Significance
Annually, more than 250,000 Americans suffer from a peripheral nerve injury, which results in a loss of function and a compromised quality of life. The current clinical gold standard to bridge long, nonhealing nerve gaps, the autograft, has several drawbacks. Therefore, there is a clear and urgent unmet clinical need for an alternative approach that can match or exceed autograft performance. Here we investigated the regenerative effect of fractalkine, a chemokine that preferentially recruits reparative monocytes in the synthetic nerve conduit. Our method of bridging gaps enhanced axonal regeneration and muscle reinnervation and showed results comparable to those observed in autografts.
Keywords: immunomodulation, nerve repair, monocyte, macrophage, fractalkine
Abstract
Injuries to the peripheral nervous system are major sources of disability and often result in painful neuropathies or the impairment of muscle movement and/or normal sensations. For gaps smaller than 10 mm in rodents, nearly normal functional recovery can be achieved; for longer gaps, however, there are challenges that have remained insurmountable. The current clinical gold standard used to bridge long, nonhealing nerve gaps, the autologous nerve graft (autograft), has several drawbacks. Despite best efforts, engineering an alternative “nerve bridge” for peripheral nerve repair remains elusive; hence, there is a compelling need to design new approaches that match or exceed the performance of autografts across critically sized nerve gaps. Here an immunomodulatory approach to stimulating nerve repair in a nerve-guidance scaffold was used to explore the regenerative effect of reparative monocyte recruitment. Early modulation of the immune environment at the injury site via fractalkine delivery resulted in a dramatic increase in regeneration as evident from histological and electrophysiological analyses. This study suggests that biasing the infiltrating inflammatory/immune cellular milieu after injury toward a proregenerative population creates a permissive environment for repair. This approach is a shift from the current modes of clinical and laboratory methods for nerve repair, which potentially opens an alternative paradigm to stimulate endogenous peripheral nerve repair.
Peripheral nervous system (PNS) injuries lead to long-term disability and decreased function in ∼2.8% of all trauma patients (1), and are often followed by neuropathic pain that significantly affects the quality of life for individuals suffering from these injuries. Many of the current surgical techniques for repairing these nerve injuries were developed during World Wars I and II in the first half of the 20th century. With the advent of microsurgical techniques, some progress has been achieved in the field of nerve repair (2), however bridging of long peripheral nerve gaps remains a continuing clinical challenge (3).
After nerve trauma, the standard clinical operating procedure is to oppose the two nerve ends and, when possible, suture them together. If the gap is large enough that tensionless apposition is not possible, an autologous nerve graft (autograft) (3, 4), typically the patient’s own sural nerve, or, more recently, a cadaver-derived graft (allograft) is used to bridge the nerve gap (5, 6). Autografts are biocompatible, nontoxic, and supportive of the structures that promote axonal adhesion/extension. Although they are currently the best clinical bridges available, they suffer from some major drawbacks such as the need for a secondary surgery, limitations in the availability of disposable nerve segments, and the possibility of neuroma formation (3). Allograft use affords improved presurgical sourcing and preparation but also entails a higher chance of immunogenicity and rejection. Moreover, both autograft and cadaver-derived allografts present challenges such as the presence of inhibitory chondroitin sulfate proteoglycans, which reduce their performance as bridges and therefore require extra graft tissue processing (7, 8). Also, multiple lengths of nerve graft are often needed to bridge the gap between the injured nerve stumps. Ultimately, even with successful autografting, only 40% of patients regain useful function (9). Therefore, there is a clear, urgent, and unmet clinical need for an alternative approach that can match or exceed autograft performance.
Given the myriad advances in the fields of regenerative medicine and materials engineering, many autograft alternatives have been explored (10–12). In both clinical and research settings, use of synthetic and natural guidance channels to bridge nerve gaps has been shown to improve nerve regeneration in small gaps (less than 10 mm in rat and 30 mm in human), but these constructs fail when the gaps are longer (11). Some tissue engineering strategies have shown promise, including the design of novel nerve conduits (3, 13), the addition of fillers within nerve conduits (14, 15), transplantation of cells (16, 17), local delivery of neurotrophic factors (18, 19), and application of topographical cues (20–22); however, none of these techniques has been able to match the autograft’s performance for long nerve gap repair (11, 12). Therefore, despite concerted effort over the last several decades, no suitable replacement for autografts has been found (3).
After PNS injuries, neurons respond rapidly by changing their activities and promoting a regenerative phenotype. At the distal nerve stump, Schwann cells (SCs) adopt a reparative phenotype. SCs, as well as infiltrating and resident macrophages, remove inhibitory debris, enabling new axons to sprout into the degenerated nerve directed by bands of Büngner (11). Although monocytes and their descendants (in particular, macrophages) have long been known to play an essential role in the degenerative process, only recently has their importance in positively influencing regeneration been recognized (23–26). Monocytes are abundant during nerve degeneration and regeneration and modulate the sequence of cellular events which can determine the outcome of the healing process (24).
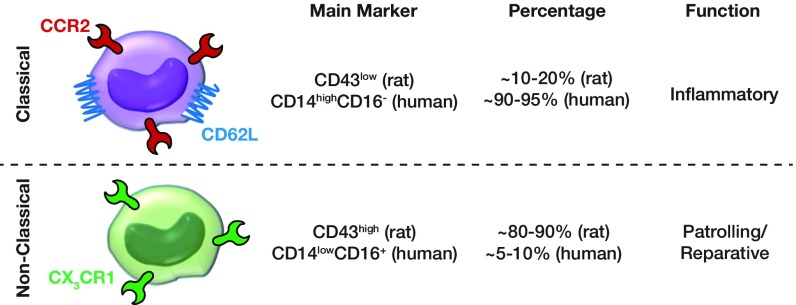
After inflammatory insult, macrophages that accumulate at the site of injury appear to be derived largely from circulating monocytes (27, 28); however, the precise kinetics and mechanisms of recruitment of blood monocyte populations to injured nerve tissues during normal repair remain to be elucidated (29, 30). The ability of monocytes to move to the site where they are needed is central to their function both in promoting immune defense and in tissue regeneration (29, 30). Entry of monocytes into the distal site of an injured nerve is enabled through up-regulation and release of a major monocyte chemokine, monocyte chemoattractant protein (CCL2) by SCs, which reaches its maximum 1 d after injury (31, 32). Besides CCL2, the CX3CR1 ligand (fractalkine) can also recruit monocytes through the CX3CR1 receptor (Fig. 1) (33, 34).
Fig. 1.
Monocyte subtypes, receptors, markers, population percentage, and function.
In rats, two major subsets of monocytes have been identified based on the expression of their chemokine receptors and CD43 levels (Fig. 1) (34–36): a CD43lowCCR2+CX3CR1low inflammatory subtype of monocyte recruited to inflamed tissues and a CD43highCCR2−CX3CR1high antiinflammatory subtype (patrolling and reparative) recruited to healing tissues (36, 37). Inflammatory monocytes phagocytose debris and clear damaged cells, whereas antiinflammatory monocytes promote tissue regeneration (37). Even though the antiinflammatory monocytes are the larger population in rat blood under homeostatic conditions (80–90%), they constitute the smaller population in human blood (5–10%) (Fig. 1) (34).
These two subtypes of monocytes can be recruited to injured tissues, where they can subsequently differentiate into classically activated (M1) or alternatively activated (M2) macrophages (37). These two phenotypes of macrophages represent a simplistic discrete depiction of a continuous spectrum between two activation states (38). Generally, M1 macrophages produce proinflammatory cytokines as well as high levels of oxidative metabolites, and M2 macrophages make the environment supportive for tissue repair by producing antiinflammatory cytokines that facilitate matrix remodeling and angiogenesis (39, 40).
Although recruited monocytes differentiate to macrophages, recent studies have shown that monocytes also may have their own independent activities that are still to be elucidated (41, 42). For example, recent studies suggest that monocytes can promote arteriogenesis and angiogenesis (41), and thus they can act as short-lived effector cells within tissues (42). The mechanisms by which the antiinflammatory subset of monocytes/macrophages is either selectively recruited to or converted in situ at the site of peripheral nerve injury have not been investigated systematically (43).
The plasticity of monocytes/macrophages makes them an attractive target for modulation in the context of immunoengineering nerve repair. A prior short-term (3-wk) study demonstrated that direct modulation of macrophages toward a prohealing phenotype, using interleukin 4 (IL-4), results in an increase in SC recruitment and axonal growth (23). The premise herein is that preferential recruitment of antiinflammatory reparative monocytes to the site of injury will more effectively bias the immune microenvironment toward a prohealing response and in turn set off a regenerative biochemical cascade involving SCs and neuronal processes that leads to improved repair (Fig. 2). Since CX3CR1 receptor is mainly expressed on antiinflammatory reparative monocytes (Fig. 1) (30, 34), exogenous fractalkine, the ligand for CX3CR1, can be used to preferentially recruit these monocytes to the site of nerve injury and thus increase the subsequent ratio of prohealing to proinflammatory macrophages during the regeneration process.
