regulating the promoter that controls P1A269 expression or by introducing amino acid mutations in P1A269.
This study also found that w1-pndP1A269/P1A269 larvae spun cocoons that were nearly fibroin-free and composed almost entirely of sericin. A previous study has shown that silkworm PSGs that express Bcl-2–associated X protein become ablated (17), although this study did not present information on the cocoons and the development of the manipulated individual after pupation. Furthermore, a separate report describing T7 RNA polymerase-modified PSGs of transgenic silkworms showed decreased but detectable FibL and FibH expression (33), whereas another study showed that silkworms with transcription activator-like effector nuclease-mediated FibH gene disruption produced thin-layered cocoons containing no FibH but a normal level of FibL (34). The Nd silkworm strain has a natural genetic mutation that affects silk glands and produces small quantities of cocoons that have very high amounts of sericin and varying percentages of fibroin (22). This report of bioengineering of silkworms used a cytotoxin that resulted in the suppression of PSG function to produce both FibH and FibL, as well as production of cocoons composed solely of sericin.
Intact, soluble sericin was successfully extracted from the w1-pndP1A269/P1A269 cocoons to prepare hydrogels (21) (SI Appendix, Fig. S12). These gels can also be fabricated into creams, sheets, sponges, or other 3D shapes for numerous biomedical applications (15). Moreover, our observations of EB5 cell proliferation on sericin hydrogels demonstrated that they can act as a scaffold to support cell growth and can mimic extracellular matrices to provide cell growth factors (Fig. 3). In this study, we used a sericin hydrogel that incorporated LIF-encapsulating polyhedra in which LIF activity could be effectively protected against protein denaturation by the presence of ethanol used for sericin gelation. Our results suggest that the LIF could be released from the polyhedron microcrystals, diffuse through the hydrogel in an active state, and be released into the medium, continually stimulating the proliferation of undifferentiated EB5 cells. Thus, other polyhedron-encapsulated cytokines could also potentially be used in sericin hydrogels.
Absorbable collagen sponges or atelocollagen sponges, both of which incorporated polyhedron-encapsulated bone morphogenetic protein-2, have been shown to be effective for the complete and rapid healing of critically sized bone defects in mammals, highlighting the effectiveness of combining polyhedron-encapsulated cytokines with biocompatible 3D substitutes to fabricate extracellular matrices that can control in vivo cell activities (26, 28, 29). However, scaffolds containing mammal-derived components, such as collagen or coagulation factor XIII, may present risks when used in human bodies because they could be contaminated by prions or viruses. We previously generated a transgenic silkworm that produced polyhedron-encapsulated basic fibroblast growth factor (FGF-2) inside the PSGs, allowing FGF-2 to remain in an active state in the larvae (14). These PSGs could be fabricated into fibrous materials that stimulate NIH 3T3 cell proliferation, again suggesting that polyhedron-encapsulated cytokines produced in silkworm silk glands can be useful for producing pathogen-free artificial extracellular matrices (14). In the future, further breeding could be performed to generate a transgenic silkworm with a middle silk gland that produces sericin incorporating polyhedron-encapsulated cytokines, from which pathogen-free artificial extracellular matrices could be fabricated for use in both ex vivo and in vivo applications. These silkworms could produce the functional proteins in their silk glands that can serve as effective, inexpensive substitutes for collagen or other candidate drug delivery biomaterials. Moreover, the production of these transgenic silkworms can be easily and economically scaled, particularly given the strong industrial foundation of the silk industry in Japan. Numerous studies have evaluated the use of manufactured silk proteins as implantable materials that mimic extracellular matrices, because proteinaceous silk materials can be physically and chemically manipulated, are biocompatible and biodegradable, and are already used as silk fibers for surgical sutures (16, 35). Our findings demonstrate that bioengineering by the targeted P1A expression successfully produced silkworms with a biologically useful trait that has significant application potential.
Materials and Methods
Expression of Truncated P1A in the PSGs of the Transgenic Silkworms.
