Abstract
Aripiprazole was the first antipsychotic developed to possess agonist properties at dopamine D2 autoreceptors, a groundbreaking strategy that presented a new vista for schizophrenia drug discovery. The dopamine D2 receptor is the crucial target of all extant antipsychotics, and all developed prior to aripiprazole were D2 receptor antagonists. Extensive blockade of these receptors, however, typically produces extrapyramidal (movement) side effects which plagued first-generation antipsychotics, such as haloperidol. Second-generation antipsychotics, such as clozapine, with unique polypharmacology and D2 receptor binding kinetics, have significantly lower risk of movement side effects, but can cause myriad additional ones, such as severe weight gain and metabolic dysfunction. Aripiprazole’s polypharmacology—characterized by its unique agonist activity at dopamine D2, D3 and serotonin 5-HT1A receptors as well as antagonist activity at serotonin 5-HT2A receptors—translates to successful reduction of positive, negative, and cognitive symptoms of schizophrenia, while also mitigating risk of weight gain and movement side effects. New observations, however, link aripiprazole to compulsive behaviors in a small group of patients, an unusual side effect for antipsychotics. In this review, we discuss the chemical synthesis, pharmacology, pharmacogenomics, drug metabolism, and adverse events of aripiprazole, and we present a current understanding of aripiprazole’s neurotherapeutic mechanisms, as well as the history and importance of aripiprazole to neuroscience.
Keywords: aripiprazole, schizophrenia, dopamine, serotonin, D2, 5-HT1A, 5-HT2A, 5-HT2B, receptors
Graphical abstract
INTRODUCTION
Schizophrenia—characterized by positive symptoms (delusions, hallucinations, disorganized speech and behavior), negative symptoms (catatonia, blunted affect, apathy, and anhedonia), and cognitive symptoms (deficits in executive function and working memory)—is a devastating psychiatric disorder that affects approximately 1% of the population.1 A key early finding was an association between positive symptoms of psychosis and an overactive striatal dopamine system,2–5 which lead to the enduring “dopamine hypothesis” of schizophrenia. Specifically, persons with schizophrenia show elevated baseline and psychostimulant-induced striatal dopamine release2, 6–7 and are hypersensitive to dopaminergic psychostimulants.8 Also, acutely psychotic patients, as well as patients prodromal for schizophrenia, display increased presynaptic striatal dopamine synthesis (measured by increased uptake of radiolabeled L-dihydroxyphenylalanine (L-DOPA), dopamine’s precursor).3, 9 Despite these associations, most studies do not observe a correlation between schizophrenia and the availability of the dopamine transporter (DAT).10–12 Also, though increases in dopamine D2 receptor density in schizophrenia are observed,13 interpreting the findings is often confounded by prior antipsychotic treatment, which itself increases D2 receptor density.14 Other alterations in the dopamine system are reported, such as increases in D2 receptor homodimers and D2 receptors existing in high-affinity (active) conformations,3 but evidence regarding these observations, presently, is not definitive.
Most antipsychotic medications that are effective at reducing positive symptoms block D2 receptors, which are highly expressed in the striatum, to recalibrate dopamine signaling and remodel dopaminergic circuits.15–17 Neuroimaging studies demonstrate that clinical improvement in positive symptoms using first-generation antipsychotics (FGAs), such as chlorpromazine and haloperidol, requires approximately 65% striatal D2 receptor occupancy.18–20 However, grave drawbacks of FGAs are extrapyramidal side effects (EPS)—motor abnormalities such as rigidity, muscle spasms, tremors, restlessness, and involuntary movements (e.g. tardive dyskinesia)—20–24 and increased serum prolactin levels (hyperprolactinemia), which can lead to lactation, decreased bone density, and disturbances in sex hormones.25 EPS are caused by a blockade of nigrostriatal D2 receptors, and hyperprolactinemia is caused by blockade of tuberoinfundibular D2 receptors, which normally function to suppress prolactin secretion from the anterior pituitary gland.26–27 Closely titrating the dose of FGAs is necessary to reduce the likelihood or severity of EPS,28 whereas reducing hyperprolactinemia may require switching to a second-generation antipsychotic (SGA).29
SGAs, including clozapine and quetiapine, are clinically as effective as FGAs,30 and they are associated with a lower incidence of EPS and hyperprolactinemia.26, 31 Many hypotheses have been put forward to explain the neuropharmacology that underlies these characteristics. In contrast to FGAs, positron emission tomography indicates SGAs can be effective when occupying less than 60% of striatal D2 receptors—a major finding that overturned the seemingly ineluctable paradigm that antipsychotic efficacy is directly proportional to striatal D2 receptor occupancy.20, 32–36 Though, differences in ligand-receptor on/off binding kinetics may also be a causal factor, i.e. effective doses of SGAs and FGAs may occupy similar numbers of striatal D2 receptors, but SGAs may dissociate more quickly than FGAs from D2 receptor binding sites, as is documented with quetiapine and clozapine.37–38 A rapid off-rate would likely permit endogenous dopamine to maintain an adequate level of D2 receptor signaling to prevent hyperprolactinemia and EPS. Whereas tight binding of FGAs to D2 receptors may decrease endogenous D2 receptor signaling to a level that causes these side effects.
Additionally, it has been argued that antagonism of serotonin 5-HT2A receptors by SGAs contributes to their efficacy, reducing the need for relatively extensive D2 receptor occupancy, consequentially reducing side effects.39–40 In support of this, studies show that selective 5-HT2A receptor antagonism attenuates amphetamine-elicited release of dopamine into the striatum while also blocking amphetamine’s psychomotor effects in non-human primates.41 Also, the selective 5-HT2A antagonist, pimavanserin, effectively treats psychosis in Parkinson’s disease without causing EPS.42–43 However, FGAs such as chlorpromazine and haloperidol have appreciable potencies at 5-HT2A that are comparable to some SGAs, challenging this hypothesis.44–45 Other targets, such as muscarinic and 5-HT1A receptors may contribute to lower EPS risk of newer antipsychotics.40, 46–48 The pharmacology of amisulpiride, however, casts doubt on this hypothesis—it has very low affinity at 5-HT1A, and at each of the muscarinic receptors (and at 5-HT2A receptors),49 yet has low EPS liability.50
From a neural systems perspective, there is evidence that SGAs, like olanzapine and clozapine, target the mesolimbic (ventral striatum) dopamine system, sparing the nigrostriatal (dorsal striatum) dopamine system that is intimately involved in motor processing, which may underlie clozapine’s low EPS characteristics.51–52 Nevertheless, some SGAs also have added risk of causing obesity and metabolic dysfunction (e.g., diabetes, high cholesterol).50, 53–54 Exploration of the precise neurobiological and neuropharmacological mechanisms underlying distinct effects of individual antipsychotics remains vigorous.
Due in part to the aforementioned side effects and others caused by both FGAs and SGAs, including sedation and emotional dampening,55 approximately two-thirds of patients with a psychotic disorder are noncompliant with their antipsychotic medications.56–57 Finally, antipsychotic drugs have limited efficacy in approximately one-third of patients,58 and treatment of negative and cognitive symptoms in schizophrenia remains a challenge—likely because they involve different neural systems and mechanisms than positive symptoms. For example, in contrast to a hyperactive striatal dopamine system underlying positive symptoms, a hypoactive mesocortical dopamine system is proposed to underlie negative and cognitive symptoms.5, 59–61
This review discusses the prototypical third-generation antipsychotic (TGA), aripiprazole, distinguished by its agonist pharmacology at certain receptor targets, most notably D2 autoreceptors.62–65 This pharmacology was the first of its kind for an approved antipsychotic medication. Furthermore, aripiprazole can be effective at attenuating negative and cognitive symptoms, in addition to positive symptoms, of schizophrenia in some patients and has lower EPS liability than FGAs (e.g., haloperidol), lower weight gain and metabolic liabilities than SGAs (e.g., clozapine), and does not cause hyperprolactinemia.50, 66–69 Though, aripiprazole is not a panacea, having limited effects in some patients, and new reports suggest a link between aripiprazole and impulse control deficits, a unique side effect for an antipsychotic, which we briefly discuss. Aripiprazole opened new vistas for exploration of the neurobiological underpinnings of psychotic symptoms and of side-effects caused by antipsychotics, and it inspired a paradigm shift from an antagonist-based to an agonist-based approach for antipsychotic drug discovery.
CHEMICAL PROPERTIES AND CHEMICAL SYNTHESIS
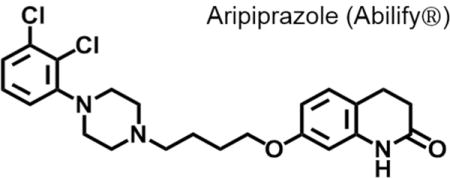
Aripiprazole, 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one, is an achiral quinolinone derivative (CAS No: [129722-12-9]; Figure 1). It possesses a single hydrogen bond donor and five acceptors, has a molecular weight of 448.4 g/mol, and a Log P value of 4.55. These physicochemical properties comply with Lipinski’s rule of five and provide the compound with high bioavailability, protein binding, and an acceptable metabolic profile.70–71 Otsuka Pharmaceutical patented aripiprazole in 1988 [US patent 4,734,416 filed in 1979], along with many other carbostyril derivatives and salts thereof, as potential antihistamine and central nervous system controlling agents.72–73 However, the patent did not explicitly mention aripiprazole by name, nor did it describe a synthetic procedure used for it.