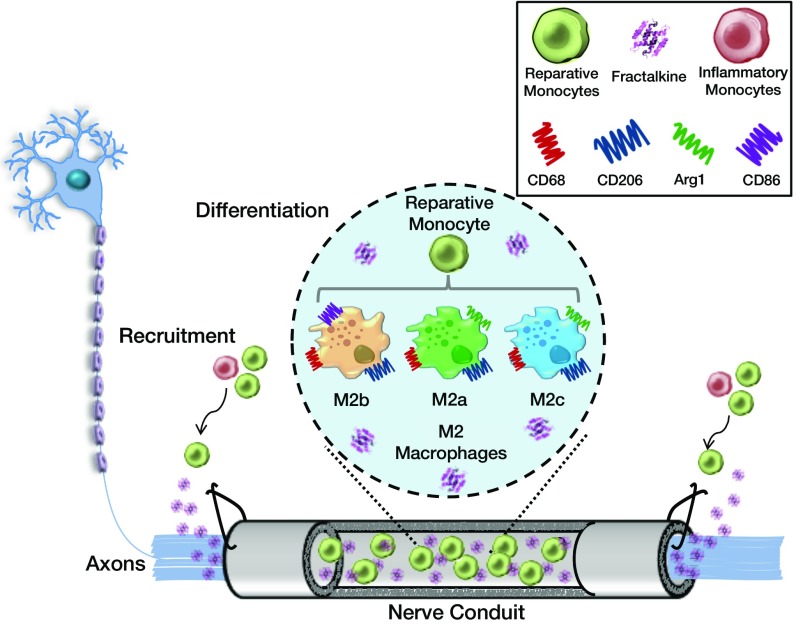
Fig. 2.
Schematic for immunoengineering nerve repair: recruitment and differentiation. This figure illustrates the response of circulating monocytes to the delivery of fractalkine at the site of nerve injury. A scaffold tube is sutured to the injured ends of the axotomized nerve and begins elution of fractalkine. Fractalkine recruits circulating monocytes, specifically reparative ones, to the site of injury, where further biochemical cues will potentially instruct the monocytes to differentiate into one of three potential prohealing macrophage phenotypes that enhance nerve repair: CD68+CD206+CD86+ (M2b) or CD68+Arg1+/CD206+(M2a or M2c).
It has been shown that a marked increase in fractalkine mRNA was observed following facial nerve axotomy (44). In addition, the expression of the fractalkine receptor CX3CR1 in macrophages has been shown to increase not only in the sciatic nerve proximal to the site of injury but also in the dorsal root ganglion after the sciatic nerve section (axotomy) (45). Fractalkine administration after ischemic stroke also has been shown to increase neuronal survival and to contribute to angiogenesis through promotion of endothelial cell proliferation, thus leading to better functional recovery (46). Fractalkine does not bind to any known mammalian chemokine receptors except CX3CR1 and in fact is the only identified ligand to bind and activate the CX3CR1 receptor (47). Thus, the typical promiscuity that characterizes the chemokine superfamily is not present for fractalkine. This specificity suggests that the roles played by fractalkine and CX3CR1 are nonredundant and are critical for nerve regeneration (45).
This study investigated if local delivery of fractalkine within the lumen of a nerve conduit early after peripheral nerve injury led to enhanced nerve repair relative to IL-4–induced nerve repair (23) and traditional autografts.
Results
Fractalkine Increases SC and Endothelial Cell Populations in Scaffolds.
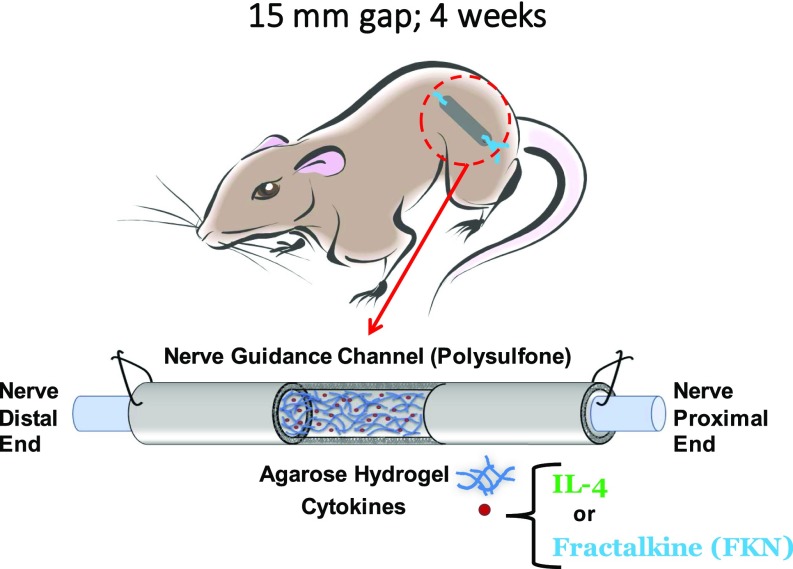
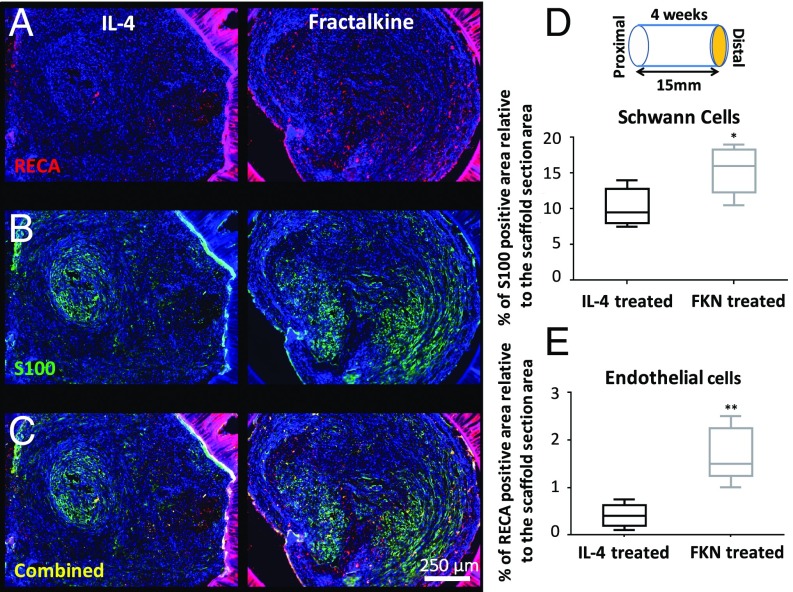
A 15-mm gap model was constructed by grafting a transected nerve 1 mm into the ends of a 17-mm porous conduit filled with 1% (wt/vol) agarose hydrogel which was mixed with 10 µg/mL of either IL-4 or fractalkine (Fig. S1). We chose 1% agarose because of its structural properties that support axonal elongation (48). The IL-4 agarose scaffold was used as a benchmark because it has been shown previously to approach the regeneration potential of autografts (23, 26). The effect of short-term fractalkine delivery (<1 d release) (Fig. S2) on SCs (Fig. 3B) and endothelial cells (Fig. 3A) was investigated 4 wk after the conduit implantation. The 4-wk time point was the earliest among our previous studies that resulted in regenerated axons reaching the distal end of the 15-mm gap (23, 49). SCs and endothelial cells were studied to assess the effect of the therapeutics on changing the conduit environment’s permissiveness for growth. As shown in Fig. 3, fractalkine delivery significantly increased both SC infiltration (determined by S100+ staining) (Fig. 3D) and endothelial cells’ migration [determined by rat endothelial cell antibody (RECA+) staining] (Fig. 3E) relative to the benchmark IL-4 control at this early time point (Fig. S3 for higher-magnification images). It should be emphasized that the RECA staining used here is reactive only to vascular endothelium and does not react with other cells such as leukocytes, fibroblasts, and nonendothelial stromal cells.
Fig. S1.
Schematic of the rat 4-wk study.
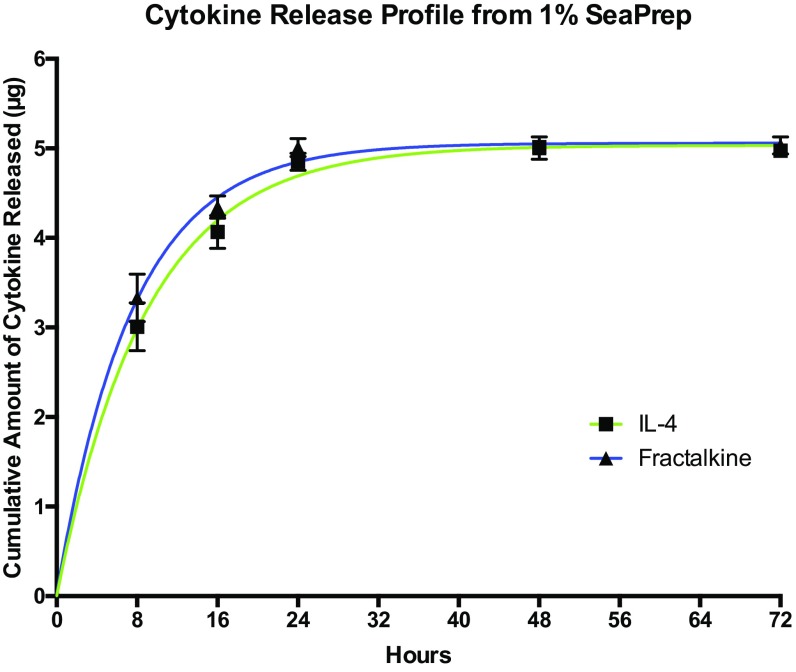
Fig. S2.
