Significance
Gliomas infiltrate into surrounding healthy brain tissue. Microsurgical resection aims for maximal tumor resection while minimizing morbidity. Surgical margins are defined based on the surgeon’s experience, visual observation, and neuronavigation. Surgical margin assessment is rarely undertaken intraoperatively due to time constraints and unreliability of such evaluation. Routine, pathologic intraoperative examination provides no molecular information. Molecular measurements using mass spectrometry can be made rapidly on tissue during surgery to identify tissue types, estimate tumor infiltration, and recognize the presence of prognostic mutations by monitoring oncometabolites and phospholipids. This intraoperative study demonstrates the power of mass spectrometry in assessing diagnostic and prognostic information on discrete surgeon-defined points along the resection margins to improve tumor resection, even in regions without MRI contrast enhancement.
Keywords: ambient ionization, neurological smears, tumor infiltration, lipids, glioma
Abstract
Intraoperative desorption electrospray ionization-mass spectrometry (DESI-MS) is used to characterize tissue smears by comparison with a library of DESI mass spectra of pathologically determined tissue types. Measurements are performed in the operating room within 3 min. These mass spectra provide direct information on tumor infiltration into white or gray brain matter based on N-acetylaspartate (NAA) and on membrane-derived complex lipids. The mass spectra also indicate the isocitrate dehydrogenase mutation status of the tumor via detection of 2-hydroxyglutarate, currently assessed postoperatively on biopsied tissue using immunohistochemistry. Intraoperative DESI-MS measurements made at surgeon-defined positions enable assessment of relevant disease state of tissue within the tumor mass and examination of the resection cavity walls for residual tumor. Results for 73 biopsies from 10 surgical resection cases show that DESI-MS allows detection of glioma and estimation of high tumor cell percentage (TCP) at surgical margins with 93% sensitivity and 83% specificity. TCP measurements from NAA are corroborated by indirect measurements based on lipid profiles. Notably, high percentages (>50%) of unresected tumor were found in one-half of the margin biopsy smears, even in cases where postoperative MRI suggested gross total tumor resection. Unresected tumor causes recurrence and malignant progression, as observed within a year in one case examined in this study. These results corroborate the utility of DESI-MS in assessing surgical margins for maximal safe tumor resection. Intraoperative DESI-MS analysis of tissue smears, ex vivo, can be inserted into the current surgical workflow with no alterations. The data underscore the complexity of glioma infiltration.
We describe the rapid analysis of neurological tissue smears by desorption electrospray ionization-mass spectrometry (DESI-MS) in the operating room (OR) from 10 subjects who underwent glioma resection. Biopsied tissue specimens from surgeon-defined positions in the tumor and the walls of the resection cavity were smeared onto glass microscope slides and sprayed with charged solvent droplets to extract molecules from the unprocessed tissue while the splashed secondary droplets were vacuumed into a customized ion-trap mass spectrometer modified for use in the OR at Indianapolis IU (Indiana University) Health Methodist Hospital. Three separate items of information were sought from the DESI mass spectra: (i) tissue type, specifically whether glioma, white brain matter, gray brain matter, or mixtures of these types; (ii) isocitrate dehydrogenase (IDH) status, i.e., whether or not this enzyme carries a characteristic mutation, the presence of which is associated with more favorable prognosis; and (iii) the tumor cell percentage (TCP) in the sampled biopsy as a measure of tumor infiltration (the latter is arguably the most actionable intraoperatively and the one for which the least information is currently available).
The infiltrative nature of most gliomas, as well as visual and textural similarities between infiltrative regions and normal brain parenchyma, poses a substantial challenge to the neurosurgeon during tumor resection. Microsurgical resection aims to maximize tumor excision while minimizing morbidity (1, 2) as the extent of resection affects both overall and progression-free survival. Improved survival and quality of life correlates with maximal resection of both low- and high-grade gliomas (3). Importantly, tumor recurrence and malignant progression is likely to occur from residual tumor within 0.5 cm of the resection (i.e., surgical) margin, viz., the point at which resection ceases (3). Stereotactic volumetric resection is usually based on preoperative structural and functional MRI used to determine tumor location, size, surgical approach, and to estimate the extent of resection achievable. Intraoperative MRI has been implemented to mitigate anatomical shifts and improve resection; however, MRI costs and technical hurdles, such as prolonged surgery time for image acquisition, limit its practical and routine implementation (1).