Figure 1.
Chemical structures of aripiprazole (OPC-14597, left) and its predecessor OPC-4392 (right).
The first synthetic procedure and reference to its antipsychotic activity was described by Oshiro et al. in Otsuka’s 1991 patent [US patent 5,006,528 filed in 1989].74 In 1998, Otsuka scientists described a similar synthesis for the free base, but with slightly different conditions (Scheme 1).75 The synthesis begins with the alkylation of 7-hydroxy-3,4-dihydro-2(1H)-quinolinone by stirring it with 1,4-dibromobutane (3 molar equivalents (mol. equiv.)) in the presence of potassium carbonate (1 mol. equiv.) in dimethylformamide at 60 °C for four hours to give 7-(4-bromobutoxy)-3,4-dihydro-2(1H)-quinolinone. The reaction mixture is then diluted with an equal volume of water, and the organic phase is extracted with ethyl acetate. After rotary evaporation, the resulting product is recrystallized in ethyl alcohol.74–75 The product is subsequently combined with sodium iodide (2 mol. equiv.) in acetonitrile and refluxed for 30 minutes before cooling to room temperature. Next, 1-(2,3-dichlorophenyl)piperazine (1.5 mol. equiv., prepared based on76), and triethylamine (2 mol. equiv.) are added to the reaction mixture, and refluxed for another four hours. The resulting precipitate is filtered and discarded. The filtrate is evaporated in a low-pressure environment, and dissolved in ethyl acetate. Then, it is washed, dried, and subjected to rotary evaporation to yield a resin. The resin is recrystallized in ethyl alcohol to provide the free base of aripiprazole as a white powdery substance. The powder may then be dissolved in ethyl alcohol with acid to yield a variety of salts. Other compounds, such as OPC-4392 (Figure 1), aripiprazole’s predecessor, were prepared using similar procedures by condensing (4-bromobutoxy)-2(1H)-quinolinone (or a structural analog) with various phenylpiperizines;74–75 the procedure has since been optimized.77
Scheme 1.
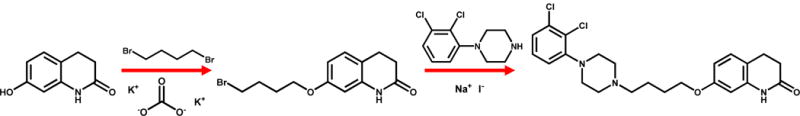
Synthesis of aripiprazole reported in the primary literature.
MANUFACTURING INFORMATION
Aripiprazole was approved by the FDA for schizophrenia on November 15th, 2002, as an oral tablet formulation in the dose range of 2 to 30 mg. PubMed-indexed publications with “aripiprazole” in their abstract rapidly increased around this time, peaking at ~300/year in 2008, then plateauing to present day (Figure 2). Aripiprazole was originally manufactured by Otsuka Pharmaceutical and was co-marketed with Bristol Meyers-Squibb under the brand name Abilify®. The following formulations have since been approved: orally disintegrating tablets, oral solution, and an aqueous solution for intramuscular injection. The original patent expired April, 2015, and generic 2 to 30 mg oral tablets have subsequently been produced by numerous manufacturers (Teva, Torrent, Hetero Labs, Alembic, and Ajanta, among others). Consistent annual sales figures for Abilify® are unavailable, however, global sales in 2013 and 2014 were approximately $7.824 and $9.285 billion, respectively,78 illustrating the drug’s enormous financial success.
Figure 2.
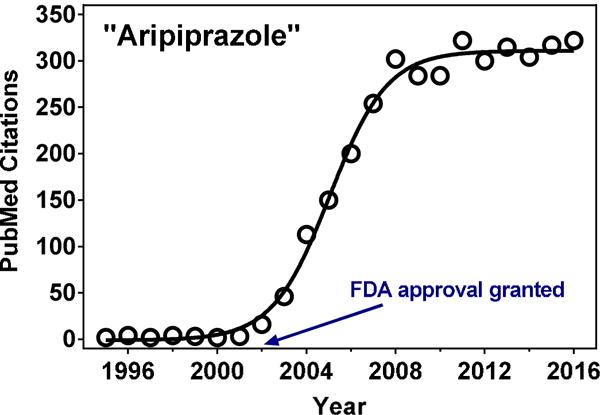
PubMed-indexed citations containing “aripiprazole” in their Abstract (1995–2016).
APPROVED INDICATIONS AND DOSING
Aripiprazole, oral formulation, is approved for the following indications: 1) schizophrenia in adults and adolescents (13–17 years); 2) bipolar I disorder (manic and mixed episodes) in adults and pediatric patients (10–17 years); 3) major depressive disorder in adults, as an adjunctive therapy; 4) irritability associated with autism spectrum disorder in pediatric patients (6–17 years); and 5) vocal and motor tics associated with Tourette’s syndrome in pediatric patients (6–18 years).70 Additionally, the intramuscular formulation is approved to treat agitation associated with schizophrenia or bipolar mania in adults.70 Generally, children are started on a 2 mg/day dose of aripiprazole, and may be titrated up to 10 mg/day, depending on the disorder and severity.70 Adults on the other hand are usually started on 10 mg/day, and may be titrated up to a maximum of 30 mg/day depending on their response.70
PHARMACOKINETICS AND PHARMACOGENOMICS
Aripiprazole displays linear kinetics, with a bioavailability of 87% (independent of low-fat food intake).70, 79 The maximum plasma concentration occurs 3–5 hours following administration, and a steady-state is achieved after approximately 14 days of daily dosing.70, 79 At steady-state, the volume of distribution is 404 L due to extensive binding to plasma proteins (>99%).70, 79 In vitro studies using human liver microsomes and recombinant cytochrome P450 enzymes indicate that aripiprazole is metabolized predominantly via phase I mechanisms by both CYP3A4 and CYP2D6 to yield dehydrogenation and hydroxylation products, while CYP3A4 also mediates N-dealkylation (Figure 3).70 Phase II metabolism is also present, albeit to a lesser extent (FDA document NDA No. 21-436).80 Interestingly, the major circulating metabolite is dependent upon the frequency of administration. With acute dosing, the phase II product BMS-337041 is the most abundant circulating metabolite, whereas the phase I product dehydroaripiprazole is more prevalent after chronic dosing. In addition to compounds provided in Figure 3, McEnvoy et al. report a motley of aripiprazole metabolites from LC-MS/MS analysis of human urine, including iminium ion, epoxide, and glutathione species.80
Figure 3.
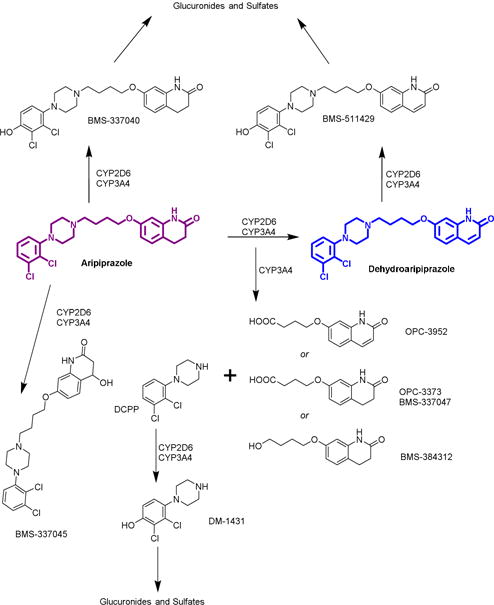
Structures of phase I metabolites of aripiprazole (adapted from FDA document NDA No. 21-436). Dehydroaripiprazole is the major metabolite after chronic dosing, and is pharmacologically active.
Aripiprazole is metabolized almost equally along both CYP3A4 and CYP2D6 pathways (1:1) in extensive metabolizers, however, this ratio approaches 3:1 in intermediate metabolizers.81–82 At steady-state the average elimination half-life of aripiprazole is approximately 75 hours, and the primary metabolite, dehydroaripiprazole, is 95 hours.70 Considering that dehydroaripiprazole is pharmacologically active at D2 receptors and exists at a concentration that is ~40% of the plasma concentration of aripiprazole, it likely contributes to the sustained pharmacologic effects of aripiprazole.70, 83
Serum concentrations of aripiprazole at therapeutic doses range between 150–300 ng/mL,83 but they can vary substantially between patients administered equivalent doses. This is likely due to polymorphisms in the CYP2D6 gene. For example, in individuals with little to no CYP2D6 activity, the elimination half-life of aripiprazole extends from ~95 to 146 hours.70, 81–83 Also, psychiatric patients are often poly-medicated, which likely impacts aripiprazole concentrations. In particular, aripiprazole is often prescribed as an adjunctive antidepressant; since many antidepressants are also metabolized by CYP2D6 and CYP3A4 (e.g., paroxetine, fluvoxamine, fluoxetine), the potential for increased serum concentrations of these drugs is inherent.70, 83 Patients also taking medications that are inhibitors or inducers of CYP3A4 and/or CYP2D684 should undergo close therapeutic drug monitoring.