In this study, we established the transgenic silkworm line w1-pndP1A269/P1A269, in which the PSGs express the designated P1A269 protein, which consists of amino acids 2–269 of P1A plus the N- and C-terminal tags (SI Appendix, SI Materials and Methods). P1A269 was detected in the PSGs by immunoblotting. Briefly, aliquots of PSG samples were dissolved in SDS/PAGE sample buffer (Nacalai Tesque) and boiled for 5 min at 95 °C. Each protein sample (16 μg) was then electrophoresed in a gel, blotted onto a Hybond-P membrane (GE Healthcare), and incubated overnight with HRP-conjugated anti-FLAG antibody for detection of the C-terminal tag (1:3,000; Sigma–Aldrich) in PBS(−) containing 5% Blocking One (Nacalai Tesque). The membranes were washed, and antibody-specific bands were developed using ECL Western Blotting Detection reagents (GE Healthcare). Protein samples from isolated P1A269-encapsulating polyhedra (15 × 104 cubes; SI Appendix, SI Materials and Methods) and Sf21 and BM-N cells expressing P1A269 at 48 h posttransfection were also analyzed as positive controls.
Analysis of Fibroin and Sericin Protein Contents of the Cocoons.
The protein contents of the cocoons were similarly analyzed by SDS/PAGE, as previously described (36). Six randomly chosen cocoon shells (10 mg) from each of the w1-pnd+/+, w1-pndP1A269/P1A269, and KWP1A269/P1A269 silkworms (SI Appendix, SI Materials and Methods) were dissolved in 200 μL of 8 M LiBr, diluted 60-fold in 8 M urea solution, mixed with a half-volume of 6 × SDS/PAGE sample buffer, and incubated for 30 min at room temperature. The protein concentration in each sample was then measured using a Protein Quantification Assay Kit (Takara Bio). Protein samples (4 μg) were electrophoresed on an e-PAGEL 5–20% gradient gel (ATTO) and detected by Coomassie Brilliant Blue staining and by immunoblotting using an HRP-conjugated FibL-specific antibody (13).
Quantitative RT-PCR Analysis of the PSG mRNA.
The values of expression levels for FibH and FibL mRNAs in PSGs were analyzed using quantitative RT-PCR. In brief, total RNAs from the PSGs of fifth-instar larvae were isolated using ISOGEN II RNA extraction reagent (Nippon Gene). Each RNA sample (1 ng) was analyzed using a SuperScript III Platinum SYBR Green One-Step RT-PCR Kit (Life Technologies) with standard reagents. The primer sequences used in this analysis are listed in SI Appendix, Table S2. Amplification of the DNA was determined using a LightCycler Nano System (Roche Diagnostics). The values of expression levels for FibH and FibL mRNAs were normalized to the value of the expression level of 18S rRNA.
Preparation of Sericin Hydrogels and ES Cell Culture.
Sericin hydrogels were prepared using a modified version of a previously reported method (21). Briefly, fresh cocoon shells (600 mg) were dissolved in 6 M LiBr aqueous solution (24 mL) overnight at 35 °C. The mixture was then adjusted to pH 8 with 1 M Tris⋅HCl buffer (pH 9.0, 6 mL) and was thoroughly dialyzed in sterilized pure water to yield a 1% (wt/vol) sericin solution. The sericin solution was mixed with 10% ethanol, resulting in a hydrogel forming in the well of a 24-well culture plate (AGC Techno Glass) after 1 h at 4 °C.
To prepare hydrogels incorporating polyhedra, LIF-encapsulating polyhedra or empty polyhedra (2.5 × 104 cubes per well; SI Appendix, SI Materials and Methods) were mixed with the sericin solution before the addition of ethanol. To saturate the hydrogel with medium and remove ethanol before initiating cultivation, it was overlaid with a sufficient volume of Glasgow minimum essential medium (Invitrogen) containing 20 μg/mL blasticidin (Invitrogen), as previously described (24, 30). Undifferentiated mouse ES cells (EB5) were derived from E14tg2a (American Type Culture Collection) by inserting the blasticidin S deaminase gene under the control of an Oct-3/4 promoter and were maintained in a medium containing blasticidin, as previously described (24, 30). The EB5 cells (0.5 × 104 cells per well) were seeded on (i) a hydrogel that lacked polyhedra, (ii) a hydrogel incorporating empty polyhedra, or (iii) a hydrogel incorporating LIF-encapsulating polyhedra in the medium without rhLIF (Merck–Millipore). EB5 cells were also seeded on a hydrogel lacking polyhedra in the medium containing 10 ng/mL rhLIF. They were then incubated at 37 °C for 7 d.
After the cells were fixed in 4% paraformaldehyde, cellular ALP activity was detected with a Vector Blue Alkaline Phosphatase Subtraction Kit (Vector Laboratories). After cultivation, cells were also dissolved in Cell Lysis Buffer (800 μL per well; MBL) and chilled on ice for 10 min. The cell suspensions were then centrifuged at 7,700 × g for 10 min. The supernatants (20 μL) were mixed with the substrate (100 μL) for an ALP activity assay using the LaboAssay ALP method (Wako Pure Chemicals) on a microtiter plate. After incubation for 15 min at 37 °C, the reaction was terminated by adding 80 μL of 0.2 M NaOH and measuring the absorbance of the mixture at 405 nm using a Bio-Rad Model 680 Microplate Reader (Bio-Rad Laboratories).