Cytokine release profile from 1% SeaPrep.
Fig. 3.
The effect of early fractalkine release on SCs and endothelial cells. (A) Combined RECA (red), and DAPI (blue) staining. (B) Combined S100 (green) and DAPI (blue) staining. (C) Combined S100 (green), RECA (red), and DAPI (blue) staining. (D) Quantitative analysis of S100+ staining of SCs at the distal end of the conduit (P = 0.0243, two-tailed t test). (E) Quantitative analysis of RECA+ staining of endothelial cells at the distal end of the conduit (P = 0.0016, two-tailed t test) (n = 5). The fractalkine-treated scaffold significantly enhanced both SC infiltration and endothelial cell presence inside the nerve conduit in comparison with the IL-4–treated scaffold 4 wk after implantation. FKN, fractalkine.
Fig. S3.
Magnified fluorescent images of (A) RECA-S100, (B) CD68-arginase, and (C) CD86-CD206.
Fractalkine Improves the Ratio of Prohealing to Proinflammatory Macrophages.
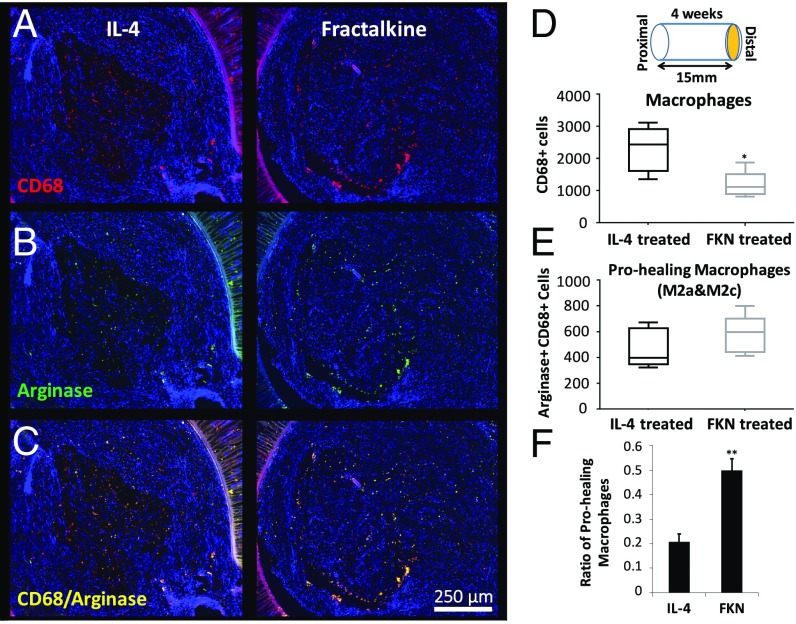
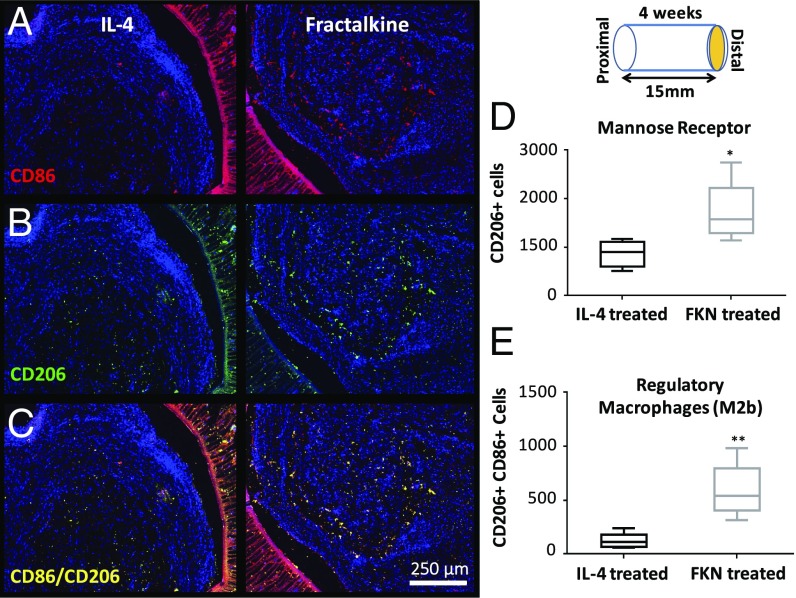
There is an important correspondence between the number of regenerated axons and the profile of immune cells within the nerve conduit (23). As an indicator of the local immune state, the number and phenotype of macrophages were evaluated 4 wk after nerve conduit implantation using established markers (Figs. 4 and 5; see Fig. S3 for higher-magnification images) (21, 50–52). The number of macrophages at the distal end of the nerve conduit was significantly lower in the fractalkine scaffold than in the IL-4 scaffold (Fig. 4D). However, the number of prohealing macrophages (M2 macrophage subtypes M2a and M2c) as determined by CD68/Arginase costaining was not significantly different in these two conditions (Fig. 4E); therefore, the ratio of prohealing macrophages to the total number of macrophages (Fig. 4F) was significantly higher in the fractalkine scaffold than in the IL-4 scaffold. Also, the number and intensity of mannose receptors (CD206), an antiinflammatory/wound-healing marker (Fig. 5B), were significantly higher in the fractalkine scaffold than in the IL-4 control (Fig. 5D). Furthermore, the number of CD206+CD86+ cells (an indication of regulatory M2b macrophages) (Fig. 5C) was significantly higher in the fractalkine scaffold than in the control IL-4 scaffold (Fig. 5E). M2a and M2c macrophages appear during the early stage of healing process, whereas M2b macrophages, which have both pro- and antiinflammatory functions, can be induced toward the end of regeneration process (53).
Fig. 4.
The effect of early fractalkine release on the number and phenotype of macrophages. (A) Combined CD68 (red), and DAPI (blue) staining. (B) Combined arginase (green) and DAPI (blue) staining. (C) Combined arginase (green), CD68 (red), and DAPI (blue) staining. (D) Quantitative analysis of total macrophage numbers at the distal end by using the CD68 marker (P = 0.015, two-tailed t test). (E) Quantitative analysis of prohealing macrophages at the distal end by double staining with CD68 and arginase (P = 0.288, two-tailed t test). (F) The ratio of prohealing macrophages to the total number of macrophages (P = 0.0019) (n = 5). Four weeks after implantation the fractalkine-treated scaffold has significantly fewer macrophages but a higher ratio of prohealing macrophage than the IL-4–treated scaffold. This figure is reproduced in part from ref. 66. FKN, fractalkine.
Fig. 5.
The effect of early fractalkine release on mannose receptor expression and regulatory macrophages. (A) Combined CD86 (red) and DAPI (blue) staining. (B) Combined CD206 (green) and DAPI (blue) staining. (C) Combined CD86 (red), CD206 (green), and DAPI (blue) staining. (D) Quantitative analysis of mannose receptor-expressing cells at the distal end assessed by the marker CD206 (P = 0.022, two-tailed t test). (E) Quantitative analysis of regulatory macrophages (M2b) at the distal end by double staining with CD86 and mannose receptor (P = 0.003, two-tailed t test) (n = 5). Four weeks after implantation, the fractalkine-treated scaffold contains a higher number of mannose receptor-expressing cells and regulatory macrophages than the IL-4–treated scaffold. FKN, fractalkine.
Although Figs. 4B and 5B demonstrate the same trend of antiinflammatory markers, it is important to note that there is a subtle difference between the graphs in Figs. 4E and 5D; Fig. 4E represents the number of subset-specific macrophages (M2a and M2c) using costaining of arginase/CD68 surface markers, while Fig. 5D portrays the number of all cells, not just macrophages, that have stained positive for the CD206 surface marker.
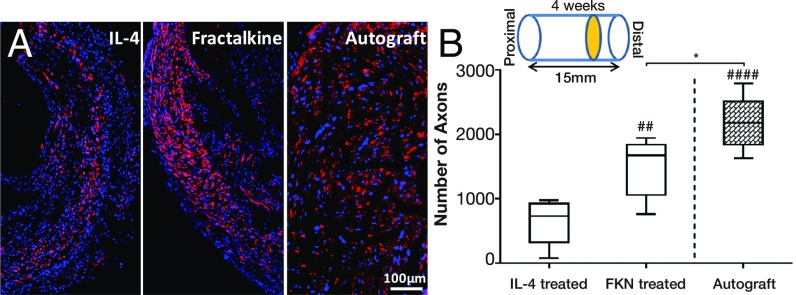
Fractalkine Dramatically Increases the Number of Regenerated Axons.