The standard diagnostic approach of intraoperative consultation, pathology, provides information on tumor type and grade. Intraoperative histopathological evaluation of tissue resected from surgical margins to estimate residual tumor infiltration is uncommon, partly because preparation and analysis of tissue sections or smears is laborious and time-consuming, and partly due to the inherent difficulty in assessing degree of infiltration in suboptimal preparations. Therefore, the amount of residual tumor near the resection margins is not usually measured during surgery and is only assessed postoperatively and indirectly by MRI (1, 4).
Following the publication of the 2016 WHO classification of central nervous system tumors (5), immunohistochemistry and molecular testing are also performed on sampled neurological tissue to evaluate several diagnostic and prognostic markers (e.g., IDH mutation and 1p/19q codeletion) but results again are available only postoperatively. The need for near-real-time assessment of tumor pathology, genetics, and margin status during surgical resection in predicting patient outcome would suggest advantages to a multimodal approach in which imaging and diagnostic technologies are coupled.
The analysis of neurological tissue smears using DESI-MS has the potential to become an ex vivo diagnostic strategy that can provide information on each of three points that we discuss in turn: (i) Tissue type, specifically glioma, white brain matter, gray brain matter, or a mixture of these. The use of ambient ionization MS methods, specifically DESI (6) and rapid evaporative ionization mass spectrometry (REIMS) (7), to distinguish diseased from healthy tissue based on membrane-derived phospholipid signatures is now well established. Note that an alternative method of molecular diagnosis of neurological tissue in the form of Raman spectroscopy is being developed concurrently (8, 9). Both DESI-MS and REIMS have been applied to different organs (7, 10–14), generally with excellent disease discrimination via comparison with spectra of pathology-defined reference material. REIMS requires the use of special surgical procedures but gives real-time information. DESI gives results in a few minutes but is applicable to any surgical method, with no alteration in procedures, and the same tissue smears can be stained after analysis and blindly evaluated by an expert pathologist for validation. Whereas mass spectral profiles (i.e., chemical signatures) of tissue sections allow accurate differentiation between gliomas and normal brain parenchyma (15–20), we emphasize in this intraoperative study the characterization of tissue smears, especially the recognition of those of mixed compositions. (ii) IDH status. It has previously been shown that the presence of IDH mutation, a prognostic marker in gliomas (21, 22), can be inferred by the MS detection of 2-hydroxyglutarate (2HG) in tissue sections (20). The same discriminating information is sought here in tissue smears––which can be prepared and analyzed inside the OR more easily and rapidly than tissue sections (19, 23)––and this is the approach used in this intraoperative study. (iii) Percentage of tumor cells. Encouraging preliminary results on tissue sections indicated that it might be possible to estimate TCP in tissue via measurements of N-acetylaspartate (NAA). However, this highly important objective needed to be tested in the relevant intraoperative environment, especially on tissue resected near surgical margins for identification of residual tumor, and that is a significant aim of this study.
Results and Discussion
General Observations.
Intraoperative DESI-MS was performed in such a way as to mimic one foreseeable implementation in which a single mass spectrometer is moved into an OR setting just before surgery, powered on, and is ready for use within ∼30 min. The chosen unit mass resolution linear ion-trap mass spectrometer used (17–19) performed well without significant electronic, vacuum, or even mass calibration problems over the 15-mo duration of the study (SI Appendix, Table S1). Fresh tissue samples were smeared and analyzed by DESI-MS within 3 min, a timeframe that addresses surgical needs. In the case of all surgeries, DESI-MS analysis of multiple biopsies was completed long before any intraoperative pathologic consultation findings were reported back to the neurosurgeon by the neuropathologist. The observed timing of in situ, quasi–real-time analysis by DESI-MS supports the fact that multiple measurements––made at discrete points selected by the neurosurgeon––are reliable and have the potential to provide the surgeon with additional information to guide resection maneuvers, particularly near critical anatomical structures.
Assessment of Disease State, TCP, and Isocitrate Dehydrogenase Mutation Status in Tumor Cores.
