Significance
Different environmental stimuli can lead animals to go into an emergency state and experience stress; but can an individual notice the stress experienced by other members of its social group and develop a similar physiological reaction? We demonstrate that such a form of cross-over of stress can actually occur in wild animal populations. Gull chicks that grew up with experimentally stressed siblings showed increased secretion of stress hormones. In the short term the cross-over of stress seemed to be favorable, improving chicks’ antipredator behavior, but in the long term the chicks grew slowly and attained a reduced adult size, showed increased accumulation of cell damage, and developed a poor-quality juvenile plumage. The cross-over of stress can be an important but complex selective force.
Keywords: glucocorticoids, group living, phenotypic programming, social environment, stress cross-over
Abstract
Recent data suggest that, in animals living in social groups, stress-induced changes in behavior have the potential to act as a source of information, so that stressed individuals could themselves act as stressful stimuli for other individuals with whom they interact repeatedly. Such form of cross-over of stress may be beneficial if it enhances adaptive responses to ecological stressors in the shared environment. However, whether stress can be transferred among individuals during early life in natural populations remains unknown. Here we tested the effect of living with stressed siblings in a gull species where, as in many vertebrates, family represents the basic social unit during development. By experimentally modifying the level of stress hormones (corticosterone) in brood mates, we demonstrate that the social transfer of stress level triggers similar stress responses (corticosterone secretion) in brood bystanders. Corticosterone-implanted chicks and their siblings were faster in responding to a potential predator attack than control chicks. In gulls, fast and coordinated reactions to predators may increase the chances of survival of the whole brood, suggesting a beneficial fitness value of cross-over of stress. However, our data also indicate that living with stressed brood mates early in life entails some long-term costs. Near independence, fledglings that grew up with stressed siblings showed reduced body size, high levels of oxidative damage in lipids and proteins, and a fragile juvenile plumage. Overall, our results indicate that stress cross-over occurs in animal populations and may have important fitness consequences.
Interacting with others in the social environment has important fitness consequences in group-living animals (1), and the behavior of other individuals in the group may generate important information about the prevailing environmental conditions (socially acquired information) (2, 3). Environmental stressors can activate the endocrine and neuroendocrine stress response, a highly conserved reaction in vertebrates that promotes several physiological and behavioral changes (4–6). Such adaptive response helps to cope with stressors and may potentially act as a source of information for other group members about the current conditions in the shared environment. Indeed, some evidence suggests that the behavior of stressed individuals may have consequences for their social partners (7, 8). However, whether stress responses may be transferred among group members is unclear, and their possible consequences have not hitherto been investigated.
Stressors trigger the hypothalamic–pituitary–adrenal (HPA) axis, a neuroendocrine pathway by which stress hormones (glucocorticoids) are released from the adrenal cortex to the general circulation (reviewed in ref. 9). The HPA axis integrates internal and external (environmental) stimuli, and the downstream release of glucocorticoids translates and transmits such information to different organs, potentiating an adaptive response (10, 11). Thus, the secretion of stress hormones leads to a rapid redistribution of energy resources away from nonvital functions and organs and to changes in behavior that help animals cope with the stressor (reviewed in refs. 9 and 12). For instance, exposure to predators causes elevation of stress hormones, which in turn enhances antipredator responses such as increased vigilance and fast reaction against predators (13, 14), thereby promoting short-term survival (4).
Empirical studies suggest that the use of socially acquired information is an important process involved in antipredator behavior or resource acquisition, and has an inherent adaptive value (2). In this context, stress-induced changes in behavior or state may act as a source of information for other group members not directly exposed to the stressful stimuli and may potentially lead to a horizontal transfer or cross-over of stress. The cross-over of stress is a well-known phenomenon in the field of human psychology, and some recent evidence suggests that the cross-over of stress could be a biological phenomenon more important than previously thought in animal social groups (e.g., 7, 10).
The social environment has important consequences also for health and fitness (15). Despite the possible benefits of stress cross-over, a protracted stress exposure can be damaging (16, 17), particularly when occurring during early stages of development (18–20). Early exposure to stressful conditions can, for instance, negatively affect growth rates (11, 16), the development of key body structures [i.e., feathers in birds (21)], and promote the accumulation of oxidative damage (22). To fully understand the role of stress cross-over in animal populations, it is fundamental to assess the costs and benefits, if any, of such phenomena.