Genetic association studies show that polymorphisms in several genes impact antipsychotic efficacy.85–87 For example, the Taq1A (rs1800497) polymorphism, a transition mutation (C→T) that generates the A1 allele of D2DR, is associated with an increased therapeutic response to aripiprazole in Asian patients.88 This association, however, is not observed in other ethnicities.85, 89 Multiple neuroimaging studies show that healthy volunteers, of European descent, who are either homozygous or heterozygous for the A1 allele exhibit less striatal D2 receptor availability.90–92 Two other D2DR polymorphisms, Ser311Cys (rs1801028) and –141C Ins/Del (rs1799732), also appear to affect antipsychotic drug response. The Ser311Cys polymorphism associates with a favorable response to risperidone in Han Chinese populations.93 Conversely, some studies show that-141 Del carriers tend to have a poorer response to several antipsychotics, but not aripiprazole.85–86, 94 Also, the C-1019G polymorphism in HTR1A, and A-1438G-T102C (rs6311/rs6313) in HTR2A (genes encoding the 5-HT1A and 5-HT2A receptors, respectively) associate with decreased efficacy of aripiprazole to treat negative and cognitive symptoms in schizophrenic patients of Han Chinese descent.95–96 Furthermore, other polymorphisms in HTR2A and DRD2 may interact to facilitate aripiprazole’s antipsychotic efficacy.97 Unfortunately, many of these experiments have small effect sizes, and results depend upon the ethnic population studied and how “clinical response” is defined. Thus, pharmacogenomics currently provides insufficient data to guide personalized medicine for schizophrenia.
PHARMACOLOGY AND ADVERSE EVENTS
Like other antipsychotics, aripiprazole’s pharmacology is complex (Table 1),62, 98 and is representative of the polypharmacology—many targets—approach to treat psychotic disorders. Aripiprazole has high affinity (defined here as Ki < 30 nM) at serotonin 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, and 5-HT7, dopamine D2 and D3, adrenergic α1a, and histamine H1 receptors. Aripiprazole is distinguished from earlier antipsychotics by its partial agonist activity at D2, D3, 5-HT1A, and 5-HT2C receptor targets62, 64, 98–101. In vitro, aripiprazole is a neutral antagonist or very weak partial agonist at 5-HT2A and 5-HT7, and is an inverse agonist at 5-HT2B receptors.62 According to the prescribing information for Abilify®, aripiprazole is an “α1 antagonist.” Its functional effects at H1 receptor have not been reported in scientific literature, to our knowledge. Though, since histamine activation effects are not typically reported, and since aripiprazole was part of a series of compounds designed to be antihistamines, it is likely an H1 antagonist.
Table 1.
Aripiprazole: Receptor Affinity Profile
| Receptor Target | Ki, nM | Radioligand and Source |
|---|---|---|
| “High” Affinity (Ki < 30 nM) | ||
| Serotonin 5-HT2B | 0.4 | [3H]LSD* |
| Dopamine D2 | 0.95 | [3H]NMSP, PDSP Certified |
| Dopamine D3 | 5.4 | [3H]NMSP* |
| Serotonin 5-HT1A | 5.6 | [3H]8-OH-DPAT, PDSP Certified |
| Serotonin 5-HT2A | 8.7 | [3H]Ketanserin* |
| Serotonin 5-HT7 | 10 | [3H]LSD, PDSP Certified |
| Serotonin 5-HT2C-INI | 22 | [125I]DOI* |
| Adrenergic α1a | 25 | [125I]HEAT, PDSP Certified |
| Histamine H1 | 29 | [3H]Pyrilamine, PDSP Certified |
| “Moderate” Affinity (Ki = 30–300 nM) | ||
| Adrenergic α1b | 34 | [125I]HEAT, PDSP Certified |
| Adrenergic α2c | 38 | [3H]Clonidine, PDSP Certified |
| Serotonin 5-HT1D | 63 | [3H]GR-125743, PDSP Certified |
| Adrenergic α2a | 74 | [3H]Clonidine, PDSP Certified |
| Adrenergic α2b | 102 | [3H]Clonidine, PDSP Certified |
| Adrenergic β1 | 141 | [125I]Pindolol, PDSP Certified |
| Adrenergic β2 | 163 | [125I]Pindolol, PDSP Certified |
| “Low” Affinity (Ki > 300 nM) | ||
| Dopamine transporter, D1, D4, D5, serotonin transporter, 5-HT1B, 5-HT1E, 5-HT3, 5-HT5a, 5-HT6, muscarinic M1, M2, M3, M4, M5, nicotinic α7, α1β2, α2β2, α2β4, α3β2, histamine H2, H4, norepinephrine transporter– all PDSP Certified. Δ-opioid*, β-opioid*, κ-opioid* | ||
Ki values obtained from the NIMH Psychoactive Drug Screening Program database, http://pdsp.med.unc.edu/pdsp.php; search conducted 02/17/2017. Affinities were determined by displacement of radioligand from human cloned receptors. *Shapiro et al., 2003
At effective doses, aripiprazole occupies up to 90% of D2 receptors, and also occupies 5-HT1A and 5-HT2A receptors but to a lesser extent.22, 102 As a D2 receptor partial agonist with moderate intrinsic activity (as characterized in some in vitro assays), aripiprazole may block postsynaptic D2 receptors in neural systems with high dopaminergic tone, i.e. the striatal dopamine system of schizophrenic patients, which may account for its effects on positive symptoms. Conversely, it may activate postsynaptic D2 receptors in neural systems with low dopaminergic tone, i.e. the mesocortical system in schizophrenic patients, which may account for its effects on negative and cognitive symptoms.65, 99, 103–104 Other proposed pharmacological mechanisms focus on aripiprazole’s full agonism at presynaptic D2 autoreceptors in vivo in animal models.62, 64, 98–99, 103, 105 D2 autoreceptors modulate dopamine neurotransmission via a negative feedback mechanism. For example, at relatively high dopamine concentrations (or during phasic dopamine release), presynaptic D2 autoreceptors are activated to decrease dopamine synthesis and release, and somatodendritic D2 autoreceptors decrease neuronal firing rate.106–109 Striatal dopamine neurons express high levels of D2 autoreceptors, whereas they are scantly expressed in the mesocortical dopamine pathway.110–111 Thus, aripiprazole, by acting as a D2 autoreceptor agonist, may decrease dopaminergic tone selectively in the striatum. Combined with antagonist activity at postsynaptic D2 receptors in vivo,105 this pharmacology may contribute to its unique clinical effects. Finally, aripiprazole’s functional effects at the D2 receptor vary depending on signaling pathway, cell type, and cellular context62, 64, 98, 112–113 providing evidence that it may be a biased agonist at the D2 receptor in vivo62, 64, 98, 112–113. How its unique intracellular signaling effects impact clinical symptoms is not known.
Despite extensive D2 receptor occupancy, aripiprazole exhibits a relatively low risk for EPS and no risk of hyperprolactinemia—both caused by chronic D2 receptor blockade.22, 114–115 Aripiprazole may even reduce prolactin secretion in certain patients, potentially via its D2 receptor partial agonist effects.50 Besides partial agonist activity at D2 receptors, aripiprazole’s agonist activity at 5-HT1A receptors, from partial to full agonist depending on cellular system, may also contribute to its efficacy and reduced side effects, relative to FGAs.46–47, 62, 101, 116–118 Aripiprazole activates somatodendritic 5-HT1A receptors, reducing serotonin release and subsequently increasing dopamine release in the cortex, which may translate to treat negative and cognitive symptoms of schizophrenia.119–120 Similarly, SGAs, such as clozapine and ziprasidone, that can attenuate negative and cognitive symptoms are also 5-HT1A partial agonists121–123 and enhance central dopamine release via activation of 5-HT1A receptors.124–125 More recently, it was shown that the ability of clozapine to reverse phencyclidine-elicited cortical desynchronization—a model of neural activity underlying negative symptoms— requires activation of 5-HT1A receptors.126
Relative to SGAs, such as clozapine and olanzapine, aripiprazole has a lower propensity to induce weight gain.50, 127 SGAs are potent 5-HT2C and H1 antagonists or inverse agonists—likely contributing to their obesity side effects.127 Aripiprazole, however, is a partial agonist at the 5-HT2C receptor, and the selective 5-HT2C agonist, lorcaserin (Belviq®), is effective at decreasing weight in humans.127–129 Nevertheless, some patients taking aripiprazole gain weight, and its putative antagonist activity at histamine H1 receptors (Ki ~29 nM, Table 1), which is associated with weight gain,45, 62 may offset beneficial effects of 5-HT2C partial agonism. Much less is known about the contribution of aripiprazole’s other targets—including 5-HT2B (which it binds with highest affinity), 5-HT7, D3, and adrenergic α1a—to its mechanism of action, though each of these targets warrants further investigation. For example, new evidence links inactivation or knockout of 5-HT2B receptors to impulsivity both preclinically and clinically.130–131 This is intriguing in light of recent reports of serious impulse control deficits and compulsive behaviors in a small group of patients taking aripiprazole.132 Although immediate focus moved to aripiprazole’s dopamine agonist activity, aripiprazole’s highly potent inverse agonist activity at 5-HT2B receptors may also contribute to impulsive and compulsive behavior.
