Abstract
Primary sensory neurons are responsible for transmitting sensory information from the peripheral to the central nervous system. Their responses to incoming stimulation become greatly enhanced and prolonged following inflammation, giving rise to exaggerated nociceptive responses and chronic pain. The inflammatory mediator, prostaglandin E2 (PGE2), released from the inflamed tissue surrounding the terminals of sensory neurons contributes to the abnormal pain responses. PGE2 acts on G protein-coupled EP receptors to activate adenylyl cyclase, which catalyzes the conversion of adenosine triphosphate to cyclic adenosine 3′,5′-monophosphate (cAMP). Under normal conditions, cAMP activates primarily protein kinase A. After inflammation, cAMP also activates the exchange proteins activated by cAMP (Epacs) to produce exaggerated PGE2-mediated hyperalgesia. The role of cAMP-Epac signaling in the generation of hypersensitivity is the topic of this review.
Keywords: Epac, protein kinase C, filamentous-actin, purinergic P2X3 receptor, dorsal root ganglion, nociception, inflammation, A-kinase anchor proteins
Introduction
Role of primary sensory neurons in chronic pain
Pain associated with injuries and/or diseases is the most common reason for patients to seek help from physicians.1 Acute pain resulting from tissue damage, e.g. a sprained ankle or hot stove burn, serves important physiological functions. It warns patients that injuries and/or diseases have occurred and necessary steps, e.g. avoidance of damaging stimuli or medical attention, need to be taken to rectify the situation. The problem comes when pain is no longer proportional to the severity of injury. Innocuous stimuli, e.g. light touches, produce painful responses (allodynia); normally, painful stimuli evoke excessive pain (hyperalgesia). Pain persists for many (> six) months and the severity of pain worsens even though signs of injuries subside.2–4 Chronic pain results in a great deal of patient suffering. In order to find effective ways to treat chronic pain, it is necessary to understand the mechanisms underlying nociceptive sensitization after tissue inflammation and nerve injuries.
Studying responses of primary dorsal root ganglion (DRG) sensory neurons (nociceptors) to injuries is of great interest because these neurons directly participate in transmission and processing of nociceptive information.5,6 Following inflammation, inflammatory mediators, e.g. prostaglandin E2 (PGE2) and bradykinin, are released from injured tissues to produce hyperexcitability in nociceptors.7–9 This gives rise to hyperalgesia—a common symptom of prevalent diseases, e.g. arthritis, bowel inflammation, and diabetes. Understanding how sensory neurons process information under inflammation and nerve injury conditions will help us find potentially effective strategies to relieve chronic pain.
Cyclic adenosine 3′,5′-monophosphate in sensory signaling
PGE2 has diverse biological functions.10 Following its release after inflammation, PGE2 activates G protein-coupled EP1-EP4 receptors in DRGs and produces a variety of effects.7,10–13 EP1 is coupled to Gq/G11, and EP2 is coupled to Gs. EP3 and EP4 are coupled to Gs or Gi.14,15 Those coupled to Gs lead to the activation of cell membrane-located adenylyl cyclases (ACs). Cyclic adenosine 3′,5′-monophosphate (cAMP), which is synthesized from adenosine triphosphate (ATP) by membrane-located ACs, exerts its effects through activation of cAMP-dependent protein kinase A (PKA) to directly phosphorylate target proteins16,17 or through actions on cyclic nucleotide-gated ion channels.18
Another set of cAMP target proteins, i.e. exchange proteins activated by cAMP (Epacs) (also called cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs)) were recently discovered.19,20 Unlike PKA, Epacs exert their effects through activation of small Ras-family of G proteins such as Rap1 and Rap2.21,22 Epac-mediated signaling has been found to regulate many physiological processes and contribute to a variety disease states, including chronic pain and cardiac stresses.23–25
This review will focus on studies of mechanisms underlying modulation of the activity of sensory neurons by cAMP through the activation of Epacs and the prominent roles of these GEFs in mediating nociceptor sensitization after injury.
Mechanism of activation of the cAMP target proteins, Epacs
PKA consists of four subunits—two catalytic and two regulatory subunits. Binding of cAMP to the regulatory units causes the release of catalytic subunits which phosphorylate their target proteins.26
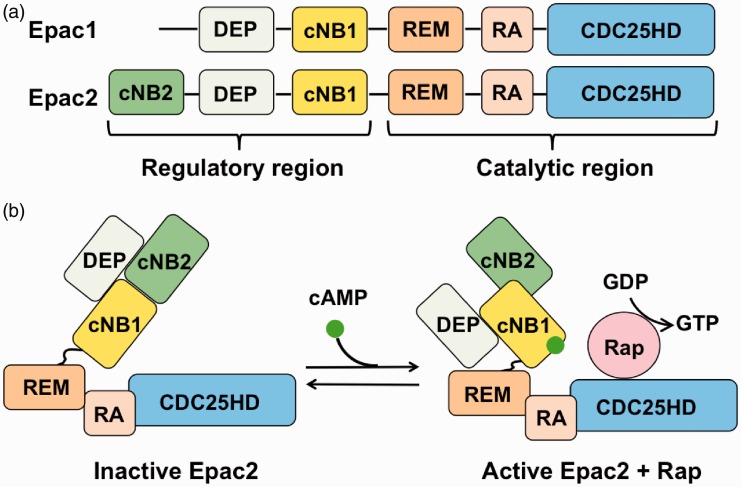
The process of Epac activation is different.19,21,23,27,28 Epacs consists of an N-terminal regulatory region and a C-terminal catalytic region (Figure 1(a)). The regulatory region consists of either one (for Epac1) or two (for Epac2) cAMP nucleotide-binding (cNB) domains and a Dishevelled-Egl-10-Pleckstrin (DEP) domain for membrane localization. The catalytic region consists of a Ras exchange motif (REM) domain for stabilization of the catalytic helix of a CDC25-homology domain (CDC25HD) and a Ras association (RA) domain, which is a protein interaction motif. RA connects to a CDC25HD, which is responsible for guanine nucleotide exchange activity. In the absence of cAMP, cNBs cover the CDC25HD domain, thus preventing Rap to bind (autoinhibition) (Figure 1(b)).27 Following cAMP binding, CDC25HD domain is exposed, allowing it to activate Rap, i.e. conversion of Rap-guanosine diphosphate (GDP) to Rap-guanosine triphosphate (GTP).