Here we first tested whether stress responses are transferred among family members during early development of a long-lived social seabird, the yellow-legged gull (Larus michahellis). As in many animals with prolonged parental care, interactions among siblings are the most common social relationship exhibited by gull chicks during early life (23) (Fig. S1). We created experimental broods in which two of the three siblings were exposed to increased levels of stress hormones by corticosterone implants, and control broods in which two chicks received a sham (empty) implant. Corticosterone is the main stress hormone present in birds (6). This experimental design allowed us to test the effects of growing up with stressed siblings in the nonimplanted counterparts. We examined the effect of stress cross-over on chick phenotype (corticosterone level, growth, and behavior) during development. We first evaluated whether stress cross-over early in life has similar programming effects on phenotype to those directly exposed to stress (corticosterone-implanted). We expected that the cross-over of stress may bring short-term benefits to brood mates of the corticosterone-implanted chicks, such as enhanced antipredator behavior, but at the cost of long-term negative consequences in body size, oxidative damage, and plumage quality.
Fig. S1.
Photographs of gull chicks during their postnatal development. (A and B) Chicks brooding (A) and showing antipredator response (B), crouching together during the first week of life. (C) Full-grown chick recaptured at 30 d of age.
Results
Early Postnatal Effects.
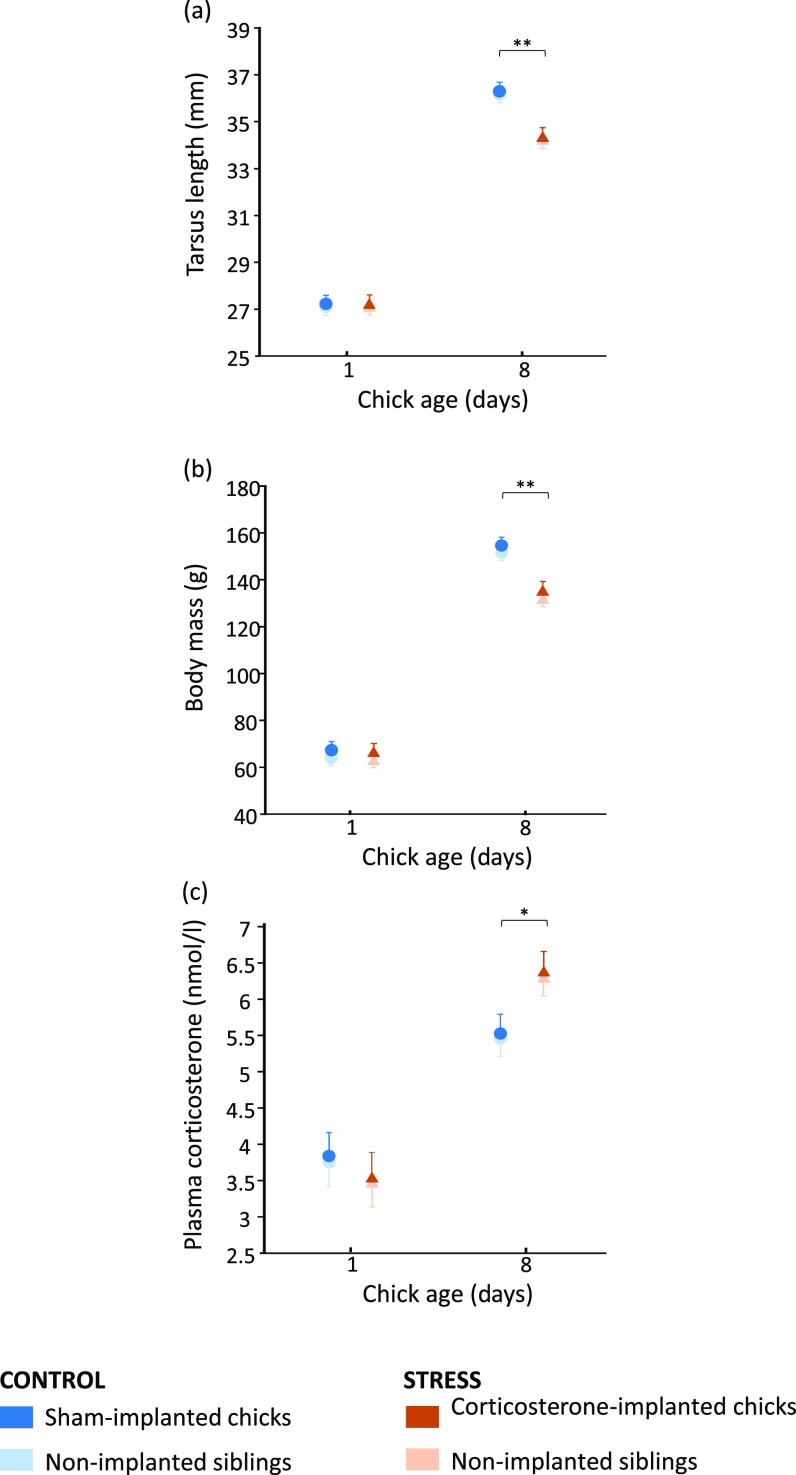
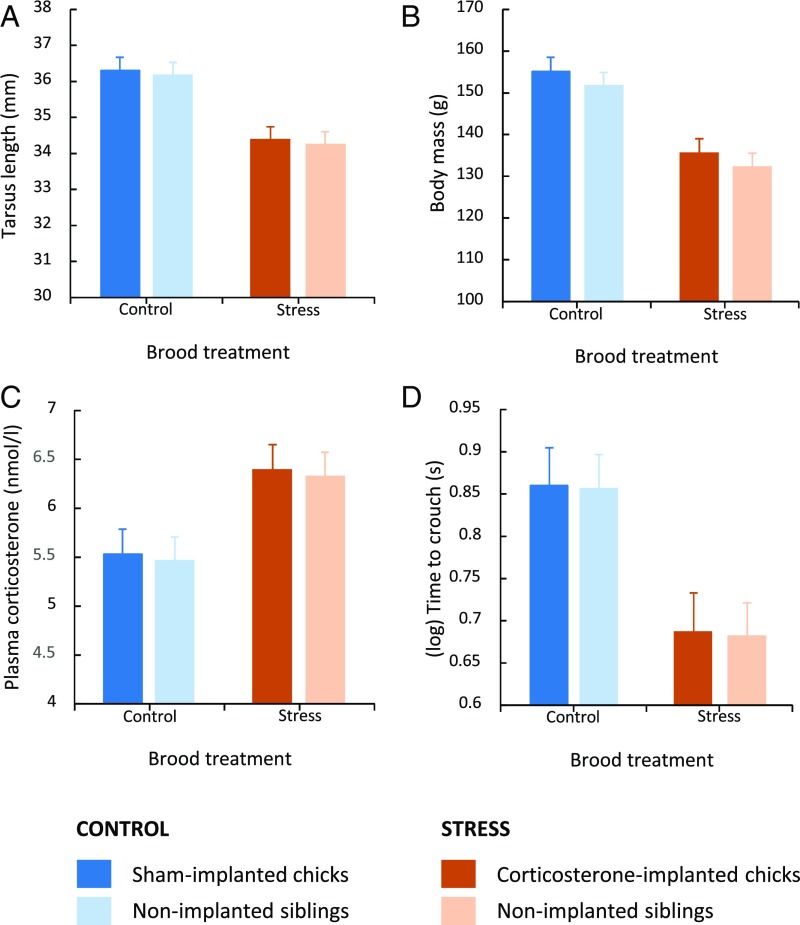
The rate of growth during the first 8 d after hatching in tarsus length and body mass was significantly lower in the chicks implanted with corticosterone and their nonimplanted brood mates (i.e., chicks in experimental broods; hereafter “stress group”; Table 1 and Fig. S2). As a result, at 8 d of age, chicks in the stress group were lighter and structurally (bone size) smaller than chicks in the control group (P ≤ 0.02 in all Tukey’s post hoc tests; Fig. 1 A and B). Interestingly, corticosterone-implanted and nonimplanted chicks in the stress broods showed a similar reduction in growth (Tukey’s post hoc tests: tarsus, P = 0.480; body mass, P = 0.480; Fig. 1 A and B and Fig. S2). Moreover, the stress group showed an increase in basal corticosterone levels (Table 1 and Fig. S2). Thus, at 8 d of age, chicks in the stress group had higher corticosterone levels than chicks in the control group (P ≤ 0.011 in all Tukey’s post hoc tests), and corticosterone-implanted or nonimplanted chicks in the stress group showed a similar corticosterone level (Tukey’s post hoc test: P = 0.608; Fig. 1C). Sex was never significant in the models (Table 1), and there were no significant two-way interactions between fixed factors (all P > 0.106). To evaluate whether these effects were mediated by nutritional differences, we measured plasma triglycerides and proteins, both very sensitive markers of nutritional status in gulls (24). However, these markers did not differ between the experimental groups at ages 1 and 8 d (P > 0.25 in all pairwise comparisons; Table S1).
Table 1.
Summary of linear mixed models for the effects of treatments and covariates on tarsus length, body mass, and basal corticosterone levels of yellow-legged gull chicks between 1 and 8 d of age
| Variable | Tarsus length | Body mass | Plasma corticosterone | |||||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 34.609 | 134.073 | 6.354 | |||||||||
| Age, day 8 | 7.129 | 1,133.33 | 1120.661 | <0.001 | 68.882 | 1,132.91 | 974.144 | <0.001 | 2.852 | 1,96.48 | 89.684 | <0.001 |
| Brood treatment, control | 1.936 | 1,51.86 | 5.538 | 0.023 | 19.522 | 1,49.98 | 7.766 | 0.008 | −0.860 | 1,49.609 | 0.850 | 0.361 |
| Chick manipulation, nonmanipulated | 0.128 | 1,138.58 | 0.267 | 0.605 | 3.409 | 1,139.26 | 1.790 | 0.183 | 0.067 | 1,82.28 | 0.095 | 0.758 |
| Sex, female | −0.0209 | 1,178.58 | 0.507 | 0.481 | −1.012 | 1,177.71 | 0.120 | 0.729 | −0.196 | 1,114.90 | 0.635 | 0.427 |
| Chick order, first | −0.533 | 1,139.80 | 4.610 | 0.034 | −2.969 | 1,140.49 | 1.35 | 0.246 | 0.127 | 1,82.39 | 0.343 | 0.560 |
| Age × brood treatment | −1.949 | 1,133.33 | 16.253 | <0.001 | −18.744 | 1,132.92 | 13.948 | <0.001 | 1.148 | 1,96.22 | 5.705 | 0.019 |
Nonsignificant interactions were removed from full models, and significant terms are highlighted in bold.
Fig. S2.
Brood mates of corticosterone-implanted chicks showed reduced growth, increased basal glucocorticoid secretion, and shortened time to respond to predation risk. Tarsus length (A), body mass (B), and basal corticosterone level (square root-transformed) (C) during the first 8 d after hatching in yellow-legged gull chicks from control (blue circles) and stress (orange triangles) broods. Within broods, implanted chicks are represented by dark symbols whereas nonimplanted chicks are represented by light symbols. In all analyses, age × brood treatment × chick manipulation was not significant (tarsus length: F1,127.24 = 0.125, P = 0.724; body mass: F1,125.04 = 0.056, P = 0.814; corticosterone level: F1,73.30 = 0.086, P = 0.770). Data show the estimated marginal mean ± SE. Significant differences between groups: *P ≤ 0.05, **P ≤ 0.01.
Fig. 1.
Brood mates of corticosterone-implanted chicks showed reduced growth, increased basal glucocorticoid secretion, and a faster reaction time to predation risk. Tarsus length (A), body mass (B), and basal corticosterone level (root-squared) (C) at 8 d of age, and antipredator behavior (log-transformed time to crouch) (D) at 9 d of age in implanted (dark bars) and nonimplanted (light bars) yellow-legged gull chicks from control (blue) and stress (orange) broods. In each brood, two of three chicks were s.c. implanted between the shoulders with a surgical silastic implant filled with corticosterone (stress broods) or left empty (control broods), and the remaining sibling was left without being manipulated (nonimplanted). Data show estimated marginal mean ± SE (Fig. S2 for the comparison between initial and final values of each variable).