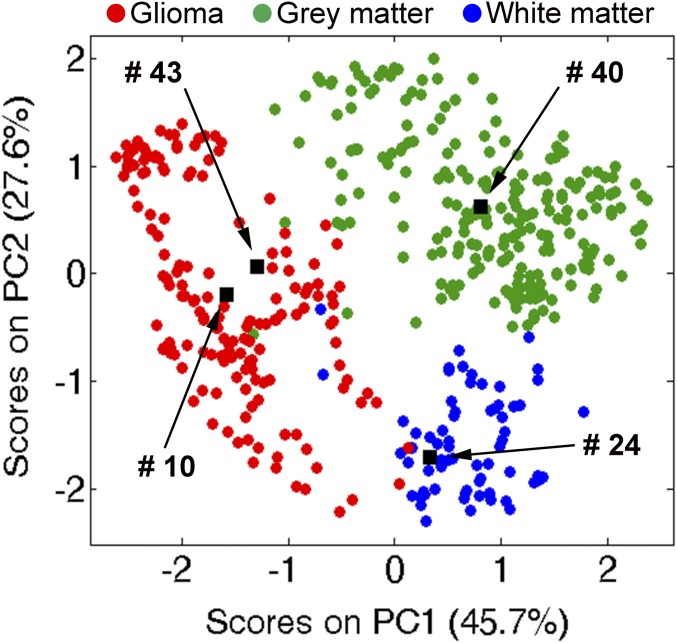
In current practice, biopsies obtained from the tumor core, prepared either as frozen sections or as smears, are evaluated by the pathologist for tumor type, grade, and assessment of IDH mutation. Information on tumor type and grade (i.e., low vs. high) is supplied to the neurosurgeon via telephone––ideally in less than 20 min––whereas IDH assessment requires more time and is only available postsurgery. Intraoperative DESI-MS was designed to predict disease state (i.e., glioma, gray matter, white matter, or mixed states) and TCP (i.e., TCP relative to normal cells). The results of earlier DESI-MS studies suggested also the ability to predict tumor grade and subtype (15). DESI-MS predictions of disease state from tumor core margin smears were compared with data from a library of MS spectra of known tissue types. The comparison was made using principal component (PC1, PC2) representations of the mass spectrum in a fashion analogous to that used earlier for tissue sections (17). Representative data are shown in Fig. 1. The results of these and many other such assignments matched one-to-one those of pathology (Tables 1 and 2). There are two semantic disparities (smears 27 and 28), viz., gliomas infiltrated with ∼50% tumor cells can be logged either as glioma or as white matter with medium TCP based on this chemical information (SI Appendix, Table S2). Intraoperative DESI-MS should ideally complement pathology, and the agreement between chemical and pathological diagnoses for tumor core biopsies validates this expectation.
Fig. 1.
Chemical predictions of disease state for smears 10 (glioma, 99% TCP), 24 (white matter, 37% TCP), 40 (gray matter, 15% TCP), and 43 (glioma, 69% TCP). Projections of the tissue smears (black objects) are imposed on the principal component analysis (PCA) score space created from a reference DESI-MS spectral library (17); green, gray matter; blue, white matter; red, glioma.
Table 1.
Association between chemical predictions of disease state and pathology for tumor cores
| Chemical evaluation of disease state | |||
| Pathological evaluation | Gray matter | White matter | Glioma |
| Infiltrated tissue | 0 | 3 | 1 |
| Glioma | 0 | 2 | 22 |
Table 2.
Association between chemical predictions of TCP vs. pathology for tumor cores
| Chemical evaluation of tumor cell percentage | |||
| Pathological evaluation | Low (<33%) | Medium (34–67%) | High (>67%) |
| Low | 0 | 2 | 2 |
| Medium | 0 | 2 | 1 |
| High | 0 | 0 | 21 |
TCP prediction by DESI-MS supplements the disease state information. Many tumor core biopsy smears have high (>67%) TCP, matching pathology and expectations given that high-grade gliomas tend to be hypercellular. Exceptionally, smear 24 from case 3 (astrocytoma WHO grade III; SI Appendix, Table S1) was infiltrated tissue with low TCP by pathology and was predicted by DESI-MS as white matter with 37% TCP (SI Appendix, Table S2). SI Appendix, Fig. S1 depicts the chemical diversity of smear 24 in comparison with another tumor core biopsy (smear 43) from a WHO grade IV tumor with high TCP. Two smears were prepared from nine tumor core biopsies by splitting the tissue in half. Encouragingly, the disease state was the same and the NAA-based predicted TCP agreed to within 15% (SI Appendix, Table S2).