Axons at the distal end of the scaffold were visualized by immunostaining for neurofilament 160 (NF160) following nerve conduit explantation. Four weeks after implantation of the nerve conduit, the quantity of NF160 immunoreactive axons at the distal ends of the fractalkine scaffolds was significantly greater than in the IL-4–treated cohort (Fig. 6A and Fig. S4), i.e., nearly twice that found in the IL-4 scaffolds and very close to that observed in autografts (Fig. 6B). Although 1% agarose (SeaPrep) has been shown to be the optimal concentration to support axonal elongation (48), it had a detrimental effect on the distribution and homogeneity of regenerated axons at early time points, as evidenced by the lack of regeneration in the center of the nerve cable (Fig. S4). This detrimental effect has been observed in a previous study with an even lower concentration of agarose (26) and is believed to be related to the higher stiffness at the center of the hydrogel caused by cell-mediated syneresis (54). Nevertheless, this central cavity is observed to be filled with regenerating axons at the later time points.
Fig. 6.
The effect of fractalkine release on axonal growth. (A) Immunohistochemical staining of axons (red) and DAPI (blue) at the distal end of nerve stump. (B) The number of regenerated axons at the distal end of the fractalkine- vs. the IL-4–treated scaffold in comparison with the autograft 4 wk after injury. *P < 0.05; ##P < 0.01; ####P < 0.0001 (one-way ANOVA) (n = 5). Fractalkine-treated scaffold dramatically increases the number of regenerated axons relative to IL-4, reaching very close to the number of regenerated axons in autograft.
Fig. S4.
Low-magnification fluorescent images of NF160-DAPI staining, 4-wk study.
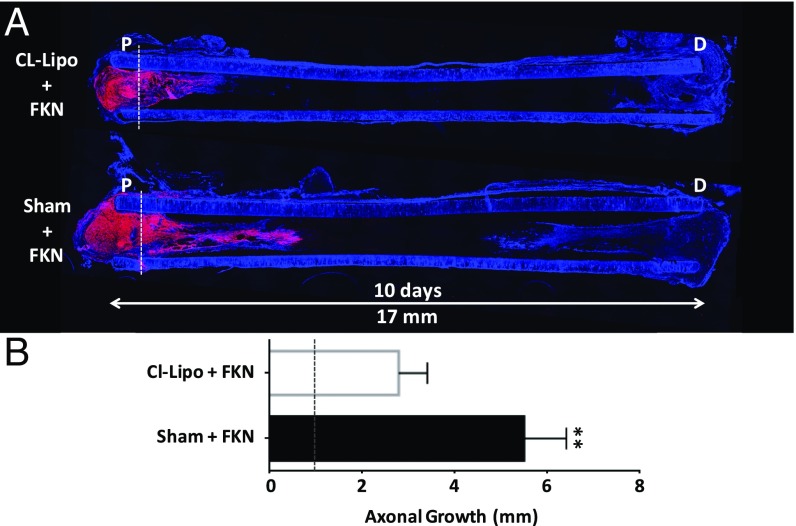
Depletion of Monocytes Diminishes the Healing Effect of Fractalkine.
To investigate whether fractalkine’s effect on nerve regeneration was mediated by its action on monocytes, clodronate liposome (CL-Lipo) treatment, a well-established methodology to deplete circulating monocytes and subsequently the number of infiltrating macrophages at the site of injury (55–57), was used. Animals were injected with CL-Lipo once, 48 h before nerve conduit implantation. Rats with fractalkine scaffolds with CL-Lipo injection were compared with rats with fractalkine scaffold with sham PBS injections. As shown in Fig. 7, depleting circulating monocytes and consequently infiltrating macrophages during the release of fractalkine from the nerve conduit (Fig. S2) (23) diminishes the axonal growth-stimulating effect of fractalkine (Fig. 7A). After 10 d, the advance of axons toward the distal end of the conduit in the macrophage/monocyte-depleted samples was limited largely to the proximal stump of the conduit, less than 2 mm from the initial placement of the transected nerve sutured within the nerve conduit (Fig. 7B). On the other hand, the average axonal growth within the fractalkine nerve conduits in sham-injected animals was considerably longer than in CL-Lipo–injected animals (4.5 mm growth considering its initial positioning at 1 mm inside the conduit) (Fig. 7B).
Fig. 7.
CL-Lipo study. (A) Axonal regeneration (stained red with NF160 antibody) is demonstrated using longitudinally sectioned scaffolds in the CL-Lipo–treated animal vs. the nontreated animal (n = 4). Both scaffolds contain fractalkine. (B) Quantification of the length of axonal growth 10 d after scaffold implantation (P = 0.003, two-tailed t test). Dotted line in both A and B indicates the position of nerve on day 0. Partial depletion of monocytes using a single injection of CL-Lipo 48 h before nerve conduit implantation significantly reduces the amount of axonal growth, indicating the central role that monocyte presence plays in the fractalkine-treated scaffold (blue = DAPI). This image is reproduced in part from ref. 66. FKN, fractalkine.
Electrophysiological Analyses Indicates Muscle Reinnervation Caused by Fractalkine.
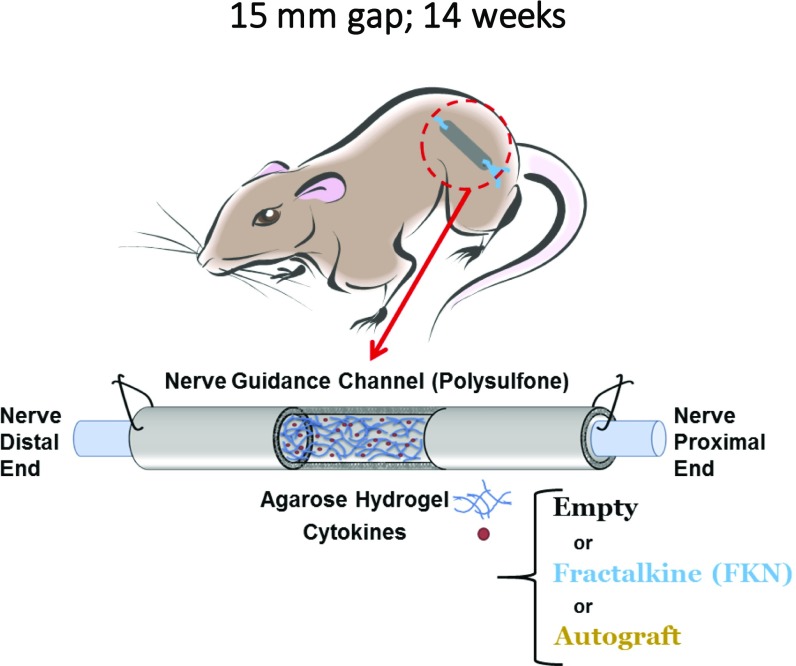
Because of the superior regeneration with fractalkine delivery relative to IL-4 delivery, a longer-term (14-wk) study was undertaken to evaluate relative functional recovery after fractalkine or autograft intervention in critically sized nerve gaps with a negative control (nerve conduit filled with 1% agarose without any immunomodulatory factor) (Fig. S5).
Fig. S5.
Schematic of the rat 14-wk study.
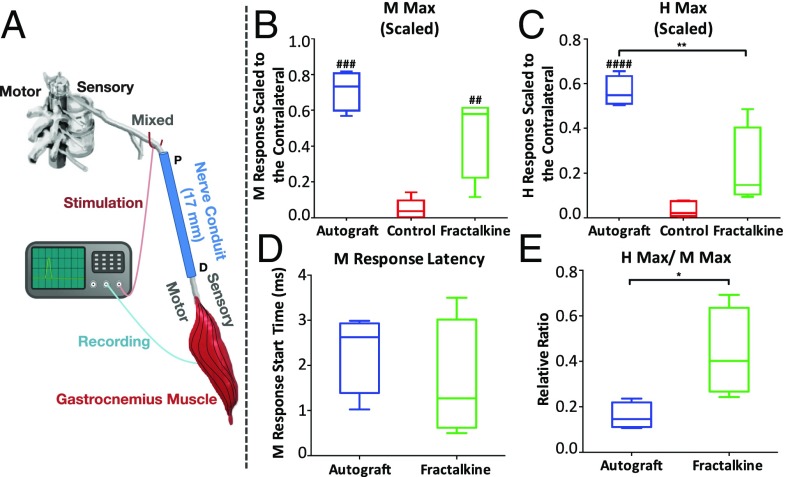
Two electromyographic (EMG) potentials were recorded in response to sciatic nerve stimulation: a short-latency direct muscle response (M-wave) produced by stimulating motor axons and a slightly longer latency reflex (H-reflex) produced by activating motoneurons synaptically via stimulation of sensory axons in the sciatic nerve. By scaling the maximum amplitude of the M-wave (M Max) in reinnervated gastrocnemius muscles to the corresponding measures from the intact contralateral gastrocnemius muscles, the relative effectiveness of axon regeneration and muscle reinnervation in the different treatment groups could be compared (Fig. 8). When scaled to the corresponding contralateral EMG signals, the M Max median was larger in autografted rats (0.73) than in those with fractalkine conduits (0.58), and both were larger than in the control group (0.04) (Fig. 8B). Although there were significant differences between these scaled M Max amplitudes in autograft and control rats (P < 0.001) and between the fractalkine and control rats (P < 0.01), there was no statistically significant difference between the autograft and fractalkine groups (Fig. 8B). In contrast, the difference in the scaled maximum H-reflex amplitudes (H Max) between the autograft and fractalkine groups is statistically significant (P < 0.01) (Fig. 8C). The medians of H Max (scaled to the contralateral side) for the autograft, fractalkine, and control groups were 0.55, 0.15, and 0.02, respectively (Fig. 8C). No statistically significant differences in latency to the start of the EMG response were found between the fractalkine and the autograft groups (Fig. 8D).