In preclinical studies of rats and rabbits, teratogenic and developmental toxicity effects of aripiprazole are observed at doses 2–11 times the maximum recommended human dose (based on area under the curve comparisons) (FDA document NDA No. 21-436). These findings guided designation of aripiprazole to pregnancy risk category C. However, the evidence for serious adverse fetal events in humans remains weak due to a lack of well-designed, clinical trials. A recent, prospective, cohort study assessed the risk of major malformations in infancy following exposure to aripiprazole in the first trimester of pregnancy. Results show an absolute risk of 3.13% (N = 96), not different from the risk associated with exposure to any SGA antipsychotic, 1.3% (N = 312), or unexposed infants, 0.6% (N = 177).133 Though, small sample sizes and wide confidence intervals may have obfuscated actual differences. These data corroborate an earlier study in pregnant women taking aripiprazole (N = 86 vs 172 unexposed).134 Although this latter study reports a significantly increased rate of pre-term delivery (odds ratio = 2.30; 95% CI 0.32–16.7) and restricted fetal growth (odds ratio = 2.97; 95% CI 1.23–7.16) in the aripiprazole group. The aripiprazole dosing regimen, however, was not controlled. Furthermore, approximately one third of patients taking aripiprazole reported also smoking tobacco and drinking alcohol while pregnant, known risk factors for prematurity and restricted fetal growth.134–135 Aripiprazole is detected in human breast milk, and women taking aripiprazole are advised not to breastfeed.136 Considering the increased risk of a psychiatric relapse and of adverse pregnancy outcomes in schizophrenic patients, (e.g. stillbirths)137 a reduction in dose or a postponement of treatment should be assessed on a case-by-case basis.
The most commonly reported side effects of aripiprazole are akathisia in adults and tremors in adolescents with schizophrenia.70, 83, 138–140 Also, adjunctive treatment with antidepressants carries a black box warning of an increased risk of suicidal thoughts and behavior in patients 24 years of age and younger.70, 141 Elderly patients treated off-label for dementia-related psychosis are at an increased risk of death, and a significant dose-response relationship exists between aripiprazole and cerebrovascular events in the elderly.70, 142–144 Other side effects include dizziness, drowsiness, sedation, insomnia, somnolence, weight gain, drooling, restlessness, anxiety, and headaches.70, 138, 145
HISTORY AND IMPORTANCE TO NEUROSCIENCE
Pharmacotherapy for schizophrenia began in the early 1950s with the discovery that the FGA chlorpromazine produced a powerful, non-narcotic, calming effect, reducing agitation in hospital patients undergoing surgery. Not long afterwards it was tested in psychotic patients, and proved tremendously effective, so much so that it freed many from institutionalization, and spawned the “psychopharmacological revolution”.146 Chlorpromazine and later SGAs, such as clozapine, were found to be dopamine receptor antagonists, inspiring the dopamine hypothesis of schizophrenia. This hypothesis has withstood the test of time. Despite intensive research efforts to discover new drug targets for schizophrenia, all approved antipsychotics share activity at dopamine receptors as an essential part of their pharmacology. FGAs and SGAs block dopamine D2 receptors, and vanquish positive symptoms of schizophrenia that result from hyperactivation of the striatal dopamine system. They are less effective at treating negative and cognitive symptoms, which are believed to result from hypoactivation of the mesocortical dopamine system. Though, a careful clozapine treatment regimen can alleviate some negative and cognitive symptoms, which may be due to its unique pharmacology at serotonin receptors. [Pointedly, clozapine remains the most effective antipsychotic, yet because of the risk of agranulocytosis, a potentially fatal condition caused by suppression of white blood cells,147 it is only approved for treatment-resistant schizophrenia and for reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.50, 148] FGAs and SGAs carry inherent serious side effect risks. Namely, FGAs are fraught with EPS and hyperprolactinemia issues, and many SGAs cause metabolic syndrome, obesity, and type II diabetes.69, 149–150
Side effect issues of FGAs and SGAs and their limited efficacy in treating negative and cognitive symptoms in schizophrenia provided the impetus and the opportunity for drug discovery targeting novel biological targets or pharmacological mechanisms, which seeded the discovery of aripiprazole. In the late 1970s, Otsuka scientists were exploring 2(1H)-quinolinone derivatives for anti-histamine functionality devoid of central nervous system side effects when they fortuitously discovered compounds with antipsychotic activity in preclinical tests.151 Compounds were derivatized into a series of (4-phenyl-1-piperazinyl)alkoxy-2(1H)-quinolinone molecules. Lead candidates, such as OPC-4392, were selected based on their agonist activity at presynaptic D2 autoreceptors, a then novel, alternative approach for reducing dopaminergic activity compared to direct blockade of postsynaptic D2 receptors.151–152 This rationale was based, in part, on observations that D2 autoreceptors are predominantly expressed in the striatal dopamine system. Activation would reduce dopamine synthesis there, without largely impacting cortical dopamine activity.
OPC-4392’s D2 autoreceptor agonist activity was assessed ex vivo and in vivo. Ex vivo, in rat striatal slices, OPC-4392 dose-dependently inhibited L-DOPA formation—an indirect measure of inhibition of tyrosine hydroxylase, which is the key enzyme for dopamine synthesis. This effect did not involve direct suppression of tyrosine hydroxylase activity, and it was reversed by the D2 receptor antagonist, sulpiride, suggesting a presynaptic D2 receptor mechanism.153 In vivo, in anesthetized rats, OPC-4392 inhibited ventral tegmental area (VTA) dopamine spikes elicited by nucleus accumbens stimulation (antidromic effects), an effect blocked by simultaneous application of a D2 receptor antagonist, providing evidence of D2 autoreceptor agonist effects in the ventral striatum.154 In addition to dopamine autoreceptor agonist effects, OPC-4392 was also purported to block postsynaptic D2 receptors, based on observations that it inhibited behaviors in mice elicited by the D2 receptor agonist apomorphine and reversed the inhibitory effect of apomorphine on acetylcholine release in rat striatal slices.155
OPC-4392 showed efficacy in preclinical behavioral models of psychosis at doses that did not induce catalepsy, a model of EPS.151 For example, it attenuated jumping behavior in mice treated with the dopamine precursor L-DOPA and dopamine releaser, methamphetamine. OPC-4392 also prevented lethality caused by high dose methamphetamine. Despite the promising preclinical data, clinical development for schizophrenia was halted. “Unpublished observations” from clinical studies included an improvement in negative symptoms without EPS, but an aggravation of positive symptoms due to an “activation” effect.105 Nevertheless, these clinical studies corroborated a link between dopamine autoreceptor agonism and efficacy to treat negative symptoms with low EPS liability, found earlier using terguride.156
Beginning with OPC-4392, structure activity relationships were used to increase antagonist potency at postsynaptic dopamine receptors to treat positive symptoms, while maintaining autoreceptor agonism to treat negative symptoms.105 By replacing the –propoxy linker in OPC-4392 with –butoxy, the 2,3-dimethyl moiety with 2,3-dicholoro, and by changing the carbostyril moiety to 3,4-dihydrocarbostyril, aripiprazole (OPC-14597) was discovered. The approach for developing aripiprazole closely paralleled OPC-4392, which was used as a prototype to guide the preclinical development of aripiprazole. Relative to OPC-4392, aripiprazole has nearly equipotent agonist activity at presynaptic dopamine autoreceptors in vivo, but has increased antagonist potency at postsynaptic dopamine receptors.105
Its dopamine autoreceptor agonist activity was determined by its efficacy to block increases in DOPA caused by reserpine and by gamma-butyrolactone in several brain regions; effects that were reversed by haloperidol. Moreover, like OPC-4392, direct application of aripiprazole inhibited VTA dopamine neurons, an effect blocked by a D2-selective receptor antagonist, domperidone, but not a D1-selective receptor antagonist, SCH-23390, suggesting agonist activity at D2 autoreceptors.157 A recent clinical neuroimaging study, however, shows that aripiprazole does not affect dopamine synthesis, even at doses that occupy up to 79% of striatal D2 receptors, challenging the conclusion that activation of D2 autoreceptors is a critical mechanism for its antipsychotic effects.158 The doses, 3–9 mg oral, used in this study are lower than the typical prescribed, starting dose for adults with schizophrenia, 10 mg. Also, this study involved a single, acute treatment in healthy subjects, so conclusions should be taken with caution.
Aripiprazole’s antagonist activity at postsynaptic receptors was inferred in preclinical studies by its failure to induce both locomotion in mice treated with reserpine, and contralateral rotations in rats with striatal 6-hydroxydopamine lesions—both behaviors are observed after postsynaptic dopamine receptor activation. Additionally, aripiprazole inhibited apomorphine induced stereotypy, locomotion, and ipsilateral rotations in a kainic acid striatal lesion model at potencies >10-fold relative to OPC-4392, suggesting more potent antagonist activity at postsynaptic D2 receptors. Aripiprazole’s ED50 doses in these models were substantially less than its ED50 for inducing catalepsy, together suggesting antipsychotic activity without EPS.105
In the same year that these preclinical results were published, Otsuka reported aripiprazole’s efficacy in both positive and negative symptoms of schizophrenia with a low risk of EPS, minor weight gain, and without significant prolactin elevation, results later corroborated.66, 159 As clinical data on aripiprazole’s efficacy accumulated in the following decades, it was found to be effective in treating numerous neuropsychiatric disorders (described under Approved Indications).