Table S1.
Summary of linear mixed models for the effects of stress treatment and covariates on plasma triglyceride and protein content of yellow-legged gull chicks between 1 and 8 d of age
| Variable | Triglycerides | Proteins | ||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 1.130 | 4.923 | ||||||
| Age, day 8 | −0.092 | 1,118.68 | 3.305 | 0.072 | 0.012 | 1,86.54 | 0.070 | 0.791 |
| Brood treatment, control | −0.062 | 1,49.06 | 1.352 | 0.251 | 0.034 | 1,52.41 | 0.279 | 0.600 |
| Chick manipulation, implanted | 0.039 | 1,128.16 | 0.590 | 0.444 | 0.005 | 1,47.28 | 0.012 | 0.915 |
| Sex, female | −0.099 | 1,136.35 | 3.576 | 0.061 | 0.037 | 1,81.07 | 0.429 | 0.514 |
| Chick order, first | −0.013 | 1,126.69 | 0.071 | 0.790 | −0.023 | 1,47.13 | 0.202 | 0.655 |
Nonsignificant interactions were removed from full models. Mean ± SE.
The stress treatment affected the chicks’ antipredator behavior [brood treatment (corticosterone vs. sham): F42.06 = 8.60, P = 0.005; chick manipulation (implanted vs. nonimplanted): F1,32.90 = 0.150, P = 0.701; brood treatment × chick manipulation: F1,33.09 = 0.272, P = 0.605; Fig. 1D). Chicks from the stress group, irrespective of whether they were corticosterone-implanted or not, were faster in crouching and hiding after listening to adult alarm calls in comparison with chicks from the control group (time to crouch; mean ± SE; control, 7.16 ± 0.95 s; stress, 4.30 ± 0.38 s; Fig. 1D). There was no effect of sex (F1,52.88 = 0.397, P = 0.531) or chick order (F1,29.26 = 0.509, P = 0.481) on the antipredator behavior. None of the three- and two-way interactions between fixed factors was significant in the models (all P > 0.089).
Late Postnatal Effects.
At the end of the growth period, 30 d after hatching, we recaptured nonimplanted fledglings. Chicks that grew up with corticosterone-implanted siblings were still lighter (F1,23 = 5.361, P = 0.030; Table S2) and structurally smaller (tarsus: F1,23 = 7.963, P = 0.010; Table S2) than chicks with nonstressed siblings (Fig. 2 A and B). By that age, however, the difference in basal corticosterone level between the experimental groups had disappeared (brood treatment: F1,23 = 0.456, P = 0.506). The sex of the chicks and its interaction with treatment were not significant (sex: F1,23 = 1.543, P = 0.227; brood treatment × sex: F1,22 = 1.069, P = 0.312). Similarly, the levels of plasma triglycerides and proteins did not differ between experimental groups (P > 0.152 in both cases), and no other variable included in the models was significant (Table S3).
Table S2.
Summary of the linear models for the effects of stress treatment and covariates on tarsus length, body mass, and basal corticosterone levels in nonimplanted yellow-legged gull chicks at 30 d of age
| Variable | Tarsus length | Body mass | Plasma corticosterone | |||||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 59.439 | 584.366 | 4.866 | |||||||||
| Brood treatment, control | 4.484 | 1,23 | 7.963 | 0.010 | 126.318 | 1,23 | 5.361 | 0.030 | −0.271 | 1,23 | 0.456 | 0.506 |
| Sex, female | −2.688 | 1,23 | 2.546 | 0.124 | −34.734 | 1,23 | 0.422 | 0.523 | 0.488 | 1,23 | 1.543 | 0.227 |
| Chick order, first | −1.020 | 1,23 | 0.350 | 0.560 | −20.773 | 1,23 | 0.144 | 0.708 | 0.561 | 1,23 | 1.946 | 0.176 |
Nonsignificant interactions were removed from full models, and significant terms are highlighted in bold.
Fig. 2.
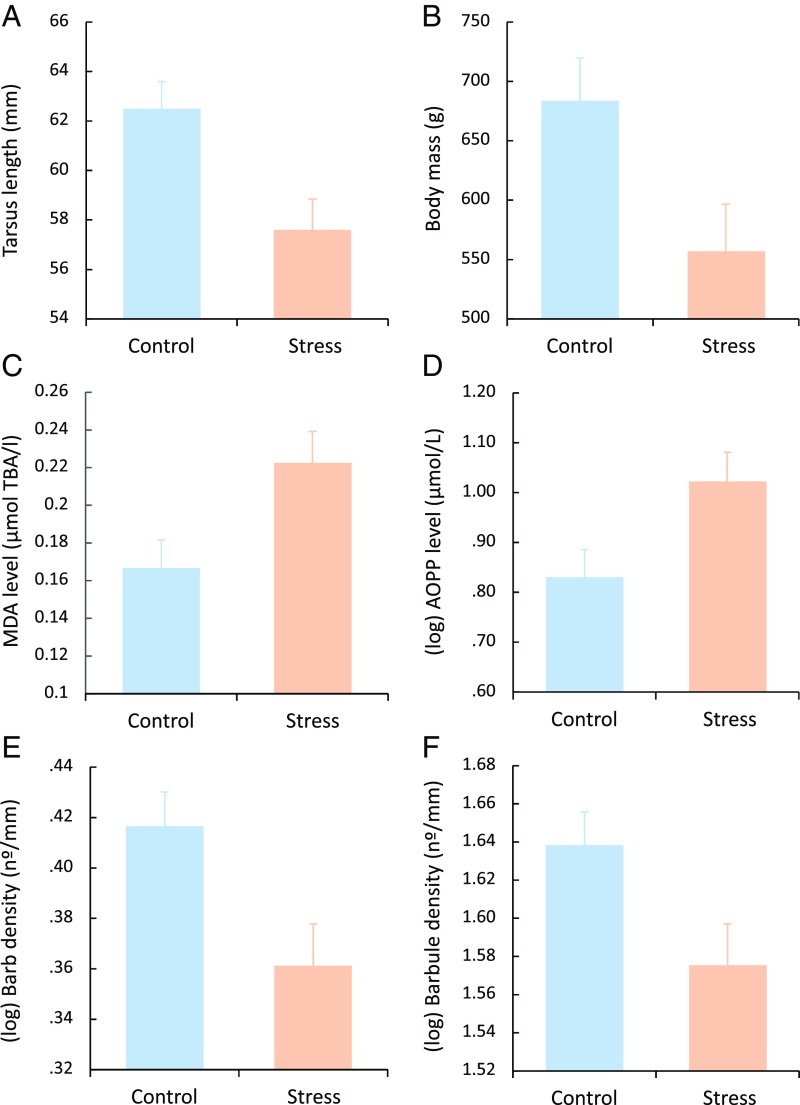
Brood mates of corticosterone-implanted chicks were smaller, had increased levels of oxidative damage, and developed a more fragile juvenile plumage. Tarsus length (A), body mass (B), lipid peroxidation level (MDA) (C), advanced oxidation protein product (AOPP) level (D), barb density (E), and barbule density (F) in nonimplanted yellow-legged gull fledglings (30 d of age) from control (blue) and stress (orange) broods; note that none of these birds received an implant. Data show the estimated marginal mean ± SE. TBA, thiobarbituric acid reactive substances.
Table S3.
Summary of the linear models for the effects of stress treatment and covariates on plasma triglyceride and protein content in nonimplanted yellow-legged gull chicks at 30 d of age
| Variable | Triglycerides | Proteins | ||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 1.167 | 5.038 | ||||||
| Brood treatment, control | 0.094 | 1,21 | 0.286 | 0.598 | −0.169 | 1,22 | 2.199 | 0.152 |
| Sex, female | −0.025 | 1,21 | 0.021 | 0.886 | 0.111 | 1,22 | 0.947 | 0.341 |
| Chick order, first | −0.007 | 1,21 | 0.001 | 0.971 | −0.051 | 1,22 | 0.191 | 0.666 |
Nonsignificant interactions were removed from full models.