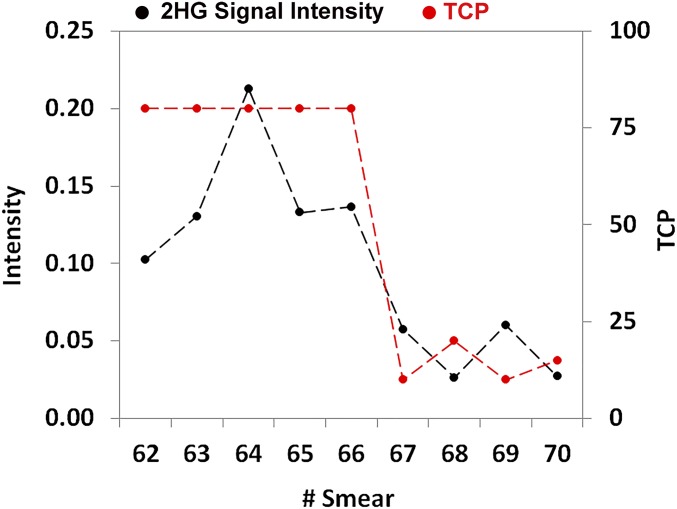
IDH mutation status was assessed intraoperatively via negative-ion DESI-MS detection of 2HG (m/z 147) followed by fragmentation using collision-induced dissociation for structural confirmation. IDH mutation status is currently only determined via immunohistochemistry and genetic testing postoperatively, even though its intraoperative assessment could have surgical utility. More aggressive resection of IDH-mutant tumors, which are associated with better prognosis (21, 22), significantly improves overall and progression-free survival, whereas more aggressive resection of wild-type tumors does not result in such benefits (24). The oncometabolite 2HG was detected in the tumor core biopsies from cases 1, 3, 5, 7, and 9; these were determined postoperatively to be IDH-mutant (SI Appendix, Table S1). In the mutant tumors, the 2HG signal intensity varied between smears, grossly correlating with the degree of tumor infiltration (Fig. 2), as previously observed by DESI-MS for tissue sections (20). Independent studies confirmed a positive correlation between 2HG concentration and tumor cellularity but showed no significant differences between tumor types and grades (24). No 2HG was detected for wild-type tumors (SI Appendix, Fig. S2).
Fig. 2.
Signal intensity of 2HG (m/z 147) normalized to the total ion counts in smears 62–70 for case 9. Secondary axis shows TCP as estimated by pathology in the same smears.
Assessment of Disease State and TCP at Discrete Points near Resection Margins.
Forty-four of 80 biopsies were obtained near surgical margins (within 0.5 cm from where tumor resection ceased). The visualization of the pseudomargin of gliomas and their margin status can be difficult and unreliable. It is also highly unusual and inefficient to use intraoperative pathologic consultation to determine where to cease resection. Postoperative MRI normally assesses the approximate volume of residual tumor. The absence of residual contrast enhancement around the resection cavity is interpreted as macroscopic or gross total resection (4). We have developed two models for estimating residual tumor at discrete points resected at the discretion of the surgeon. One is based on NAA signal intensity whereas the other is a more indirect measure based on the lipid profile of the tissue. Note, although we provide prediction of disease state from smears of margin biopsies, the pathologist does not routinely or exclusively use such samples for the determination of tumor type, grade (such metrics are determined using tumor core biopsies), or margin status when applicable.
N-acetylaspartate model for TCP predictions.
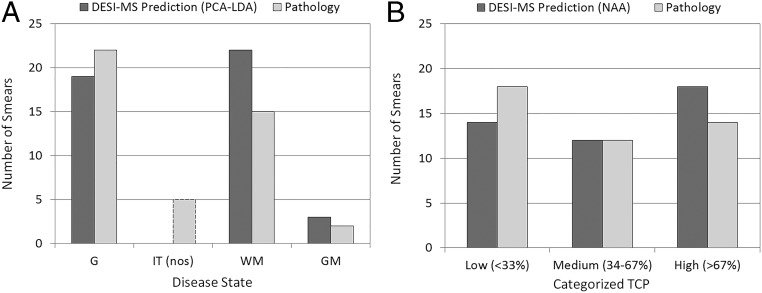
A roughly exponential decrease of NAA signal intensity was observed vs. pathologically measured increase in TCP in tissue sections. This observation has now been extended and confirmed in tissue smears analyzed intraoperatively (SI Appendix, Fig. S3). TCP predictions based on NAA of biopsied tissue near surgical margins ranged between 0% and 100%. Notably, about one-half of the margin tissue smears were infiltrated with more than 50% tumor cells. Fig. 3 shows the frequency of low, medium, and high TCP, as well as of the disease state predictions, both compared with histopathological results. Agreement is good. The absence of a signal for NAA in tissue––when a distinct glioma lipid profile is present––is specific for high-density gliomas (SI Appendix, Table S3). Sensitivity is also high, as just three smears (69, 77–79) showed low NAA signal (TCP estimates between 73–74%; SI Appendix, Table S2) when pathology determined less than 10% tumor cells. The presence of vascular proliferation was noted in these smears. The availability of an intraoperative tool for identification of high (>50%) residual tumor infiltration at surgical margins is essential for neurosurgeons to refine surgical maneuvers. Safe removal of high tumor infiltration is a primary goal of neurosurgery whereas areas of low infiltration are more likely the target of coadjuvant postsurgical therapies. Our current qualitative methodology is less accurate in differentiating between medium and low TCP (SI Appendix, Table S3). The literature suggests that NAA varies with age, gender, and other biological factors (25) that were not controlled in this small study. Biological variability affecting agreement between chemical and pathological estimations manifests itself when NAA is present in the tissue; i.e., in low- and medium-infiltrated tissue.