The discovery of aripiprazole broke ground, because of its novel agonist activities at D2 autoreceptors that translated to efficacy for positive, negative, and cognitive symptoms with reduced side effects. Remarkably, or as if aligned with the history of antipsychotic drug discovery in general, it wasn’t until after clinical trials for schizophrenia that aripiprazole’s pharmacological activity at 5-HT1A receptors was discovered. Clozapine, a partial agonist at 5-HT1A, shares a similar history. These discoveries were important for neuroscience, as they helped unveil the interaction between 5-HT1A receptors and cortical dopamine neurotransmission, and they guided rational antipsychotic drug discovery targeting 5-HT1A receptors to reduce negative and cognitive symptoms. Since aripiprazole, other antipsychotics designed to possess 5-HT1A agonist activity have been approved, including cariprazine, asenapine, and brexpiprazole.
Schizophrenia pathophysiology is more than appreciably complex, involving genetic and environmental factors interacting with neurodevelopmental factors, and basic science remains crucial for uncovering new biological targets for schizophrenia drug discovery. There has been much research into other neural circuits impacted in the disease, such as the glutamate and acetylcholine systems.160–163 Moreover, basic science has led to the discovery of new biological mechanisms for existing antipsychotics. For example, new research shows that aripiprazole inhibits microglia activation, an anti-inflammatory effect that may have clinical utility for schizophrenia.164–166 Aripiprazole has inspired and continues to inspire new questions regarding the neurobiological mechanisms of schizophrenia.
Acknowledgments
FUNDING
Author C.E.C. received funding from NIH/NIDA grant, 1R21DA040907-01.
ABBREVIATIONS
- EPS
extrapyramidal side effects
- FGA
first generation antipsychotic
- SGA
second generation antipsychotic
- TGA
third generation antipsychotic
REFERENCES CITED
- 1.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppa T, Harkanen T, Koskinen S, Lonnqvist J. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64(1):19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–7. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 3.Seeman MV, Seeman P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:155–60. doi: 10.1016/j.pnpbp.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson A. Does dopamine have a role in schizophrenia? Biol Psychiatry. 1978;13(1):3–21. [PubMed] [Google Scholar]
- 5.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–40. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–9. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- 9.Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, Valmaggia L, Allen P, Murray R, McGuire P. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16(9):885–6. doi: 10.1038/mp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt GJ, Frodl T, Dresel S, la Fougere C, Bottlender R, Koutsouleris N, Hahn K, Moller HJ, Meisenzahl EM. Striatal dopamine transporter availability is associated with the productive psychotic state in first episode, drug-naive schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2006;256(2):115–21. doi: 10.1007/s00406-005-0618-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt GJ, Meisenzahl EM, Frodl T, La Fougere C, Hahn K, Moller HJ, Dresel S. The striatal dopamine transporter in first-episode, drug-naive schizophrenic patients: evaluation by the new SPECT-ligand[99mTc]TRODAT-1. J Psychopharmacol. 2005;19(5):488–93. doi: 10.1177/0269881105056530. [DOI] [PubMed] [Google Scholar]
- 12.Laakso A, Vilkman H, Alakare B, Haaparanta M, Bergman J, Solin O, Peurasaari J, Rakkolainen V, Syvalahti E, Hietala J. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157(2):269–71. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- 13.Kestler LP, Walker E, Vega EM. Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behavioural pharmacology. 2001;12(5):355–71. doi: 10.1097/00008877-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ginovart N, Wilson AA, Hussey D, Houle S, Kapur S. D2-receptor upregulation is dependent upon temporal course of D2-occupancy: a longitudinal [11C]-raclopride PET study in cats. Neuropsychopharmacology. 2009;34(3):662–71. doi: 10.1038/npp.2008.116. [DOI] [PubMed] [Google Scholar]
- 15.Sebel LE, Graves SM, Chan CS, Surmeier DJ. Haloperidol Selectively Remodels Striatal Indirect Pathway Circuits. Neuropsychopharmacology. 2017;42(4):963–973. doi: 10.1038/npp.2016.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol Psychiatry. 2001;50(10):729–42. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188(4194):1217–9. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 18.Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45(1):71–6. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- 19.Wiesel FA, Farde L, Nordstrom AL, edvall G. Central D1- and D2-receptor occupancy during antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(5):759–67. doi: 10.1016/0278-5846(90)90046-j. [DOI] [PubMed] [Google Scholar]
- 20.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49(7):538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27(2):248–59. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, Bies RR, Suzuki T, Remington G, Pollock BG, Mizuno Y, Mimura M, Uchida H. Tardive dyskinesia in relation to estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Schizophr Res. 2014;153(1–3):184–8. doi: 10.1016/j.schres.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews M, Gratz S, Adetunji B, George V, Mathews M, Basil B. Antipsychotic-Induced Movement Disorders: Evaluation and Treatment. Psychiatry. 2005;2(3):36–41. [PMC free article] [PubMed] [Google Scholar]
- 25.Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci. 2013;6(3):168–75. doi: 10.4103/0974-1208.121400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–73. doi: 10.1016/s0920-9964(98)00158-3. [DOI] [PubMed] [Google Scholar]
- 27.Jung DU, Seo YS, Park JH, Jeong CY, Conley RR, Kelly DL, Shim JC. The prevalence of hyperprolactinemia after long-term haloperidol use in patients with chronic schizophrenia. J Clin Psychopharmacol. 2005;25(6):613–5. doi: 10.1097/01.jcp.0000186738.84276.9f. [DOI] [PubMed] [Google Scholar]
- 28.Caroff SN, Hurford I, Lybrand J, Campbell EC. Movement disorders induced by antipsychotic drugs: implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29(1):127–48, viii. doi: 10.1016/j.ncl.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones A, Jones M. Managing patients with antipsychotic drug-induced hyperprolactinaemia. Nurs Stand. 2008;23(6):48–55. doi: 10.7748/ns2008.10.23.6.48.c6709. quiz 56. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness, I Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 31.Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry. 2008;21(2):151–6. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- 32.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47(1):27–38. [PubMed] [Google Scholar]
- 33.Pilowsky LS, Busatto GF, Taylor M, Costa DC, Sharma T, Sigmundsson T, Ell PJ, Nohria V, Kerwin RW. Dopamine D2 receptor occupancy in vivo by the novel atypical antipsychotic olanzapine–a 123I IBZM single photon emission tomography (SPET) study. Psychopharmacology (Berl) 1996;124(1–2):148–53. doi: 10.1007/BF02245615. [DOI] [PubMed] [Google Scholar]
- 34.Nyberg S, Olsson H, Nilsson U, Maehlum E, Halldin C, Farde L. Low striatal and extra-striatal D2 receptor occupancy during treatment with the atypical antipsychotic sertindole. Psychopharmacology (Berl) 2002;162(1):37–41. doi: 10.1007/s00213-002-1083-5. [DOI] [PubMed] [Google Scholar]
- 35.Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57(6):553–9. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- 36.Farde L, Nordstrom AL. PET analysis indicates atypical central dopamine receptor occupancy in clozapine-treated patients. Br J Psychiatry Suppl. 1992;(17):30–3. [PubMed] [Google Scholar]
- 37.Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiatry. 1999;156(6):876–84. doi: 10.1176/ajp.156.6.876. [DOI] [PubMed] [Google Scholar]
- 38.Seeman P. Clozapine, a fast-off-D2 antipsychotic. ACS Chem Neurosci. 2014;5(1):24–9. doi: 10.1021/cn400189s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goyer PF, Berridge MS, Morris ED, Semple WE, Compton-Toth BA, Schulz SC, Wong DF, Miraldi F, Meltzer HY. PET measurement of neuroreceptor occupancy by typical and atypical neuroleptics. J Nucl Med. 1996;37(7):1122–7. [PubMed] [Google Scholar]
- 40.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21(2 Suppl):106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 41.Murnane KS, Andersen ML, Rice KC, Howell LL. Selective serotonin 2A receptor antagonism attenuates the effects of amphetamine on arousal and dopamine overflow in non-human primates. J Sleep Res. 2013;22(5):581–8. doi: 10.1111/jsr.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH. Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35(4):881–92. doi: 10.1038/npp.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbas A, Roth BL. Pimavanserin tartrate: a 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother. 2008;9(18):3251–9. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- 44.Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology. 1997;16(2):93–110. doi: 10.1016/S0893-133X(96)00187-X. discussion 111–35. [DOI] [PubMed] [Google Scholar]