Fig. 8.
Electrophysiological analysis. (A) Schematic demonstrating proximal stimulation of sciatic nerve motor axons and recording of the EMG signal from reinnervated gastrocnemius muscle 14 wk after scaffold implantation. (B and C) M Max (B) and H Max (C) amplitudes in the autograft group vs. the control scaffold and the fractalkine-loaded scaffold groups. (D and E) M-response latency (D) and H Max/M Max ratio (E) in the autograft vs. the fractalkine-loaded scaffold groups. *P < 0.05; ##P < 0.01; ###P < 0.001; ####P < 0.0001 (one-way ANOVA or two-tailed t test) (n = 6). M Max in rats with the fractalkine-loaded scaffold is significantly larger than in control rats and very closely approaches the autograft performance. H Max in rats with the fractalkine-loaded scaffold is still significantly lower than autograft, whereas the H Max/M Max ratio in rats with the fractalkine-loaded scaffold is significantly larger than autograft.
The ratio of H Max to M Max was also studied for these groups of animals. This ratio represents a measure of the extent of recruitment of all motoneurons whose axons have successfully reinnervated the gastrocnemius muscle into the H-reflex. In self-reinnervated muscle, this ratio is only about half as large (59%) as in intact muscle (58). Because a majority of negative control conduits did not show any positive M or H response, the magnitude of the H Max/M Max ratio measured from reinnervated muscles was analyzed and reported only in the autograft and fractalkine groups (Fig. 8E). Rats in the fractalkine group had a significantly higher scaled H Max/M Max ratio than animals in the autograft group (P < 0.05) (Fig. 8E). The median H Max/M Max ratios for the autograft and fractalkine groups are 0.14 and 0.40, respectively, indicating that the efficacy of this reflex is restored more in the fractalkine group than in the autograft group.
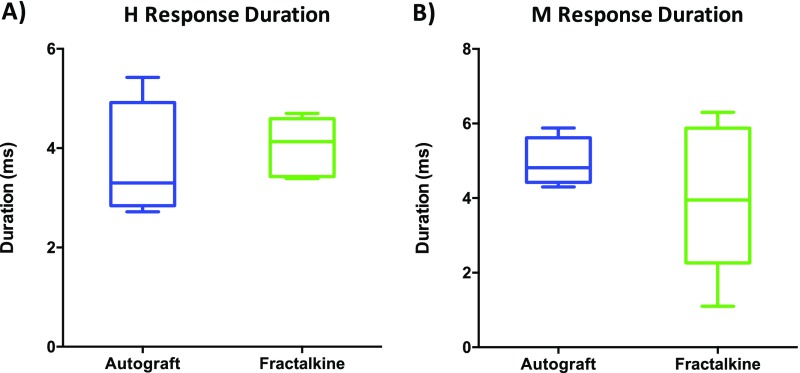
Last, the H and M response duration in autograft and fractalkine groups were compared (Fig. S6). No significant difference was observed in the latency profiles of these two groups (Fig. S6); however, the absence of significant differences among the groups may be a result of the inherent difficulty in quantifying this measure accurately (see Supporting Information for more detail).
Fig. S6.
Graphs of H latency (A) and M latency (B).
Histological Analyses at 14 wk Further Indicate Enhanced Nerve Regeneration Caused by Fractalkine.
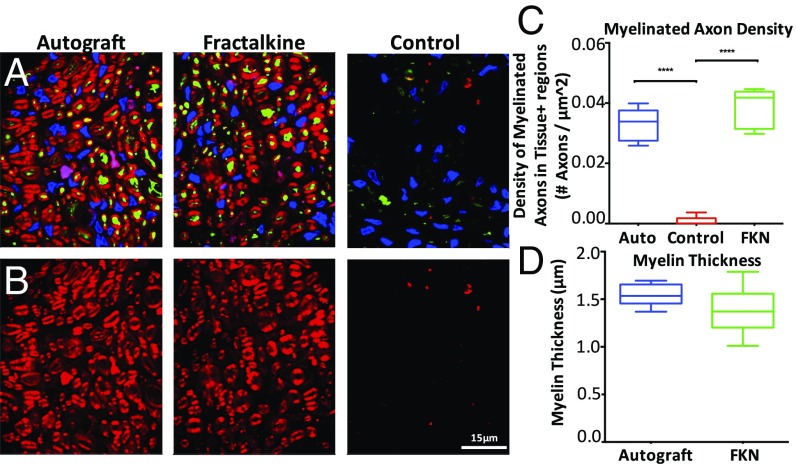
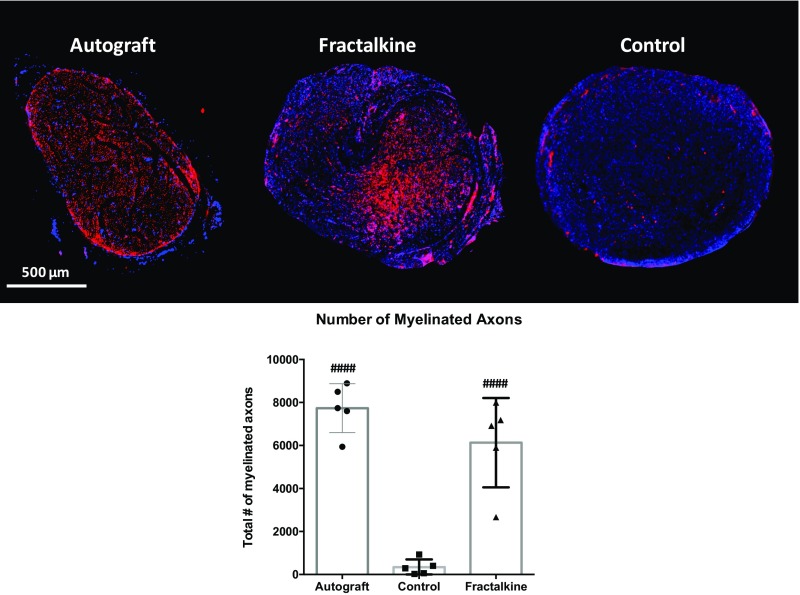
To support our functional analyses with anatomical data, additional histological analyses were performed at the 14-wk time point (Fig. 9 A and B and Fig. S7). Along with a standard axonal count, myelin thickness and myelinated axon density were measured. Despite the very different spatial appearance of the regenerating axons in the autograft and fractalkine groups, there was no significant difference in the mean numbers of regenerating axons in these two groups (Fig. S7). The numbers of myelinated axons at the distal ends of scaffolds were significantly higher in both the autograft (7,736 ± 1,136) and fractalkine (6,131 ± 2,078) groups than in the control (342 ± 363) group (P < 0.0001). However, the distribution of regenerated axons was significantly distinct in the different groups and was most homogeneous in the autograft group (Fig. S7). The myelinated axon density in the tissue-positive regions of nerve cable was also significantly larger in the autograft (3.29 ± 0.53 E-2 axons/μm2) and fractalkine (3.88 ± 0.61 E-2 axons/μm2) groups than in the control (8.66 ± 13.6 E-4 axons/μm2) group (P < 0.0001), but the differences observed between the autograft and fractalkine groups were not significant (Fig. 9C). The average thickness of myelin in the autograft group (1.547 ± 0.04 μm) was greater than that found in sections in the fractalkine group (1.385 ± 0.09 μm), but these differences were not significant (Fig. 9D).
Fig. 9.