Growing up with stressed siblings affected the fledglings’ oxidative status (Table 2); chicks that lived with corticosterone-implanted siblings had a higher level of lipid peroxidation (Fig. 2C) and advanced oxidation protein products (Fig. 2D) in comparison with nonimplanted chicks from the control group. The experimental treatment did not affect the antioxidant capacity of plasma or glutathione peroxidase level (Table 2). The sex of the chick and the rest of the variables included in the models of oxidative stress markers were not significant (Table 2).
Table 2.
Summary of the linear models for the effects of stress treatment and covariates on antioxidant defenses and oxidative damage in nonimplanted yellow-legged gull fledglings
| Variable | TAC | GPx | AOPP | MDA | ||||||||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 2.697 | 2,337.923 | 0.942 | 0.178 | ||||||||||||
| Brood treatment, control | 0.990 | 1,20 | 1.707 | 0.206 | 368.200 | 1,23 | 2.721 | 0.113 | −0.193 | 1,22 | 5.605 | 0.027 | −0.056 | 1,20 | 5.858 | 0.025 |
| Sex, female | −0.521 | 1,20 | 0.482 | 0.495 | −816.394 | 1,23 | 13.910 | 0.001 | 0.110 | 1,22 | 1.830 | 0.190 | 0.006 | 1,20 | 0.072 | 7.792 |
| Chick order, first | −0.235 | 1,20 | 0.094 | 0.762 | −224.794 | 1,23 | 1.005 | 0.327 | 0.050 | 1,22 | 0.368 | 0.550 | 0.037 | 1,20 | 2.400 | 0.137 |
| UA | 0.114 | 1,20 | 0.426 | 0.521 | ||||||||||||
| TRIG | 0.001 | 1,20 | 3.234 | 0.087 | ||||||||||||
Nonsignificant interactions were removed from full models, and significant terms are highlighted in bold.
Finally, our data also revealed that birds that grew up with stressed siblings developed weaker wing feathers, as suggested by the lower density of barbs and barbules in the primary covers (barb density: F1,19 = 6.307, P = 0.021; barbule density: F1,19 = 4.708, P = 0.043; Fig. 2 E and F). The social interaction with stressed siblings also tended to affect body feathers, but the differences between the two treatments were not significant (Table S4).
Table S4.
Summary of the linear models for the effects of stress treatment and covariates on feather quality in wing and body feathers of nonimplanted yellow-legged gull chicks at 30 d of age
| Variable | Primary covers (wing feathers) | Scapulars (body feathers) | ||||||||||||||
| Barb density (TAC) | Barbule density | Barb density | Barbule density | |||||||||||||
| Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | Estimate | dfn,d | F | P | |
| Intercept | 0.369 | 1.567 | 0.336 | 1.522 | ||||||||||||
| Brood treatment, control | 0.055 | 1,19 | 6.307 | 0.021 | 0.063 | 1,19 | 4.708 | 0.043 | 0.031 | 1,19 | 3.888 | 0.063 | −0.025 | 1,19 | 2.204 | 0.154 |
| Sex, female | 0.004 | 1,19 | 0.042 | 0.841 | 0.036 | 1,19 | 1.689 | 0.209 | 0.002 | 1,19 | 0.019 | 0.998 | 0.002 | 1,19 | 0.010 | 0.923 |
| Chick order, first | −0.021 | 1,19 | 0.952 | 0.342 | −0.019 | 1,19 | 0.442 | 0.514 | −4.849e5 | 1,19 | 0.000 | 0.892 | −0.009 | 1,19 | 0.323 | 0.577 |
Nonsignificant interactions were removed from full models, and significant terms are highlighted in bold.
Discussion
Our findings reveal that experimental elevation of stress level in gull chicks can trigger stress responses in brood bystanders. This phenomenon was clearly evidenced by the increased secretion of glucocorticoid hormones in nestlings that were reared with experimentally stressed siblings. Increased basal corticosterone levels enhanced antipredator behavior but negatively affected growth in the implanted chicks. These effects of corticosterone implants were exactly mimicked in their social brood mate, suggesting that physiological and behavioral responses to stress may be acquired following exposure to stressed individuals in the social environment. We also provide strong evidence indicating that such form of horizontal transfer of stress has negative consequences for the affected individuals. Near independence, fledglings that grew up with stressed siblings were lighter, had a reduced skeletal size, showed increased levels of oxidative damage in several macromolecules, and developed a more fragile juvenile plumage than the control fledglings. In summary, our findings strongly suggest that the cross-over of stress may be a biological phenomenon more common than previously thought in vertebrates, and also highlight the critical role that the social environment may have on individual fitness.
Nonimplanted chicks had nearly identical basal corticosterone level to that seen in the corticosterone-implanted sibling at 8 d of age, suggesting that living with stressed siblings induces an up-regulation of the HPA axis. The transmission of stress from one chick to another may be caused by different mechanisms. In the first week of life, gull chicks in a brood closely interact with each other in many social contexts; together they explore the territory and respond to parental behavior and environmental threats, such as predators (23, 25) (Fig. S1B). Because corticosterone implants are likely to reduce begging displays (26), promote neophobic and fearful behaviors (16, 27), or even alter chicks’ odor (28), corticosterone-implanted chicks may have acted as a source of information for their (nonimplanted) sibling, affecting their physiology and development. Previous studies suggest that the use of socially acquired information (e.g., behavior or states of conspecifics) may be a widespread phenomenon in animal social groups and a major driving force in social evolution (2). The cross-over of stress may also be the result of empathic-like reactions. The existence of this type of mechanism involving the recognition of others’ emotional state has been described in humans, apes, and some rodents (8, 29), but the evidence is less clear in birds (but see refs. 30 and 31). Alternatively, stressed chicks could have generated stress on their parents (32), resulting in reduced parental care and indirectly increasing corticosterone level in their nonimplanted siblings (32). However, this possibility seems unlikely because chicks from the control and stress groups showed similar nutritional status. Regardless of the mechanism, our results demonstrate stress transmission (either direct or indirect) among family members.
Corticosterone implants shortened the time taken by gull chicks to react against a potential threat (i.e., predator). This supports previous studies in other vertebrate species (e.g., 13, 14) and emphasizes the adaptive value of stress responses for promoting short-term survival (9). Importantly, our results suggest that stress transfer from brood mates experiencing an external stressor (predator) in the shared environment may enhance the chance of coping with this stressor (antipredator behavior) in brood mates that did not directly receive the stimuli. The coordinated crouching behavior of a brood probably reduces the predation risk of the whole brood (33), suggesting an important adaptive value of cross-over of stress. In some social species, the cross-over of stress may also be important for the persistence of chronic stress in populations under high predation risk, although the majority of living individuals do not directly interact with predators (e.g., 34). Information transmission about environmental stressors, such as predators, is a key factor underpinning group living and affecting collective behavior (35, 36). The transfer of stress among members of the same social group has important implications for understanding the evolution of social living, providing a mechanism through which social factors may influence the collective behavior of social groups.
The cross-over of stress had, however, some negative consequences for gull chicks. The elevated corticosterone levels seen in stressed chicks (corticosterone-implanted chicks and their nonimplanted siblings) impaired growth, as occurs in many taxa (reviewed in ref. 16). Corticosterone reduces and inhibits the production of important growth-related hormones such as growth hormone, somatomedin, and insulin-like growth factors, increases muscle protein degradation, and induces a general metabolic shift (see ref. 37 for a complete review of mechanisms). These effects were long-lasting, as evidenced by reduced skeletal size and body mass at age 30 d in siblings of the corticosterone-implanted chicks, although the effect on corticosterone levels disappeared at this age. Most yellow-legged gull chicks reach the adult skeletal size by this age and, therefore, the reduced size shown in socially stressed individuals is likely to have long-term fitness consequences (38).