- 45.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28(3):519–26. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 46.Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Investig Drugs. 2010;11(7):802–12. [PubMed] [Google Scholar]
- 47.Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl) 2011;216(4):451–73. doi: 10.1007/s00213-011-2247-y. [DOI] [PubMed] [Google Scholar]
- 48.Arnt J, Skarsfeldt T. Do Novel Antipsychotics Have Similar Pharmacological Characteristics? A Review of the Evidence. Neuropsychopharmacology. 1998;18(2):63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 49.Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacology (Berl) 2009;205(1):119–28. doi: 10.1007/s00213-009-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 51.Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53(1):119–33. [PubMed] [Google Scholar]
- 52.Kessler RM, Ansari MS, Riccardi P, Li R, Jayathilake K, Dawant B, Meltzer HY. Occupancy of striatal and extrastriatal dopamine D2/D3 receptors by olanzapine and haloperidol. Neuropsychopharmacology. 2005;30(12):2283–9. doi: 10.1038/sj.npp.1300836. [DOI] [PubMed] [Google Scholar]
- 53.Komossa K, Rummel-Kluge C, Hunger H, Schmid F, Schwarz S, Duggan L, Kissling W, Leucht S. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;3:CD006654. doi: 10.1002/14651858.CD006654.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha KB, Bo L, Zhao S, Xia J, Sampson S, Zaman RU. Chlorpromazine versus atypical antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2016;4:CD010631. doi: 10.1002/14651858.CD010631.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moritz S, Andreou C, Klingberg S, Thoering T, Peters MJ. Assessment of subjective cognitive and emotional effects of antipsychotic drugs. Effect by defect? Neuropharmacology. 2013;72:179–86. doi: 10.1016/j.neuropharm.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 56.Oehl M, Hummer M, Fleischhacker WW. Compliance with antipsychotic treatment. Acta Psychiatr Scand Suppl. 2000;(407):83–6. doi: 10.1034/j.1600-0447.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 57.Bellack AS. Scientific and consumer models of recovery in schizophrenia: concordance, contrasts, and implications. Schizophr Bull. 2006;32(3):432–42. doi: 10.1093/schbul/sbj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindenmayer JP. Treatment refractory schizophrenia. Psychiatr Q. 2000;71(4):373–84. doi: 10.1023/a:1004640408501. [DOI] [PubMed] [Google Scholar]
- 59.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174(1):3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 60.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- 61.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–96. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–11. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 63.Tadori Y, Forbes RA, McQuade RD, Kikuchi T. Characterization of aripiprazole partial agonist activity at human dopamine D3 receptors. Eur J Pharmacol. 2008;597(1–3):27–33. doi: 10.1016/j.ejphar.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302(1):381–9. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 65.Keltner NL, Johnson V. Biological perspectives. Aripiprazole: a third generation of antipsychotics begins? Perspect Psychiatr Care. 2002;38(4):157–9. doi: 10.1111/j.1744-6163.2002.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 66.Kane JM, Carson WH, Saha AR, McQuade RD, Ingenito GG, Zimbroff DL, Ali MW. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–71. doi: 10.4088/jcp.v63n0903. [DOI] [PubMed] [Google Scholar]
- 67.Lieberman JA. Dopamine partial agonists: a new class of antipsychotic. CNS Drugs. 2004;18(4):251–67. doi: 10.2165/00023210-200418040-00005. [DOI] [PubMed] [Google Scholar]
- 68.Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH, Ali M, Marcus R. The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology (Berl) 2006;187(3):312–20. doi: 10.1007/s00213-006-0428-x. [DOI] [PubMed] [Google Scholar]
- 69.Cai HL, Tan QY, Jiang P, Dang RL, Xue Y, Tang MM, Xu P, Deng Y, Li HD, Yao JK. A potential mechanism underlying atypical antipsychotics-induced lipid disturbances. Transl Psychiatry. 2015;5:e661. doi: 10.1038/tp.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Otsuka Pharmaceutical Co, L. Abilify® Prescribing Information. 2014 [Google Scholar]
- 71.Spina E, de Leon J. Metabolic drug interactions with newer antipsychotics: a comparative review. Basic Clin Pharmacol Toxicol. 2007;100(1):4–22. doi: 10.1111/j.1742-7843.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- 72.Banno K, Fujioka T, Oshiro Y, Nakagawa K. Pharmaceutically useful carbostyril derivatives. 1988;(4) [Google Scholar]
- 73.OTSUKA PHARMACEUTICAL CO., LTD. v. SANDOZ, INC. United States Court of Appeals, Federal Circuit. 2010;678:1280. F.3d. [Google Scholar]
- 74.Oshiro Y, Sato S, Kurahashi N. Carbostyril Derivatives. 1991;(5) [Google Scholar]
- 75.Oshiro Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, Tottori K, Uwahodo Y, Nishi T. Novel antipsychotic agents with dopamine autoreceptor agonist properties: synthesis and pharmacology of 7-[4-(4-phenyl-1-piperazinyl)butoxy]-3,4-dihydro-2(1H)-quinolinone derivatives. J Med Chem. 1998;41(5):658–67. doi: 10.1021/jm940608g. [DOI] [PubMed] [Google Scholar]
- 76.Pollard CB, Wicker TH. Derivatives of Piperazine. XXIV. Synthesis of 1-Arylpiperazines and Amino Alcohol Derivatives. Journal of the American Chemical Society. 1954;76(7):1853–1855. [Google Scholar]
- 77.Les A, Badowska-Roslonek K, Laszcz M, Kamienska-Duda A, Baran P, Kaczmarek L. Optimization of aripiprazole synthesis. Acta Pol Pharm. 2010;67(2):151–7. [PubMed] [Google Scholar]
- 78.MIDAS IH. Top 20 Global Products 2014. 2014 [Google Scholar]
- 79.Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44(2):179–87. doi: 10.1177/0091270003261901. [DOI] [PubMed] [Google Scholar]
- 80.Bauman JN, Frederick KS, Sawant A, Walsky RL, Cox LM, Obach RS, Kalgutkar AS. Comparison of the bioactivation potential of the antidepressant and hepatotoxin nefazodone with aripiprazole, a structural analog and marketed drug. Drug Metab Dispos. 2008;36(6):1016–29. doi: 10.1124/dmd.108.020545. [DOI] [PubMed] [Google Scholar]
- 81.Kubo M, Koue T, Inaba A, Takeda H, Maune H, Fukuda T, Azuma J. Influence of itraconazole co-administration and CYP2D6 genotype on the pharmacokinetics of the new antipsychotic ARIPIPRAZOLE. Drug Metab Pharmacokinet. 2005;20(1):55–64. doi: 10.2133/dmpk.20.55. [DOI] [PubMed] [Google Scholar]
- 82.Ji Y, Skierka JM, Blommel JH, Moore BE, VanCuyk DL, Bruflat JK, Peterson LM, Veldhuizen TL, Fadra N, Peterson SE, Lagerstedt SA, Train LJ, Baudhuin LM, Klee EW, Ferber MJ, Bielinski SJ, Caraballo PJ, Weinshilboum RM, Black JL., 3rd Preemptive Pharmacogenomic Testing for Precision Medicine: A Comprehensive Analysis of Five Actionable Pharmacogenomic Genes Using Next-Generation DNA Sequencing and a Customized CYP2D6 Genotyping Cascade. J Mol Diagn. 2016;18(3):438–45. doi: 10.1016/j.jmoldx.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirschbaum KM, Muller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry. 2008;9(3):212–8. doi: 10.1080/15622970701361255. [DOI] [PubMed] [Google Scholar]
- 84.Hiemke C, Pfuhlmann B. Interactions and monitoring of antipsychotic drugs. Handb Exp Pharmacol. 2012;212:241–65. doi: 10.1007/978-3-642-25761-2_10. [DOI] [PubMed] [Google Scholar]
- 85.Vehof J, Burger H, Wilffert B, Al Hadithy A, Alizadeh BZ, Snieder H, investigators, G Clinical response to antipsychotic drug treatment: association study of polymorphisms in six candidate genes. Eur Neuropsychopharmacol. 2012;22(9):625–31. doi: 10.1016/j.euroneuro.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Zhang JP, Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol. 2011;7(1):9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang JP, Robinson DG, Gallego JA, John M, Yu J, Addington J, Tohen M, Kane JM, Malhotra AK, Lencz T. Association of a Schizophrenia Risk Variant at the DRD2 Locus With Antipsychotic Treatment Response in First-Episode Psychosis. Schizophr Bull. 2015;41(6):1248–55. doi: 10.1093/schbul/sbv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kwon JS, Kim E, Kang DH, Choi JS, Yu KS, Jang IJ, Shin SG, group, A. s Taq1A polymorphism in the dopamine D2 receptor gene as a predictor of clinical response to aripiprazole. Eur Neuropsychopharmacol. 2008;18(12):897–907. doi: 10.1016/j.euroneuro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 89.Zhang JP, Lencz T, Malhotra AK. D2 receptor genetic variation and clinical response to antipsychotic drug treatment: a meta-analysis. Am J Psychiatry. 2010;167(7):763–72. doi: 10.1176/appi.ajp.2009.09040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, Hietala J. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3(3):256–60. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 91.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4(3):290–6. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 92.Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN, Court JA. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7(6):479–84. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 93.Lane HY, Lee CC, Chang YC, Lu CT, Huang CH, Chang WH. Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int J Neuropsychopharmacol. 2004;7(4):461–70. doi: 10.1017/S1461145704004389. [DOI] [PubMed] [Google Scholar]