Histological analysis of myelin and axon density at the late time point. (A) Representative pseudocolored fluorescent images showing NF160 (green), FluoroMyelin (red), and DAPI (blue) staining of scaffold cross-sections for autograft, fractalkine, and control groups. (B) Representative pseudocolored fluorescent images showing FluoroMyelin (red) staining of scaffold cross-sections at the 14-wk time point for autograft, fractalkine, and control groups, respectively. (C) Density of myelinated axons (normalized to the combined area of tissues positively stained for any of NF160, FluoroMyelin, or DAPI, not to the total image area) comparing autograft, fractalkine, and control (n = 8). The densities in the autograft and fractalkine groups are not significantly different, but both are significantly higher than in the control group (****P < 0.0001). (D) Comparison of myelin thickness surrounding regenerated axons in autograft and fractalkine (n = 8). The average thickness of myelin is greater in the autograft group than in the fractalkine group, but these differences are not statistically significant. FKN, fractalkine.
Fig. S7.
(Upper) Low-magnification fluorescent images of NF-160-DAPI staining. (Lower) Number of regenerated axons in the autograft, control, and fractalkine groups: 14-wk study. Statistical significance was observed for both autograft and fractalkine relative to control, but differences were not statistically significant between autograft and fractalkine. ####P < 0.0001.
Discussion
Autografts are the current standard for bridging long peripheral nerve gaps, but several drawbacks limit their use. Currently, as an alternative to autografts, synthetic biomaterial-based nerve conduits have been developed that are capable of bridging short gaps (11, 13, 21, 59, 60). However, these nerve conduits have not been very successful in bridging critically sized nerve gaps. Subsequently, functional recovery is rarely achieved in those gaps (11). The approach in this study was to enhance nerve regeneration across long gaps by preferentially recruiting a subset of reparative monocytes to the site of injury (Fig. 2).
In this study, we show that fractalkine, as compared with IL-4, significantly enhances the ratio of prohealing macrophages to the total number of macrophages 4 wk after scaffold implantation (Fig. 4F) even though the number of macrophages is lower in the fractalkine scaffolds than in the IL-4 scaffolds (Fig. 4D). We have previously demonstrated the direct correlation between the ratio of prohealing macrophages and the number of regenerated axons (23). The results of this study confirm that the phenotype of macrophages, and not their number, correlates with the regeneration outcome in the later stages of nerve repair. It is believed that, when the difference in the number of macrophages is not substantial, the ratio of prohealing to proinflammatory macrophages determines the permissiveness of the conduit environment for growth (23, 26). The criterion for permissiveness used was the quantity of infiltrated SCs and endothelial cells in the conduits a few weeks after implantation (Fig. 3 A–C). Based on this criterion, the fractalkine scaffold outperformed the IL-4 scaffold significantly (Fig. 3 D and E).
Given the data presented here, it is clear that scaffolds containing fractalkine result in a dramatically higher number of axons at the distal end of the nerve conduit than scaffolds containing IL-4 (Fig. 6). Further, it was confirmed that the reparative effect of fractalkine was enabled through monocyte/macrophages because systematic depletion of monocytes from the blood with CL-Lipo during fractalkine release inside the nerve conduit diminished the positive reparative effect of fractalkine (Fig. 7). Hence, this study strongly highlights the important role that monocytes and macrophages play during the nerve regeneration process enhanced by fractalkine. Moreover, because fractalkine recruits CX3CR1+ monocytes (33), which include only reparative monocytes in rats (34), the regenerative effect of fractalkine seems to occur via the contribution of this monocyte subtype.
The extent of axon regeneration and muscle reinnervation in different treatment groups was studied by analyzing different characteristics of the M-wave and H-reflex responses elicited by stimulus to the sciatic nerve and recorded in the gastrocnemius muscle. As described by Palmieri, et al. (61), the measurement of H Max and M Max provides a valuable tool for the evaluation of nerve health in clinical diagnostic use. Similarly, these values can be used to evaluate the success of healthy reinnervation. Eight weeks after injury, a chronically denervated distal stump loses its capability to support axonal growth, and after 6 mo the stump becomes completely incapable of supporting reinnervation (62). It is critical for axons to reach their target muscle during this 8-wk to 6-mo regenerative time window (62, 63). Therefore, our electrophysiological analyses of affected nerve/muscle (sciatic nerve/gastrocnemius muscle) were conducted 14 wk after the nerve transection.
The nearly complete return of the H Max/M Max ratio to pretransection values in rats from our fractalkine-treated group is one of the more striking findings of this study. The H Max/M Max ratio represents a measure of the extent of recruitment of all motoneurons whose axons have successfully reinnervated the gastrocnemius muscle into the H-reflex. In 10 intact, healthy rats the average H Max/M Max ratio (± SEM) was 0.44 (0.069) (64). In sciatic nerve transections repaired using the fractalkine scaffold, the mean H Max/M Max ratio was 0.40 (Fig. 8E), a value of 91% when scaled to the Hmax/Mmax ratio found in intact rats. This H Max/M Max ratio was more than twice that observed in autograft-treated rats (Fig. 8E) and even higher than in rats with cut sciatic nerves that had been repaired by end-to-end anastomosis (64). We interpret these findings as evidence that the fractalkine treatments influenced the regeneration not only of motor and sensory axons in the periphery but also of circuitry in the spinal cord.
The scaled M Max values were significantly greater in rats in which sciatic nerve transections were repaired using either autografts or scaffolds containing the fractalkine protein than in rats with transections repaired with the control scaffolds filled only by agarose hydrogel (Fig. 8B). Late-time-point histological analyses also corroborate the electrophysiological findings about the overall quality of the regenerative nerve cable (Fig. 9 and Fig. S7). We interpret these data to mean that motor axon regeneration was enhanced significantly in both the autograft and fractalkine groups. Because differences in M-wave amplitudes in the autograft and fractalkine groups were not significant, we conclude that our fractalkine-treated scaffolds represent a synthetic alternative that closely matched an important aspect of autograft electrophysiological performance, the M-wave response. It is important to recall that the autograft contains SCs, whereas the fractalkine group recruits endogenous cells and does not have any autogenic transplants similar to the autograft. It is also important to note that in this nerve gap an agarose-only nerve-guidance channel elicits no axon regeneration, because the experimental model is a critically sized gap.
Across almost all late-time-point measures, greater data variability was observed among the groups of rats that had the sciatic nerve repaired using the scaffold, and especially fractalkine treatment, than in the autograft group. This variability can be explained by the inherent differences in each animal's monocyte recruitment response; also, in this kind of extremely early intervention, small changes in the efficacy could result in big changes in outcome. For example, the effects of small differences in early monocyte recruitment may be amplified by other players in the cascade of regeneration such as macrophages, fibroblasts, endothelial cells, and SCs (12).
Although early engagement of macrophages and their consequent state of activation play critical roles in determining the fate of the healing process (23, 55, 65), the underlying mechanism whereby short-lived fractalkine treatment provides a sustained increase in reparative macrophages and subsequently dramatically better healing remains unclear. Additionally, the particular role of fractalkine and its receptor in the accumulation of monocytes and macrophages at injured peripheral nerves has not been elucidated. Unraveling the process of monocyte recruitment after peripheral nerve injuries could provide further insights for the development of new therapeutics based on manipulating the number and distribution of these cells as a way to enhance the proregenerative immune response (30).
Conclusion
Current treatment options available for patients with peripheral nerve injury are unsatisfactory. Off-the-shelf availability of long synthetic grafts would revolutionize the acute management of complex peripheral nerve injuries, allowing surgeons to bridge multiple nerves immediately at the time of injury. The innovative approach described here uses the endogenous capacity of the body for healing using its own innate immune cells. In contrast to the current established views regarding tissue remodeling and inflammation, strategies that promote the temporal, spatial, and phenotypical recruitment of monocytes can encourage the functional recovery of damaged tissue instead of exacerbating injury.
The data presented in this study suggest that the release of an antiinflammatory monocyte-recruiting factor, fractalkine, early after nerve injury can dramatically enhance the permissiveness of nerve conduits, stimulate axonal growth 4 wk after axotomy, and enhance electrophysiological outcomes at 14 wk. Controlling monocyte subtype recruitment, rather than preventing monocyte infiltration to the injured tissue, is a functional shift in tissue engineering approaches. The data from this study clearly attest to the effectiveness of this immunoengineering approach to nerve repair.
Materials and Methods
Scaffold Implantation.
Polysulfone tubes filled with 1% agarose (SeaPrep Lonza) mixed with 10 µg/mL rat recombinant chemokine fractalkine or IL-4 (Antigenix America, Inc.) or with agarose only were prepared (n = 13 per group) (Fig. S1). We chose 1% SeaPrep agarose because its mechanical properties and porosity had been demonstrated previously as being supportive of axonal growth as well as ensuring the complete filling of the scaffold to achieve uniform short-term release of cytokine (Fig. S2) (48). Microscissors were used to transect the sciatic nerve, and the nerve stumps were pulled 1 mm into each end of the 17-mm-long guidance scaffolds (leaving a 15-mm gap) and were fixed in place with a single 10-0 nylon suture (Ethicon). As a positive control, using a standard protocol (21), we created nerve autographs in some rats by resecting a segment of rat sciatic nerve and then flipping it to bridge the 15-mm gap (n = 8). Animals were maintained in facilities approved by the Institutional Animal Care and Use Committee (IACUC) at the Georgia Institute of Technology or Emory University and in accordance with the US Department of Agriculture, US Department of Health and Human Services, and NIH regulations and standards. IACUC at the aforementioned universities also approved all the protocols for research involving animal subjects. For details see SI Text.