The negative consequences of living with stressed siblings were also evidenced by reduced feather quality and increased oxidative damage of the fledglings. Feather microstructure is impaired by high corticosterone levels at the time of feather development (15). Although corticosterone level at 30 d of age did not differ between experimental groups, it might be possible that the effect of stress cross-over on basal corticosterone was still evident during part of the time period when feather development took place [i.e., between 12 and 40 d of age in this species (39)]. Living with stressed siblings also resulted in increased level of oxidative damage to lipids and proteins when chicks were near fledging, indicating that stress cross-over was oxidatively costly for gull fledglings. These oxidative costs support the general view of glucocorticoid hormones as modulators of oxidative status in vertebrates (22). In birds, prenatal elevation of corticosterone levels is associated with postnatal oxidative costs (40), agreeing with our result of the long-term effect of early exposure to corticosterone on oxidative damage. Importantly, high levels of oxidative damage at fledging are related to a reduction in adult survival in seabirds (41, 42).
In conclusion, our experimental manipulation clearly demonstrated that stressed animals can act by themselves as a source of stress for their siblings, and that such a form of horizontal transfer of stress may entail some possible short-term benefits by enhancing antipredator behavior, but at the cost of growth reduction, oxidative damage accumulation, and a more fragile plumage development. These costs may have important long-term fitness consequences. Future studies should, therefore, explore whether the short-term benefits could outweigh the long-term costs. We only investigated stress cross-over in the family context and in particular among siblings, but the function of social cues in the transfer of stress responses may be a widespread biological phenomenon that should be explored in other social contexts.
Materials and Methods
Study Area and General Procedures.
We performed the study between April and July 2016 in a large breeding colony of yellow-legged gulls on Sálvora Island, northwest Spain. In this species, modal clutch size is three eggs, chicks hatch asynchronously, and broods remain together until fledging (35 to 40 d of age), allowing the young birds to interact for a prolonged period. We surveyed the study area once daily during egg laying and marked 64 three-egg nests with numbered sticks. We visited each nest every day until clutch completion to mark eggs and register egg-laying order. After clutch completion, we assigned the nests randomly to two different experimental groups: “control” or “stress” broods. To disrupt any potential stress covariation between parental and offspring phenotype, we cross-fostered the whole clutch between pairs of nests that had similar laying dates (±1 d) within each experimental group. We checked each nest daily, beginning 2 d before the estimated hatching date. Because yellow-legged gull chicks are semiprecocial and can move far away from their nest, before hatching we installed a fenced enclosure around each nest to keep chicks in their territory (see ref. 43 for further details about fence dimension). At hatching, we marked all chicks with numbered leg flags made with Velcro. In some nests, two or more eggs failed to hatch (control, n = 6; stress, n = 5), so the final sample size was 53 nests (control, n = 27 nests; stress, n = 26 nests).
The study was carried out with permission granted by the authorities of Parque Nacional de las Islas Atlánticas (364/RX598377). All experimental procedures complied with the standards of animal experimentation and animal welfare established under current Spanish law (RD53/2013) and were approved by the Xunta de Galicia review board.
Experimental Manipulation of the Level of Social Stress Within a Brood.
In all broods, two chicks were s.c. implanted between the shoulders with a 10-mm surgical silastic tube (Dow Corning; BB518-58) when they were 1 d old (hereafter “implanted” chicks). In the stress broods, implants were filled with crystallized corticosterone (Sigma-Aldrich; 27840), whereas in the control broods, implants were empty (sham). Therefore, one chick per brood was kept without being manipulated (hereafter “nonimplanted” chick). The chick hatched from either the first- or the second-laid egg was randomly assigned as the nonimplanted chick in each nest. Chicks hatched from third-laid eggs were always implanted (corticosterone or sham) and kept with their siblings but not taken into account in the study. We decided to focus on only the first two hatched chicks (one implanted and one nonimplanted) for several reasons. The first two chicks have similar competitive abilities, but the third suffers a competitive disadvantage (44). Thus, by focusing on the first two chicks, we controlled the effect of sibling competition on stress hormone level (45), maximized sample size, and reduced researcher disturbance (in our study population the first two chicks showed a synchronous hatching, i.e., minimizing the number of visits per nest).
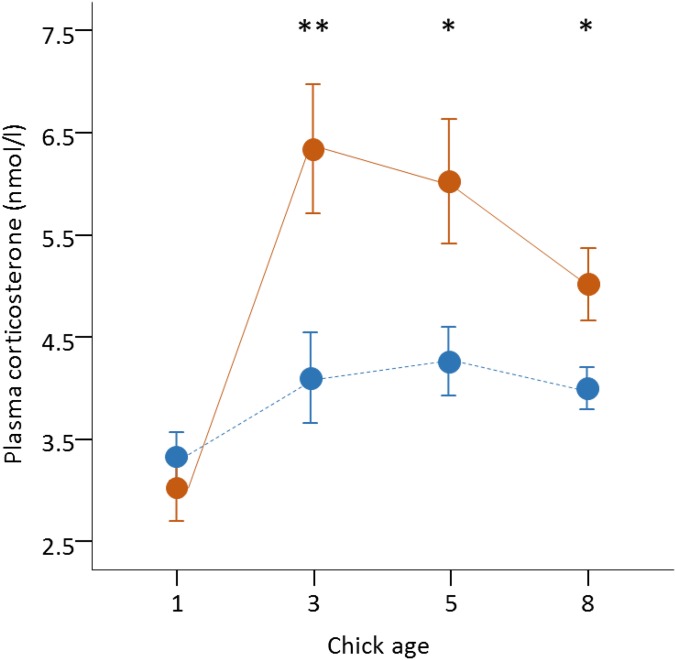
Implants were inserted under the skin through a small incision (2 to 3 mm) that was then sealed with surgical glue (Vetbond; 3M). All incisions healed well, and no bird showed any permanent detrimental effects from the surgical procedures. A pilot study confirmed that our corticosterone implants successfully elevated basal corticosterone levels for at least the following 7 d after implantation and within the normal range of variation in this colony [square-root transformed range, 3.8 to 18.5 nmol/L (43); Fig. S3].
Fig. S3.
Corticosterone implants increased basal corticosterone levels in yellow-legged gull chicks (pilot study). Basal corticosterone levels (square root-transformed; mean ± SE) during the first 8 d after hatching in yellow-legged gull chicks from control (blue) and stress (orange) broods. Significant differences between groups: *P ≤ 0.05, **P ≤ 0.01.
Sampling.
We blood-sampled and measured the first two hatched chicks in each brood at days 1 (just before the implantation) and 8 of age. We collected blood samples from the brachial vein with heparinized capillary tubes, measured their tarsus length (±0.1 mm), and weighed them (±1 g) using a Pesola spring balance. Blood samples were always collected within 3 min of capture to avoid any increase of baseline corticosterone levels as a consequence of handling (46). Blood samples were kept cold until plasma was separated from red blood cells (within a few hours after collection) and stored in liquid nitrogen. Red blood cells from day 1 were used for molecular sexing of the chicks as described (47). We assessed the antipredator response of the chicks at 9 d of age by assessing the latency to respond to adult alarm calls (SI Materials and Methods).
To avoid the stress of reduced territory size, we removed the enclosures around the nests after blood sampling the chicks at 8 d of age. We marked all of the nonimplanted chicks with a numbered plastic ring with an individual three-digit combination to facilitate their long-term identification. At 30 d of age, when nonimplanted chicks were fully grown and near fledging, we searched for them around their territories. For all nonimplanted chicks we found alive (n = 27; 14 control and 13 stress), we took a third blood sample (within 3 min of capture), measured their tarsus length, and weighed them (±5 g). We also plucked the third primary cover feather (counted from the outermost) of the left wing and one of the central scapular feathers to assess the effect of experimental manipulation on feather quality. Feathers were stored in small paper envelopes until analysis (see below). Although the majority of birds had already developed their primary covers and scapulars by 30 d of age, in four birds feathers were still growing the day of sampling and therefore the birds were not feather-sampled (one control and three stress). For each feather, we determined barb and barbule density (SI Materials and Methods).
Biochemical Analyses.