- 94.Miura I, Kanno-Nozaki K, Hino M, Horikoshi S, Ota T, Mashiko H, Niwa S, Yabe H. Influence of-141C Ins/Del Polymorphism in DRD2 Gene on Clinical Symptoms and Plasma Homovanillic Acid Levels in the Treatment of Schizophrenia With Aripiprazole. J Clin Psychopharmacol. 2015;35(3):333–4. doi: 10.1097/JCP.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 95.Chen SF, Shen YC, Chen CH. HTR2A A-1438G/T102C polymorphisms predict negative symptoms performance upon aripiprazole treatment in schizophrenic patients. Psychopharmacology (Berl) 2009;205(2):285–92. doi: 10.1007/s00213-009-1538-z. [DOI] [PubMed] [Google Scholar]
- 96.Chen SF, Shen YC. 5-HT1A C-1019G (rs6295) Predicts Aripiprazole Treatment Response Specifically for Cognitive and Depressive Symptoms in Schizophrenia. J Clin Psychopharmacol. 2017;37(1):114–118. doi: 10.1097/JCP.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 97.Blasi G, Selvaggi P, Fazio L, Antonucci LA, Taurisano P, Masellis R, Romano R, Mancini M, Zhang F, Caforio G, Popolizio T, Apud J, Weinberger DR, Bertolino A. Variation in Dopamine D2 and Serotonin 5-HT2A Receptor Genes is Associated with Working Memory Processing and Response to Treatment with Antipsychotics. Neuropsychopharmacology. 2015;40(7):1600–1608. doi: 10.1038/npp.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20(6):612–27. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 99.Mailman RB, Murthy V. Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des. 2010;16(5):488–501. doi: 10.2174/138161210790361461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oosterhof CA, El Mansari M, Blier P. Acute effects of brexpiprazole on serotonin, dopamine, and norepinephrine systems: an in vivo electrophysiologic characterization. J Pharmacol Exp Ther. 2014;351(3):585–95. doi: 10.1124/jpet.114.218578. [DOI] [PubMed] [Google Scholar]
- 101.Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441(3):137–40. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 102.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164(9):1411–7. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 103.Hirose T, Uwahodo Y, Yamada S, Miwa T, Kikuchi T, Kitagawa H, Burris KD, Altar CA, Nabeshima T. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J Psychopharmacol. 2004;18(3):375–83. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- 104.Tamminga CA, Carlsson A. Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr Drug Targets CNS Neurol Disord. 2002;1(2):141–7. doi: 10.2174/1568007024606195. [DOI] [PubMed] [Google Scholar]
- 105.Kikuchi T, Tottori K, Uwahodo Y, Hirose T, Miwa T, Oshiro Y, Morita S. 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinon e (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther. 1995;274(1):329–36. [PubMed] [Google Scholar]
- 106.Meltzer HY. Relevance of dopamine autoreceptors for psychiatry: preclinical and clinical studies. Schizophr Bull. 1980;6(3):456–75. doi: 10.1093/schbul/6.3.456. [DOI] [PubMed] [Google Scholar]
- 107.Benkert O, Muller-Siecheneder F, Wetzel H. Dopamine agonists in schizophrenia: a review. Eur Neuropsychopharmacol. 1995;5(Suppl):43–53. doi: 10.1016/0924-977x(95)00022-h. [DOI] [PubMed] [Google Scholar]
- 108.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 109.Cooper JR, Bloom FE, Robert RH. The biochemical basis of neuropharmacology. 8th. Oxford University Press; New York: 2003. p. 518. [Google Scholar]
- 110.Bannon MJ, Michaud RL, Roth RH. Mesocortical dopamine neurons. Lack of autoreceptors modulating dopamine synthesis. Mol Pharmacol. 1981;19(2):270–5. [PubMed] [Google Scholar]
- 111.Bannon MJ, Reinhard JF, Jr, Bunney EB, Roth RH. Unique response to antipsychotic drugs is due to absence of terminal autoreceptors in mesocortical dopamine neurones. Nature. 1982;296(5856):444–6. doi: 10.1038/296444a0. [DOI] [PubMed] [Google Scholar]
- 112.Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang X-p, Feng B, Jensen NH, Che X, Bai X, Frye SV, Wetsel WC, Caron MG, Javitch JA, Roth BL, Jin J. Discovery of β-Arrestin–Biased Dopamine D2 Ligands for Probing Signal Transduction Pathways Essential for Antipsychotic Efficacy. Proceedings of the National Academy of Sciences. 2011;108(45):18488–18493. doi: 10.1073/pnas.1104807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu J-M, Gainetdinov RR, Caron MG. Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proceedings of the National Academy of Sciences. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–17. doi: 10.1146/annurev.med.52.1.503. [DOI] [PubMed] [Google Scholar]
- 115.Grunder G, Fellows C, Janouschek H, Veselinovic T, Boy C, Brocheler A, Kirschbaum KM, Hellmann S, Spreckelmeyer KM, Hiemke C, Rosch F, Schaefer WM, Vernaleken I. Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry. 2008;165(8):988–95. doi: 10.1176/appi.ajp.2008.07101574. [DOI] [PubMed] [Google Scholar]
- 116.Stark AD, Jordan S, Allers KA, Bertekap RL, Chen R, Mistry Kannan T, Molski TF, Yocca FD, Sharp T, Kikuchi T, Burris KD. Interaction of the novel antipsychotic aripiprazole with 5-HT1A and 5-HT 2A receptors: functional receptor-binding and in vivo electrophysiological studies. Psychopharmacology (Berl) 2007;190(3):373–82. doi: 10.1007/s00213-006-0621-y. [DOI] [PubMed] [Google Scholar]
- 117.Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013;27(9):703–16. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 118.Di Sciascio G, Riva MA. Aripiprazole: from pharmacological profile to clinical use. Neuropsychiatr Dis Treat. 2015;11:2635–47. doi: 10.2147/NDT.S88117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Assie MB, Ravailhe V, Faucillon V, Newman-Tancredi A. Contrasting contribution of 5-hydroxytryptamine 1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther. 2005;315(1):265–72. doi: 10.1124/jpet.105.087163. [DOI] [PubMed] [Google Scholar]
- 120.Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853–61. [PubMed] [Google Scholar]
- 121.Newman-Tancredi A, Assie MB, Leduc N, Ormiere AM, Danty N, Cosi C. Novel antipsychotics activate recombinant human and native rat serotonin 5-HT1A receptors: affinity, efficacy and potential implications for treatment of schizophrenia. Int J Neuropsychopharmacol. 2005;8(3):341–56. doi: 10.1017/S1461145704005000. [DOI] [PubMed] [Google Scholar]
- 122.Millan MJ, Gobert A, Newman-Tancredi A, Audinot V, Lejeune F, Rivet JM, Cussac D, Nicolas JP, Muller O, Lavielle G. S 16924 ((R)-2-[1-[2-(2,3-dihydro-benzo[1,4] dioxin-5-Yloxy)-ethyl]-pyrrolidin-3yl]-1-(4-fluoro-phenyl)-ethanone), a novel, potential antipsychotic with marked serotonin (5-HT)1A agonist properties: I. Receptorial and neurochemical profile in comparison with clozapine and haloperidol. J Pharmacol Exp Ther. 1998;286(3):1341–55. [PubMed] [Google Scholar]
- 123.Newman-Tancredi A, Chaput C, Verriele L, Millan MJ. Clozapine is a partial agonist at cloned, human serotonin 5-HT1A receptors. Neuropharmacology. 1996;35(1):119–21. doi: 10.1016/0028-3908(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 124.Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol. 1997;338(2):R3–R5. doi: 10.1016/s0014-2999(97)81951-6. [DOI] [PubMed] [Google Scholar]
- 125.Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry. 2000;48(3):229–37. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- 126.Kargieman L, Riga MS, Artigas F, Celada P. Clozapine Reverses Phencyclidine-Induced Desynchronization of Prefrontal Cortex through a 5-HT(1A) Receptor-Dependent Mechanism. Neuropsychopharmacology. 2012;37(3):723–33. doi: 10.1038/npp.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment–pharmacological mechanisms. Pharmacol Ther. 2010;125(1):169–79. doi: 10.1016/j.pharmthera.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 128.Nguyen CT, Rosen JA, Bota RG. Aripiprazole partial agonism at 5-HT2C: a comparison of weight gain associated with aripiprazole adjunctive to antidepressants with high versus low serotonergic activities. Prim Care Companion CNS Disord. 2012;14(5) doi: 10.4088/PCC.12m01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arena Pharmaceuticals Arena Pharmaceuticals and Eisai Announce FDA Approval of BELVIQ® (lorcaserin HCl) for Chronic Weight Management in Adults who are Overweight with a Comorbidity or Obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182.