CL-Lipo Study.
The CL-Lipo suspension was purchased from Clodronate Liposomes. To prevent precipitation of liposomes, the suspension was shaken before injection. A homogeneous suspension was required to warrant an equal concentration of liposomes per milliliter. For i.v. injection, 0.1 mL of the suspension was injected per 10 g of animal weight 48 h before the implantation. Depletion of liver and splenic macrophages was complete after ∼24 h. Two experimental groups were included (n = 4): fractalkine scaffolds with CL-Lipo injection, and fractalkine scaffolds with sham PBS injection (see SI Text for more details). Scaffolds were explanted for analysis 10 d postimplantation.
Scaffold Explantation and Histological Analysis.
Unless otherwise stated, scaffolds or autografts were explanted at 4 wk or 14 wk postimplantation for histological analysis of nerve regeneration (n = 5 or 8). Because axons grow from the proximal to the distal end of the scaffold, and the Wallerian degeneration process mainly affects the distal end of the nerve (26), the last 2 mm of the distal end was used for histological analysis. Nerve tissue was sectioned from the distal end (where the scaffold started or at the distal autograft suture mark), and three sections were collected every 200 µm. Suture marks on the nerve or nerve scaffold were used to choose spatially consistent and comparable sections. The remaining parts of scaffolds or autografts were snap-frozen in liquid nitrogen and stored at −80 °C for further analysis. Explants were fixed in 4% paraformaldehyde (Sigma Aldrich), washed, and stored in 30% sucrose for 24 h. Samples were embedded in optimum cutting temperature gel (Tissue-Tek) and frozen for cryosectioning (CM30505; Leica). Autografts or scaffolds were sectioned transversely or longitudinally to a thickness of 16 μm and reacted with immunofluorescent markers to quantify the different cell types, using techniques previously described (21) and as explained further in SI Text.
Electrophysiology.
Axon regeneration and muscle fiber reinnervation were studied using evoked EMG activity in ketamine/xylazine-anesthetized animals at 14 wk posttransection (for details see SI Text, Fig. S8). The sciatic nerve was stimulated on each side of the animal in the midthigh, proximal to the surgical repair site on the injured side of the animal, and direct muscle (M-wave) and monosynaptic H-reflexes were recorded from the gastrocnemius muscle. The largest M-wave and H-reflex and their ratio were recorded, and responses on the injured side of each rat were scaled to the corresponding responses on the intact side. Autograft-, fractalkine-, and control sham-treated cohorts (n = 6) were compared. For more information, please refer to SI Text.
Fig. S8.
Electrophysiology experiment setup.
Statistical Analysis.
To determine significant differences among groups, data were analyzed using Prism 6 (GraphPad) with an unpaired Student’s t test for two-group comparisons and one-way ANOVA with pairwise Tukey’s multiple comparison test for data with more than two groups. A P value <0.05 was defined to indicate a statistically significant difference. Data are reported as mean ± SD unless otherwise specified.
Cytokine Release Profile
For the cytokine in vitro release study, rat recombinant cytokine IL-4 or fractalkine was mixed with 1% agarose (SeaPrep; Lonza) to the final concentration of 10 µg/mL of protein. Five hundred microliters of the solution was plated in the wells of a 24-well plate and kept at 4 °C for 1 h to form a solid gel. The gel then was covered with an equivalent amount of PBS, incubated at 37 °C, and covered again with new PBS at the 8-, 16-, 24-, 48-, and 72-h time points. Samples were collected from designated wells at the various time points (n = 3) and were quantified using the NanoOrange Protein Quantitation kit (Thermo Fisher Scientific). The cytokine release profile from the gel is reported in Fig. S2. This fast-release profile was expected because of the relatively large (micrometer-sized) pores of 1% agarose SeaPrep (47).
Scaffold Implantation
The scaffolds were cooled at 4 °C for 1 h. Anesthesia was induced in adult Lewis male rats (250–300 g) with inhaled isoflurane at 3–4%. Throughout surgery, the animals were maintained at 1.5–2.0% isoflurane. The muscles were reapposed with 4-0 Vicryl sutures (Ethicon), and the skin incision was clamped shut with wound clips (Braintree Scientific). After the surgery, the rats were placed under a warm light until they regained sternal recumbency and then were housed separately with access to food and water ad libitum at a constant temperature (19–22 °C) and humidity (40–50%) on a 12:12-h light/dark cycle. To prevent toe chewing, a bitter solution (Grannick’s Bitter Apple) was applied to the affected foot when necessary. Marcaine (0.25% wt/vol; 0.3 mL per animal) and Buprenorphine SR (1 mg/kg; SR Veterinary Technologies) were administered s.c. for postsurgical pain relief.
CL-Lipo Study
It should be noted that administration of saline or PNS liposomes does not represent the appropriate control for this study. Control animals should have nonactivated, nonblocked, and nonsuppressed macrophages, and most particulate compounds including liposomes may affect macrophages function by activating, blocking, or suppressing them. Therefore, the best practice to mimic depletion treatments without affecting healthy macrophages is a sham operation with injection of physiological saline. This information is adapted from the CL-Lipo supplier website (www.clodronateliposomes.org).
Scaffold Explantation and Analysis
Sections were incubated for 1 h at room temperature in a blocking solution of 4% goat serum (Gibco) in PBS, were incubated overnight at 4 °C in a mixture of primary antibody and blocking solution, and were washed and incubated for 1 h at room temperature in a solution of secondary antibody mixed in 0.5% Triton X-100 (Sigma) in PBS. Slides were washed twice more with PBS, incubated with DAPI for 10 min, and then dried and coverslipped for analysis. Primary antibodies NF160 (1:250, mouse IgG1; Sigma), S100 (1:250, rabbit IgG; DakoCytomation), RECA (1:250, mouse IgG1; Invitrogen), CD68 [1:100, mouse IgG1; AbD Serotec (ED1)], CD86 (1:100, mouse IgG1; AbD Serotec), Arginase-1 (1:250, rabbit, IgG; Cell Application), CD206 (1:100, rabbit, IgG; Santa Cruz Biotechnology), and FluoroMyelin (1:300, Invitrogen) were used. The following secondary antibodies were used: goat anti-rabbit IgG Alexa 488/594 (1:220; Invitrogen) and goat anti-mouse IgG1 Alexa 488/594 (1:220; Invitrogen). Previously established immunohistochemistry techniques for quantifying the fluorescent pixel intensity of the aforementioned antibodies were used to quantify each cell (Fig. 5A and Fig. S3) (21, 49–51). We analyzed and counted all the positively stained cells across the entirety of each of the selected cross-sections and then ran statistical analyses comparing different animals for each condition or control. Myelinated axon density and myelin thickness were calculated using custom computer code in Python 2.7.11 with SciPy 0.17.0 (myelin_quantify.py) (Dataset S1). In brief, NF160+ segments were designated as either myelinated or unmyelinated axons based on whether they were 40% or more encircled by FluoroMyelin+ tissue. To ensure consistent metrics across images, the reported density of myelinated axons is normalized to the combined area of tissues positively stained for any of NF160, FluoroMyelin, or DAPI (and thus overestimates the absolute axonal density but provides consistent relative density for the comparisons herein). Myelinated axons were analyzed further for myelin thickness by rotating a vector (1° per measurement) around the center of the axon segment and measuring the thickness of surrounding radius of FluoroMyelin+ tissue. Once an axon is analyzed, the counted FluoroMyelin+ pixels are removed from successive measurements to limit bias caused by measuring myelin insulation twice for adjacent axons. Reported thicknesses have been twice averaged, once for each myelinated axon and again for all myelinated axons within the image.
Electrophysiology
Rats were injected i.p. with ketamine (40 mg/kg) and xylazine (5 mg/kg) to induce a surgical plane of anesthesia. Additional ketamine (10 mg/kg) was administered if necessary to maintain this level of anesthesia throughout the experiment. The sciatic nerve was exposed bilaterally, and a bipolar stimulating cuff was placed around each exposed nerve just proximal to the transection site (Fig. S8). On the contralateral, intact side of each animal, the stimulating cuff was placed around the sciatic nerve at a comparable location. Bipolar fine-wire EMG electrodes were placed into the body of each gastrocnemius muscle and were connected to an optically isolated differential amplifier (Fig. 8A).