Fig. 3.
Margin smears (n = 44). (A) Frequency of DESI-MS prediction of disease state vs. pathology. G, glioma; GM, gray matter; IT (nos), infiltrated tissue (not otherwise specified); WM, white matter. DESI-MS has no IT (nos) assignments because all smears were assigned. (B) Frequency of DESI-MS prediction of TCP using NAA vs. pathology. Percentages of tumor cells are categorized as low (<33%), medium (34–67%), and high (>67%).
Lipid profile deconvolution for evaluation of tumor density.
The lipid profile detected by DESI-MS can show the presence of tumor in tissue and distinguishes the background into which the tumor infiltrates; i.e., gray or white brain matter (17). The dynamic changes of DESI-MS lipid profiles acquired from tissue sections were previously interpreted in terms of three-component mixtures of glioma, white matter, and gray matter (SI Appendix, Fig. S4). The dynamics are reflective of the infiltrative nature of gliomas into the surrounding brain parenchyma. A linear regression model was developed to determine composition of gray matter, white matter, and glioma based on a reference spectral library (see details in SI Appendix). We further speculate that the deconvolution of lipid profiles into fractions of white matter, gray matter, and glioma can indicate tumor density and corroborate NAA predictions. In the 44 margin smears analyzed, the lipid deconvolution underscored the complexity of gliomas identifying tumor infiltration into white- (e.g., smear 18) or gray matter (e.g., smear 44), into a mixture of those (e.g., smear 49), or even identifying mixtures of gray and white matter with no detectable presence of tumor cells (e.g., smear 68). SI Appendix, Fig. S5 depicts the dynamic of the lipid profiles reflecting such complexity. This determination is made even more difficult during pathological evaluation by the fact that tumor effaces normal parenchyma resulting in identification of infiltrated tissue (not otherwise specified) [(IT nos); Fig. 3]. Most tumors infiltrate into white matter, so their lipid signatures express high abundances of sulfatides related to myelination of neurons (17). Only a few smears showed infiltration into gray matter (smears 20, 26, 40, 42, 44, 45, 55, 67, and 68; SI Appendix, Table S2). In these cases, pathological evaluation confirmed the presence of gray matter infiltrated with tumor cells or mixed with white matter. As was the case for NAA, tissue near surgical margins showed high heterogeneity in lipid expression. The deconvolution of lipid profiles of the smears into their constituent tissue types accurately identified low- (14 smears of 18) and high-density gliomas (10 smears of 14), whereas less accuracy was obtained for medium-infiltrated tissue (SI Appendix, Table S4). A larger cohort of patients is necessary to develop a more accurate multifactorial regression model based on experimental results rather than using the assumption of linear combination with no interactions.
Residual tumor near surgical margins.