Statistical Analysis.
Means ± SD were calculated for all normalized cocoon shell and pupa weights, luciferase, caspase-3, and ALP activities, and values of expression levels were calculated for FibH, FibL, P1A269, and actin A3 mRNAs. The significance of differences between groups was tested using a one-way analysis of variance followed by Tukey’s test.
Supplementary Material
Acknowledgments
We thank Dr. Katsura Kojima (Institute of Agrobiological Sciences, National Agriculture and Food Research Organization) for kindly providing the antibody specific for FibL. This work was supported by Grants-in-Aid for Scientific Research (Grant 15K07794 to H.M. and Grant 26450467 to E.K.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was partly supported by a Grant-in-Aid from the Japan Society for the Promotion of Science Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (Grant S2802) and by the Core-to-Core Program, B Asia-Africa Science Platforms.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the DNA Data Bank of Japan (DDBJ) database (accession no. LC200434).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703449114/-/DCSupplemental.
References
- 1.Koyama K, et al. Presence in Pieris rapae of cytotoxic activity against human carcinoma cells. Jpn J Cancer Res. 1996;87:1259–1262. doi: 10.1111/j.1349-7006.1996.tb03141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe M, et al. Molecular cloning of an apoptosis-inducing protein, pierisin, from cabbage butterfly: Possible involvement of ADP-ribosylation in its activity. Proc Natl Acad Sci USA. 1999;96:10608–10613. doi: 10.1073/pnas.96.19.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takamura-Enya T, et al. Mono(ADP-ribosyl)ation of 2′-deoxyguanosine residue in DNA by an apoptosis-inducing protein, pierisin-1, from cabbage butterfly. Proc Natl Acad Sci USA. 2001;98:12414–12419. doi: 10.1073/pnas.221444598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanazawa T, et al. Distinct roles for the N- and C-terminal regions in the cytotoxicity of pierisin-1, a putative ADP-ribosylating toxin from cabbage butterfly, against mammalian cells. Proc Natl Acad Sci USA. 2001;98:2226–2231. doi: 10.1073/pnas.051628898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell AE, Bennett D. Targeting protein function: The expanding toolkit for conditional disruption. Biochem J. 2016;473:2573–2589. doi: 10.1042/BCJ20160240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lander ES. The heroes of CRISPR. Cell. 2016;164:18–28. doi: 10.1016/j.cell.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Orth JH, et al. Cell-free synthesis and characterization of a novel cytotoxic pierisin-like protein from the cabbage butterfly Pieris rapae. Toxicon. 2011;57:199–207. doi: 10.1016/j.toxicon.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Yamao M, et al. Gene targeting in the silkworm by use of a baculovirus. Genes Dev. 1999;13:511–516. doi: 10.1101/gad.13.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura T, et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, et al. New and highly efficient method for silkworm transgenesis using Autographa californica nucleopolyhedrovirus and piggyBac transposable elements. Biotechnol Bioeng. 2004;88:849–853. doi: 10.1002/bit.20296. [DOI] [PubMed] [Google Scholar]
- 11.Tamura T, Kuwabara N, Uchino K, Kobayashi I, Kanda T. An improved DNA injection method for silkworm eggs drastically increases the efficiency of producing transgenic silkworms. J Insect Biotechnol Sericology. 2007;76:155–159. [Google Scholar]
- 12.Sezutsu H, et al. Conservation of fibroin gene promoter function between the domesticated silkworm Bombyx mori and the wild silkmoth Antheraea yamamai. J Insect Biotechnol Sericology. 2009;78:1–10. [Google Scholar]
- 13.Sato M, et al. Production of scFv-conjugated affinity silk film and its application to a novel enzyme-linked immunosorbent assay. Sci Rep. 2014;4:4080. doi: 10.1038/srep04080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotani E, et al. Cell proliferation by silk gut incorporating FGF-2 protein microcrystals. Sci Rep. 2015;5:11051. doi: 10.1038/srep11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunz RI, Brancalhão RM, Ribeiro LF, Natali MR. Silkworm sericin: Properties and biomedical application. BioMed Res Int. 2016;2016:8175701. doi: 10.1155/2016/8175701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omenetto FG, Kaplan DL. New opportunities for an ancient material. Science. 2010;329:528–531. doi: 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumitani M, et al. Establishment of a specific cell death induction system in Bombyx mori by a transgene with the conserved apoptotic regulator, mouse Bcl-2-associated X protein (mouse Bax) Insect Mol Biol. 2015;24:671–680. doi: 10.1111/imb.12192. [DOI] [PubMed] [Google Scholar]