In plasma sampled at age 1, 8, and 30 d, we measured basal corticosterone, uric acid (UA), and triglyceride (TRIG) levels using commercially available kits, and protein levels as described (48) (details can be found in SI Materials and Methods). We also measured different biomarkers of antioxidant defense [nonenzymatic total antioxidant capacity (TAC) and glutathione peroxidase (GPx)] and lipid and protein oxidative damage [malondialdehyde (MDA) and advanced oxidation protein product (AOPP) level] in the blood samples taken from nonimplanted chicks at 30 d of age (SI Materials and Methods).
Statistical Analyses.
We used linear mixed-effect models (LMMs) to test the effect of experimental manipulations [brood treatment (control vs. stress) and chick manipulation (implanted vs. nonimplanted)] on chicks sampled at 1 and 8 d of age. Brood identity and chick identity were included as random terms. We also ran an LMM to examine the effect of experimental manipulations on chick antipredator behavior (time to crouch), including brood identity as a random term. In nonimplanted birds recaptured at 30 d of age, we analyzed the effect of stress treatment using linear models (LMs). Residuals obtained from the models were always normally distributed. We report results for full models (Table S5 for sample sizes) after removing nonsignificant interactions (49). Additional details are provided in SI Materials and Methods.
Table S5.
Summary of final sample sizes used in the statistical analyses
| Age | Tarsus | Body mass | CORT | TRIG | UA | Protein | TC | TAC | GPx | AOPP | MDA | FQ |
| Day 1 | 98 (49C, 49S) | 98 (49C, 49S) | 40 (20C, 20S) | 82 (40C, 42S) | 82 (40C, 42S) | 88 (43C, 45S) | ||||||
| Day 8 | 88 (45C, 43S) | 88 (45C, 43S) | 82 (42C, 40S) | 76 (39C, 37S) | 76 (39C, 37S) | 80 (42C, 38S) | ||||||
| Day 9 | 61 (31C, 30S) | |||||||||||
| Day 30 | 27 (14C, 13S) | 27 (14C, 13S) | 27 (14C, 13S) | 25 (13C, 12S) | 25 (13C, 12S) | 26 (13C, 13S) | 25 (13C, 12S) | 27 (14C, 13S) | 26 (13C, 13S) | 27 (14C, 13S) | 23 (13C, 10S) |
Sample sizes for each experimental group (C, control; S, stress) are indicated. Sample sizes differ among analyses because of the death or loss of chicks and/or insufficient volume of sample. Note that only nonimplanted chicks were recaptured at 30 d of age. See main text for details. CORT, corticosterone; FQ, feather quality; TC, time to crouch.
SI Materials and Methods
Pilot Study of Corticosterone Implants.
To validate the effectiveness of our corticosterone implants in increasing basal corticosterone levels in yellow-legged gull chicks, we conducted a pilot study on a sample of nonexperimental nests (n = 6 nests). Half of the nests were randomly allocated to the “control” treatment and the other half to the “stress” treatment just before hatching. As in the main text, we installed a fence enclosure around the nests (see ref. 43 for further details about fence dimension) to keep the chicks in their territories. One day after hatching, and following the same protocol as in the main text (Materials and Methods), in the stress group two chicks per nest were s.c. implanted between the shoulders with a 10-mm surgical silastic tube (Dow Corning; BB518-58) filled with crystallized corticosterone (Sigma-Aldrich; 27840), whereas the same chicks in the control group received a sham (empty) implant. One chick per brood (the first or second hatched) was always left without being manipulated to mimic the same experimental conditions as followed in the main text (see Materials and Methods for further details). Implants were inserted under the skin through a small incision (2 to 3 mm) that was then sealed with surgical glue (Vetbond; 3M). All chicks that were implanted were blood-sampled at days 1 (just before being implanted), 3, 5, and 8 of age. Blood samples were collected from the brachial vein with heparinized capillary tubes and within 3 min of capture to avoid any increase of baseline corticosterone levels as a consequence of handling. Blood samples were kept cold until they were centrifuged (within a few hours after collection). Plasma was separated from red blood cells by centrifugation (6 min at 600 × g) and stored in liquid nitrogen. We measured corticosterone concentration in plasma sampled at ages 1, 3, 5, and 8 d using commercial kits (ELISA Kit EIA-4164; DRG Diagnostics) and following the manufacturer’s instructions (see below). Three chicks (two control and one stress) died over the course of the pilot study.
To test the effectiveness of our corticosterone implants, we ran linear mixed-effect models (LMMs) with corticosterone concentration as a dependent variable (square root-transformed). In the model, we included brood treatment (control or stress), age, and their two-way interaction as fixed factors, and brood identity and chick identity as random terms. Chick (laying) order was also included in the models to control for any influence of laying order on basal corticosterone levels. The analyses confirmed the effectiveness of our treatment (Fig. S3); corticosterone-implanted chicks had higher corticosterone levels than sham-implanted chicks during the next 7 d after the implantation (age: F1,35 = 9.904, P < 0.001; brood treatment: F1,35 = 15.061, P < 0.001; age × brood treatment: F3,35 = 3.545, P = 0.024). Chick order was not significant in the model (F2,35 = 1.760, P = 0.187).
Behavioral Test.
We assessed the antipredator response of chicks at 9 d of age by following a test previously described for yellow-legged gull chicks (44), with minor modifications. The day of the behavioral test, we transported the chicks from their nests to the observation site in individual cloth bags. The observation site was placed outside the dense breeding colony to avoid disturbance by gull noise during the behavioral tests. We carried out the behavioral tests inside an enclosure with opaque plastic walls (120 × 50 × 50 cm) on the ground. The plastic walls prevented external visual stimuli but allowed the light conditions inside the arena to be similar to external levels. In the center of the arena, a speaker (BSP; 60 W) was hanging 75 cm above the ground. During the test, the chick was placed in the center of the arena, just under the speaker, and covered with a dark mesh until it was calm and quiet; the behavioral test began immediately after uncovering the chick. The test consisted of exposing the chick to a playback of adults’ alarm calls for 10 s. We selected a volume as similar as possible to that the chicks can normally hear in the colony and used the same playback and volume setting in all experimental subjects. The alarm calls used in the tests were recorded in the same breeding colony but outside the area where we carried out the experiment. The chick behavior during the test was recorded using a digital video camera (Sony; Handycam DCR-SX44) placed at a distance of 1.5 m from the arena. During the tests, the chicks did not have visual contact with the experimenters. From video records and blind to the treatment, we quantified the time to crouch of each chick after listening to the alarm calls as a measure of antipredator behavior (23, 44).
Quantification of Plasma Corticosterone Level.
We measured corticosterone concentration in plasma sampled at ages 1, 8, and 30 d using a commercially available ELISA (ELISA Kit EIA-4164; DRG Diagnostics), following the manufacturer’s instructions. Briefly, plasma samples (20 µL) were incubated with a corticosterone–horseradish peroxidase conjugate for 60 min in a microtiter plate. Afterward, the microtiter plate was washed three times and allowed to react with a substrate solution leading to a blue-green complex. The change in absorbance at 450 nm (Synergy 2 Multi-Mode Microplate Reader; BioTek Instruments) of the blue-green complex was reverse-proportional to the concentration of corticosterone. Because of the small plasma volume available at day 1 of age, we were only able to measure corticosterone levels in a subsample of birds at day 1 (n = 40; 20 stress and 20 control chicks, respectively). Samples were analyzed in duplicate, and the mean value was used in the analysis. The assay showed high repeatability (r = 0.95, F148,149 = 43.71, P < 0.001, n = 149). The cross-reactivity of the antibody with respective related substances was negligible (e.g., deoxycorticosterone 3.4%, 11-dehydrocorticosterone 1.6%, cortisol and other steroids <0.1%).
Uric Acid, Triglyceride, and Protein Content in Plasma.