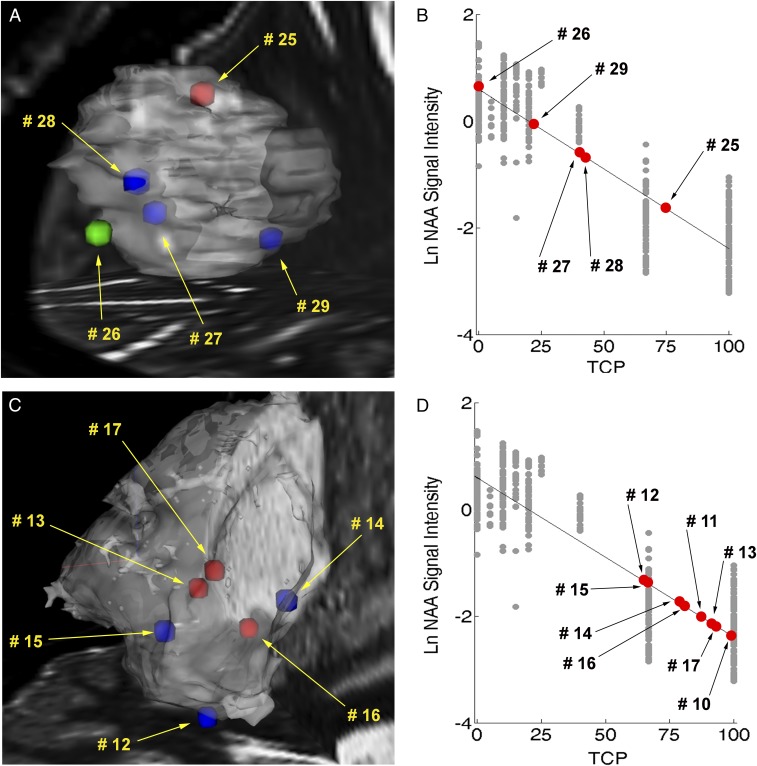
Tumor infiltration varied greatly not only in biopsied tissue resected near surgical margins but also within the epicenter of the tumor. The peculiar behavior of gliomas to infiltrate irregularly into brain matter, mostly following white-matter tracts, was observed in the multiple measurements performed by DESI-MS. We found high TCP near the margins in both high- and low-grade gliomas, with only a weak relationship between TCP and biopsy location within the tumor volume (i.e., decreasing TCP moving from tumor core to margins). Marginal points sometimes showed TCP higher than those measured inside the tumor. As extreme examples, we detail cases 2 and 4. For case 4, glioblastoma WHO grade IV, two biopsies (smears 25 and 26) obtained near surgical margins had TCP (predicted using NAA) of 75% and 0%, respectively, matching pathology (high and low TCP). Two biopsies resected inside the tumor mass showed TCP of about 40% (SI Appendix, Table S2). For case 2, dysembryoplastic neuroepithelial tumor WHO grade I, discrete biopsied tissue resected near the surgical margins showed TCP from 65% to 93% (Fig. 4). Again, marginal points showed TCP higher than discrete locations inside the tumor mass (SI Appendix, Table S2). Similar trends were found in the other cases as well (SI Appendix, Table S2). Remarkably, and unfortunately, postoperative MRI provided no evidence of residual tumor in any of the cases except 1 and 2. Limitations in estimating extent of resection by MRI exist and are largely due to lack of correlation between MRI contrast enhancement and histological composition of the tissue, difficulties in calculating tumor volume, and the occurrence of nonenhancing tumors (4). Also, the volume of the resection cavity does not provide a true indication of the residual microscopic tumor burden (3). Unresected tumor is the primary cause of recurrence and malignant progression. This is shown in cases 1 and 7, which refer to the same subject who underwent glioma resection twice within one year. The primary tumor was diagnosed as oligodendroglioma WHO grade II, using WHO 2007 terminology (SI Appendix, Table S1). The tumor was adherent to medial structures and the lateral thalamus; only partial tumor resection was performed as total resection was determined to be unsafe. Residual tumor was detected by DESI-MS in the posterior margin, with TCP ranging between 74% and 88% into the biopsied tissue (smears 6–8, case 1). A secondary tumor mass recurred from the posterior margin and progressed into glioblastoma WHO grade IV (SI Appendix, Fig. S6). Notably, the lipid signature of the recurrent tumor showed higher intensity of phosphatidylinositol (38:4), m/z 885.5 (SI Appendix, Fig. S6), which has been noted as a discriminatory marker for glioblastoma in previous DESI-MS studies (15). Also, DESI-MS identified the presence of 2HG in the primary oligodendroglioma, indicative of IDH mutation, confirmed postoperatively (SI Appendix, Table S1). Progression of oligodendrogliomas into glioblastomas occurs predominantly in IDH-mutant tissue (21, 22).
Fig. 4.
Chemical predictions of disease state and tumor cell percentage. (A) Three-dimensional mapping of chemical predictions over MRI volume reconstruction for case 4 with overlaid stereotactic positions of smears 25–29. Stereotactic positions were registered digitally to the preoperative MRI using neuronavigation in the OR. Stereotactic positions are color coded by classification using PCA and linear discriminant analysis: green, gray matter; blue, white matter; red, glioma. (B) TCP predictions via linear regression using NAA for smears 25–29. Gray objects, natural log of the NAA signal intensity (normalized to the total ion count) vs. TCP for histologically defined reference specimens with a line of regression (black line); red objects, predictions of tissue smears. The equation of the regression line was calculated as y = –0.03x + 0.59 with a Pearson correlation r of –0.89. (C) Three-dimensional mapping of chemical predictions over MRI volume reconstruction for case 2 with overlaid stereotactic positions of smears 12–17. The stereotactic image for the biopsied tissue corresponding to smears 10 and 11 was not recorded. (D) TCP predictions via linear regression using NAA for smears 10–17.