- 18.Kotani E, Muto S, Ijiri H, Mori H. Bombyx mori nucleopolyhedrovirus nucleic acid binding proteins BRO-B and BRO-E associate with host T-cell intracellular antigen 1 homologue BmTRN-1 to influence protein synthesis during infection. J Gen Virol. 2015;96:1947–1956. doi: 10.1099/vir.0.000136. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda T, Kirimura J, Matsuda M, Suzuki T. Biochemical studies on the formation of the silkprotein. I. The kinds of free amino acids concerned in the biosynthesis of the silkprotein. J Biochem. 1955;42:341–346. [Google Scholar]
- 20.Takasu Y, Hata T, Uchino K, Zhang Q. Identification of Ser2 proteins as major sericin components in the non-cocoon silk of Bombyx mori. Insect Biochem Mol Biol. 2010;40:339–344. doi: 10.1016/j.ibmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto H, Nakajima K, Takabayashi C. Preparation of elastic silk sericin hydrogel. Biosci Biotechnol Biochem. 2005;69:845–847. doi: 10.1271/bbb.69.845. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe T. Studies on the sericin cocoon: (I) Chemical properties of the domestic silkworm spinning sericin cocoon. J Seric Sci Jpn. 1959;28:251–256. [Google Scholar]
- 23.Teng X, Zhang Z, He G, Yang L, Li F. Validation of reference genes for quantitative expression analysis by real-time rt-PCR in four lepidopteran insects. J Insect Sci. 2012;12:60. doi: 10.1673/031.012.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishishita N, et al. The use of leukemia inhibitory factor immobilized on virus-derived polyhedra to support the proliferation of mouse embryonic and induced pluripotent stem cells. Biomaterials. 2011;32:3555–3563. doi: 10.1016/j.biomaterials.2010.12.063. [DOI] [PubMed] [Google Scholar]
- 25.Ijiri H, et al. Structure-based targeting of bioactive proteins into cypovirus polyhedra and application to immobilized cytokines for mammalian cell culture. Biomaterials. 2009;30:4297–4308. doi: 10.1016/j.biomaterials.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto G, et al. Bone regeneration by polyhedral microcrystals from silkworm virus. Sci Rep. 2012;2:935. doi: 10.1038/srep00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto G, et al. Control of angiogenesis by VEGF and endostatin-encapsulated protein microcrystals and inhibition of tumor angiogenesis. Biomaterials. 2014;35:1326–1333. doi: 10.1016/j.biomaterials.2013.10.051. [DOI] [PubMed] [Google Scholar]
- 28.Shimabukuro J, et al. 3D co-cultures of keratinocytes and melanocytes and cytoprotective effects on keratinocytes against reactive oxygen species by insect virus-derived protein microcrystals. Mater Sci Eng C. 2014;42:64–69. doi: 10.1016/j.msec.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto G, et al. Polyhedral microcrystals encapsulating bone morphogenetic protein 2 improve healing in the alveolar ridge. J Biomater Appl. 2015;30:193–200. doi: 10.1177/0885328215575763. [DOI] [PubMed] [Google Scholar]
- 30.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 31.Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 32.Sridhara S, Portier M-M, Daillie J. RNA polysomes and RNA synthesis in the silk glands of the silkworm Bombyx mori. Eur J Biochem. 1977;72:331–339. doi: 10.1111/j.1432-1033.1977.tb11257.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang SH, et al. Quantitative proteomic and transcriptomic analyses of molecular mechanisms associated with low silk production in silkworm Bombyx mori. J Proteome Res. 2014;13:735–751. doi: 10.1021/pr4008333. [DOI] [PubMed] [Google Scholar]
- 34.Ma S, et al. Genome editing of BmFib-H gene provides an empty Bombyx mori silk gland for a highly efficient bioreactor. Sci Rep. 2014;4:6867. doi: 10.1038/srep06867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials. 2015;71:145–157. doi: 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teramoto H, Kojima K. Production of Bombyx mori silk fibroin incorporated with unnatural amino acids. Biomacromolecules. 2014;15:2682–2690. doi: 10.1021/bm5005349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.