Amplifier gain was set at 1,000 with the output of the amplifiers bandpass filtered at 100 Hz and 1 kHz. Amplified potentials were recorded using a laboratory computer system. Spontaneous background EMG was sampled by the laboratory computer system at a rate of 10 kHz. When the average rectified background EMG voltage (sampled over a 20-ms interval) fell within a user-defined voltage window, a single stimulus was delivered to the cuff electrode. These stimuli were delivered in the form of constant voltage pulses with 0.1-ms duration. Stimulus intensity was increased gradually to cover the full range of intensities expected to elicit muscle responses, generally 0–5 V. Additionally, these stimuli were delivered no more frequently than once every 3 s to avoid any muscle fatigue or failure of nerve action potential. EMG activity was recorded from 20 ms before stimulation to 50 ms following stimuli, and the responses were saved to data files. At the end of the experiment, animals were killed with an overdose of pentobarbital sodium (90 mg/kg, i.p.).
We included readings only from the experiments in which we were able to read M and H responses. We recorded six out of six successful readings for autograft and fractalkine but were able to record only very weak responses from two of our negative control samples. For the graphs presented in Fig. 8 B and C, we simply put zeros for the four no-reading negative control samples. However, the controls were omitted in Fig. 8 D and E, because reporting zeros for the negative controls would falsely skew the results, and reporting only the successful readings would be misleading with respect to their overall electrophysiologically measured performance.
Latency Time Calculation
The latency times of these responses have very typical windows in intact nerves. Stimulation of an intact sciatic nerve in ambulatory awake rats produces an M-wave that begins ∼2 ms after stimulation and returns to prestimulus levels at 4.5 ms, whereas the H-reflex occurs between 6 and 10 ms after stimulation (64). The distribution of myelination of the axons is also indicated by the return of response latency to these time windows. The number of peaks present and the latency of the responses go hand-in-hand. Because myelination distribution is ideally working toward a pretransection uniformity, the latency time will decrease as the number of peaks decreases with myelination. Ideally, the putative response windows could be analyzed quantitatively to determine the success of remyelination in transected animals. However, factors such as overlap of the M-wave and H-reflex, as well as the change in the wave characteristics based on the placement of recording electrodes for recording (65), make it difficult to measure myelination distribution accurately. No significant difference was observed in the latency profiles of the experimental condition and controls (Fig. S6). The difficulty in quantifying this measure accurately because of the many potentially confounding variables may explain the lack of statistical significance between all three trial groups.
As mentioned above with respect to the negative controls in Fig. 8 D and E, we have omitted the negative controls from Fig. S6, because the inclusion of zero values for unsuccessful readings would falsely skew the results.
Supplementary Material
Acknowledgments
We thank Dr. Balakrishna Pai and Dr. Tarun Saxena for helpful scientific and editorial discussions; Sohee Park, Yogi Patel, and Sheridan Carroll for their technical support on electrophysiological and imaging analyses; and Dr. Giles W. Plant for his suggestions regarding myelin staining. This work was supported by NIH Grants 7R01NS093666 and 5R01NS065109.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705757114/-/DCSupplemental.
References
- 1.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 2.Millesi H. Factors affecting the outcome of peripheral nerve surgery. Microsurgery. 2006;26:295–302. doi: 10.1002/micr.20242. [DOI] [PubMed] [Google Scholar]
- 3.Bellamkonda RV. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Giusti G, et al. Return of motor function after segmental nerve loss in a rat model: Comparison of autogenous nerve graft, collagen conduit, and processed allograft (AxoGen) J Bone Joint Surg Am. 2012;94:410–417. doi: 10.2106/JBJS.K.00253. [DOI] [PubMed] [Google Scholar]
- 5.Wood MD, et al. Rat-derived processed nerve allografts support more axon regeneration in rat than human-derived processed nerve xenografts. J Biomed Mater Res A. 2014;102:1085–1091. doi: 10.1002/jbm.a.34773. [DOI] [PubMed] [Google Scholar]
- 6.Szynkaruk M, Kemp SWP, Wood MD, Gordon T, Borschel GH. Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng Part B Rev. 2013;19:83–96. doi: 10.1089/ten.TEB.2012.0275. [DOI] [PubMed] [Google Scholar]
- 7.Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]
- 8.Groves ML, et al. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Pfister BJ, et al. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 11.Mukhatyar V, Karumbaiah L, Yeh J, Bellamkonda R. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves. Adv Mater. 2009;21:4670–4679. [Google Scholar]
- 12.Mokarram N, Bellamkonda RV. Overcoming endogenous constraints on neuronal regeneration. IEEE Trans Biomed Eng. 2011;58:1900–1906. doi: 10.1109/TBME.2010.2103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madaghiele M, Sannino A, Yannas IV, Spector M. Collagen-based matrices with axially oriented pores. J Biomed Mater Res A. 2008;85:757–767. doi: 10.1002/jbm.a.31517. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain LJ, Yannas IV, Hsu HP, Spector M. Connective tissue response to tubular implants for peripheral nerve regeneration: The role of myofibroblasts. J Comp Neurol. 2000;417:415–430. doi: 10.1002/(sici)1096-9861(20000221)417:4<415::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez FJ, Verdú E, Ceballos D, Navarro X. Nerve guides seeded with autologous schwann cells improve nerve regeneration. Exp Neurol. 2000;161:571–584. doi: 10.1006/exnr.1999.7315. [DOI] [PubMed] [Google Scholar]
- 17.Nie X, et al. Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac Surg. 2007;36:32–38. doi: 10.1016/j.ijom.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Fine EG, Decosterd I, Papaloïzos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee AC, et al. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, cell response, and nerve regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y-T, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spivey EC, Khaing ZZ, Shear JB, Schmidt CE. The fundamental role of subcellular topography in peripheral nerve repair therapies. Biomaterials. 2012;33:4264–4276. doi: 10.1016/j.biomaterials.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience. 2015;302:174–203. doi: 10.1016/j.neuroscience.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokarram N, Bellamkonda RV. A perspective on immunomodulation and tissue repair. Ann Biomed Eng. 2014;42:338–351. doi: 10.1007/s10439-013-0941-0. [DOI] [PubMed] [Google Scholar]
- 27.Cortez-Retamozo V, Etzrodt M, Pittet MJ. Regulation of macrophage and dendritic cell responses by their lineage precursors. J Innate Immun. 2012;4:411–423. doi: 10.1159/000335733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yona S, Jung S. Monocytes: Subsets, origins, fates and functions. Curr Opin Hematol. 2010;17:53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 29.Bellingan GJ, et al. Adhesion molecule-dependent mechanisms regulate the rate of macrophage clearance during the resolution of peritoneal inflammation. J Exp Med. 2002;196:1515–1521. doi: 10.1084/jem.20011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrin FE, Lacroix S, Avilés-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- 32.Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Chapman GA, et al. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur J Pharmacol. 2000;392:189–195. doi: 10.1016/s0014-2999(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 34.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 35.Ahuja V, Miller SE, Howell DN. Identification of two subpopulations of rat monocytes expressing disparate molecular forms and quantities of CD43. Cell Immunol. 1995;163:59–69. doi: 10.1006/cimm.1995.1099. [DOI] [PubMed] [Google Scholar]
- 36.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 37.Awojoodu AO, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci USA. 2013;110:13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Avraham-Davidi I, et al. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med. 2013;210:2611–2625. doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison JK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes FE, et al. Intra-neural administration of fractalkine attenuates neuropathic pain-related behaviour. J Neurochem. 2008;106:640–649. doi: 10.1111/j.1471-4159.2008.05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin W, et al. Exogenous fractalkine enhances proliferation of endothelial cells, promotes migration of endothelial progenitor cells and improves neurological deficits in a rat model of ischemic stroke. Neurosci Lett. 2014;569:80–84. doi: 10.1016/j.neulet.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 47.Stievano L, Piovan E, Amadori A. C and CX3C chemokines: Cell sources and physiopathological implications. Crit Rev Immunol. 2004;24:205–228. doi: 10.1615/critrevimmunol.v24.i3.40. [DOI] [PubMed] [Google Scholar]
- 48.Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 49.Clements IP, et al. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30:3834–3846. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown BN, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 53.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 54.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 55.Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summan M, et al. Macrophages and skeletal muscle regeneration: A clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 57.Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1687–1694. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol. 2007;97:1127–1134. doi: 10.1152/jn.01035.2006. [DOI] [PubMed] [Google Scholar]
- 59.Taras JS, Nanavati V, Steelman P. Nerve conduits. J Hand Ther. 2005;18:191–197. doi: 10.1197/j.jht.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: Review. Microsurgery. 2010;30:574–588. doi: 10.1002/micr.20799. [DOI] [PubMed] [Google Scholar]
- 61.Palmieri RM, Ingersoll CD, Hoffman MA. The Hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J Athl Train. 2004;39:268–277. [PMC free article] [PubMed] [Google Scholar]
- 62.Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 63.Ma CHE, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boeltz T, et al. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol. 2013;109:2645–2657. doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ydens E, et al. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation. 2012;9:176. doi: 10.1186/1742-2094-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mokarram N, Dymanus K, Srinivasan A, English A, Bellamkonda R. Preferential recruitment of anti-inflammatory monocytes significantly enhances peripheral nerve regeneration. Front Bioeng Biotechnol. 2016;4 doi: 10.3389/conf.FBIOE.2016.01.00186. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.