Conclusions
Rapid DESI-MS analysis of tissue smears was performed inside the OR during glioma resection. The results from the first 10 patients in a projected 50-patient study indicate that the DESI-MS methodology is simple, reliable, and can be inserted into the current surgical workflow without interference. Simplicity of the instrumentation, a low-resolution ion-trap mass spectrometer, and the methodology is desirable for robustness and ruggedness. DESI-MS faithfully recapitulates basic diagnostic information (disease state and TCP) in less time than is needed for pathological evaluation. This enables multiple direct measurements on neurological tissue for examination of clinically relevant variants within the tumor and for the assessment of discrete points in the resection cavity for residual tumor. The finding that biopsies taken near each other and having similar tumor types can show vastly different TCPs speaks to the complexity and known heterogeneity of glioma invasion into adjacent tissue. This heterogeneity partly accounts for failure of single treatment paradigms. High percentages of tumor cells were found near surgical margins, even in cases where postoperative MRI showed gross total resection, corroborating the utility of DESI-MS coupled with neuronavigation to assist in maximal tumor resection, an outcome associated with better patient survival. In situations where tumor is detected but cannot be safely removed, intraoperative measurement of residual tumor can serve to focus the delivery of adjuvant postsurgical therapies. Intraoperative DESI-MS can assess the presence of IDH mutation via detection of 2HG. Intraoperative assessment of IDH mutation could differentiate low-grade glioma from reactive tissue, potentially influencing surgical decision-making process and aggressiveness of resection, predominantly based on tumor location (24). The DESI-MS methodology can be improved to become more quantitative and relevant for detection of important markers such as 2HG and NAA. Prospective randomized clinical trials will be necessary to further validate these results and define how DESI-MS can be used to expedite clinical decision-making and improve the care of brain tumor patients.
Materials and Methods
Ten subjects who underwent glioma resection were recruited in this study [Indiana University Institutional Review Board (IRB) Protocol No. 1410342262]. The Health Insurance Portability and Accountability Act authorization was obtained from each subject. Details on the patient cohort are reported in SI Appendix, Table S1. Tissue biopsies (total of 73 smears; SI Appendix, Table S2) collected from stereotactically registered positions in either the tumor core or near the surgical margins (within 0.5 cm from where tumor resection ceased) were obtained during surgical glioma resection and analyzed using DESI-MS in the OR. The number and size of the biopsies were determined at the discretion of the neurosurgeon. Tissue specimens (5–50 mm3) were smeared on standard microscope glass slides (18). The act of smearing homogenizes the tissue and allows acquisition of representative data even when examining a small fraction of the surface of the smear, thereby limiting the analysis time to a few minutes (19, 23). Scans acquired orthogonally to the smearing direction average any remaining heterogeneity of the tissue, a conclusion which is supported by DESI-MS images of tissue smears (SI Appendix, Fig. S7). DESI-MS was performed using dimethylformamide-acetonitrile (1:1 vol/vol), a solvent that preserves tissue morphology (26). MS measurements were performed using a linear ion-trap mass spectrometer (Finnigan LTQ, Thermo Scientific) modified for use in the OR; details in SI Appendix. Smears were subjected to sequential negative-mode DESI-MS (analysis time, 2.2 min) to obtain spectral signatures providing information on the presence of tumor, the extent of tumor infiltration, and the presence of IDH mutation via 2HG detection. The same smears analyzed by DESI-MS were stained and blindly evaluated by an expert neuropathologist, postoperatively, to provide information on overall diagnosis, TCP, and WHO grading when feasible. Chemical data were analyzed offline to minimize the potential influence on surgical decision-making (following the terms of approved IRB); computations are rapid, simple, and feasible in situ. Smears were diagnosed as gray brain matter, white brain matter, or glioma via pattern recognition of their DESI-MS spectral signatures using a reference DESI-MS spectral library (17). Additional information can be found in SI Appendix. TCP was predicted using the relative intensity of NAA (17) as well as from the lipid profiles. Preoperative and postoperative MRI scans, radiology, operative, and pathology reports were obtained for each case within 2 wk of surgery.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Zane Baird for construction of the modified mass spectrometer; Adam Hollerbach for 3D-printing the smear devices; clinical research nurses Jaala Hughes and Heather Cero for patient consent, providing clinical data, and IRB monitoring; and the surgical teams of A.A.C.-G. and Dr. Troy Payner for their keen cooperation. The research was supported by the National Institute of Biomedical Imaging and Bioengineering, NIH Grant R21EB015722; and the Purdue University Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706459114/-/DCSupplemental.