We measured the UA and TRIG levels of all plasma samples (days 1, 8, and 30) with commercially available kits (Biosystems, Barcelona; COD 11521 and COD 11528). The kits were based on the uricase/peroxidase method and the glycerol phosphate oxidase/peroxidase method, respectively. Plasma samples (10 µL) were run in duplicate, and the concentration of UA (mg/dL) and TRIG (mg/dL) was estimated from the sample absorbance at 520 and 505 nm, respectively. Plasma protein content (mg/mL) was determined spectrophotometrically as described in Grimsley and Pace (48). Samples were always assessed in duplicate, and the mean value was used in the analyses. All assays were repeatable (UA: r = 0.98, F182,183 = 251.88, P < 0.001, n = 183; TRIG: r = 0.96, F182,183 = 53.60, P < 0.001, n = 183; protein: r = 0.78, F193,194 = 5.99, P < 0.001, n = 194).
Quantification of Oxidative Status.
We measured different biomarkers of antioxidant defense and oxidative damage in the blood samples taken from nonimplanted chicks at 30 d of age. We measured the nonenzymatic total antioxidant capacity (TAC) of plasma using the method described by Erel (50). During the assay, plasma samples (5 μL) were diluted in acetate solution and reacted with ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) and the change in absorbance at 660 nm was measured. In the assay, ABTS is decolorized by antioxidants according to their concentration and antioxidant capacity (50). The assays were calibrated with Trolox, a water-soluble α-tocopherol derivative. Enzymatic antioxidant defenses were assessed by analyzing red blood cell (RBC) activities of glutathione peroxidase (GPx), an important intracellular enzyme that catalyzes the reduction of hydroperoxides. GPx activity was measured in RBC lysate (20 µL diluted 1:5 in PBS) using a commercial kit (Cayman Chemical; 703102) and following the manufacturer’s instructions. The method was based on the change in absorbance at 340 nm as a consequence of oxidation of NADPH to NADP+, which is directly proportional to the GPx activity of the sample. We also determined the level of lipid peroxidation (level of oxidative damage in lipids) by measuring malondialdehyde (MDA) as described previously by the thiobarbituric acid reactive substances (TBARS) spectrophotometric test (51). Briefly, plasma samples (20 µL) were mixed with a solution containing trichloroacetic acid (15% wt/vol), thiobarbituric acid (0.38% wt/vol), and hydrochloric acid (0.25 M). The mixture was heated at 90 °C for 30 min and centrifuged for 1 min at 10,000 × g, and the absorbance of the supernatant was measured at 535 nm. The total content of MDA was determined from a calibration curve made with the MDA standard and expressed as µmol TBARs/L. We also measured the concentration of advanced oxidation protein products (AOPPs) in the plasma samples. AOPP concentration was assessed by the method described by Witko-Sarsat et al. (52) but reducing the volume of reagents as specified in ref. 53. During the assay, plasma samples (40 µL) were mixed with phosphate buffer saline and incubated in the dark, and then the absorbance of the mixture was read at 340 nm. Then, 20 µL of acetic acid was added to the mixture and incubated, followed by the addition of 10 µL potassium iodide. The mixture was left to react for 30 s in the dark and the absorbance at 340 nm was read again. The AOPP concentration was calculated from a calibration curve made with chloramine T as the standard. In all of the oxidative stress assays previously described, samples were run in duplicate and the mean value was used in the analyses. All assays were repeatable (TAC: r = 0.98, F24,25 = 117.50, P < 0.001, n = 25; GPx: r = 0.97, F26,27 = 88.43, P < 0.001, n = 27; MDA: r = 86, F26,27 = 12.78, P < 0.001, n = 27; AOPP: r = 0.93, F25,26 = 35.26, P < 0.001, n = 26).
Feather Quality.
For each feather, we determined barb and barbule density following the methodology previously described (54), with some minor modifications. Feathers were placed between two microscope slides and photographed with a digital camera (Nikon; 1200F at 4 megapixels) connected to a dissecting scope (Nikon; SMZ1500) and imported into analySIS FIVE software (Olympus). Barb density was measured on the inner vane, at three different points along the rachis (bottom, middle, and top) by measuring the distance occupied by five consecutive barbs. Similarly, barbule density was estimated by measuring the distance occupied by five consecutive barbules measured at three different points (bottom, middle, and top) of three randomly selected barbs within each section of the rachis (bottom, middle, and top). For the analyses, we used the average barb and barbule density (transformed to the number of barbs or barbules per micrometer) for each feather type (primary cover and scapular).
Statistical Analyses.
We tested the effects of brood treatment on chick growth (tarsus length and body mass), basal corticosterone levels, and triglyceride and protein content of chicks between 1 and 8 d of age using LMMs. The models included brood treatment (control or stress), chick manipulation (implanted or not), age, and the sex of the chick as fixed factors. Two-way interactions between fixed factors and the three-way interaction between brood treatment, chick manipulation, and age were also tested. Brood identity and chick identity were included as random terms in the models to account for the nonindependence of chicks from the same family and samples from the same individual. We also ran an LMM to investigate the effect of treatment on chick antipredator behavior (time to crouch). This model included brood treatment, chick manipulation, sex, and their two- and three-way interactions as fixed factors and brood identity as a random term.
In nonimplanted birds recaptured at 30 d of age, we analyzed the effect of our experimental treatments on growth (tarsus length and body mass), basal corticosterone level, triglyceride and protein content, oxidative stress markers (TAC, GPx, AOPP, and MDA), and feather quality (barb and barbule density) using linear models (LMs). These models included brood treatment, sex, and their interaction as fixed factors. In the models of MDA and TAC, we also included plasma triglycerides and uric acid, respectively, as covariates to control for any potential effect of recent food intake (55, 56). Because the feather microstructure can vary substantially among feather types (57), we ran the models on barb and barbule density separately for each type of feather (wing and body feathers).
In all models, we included laying order (first or second) to control for any potential variation due to prenatal maternal effects (i.e., egg size and composition) or differences in the level of sibling competition. When needed, variables were log- (AOPP, triglycerides, barb and barbule density, and time to crouch) or square root-transformed (corticosterone levels) to improve data distribution. Residuals obtained from the models were always normally distributed. We reported results from full models after removing nonsignificant interactions (49). The Satterthwaite approximation was used for the estimation of degrees of freedom. Sample sizes among analyses can slightly differ because of the death or loss of chicks and/or insufficient volume of sample (a detailed description of sample sizes used in each analysis is provided in Table S5). Data are presented as mean ± SE, and significance level was set at P = 0.05.
Ethical Note.
The study was carried out with permission granted by the authorities of Parque Nacional de las Islas Atlánticas (364/RX598377). All experimental procedures complied with the standards of animal experimentation and animal welfare established under current Spanish law (RD53/2013) and were approved by the Xunta de Galicia review board.
Acknowledgments
We are grateful to the staff at the Parque Nacional de las Islas Atlánticas for their logistic support, particularly Pablo and Roberto. We especially thank Neil B. Metcalfe and three anonymous reviewers for constructive comments on an earlier version of the manuscript, and the lighthouse keeper Pepe for his help during the last 10 y of field work at Sálvora Island. During the study, J.C.N. was funded by a Juan de Cierva fellowship (IJCI-2014-20246). The study was also funded by MINECO (CGL2015-69338-C2-1-P).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data reported in this paper have been deposited in the Figshare digital repository, https://doi.org/10.6084/m9.figshare.4653841.v1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706164114/-/DCSupplemental.