- 130.Pitychoutis PM, Belmer A, Moutkine I, Adrien J, Maroteaux L. Mice Lacking the Serotonin Htr2B Receptor Gene Present an Antipsychotic-Sensitive Schizophrenic-Like Phenotype. Neuropsychopharmacology. 2015;40(12):2764–73. doi: 10.1038/npp.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, Belmer A, Hodgkinson CA, Dell’osso L, Suvisaari J, Coccaro E, Rose RJ, Peltonen L, Virkkunen M, Goldman D. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468(7327):1061–6. doi: 10.1038/nature09629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.FDA, FDA warns about new impulse-control problems associated with mental health drug aripiprazole (Abilify, Abilify Maintena, Aristada) 2016 https://www.fda.gov/Drugs/DrugSafety/ucm498662.htm.
- 133.Cohen LS, Viguera AC, Freeman MP, Sosinsky AZ, Savella G, Cheng L, Chitayat D, Hernandez-Diaz S. The National Pregnancy Registry for Atypical Antipsychotics: Effects of First Trimester Exposure to Aripiprazole and Quetiapine on Risk for Major Malformations. American College of Neuropsychopharmacology; Hollywood, Florida: Dec, 2016. Hollywood, Florida, 2016. [Google Scholar]
- 134.Bellet F, Beyens MN, Bernard N, Beghin D, Elefant E, Vial T. Exposure to aripiprazole during embryogenesis: a prospective multicenter cohort study. Pharmacoepidemiol Drug Saf. 2015;24(4):368–80. doi: 10.1002/pds.3749. [DOI] [PubMed] [Google Scholar]
- 135.Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy. Their effects on preterm birth. JAMA. 1986;255(1):82–4. [PubMed] [Google Scholar]
- 136.Uguz F. Second-Generation Antipsychotics During the Lactation Period: A Comparative Systematic Review on Infant Safety. J Clin Psychopharmacol. 2016;36(3):244–52. doi: 10.1097/JCP.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 137.Nilsson E, Lichtenstein P, Cnattingius S, Murray RM, Hultman CM. Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res. 2002;58(2–3):221–9. doi: 10.1016/s0920-9964(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 138.Tandon R, Marcus RN, Stock EG, Riera LC, Kostic D, Pans M, McQuade RD, Nyilas M, Iwamoto T, Crandall DT. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA) Schizophr Res. 2006;84(1):77–89. doi: 10.1016/j.schres.2005.12.857. [DOI] [PubMed] [Google Scholar]
- 139.Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012;5:CD009043. doi: 10.1002/14651858.CD009043.pub2. [DOI] [PubMed] [Google Scholar]
- 140.Selfani K, Soland VL, Chouinard S, Huot P. Movement Disorders Induced by the “Atypical” Antipsychotic Aripiprazole. Neurologist. 2017;22(1):24–28. doi: 10.1097/NRL.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 141.Ketter TA, Citrome L, Wang PW, Culver JL, Srivastava S. Treatments for bipolar disorder: can number needed to treat/harm help inform clinical decisions? Acta Psychiatr Scand. 2011;123(3):175–89. doi: 10.1111/j.1600-0447.2010.01645.x. [DOI] [PubMed] [Google Scholar]
- 142.Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, McQuade RD, Forbes A. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry. 2007;15(11):918–31. doi: 10.1097/JGP.0b013e3181557b47. [DOI] [PubMed] [Google Scholar]
- 143.Madhusoodanan S, Shah P. Management of psychosis in patients with Alzheimer’s disease: focus on aripiprazole. Clin Interv Aging. 2008;3(3):491–501. doi: 10.2147/cia.s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mittal V, Kurup L, Williamson D, Muralee S, Tampi RR. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26(1):10–28. doi: 10.1177/1533317510390351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gerasch S, Kanaan AS, Jakubovski E, Muller-Vahl KR. Aripiprazole Improves Associated Comorbid Conditions in Addition to Tics in Adult Patients with Gilles de la Tourette Syndrome. Front Neurosci. 2016;10:416. doi: 10.3389/fnins.2016.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lopez-Munoz F, Alamo C, Cuenca E, Shen WW, Clervoy P, Rubio G. History of the discovery and clinical introduction of chlorpromazine. Ann Clin Psychiatry. 2005;17(3):113–35. doi: 10.1080/10401230591002002. [DOI] [PubMed] [Google Scholar]
- 147.Drew L. Clozapine and agranulocytosis: re-assessing the risks. Australas Psychiatry. 2013;21(4):335–7. doi: 10.1177/1039856213491990. [DOI] [PubMed] [Google Scholar]
- 148.Wenthur CJ, Lindsley CW. Classics in chemical neuroscience: clozapine. ACS Chem Neurosci. 2013;4(7):1018–25. doi: 10.1021/cn400121z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–26. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 150.Ucok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World psychiatry: official journal of the World Psychiatric Association. 2008;7(1):58–62. doi: 10.1002/j.2051-5545.2008.tb00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Banno K, Fujioka T, Kikuchi T, Oshiro Y, Hiyama T, Nakagawa K. Studies on 2(1H)-quinolinone derivatives as neuroleptic agents I. Synthesis and biological activities of (4-phenyl-1-piperazinyl)-propoxy-2(1H)-quinolinone derivatives. Chem Pharm Bull (Tokyo) 1988;36(11):4377–88. doi: 10.1248/cpb.36.4377. [DOI] [PubMed] [Google Scholar]
- 152.Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacol Rev. 1983;35(1):53–68. [PubMed] [Google Scholar]
- 153.Kiuchi K, Hirata Y, Minami M, Nagatsu T. Effect of 7-(3-[4-(2,3-dimethylphenyl)piperazinyl]propoxy)-2(1H)-quinolinone (OPC-4392), a newly synthesized agonist for presynaptic dopamine D2 receptor, on tyrosine hydroxylation in rat striatal slices. Life Sci. 1988;42(3):343–9. doi: 10.1016/0024-3205(88)90644-3. [DOI] [PubMed] [Google Scholar]
- 154.Momiyama T, Sasa M, Takaori S. D-2 receptor-mediated inhibition by a substituted quinolinone derivateive, 7-{3-(4-(2,3-dimethylphenyl)piperazinyl)propoxy}-2(1h)-quinolinone (OPC-4392), of dopaminergic neurons in the ventral tegmental area. Life Sciences. 1990;47(9):761–769. doi: 10.1016/0024-3205(90)90548-6. [DOI] [PubMed] [Google Scholar]
- 155.Yasuda Y, Kikuchi T, Suzuki S, Tsutsui M, Yamada K, Hiyama T. 7-[3-(4-[2,3-dimethylphenyl]piperazinyl)propoxy]-2(1H)-quinolinone (OPC-4392), a presynaptic dopamine autoreceptor agonist and postsynaptic D2 receptor antagonist. Life Sciences. 1988;42(20):1941–1954. doi: 10.1016/0024-3205(88)90493-6. [DOI] [PubMed] [Google Scholar]
- 156.Olbrich R, Schanz H. The effect of the partial dopamine agonist terguride on negative symptoms in schizophrenics. Pharmacopsychiatry. 1988;21(6):389–90. doi: 10.1055/s-2007-1017021. [DOI] [PubMed] [Google Scholar]
- 157.Sasa M, Amano T. Unique Pharmacological Profile of a Novel Antipsychotic Drug, Aripiprazole (OPC-14597) CNS Drug Rev. 1997;3(1):24–33. [Google Scholar]
- 158.Ito H, Takano H, Arakawa R, Takahashi H, Kodaka F, Takahata K, Nogami T, Suzuki M, Suhara T. Effects of dopamine D2 receptor partial agonist antipsychotic aripiprazole on dopamine synthesis in human brain measured by PET with L-[beta-11C]DOPA. PLoS One. 2012;7(9):e46488. doi: 10.1371/journal.pone.0046488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–90. doi: 10.1001/archpsyc.60.7.681. [DOI] [PubMed] [Google Scholar]
- 160.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3(5):241–53. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 161.Hu W, MacDonald ML, Elswick DE, Sweet RA. The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci. 2015;1338:38–57. doi: 10.1111/nyas.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. A model of cholinergic/dopaminergic interactions in schizophrenia. Arch Gen Psychiatry. 1989;46(8):745–53. doi: 10.1001/archpsyc.1989.01810080075010. [DOI] [PubMed] [Google Scholar]
- 163.Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013;86(8):1122–32. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, Seki Y, Iwaki T, Hashioka S, Kanba S. Inhibitory effects of aripiprazole on interferon-gamma-induced microglial activation via intracellular Ca2+ regulation in vitro. J Neurochem. 2008;106(2):815–25. doi: 10.1111/j.1471-4159.2008.05435.x. [DOI] [PubMed] [Google Scholar]
- 165.Sato-Kasai M, Kato TA, Ohgidani M, Mizoguchi Y, Sagata N, Inamine S, Horikawa H, Hayakawa K, Shimokawa N, Kyuragi S, Seki Y, Monji A, Kanba S. Aripiprazole inhibits polyI:C-induced microglial activation possibly via TRPM7. Schizophr Res. 2016;178(1–3):35–43. doi: 10.1016/j.schres.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 166.Sobis J, Rykaczewska-Czerwinska M, Swietochowska E, Gorczyca P. Therapeutic effect of aripiprazole in chronic schizophrenia is accompanied by anti-inflammatory activity. Pharmacol Rep. 2015;67(2):353–9. doi: 10.1016/j.pharep.2014.09.007. [DOI] [PubMed] [Google Scholar]