References
- 1.Young RM, Jamshidi A, Davis G, Sherman JH. Current trends in the surgical management and treatment of adult glioblastoma. Ann Transl Med. 2015;3:121. doi: 10.3978/j.issn.2305-5839.2015.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orringer D, et al. Extent of resection in patients with glioblastoma: Limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117:851–859. doi: 10.3171/2012.8.JNS12234. [DOI] [PubMed] [Google Scholar]
- 3.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low- and high-grade glioma. J Neurooncol. 2016;130:269–282. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 4.Eidel O, et al. Tumor infiltration in enhancing and non-enhancing parts of glioblastoma: A correlation with histopathology. PLoS One. 2017;12:e0169292. doi: 10.1371/journal.pone.0169292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis DN, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 6.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Detection technologies. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 7.Balog J, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med. 2013;5:194ra93. doi: 10.1126/scitranslmed.3005623. [DOI] [PubMed] [Google Scholar]
- 8.Orringer DA, et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat Biomed Eng. 2017;1:0027. doi: 10.1038/s41551-016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jermyn M, et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med. 2015;7:274ra19. doi: 10.1126/scitranslmed.aaa2384. [DOI] [PubMed] [Google Scholar]
- 10.Eberlin LS, et al. Molecular assessment of surgical-resection margins of gastric cancer by mass-spectrometric imaging. Proc Natl Acad Sci USA. 2014;111:2436–2441. doi: 10.1073/pnas.1400274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberlin LS, et al. Pancreatic cancer surgical resection margins: Molecular assessment by mass spectrometry imaging. PLoS Med. 2016;13:e1002108. doi: 10.1371/journal.pmed.1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tata A, et al. Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci Rep. 2016;6:35374. doi: 10.1038/srep35374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calligaris D, et al. Molecular typing of meningiomas by desorption electrospray ionization mass spectrometry imaging for surgical decision-making. Int J Mass Spectrom. 2015;377:690–698. doi: 10.1016/j.ijms.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ifa DR, Eberlin LS. Ambient ionization mass spectrometry for cancer diagnosis and surgical margin evaluation. Clin Chem. 2016;62:111–123. doi: 10.1373/clinchem.2014.237172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberlin LS, et al. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 2012;72:645–654. doi: 10.1158/0008-5472.CAN-11-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberlin LS, et al. Ambient mass spectrometry for the intraoperative molecular diagnosis of human brain tumors. Proc Natl Acad Sci USA. 2013;110:1611–1616. doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarmusch AK, et al. Lipid and metabolite profiles of human brain tumors by desorption electrospray ionization-MS. Proc Natl Acad Sci USA. 2016;113:1486–1491. doi: 10.1073/pnas.1523306113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarmusch AK, et al. Differential lipid profiles of normal human brain matter and gliomas by positive and negative mode desorption electrospray ionization - mass spectrometry imaging. PLoS One. 2016;11:e0163180. doi: 10.1371/journal.pone.0163180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirro V, et al. Utility of neurological smears for intrasurgical brain cancer diagnostics and tumour cell percentage by DESI-MS. Analyst (Lond) 2017;142:449–454. doi: 10.1039/c6an02645a. [DOI] [PubMed] [Google Scholar]
- 20.Santagata S, et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci USA. 2014;111:11121–11126. doi: 10.1073/pnas.1404724111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tietze A, et al. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J Neurosurg. March 3, 2017 doi: 10.3171/2016.10.JNS161793. [DOI] [PubMed] [Google Scholar]
- 22.Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13:345. doi: 10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolman M, et al. An assessment of the utility of tissue smears in rapid cancer profiling with desorption electrospray ionization mass spectrometry (DESI-MS) J Am Soc Mass Spectrom. 2017;28:145–153. doi: 10.1007/s13361-016-1506-x. [DOI] [PubMed] [Google Scholar]
- 24.Beiko J, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-oncol. 2014;16:81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eberlin LS, et al. Nondestructive, histologically compatible tissue imaging by desorption electrospray ionization mass spectrometry. ChemBioChem. 2011;12:2129–2132. doi: 10.1002/cbic.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.