References
- 1.Nunn CL, Craft ME, Gillespie TR, Schaller M, Kappeler PM. The sociality–health–fitness nexus: Synthesis, conclusions and future directions. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140115. doi: 10.1098/rstb.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danchin E, Giraldeau L-A, Valone TJ, Wagner RH. Public information: From nosy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- 3.Dall SR, Giraldeau L-A, Olsson O, McNamara JM, Stephens DW. Information and its use by animals in evolutionary ecology. Trends Ecol Evol. 2005;20:187–193. doi: 10.1016/j.tree.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Wingfield JC. Ecological processes and the ecology of stress: The impacts of abiotic environmental factors. Funct Ecol. 2013;27:37–44. [Google Scholar]
- 5.Wingfield JC, Kitaysky AS. Endocrine responses to unpredictable environmental events: Stress or anti-stress hormones? Integr Comp Biol. 2002;42:600–609. doi: 10.1093/icb/42.3.600. [DOI] [PubMed] [Google Scholar]
- 6.Monaghan P. Organismal stress, telomeres and life histories. J Exp Biol. 2014;217:57–66. doi: 10.1242/jeb.090043. [DOI] [PubMed] [Google Scholar]
- 7.Monaghan P, Heidinger BJ, D’Alba L, Evans NP, Spencer KA. For better or worse: Reduced adult lifespan following early-life stress is transmitted to breeding partners. Proc Biol Sci. 2012;279:709–714. doi: 10.1098/rspb.2011.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westman M. Stress and strain crossover. Hum Relat. 2001;54:717–751. [Google Scholar]
- 9.Boonstra R. Equipped for life: The adaptive role of the stress axis in male mammals. J Mammal. 2005;86:236–247. [Google Scholar]
- 10.Creel S, Dantzer B, Goymann W, Rubenstein DR. The ecology of stress: Effects of the social environment. Funct Ecol. 2013;27:66–80. [Google Scholar]
- 11.Romero LM. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol Evol. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Romero ML, Butler LK. Endocrinology of stress. Int J Comp Psychol. 2007;20:89–95. [Google Scholar]
- 13.Voellmy IK, Goncalves IB, Barrette M-F, Monfort SL, Manser MB. Mean fecal glucocorticoid metabolites are associated with vigilance, whereas immediate cortisol levels better reflect acute anti-predator responses in meerkats. Horm Behav. 2014;66:759–765. doi: 10.1016/j.yhbeh.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Thaker M, Lima SL, Hews DK. Acute corticosterone elevation enhances antipredator behaviors in male tree lizard morphs. Horm Behav. 2009;56:51–57. doi: 10.1016/j.yhbeh.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Seeman TE, Crimmins E. Social environment effects on health and aging: Integrating epidemiologic and demographic approaches and perspectives. Ann N Y Acad Sci. 2001;954:88–117. doi: 10.1111/j.1749-6632.2001.tb02749.x. [DOI] [PubMed] [Google Scholar]
- 16.Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: A review. Curr Zool. 2011;57:514–530. [Google Scholar]
- 17.Selye H. Stress in Health and Disease. Butterworths; London: 2013. [Google Scholar]
- 18.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 21.DesRochers DW, et al. Exogenous and endogenous corticosterone alter feather quality. Comp Biochem Physiol A Mol Integr Physiol. 2009;152:46–52. doi: 10.1016/j.cbpa.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Costantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–456. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- 23.Tinbergen N. The Herring Gull’s World: A Study of the Social Behaviour of Birds. Collins; London: 1953. [Google Scholar]
- 24.Alonso-Alvarez C, Ferrer M. A biochemical study of fasting, subfeeding, and recovery processes in yellow-legged gulls. Physiol Biochem Zool. 2001;74:703–713. doi: 10.1086/322932. [DOI] [PubMed] [Google Scholar]
- 25.Mathevon N, Charrier I. Parent-offspring conflict and the coordination of siblings in gulls. Proc Biol Sci. 2004;271:S145–S147. doi: 10.1098/rsbl.2003.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubolini D, et al. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm Behav. 2005;47:592–605. doi: 10.1016/j.yhbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Spencer KA, Verhulst S. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata) Horm Behav. 2007;51:273–280. doi: 10.1016/j.yhbeh.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Asnani MV, Ramachandran AV. Roles of adrenal and gonadal steroids and season in uropygial gland function in male pigeons, Columba livia. Gen Comp Endocrinol. 1993;92:213–224. doi: 10.1006/gcen.1993.1157. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe S, Ono K. An experimental analysis of “empathic” response: Effects of pain reactions of pigeon upon other pigeon’s operant behavior. Behav Processes. 1986;13:269–277. doi: 10.1016/0376-6357(86)90089-6. [DOI] [PubMed] [Google Scholar]
- 31.Schwing R, Nelson XJ, Wein A, Parsons S. Positive emotional contagion in a New Zealand parrot. Curr Biol. 2017;27:R213–R214. doi: 10.1016/j.cub.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Edgar JL, Lowe JC, Paul ES, Nicol CJ. Avian maternal response to chick distress. Proc Biol Sci. 2011;278:3129–3134. doi: 10.1098/rspb.2010.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause J, Ruxton GD. Living in Groups. Oxford Univ Press; New York: 2002. [Google Scholar]
- 34.Boonstra R. Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct Ecol. 2013;27:11–23. [Google Scholar]
- 35.Krause J, James R, Franks D, Croft DP. Animal Social Networks. Oxford Univ Press; New York: 2014. [Google Scholar]
- 36.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc Natl Acad Sci USA. 2015;112:4690–4695. doi: 10.1073/pnas.1420068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scanes CG. Perspectives on the endocrinology of poultry growth and metabolism. Gen Comp Endocrinol. 2009;163:24–32. doi: 10.1016/j.ygcen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Reiss MJ. The Allometry of Growth and Reproduction. Cambridge Univ Press; Cambridge, UK: 1991. [Google Scholar]
- 39.Kadlec JA, Drury WH, Jr, Onion DK. Growth and mortality of herring gull chicks. Bird-Banding. 1969;40:222–233. [Google Scholar]
- 40.Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc Biol Sci. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguera JC, Kim SY, Velando A. Pre-fledgling oxidative damage predicts recruitment in a long-lived bird. Biol Lett. 2012;8:61–63. doi: 10.1098/rsbl.2011.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herborn KA, et al. Age, oxidative stress exposure and fitness in a long‐lived seabird. Funct Ecol. 2015;30:913–921. [Google Scholar]
- 43.Kim SY, Noguera JC, Tato A, Velando A. Vitamins, stress and growth: The availability of antioxidants in early life influences the expression of cryptic genetic variation. J Evol Biol. 2013;26:1341–1352. doi: 10.1111/jeb.12136. [DOI] [PubMed] [Google Scholar]
- 44.Diaz-Real J, Kim S-Y, Velando A. Hatching hierarchy but not egg-related effects governs behavioral phenotypes in gull chicks. Behav Ecol. 2016;27:1782–1789. [Google Scholar]
- 45.Rensel MA, Wilcoxen TE, Schoech SJ. Corticosterone, brood size, and hatch order in free-living Florida scrub-jay (Aphelocoma coerulescens) nestlings. Gen Comp Endocrinol. 2011;171:197–202. doi: 10.1016/j.ygcen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Romero LM, Reed JM. Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp Biochem Physiol A Mol Integr Physiol. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths R, Tiwari B. The isolation of molecular genetic markers for the identification of sex. Proc Natl Acad Sci USA. 1993;90:8324–8326. doi: 10.1073/pnas.90.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grimsley GR, Pace CN. Spectrophotometric determination of protein concentration. Curr Protoc Protein Sci. 2004;33:3.1.1–3.1.9. doi: 10.1002/0471140864.ps0301s33. [DOI] [PubMed] [Google Scholar]
- 49.Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP. Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol. 2006;75:1182–1189. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 50.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Costa CM, dos Santos RC, Lima ES. A simple automated procedure for thiol measurement in human serum samples. J Bras Pat Med Lab. 2006;42:345–350. [Google Scholar]
- 52.Witko-Sarsat V, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 53.Selmeci L, et al. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: A simple, fast and inexpensive automated technique. Clin Chem Lab Med. 2005;43:294–297. doi: 10.1515/CCLM.2005.050. [DOI] [PubMed] [Google Scholar]
- 54.Pap PL, Vágási CI, Bărbos L, Marton A. Chronic coccidian infestation compromises flight feather quality in house sparrows Passer domesticus. Biol J Linn Soc Lond. 2013;108:414–428. [Google Scholar]
- 55.Cohen A, Klasing K, Ricklefs R. Measuring circulating antioxidants in wild birds. Comp Biochem Physiol B Biochem Mol Biol. 2007;147:110–121. doi: 10.1016/j.cbpb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Rodríguez L, et al. Measuring oxidative stress: The confounding effect of lipid concentration in measures of lipid peroxidation. Physiol Biochem Zool. 2015;88:345–351. doi: 10.1086/680688. [DOI] [PubMed] [Google Scholar]
- 57.Grubb TC. Ptilochronology: Feather Time and the Biology of Birds. Oxford Univ Press; Cambridge, UK: 2006. [Google Scholar]