Figure 1.
Domain structure of Epacs and activation of Epac by cAMP. (a) The regulatory region contains one (for Epac1) or two (for Epac2) cAMP nucleotide binding (cNB) domains and a Dishevelled-Egl-10-Pleckstrin (DEP) domain. The carboxyl-terminal catalytic region consists of a Ras exchange motif (REM), a Ras association (RA) domain, and a CDC25-homology domain (CDC25-HD). (b) Activation of Epac (shown for Epac2). Binding of cAMP exposes CDC25HD domain, thus allowing the activation of Rap.
Epac: exchange protein activated by cAMP; cAMP: cyclic adenosine 3′,5′-monophosphate; GTP: guanosine triphosphate; GDP: guanosine diphosphate.
The major action of Epacs is to catalyze the exchange of GDP to GTP for the small G proteins, e.g. Rap1 and Rap2.19,20 Activation of Raps in turn activates a large number of effector proteins, including Rap1, phospholipase Cs (PLCs), protein kinase C (PKC)ɛ, and MAP kinases (MAPKs), to regulate various cell functions.23–25,29 It was further found that binding of cAMP to the Epacs exert dual controls on Epac activity. It releases Epacs from autoinhibition (Figure 1(b)). At the same time, the Epacs allow their DEP domains to bind to phosphatidic acid. This facilitates the translocation of Epacs from the cytoplasm to the plasma membrane and subsequent activation of membrane-located Rap1.30,31
Sensitization of nociceptors by Epacs
Epac roles in epinephrine-induced cAMP-PKCɛ signaling
There is a great deal evidence that PKCɛ plays an essential role in pain signaling.32–36 To identify the molecule mediating cAMP to PKCɛ signaling, Hucho et al.37 studied the membrane translocation of PKCɛ in cultured DRG neurons isolated from adult male rats in response to epinephrine-induced activation of β2-adrenergic receptors. They found that epinephrine promotes PKCɛ membrane translocation in IB4 (+) neurons. Increasing the level of cAMP inside cells by cholera toxin, which activates Gαs, or by forskolin, which activates AC, also induces PKCɛ translocation. The PKA-specific inhibitor CMIQ, on the other hand, has no effect on epinephrine-induced PKCɛ translocation. These results suggest that epinephrine induces cAMP-PKCɛ signaling in DRG neurons. Since the activation of Epac by the selective agonist, 8-pCPT-2-O-Me-cAMP (CPT), mimics the effect of epinephrine, the observation led to the proposal that Epac mediates the cAMP-PKCɛ signaling, i.e. cAMP-Epac-PKCɛ, in DRG neurons.37 Inhibition of phosphatidylinositol (PI)-specific PLC (PI-PLC) and phospholipase D (PLD), which are required for PKCɛ translocation and activation, was found to reduce epinephrine- and CPT-induced PKCɛ translocation. Thus, PI-PLC and PLD act downstream of Epac. In behavioral analyses, both epinephrine and CPT induce mechanical hyperalgesia and are blocked by the PKCɛ inhibitor, ɛV1-2. The same inhibitors of PI-PLC and PLD also block epinephrine- and CPT-induced mechanical hyperalgesia.37 These observations have led to the conclusion that Epac participates in the epinephrine-induced cAMP-PKCɛ-mediated hyperalgesia.
PGE2-mediated hyperalgesia
In the study of PGE2-induced hyperalgesia, Levine’s group33,38 found an important priming phenomenon after inflammation (termed hyperalgesic priming). Under normal conditions (without carrageenan treatment), injection of PGE2 into the rat hindpaw produces hyperalgesia which lasts < 4 h and is blocked by PKA inhibitors. Application of a low amount (5 µl, 1%) of carrageenan into the rat dorsal hindpaw produces visible signs of inflammation, e.g. paw swelling, skin redness, and a decrease in paw withdrawal threshold to mechanical pressure stimuli that resolve within three days. Five to 21 days after carrageenan treatment, application of PGE2 to those rat paws evokes hyperalgesia with different characteristics. PGE2-induced hyperalgesia is much enhanced38 and prolonged (> 24 h).33 The late phase (>4 h after PGE2 application) of the hyperalgesia is no longer mediated by PKA but is inhibited by PKCɛ inhibitors.35 They also found this phenomenon occurs only in male rats,39 is present in IB4 positive (IB4+) cells,40 and can be blocked by protein translation inhibitors.41
The PGE2-induced hyperalgesia in carrageenan-primed paws is different from epinephrine-induced hyperalgesia in several important ways. Unlike PGE2, epinephrine-induced hyperalgesia is mediated through PKA, PKC, and ERK under normal conditions.42,43 The epinephrine-induced hyperalgesia is neither potentiated by carrageenan treatment38,44 nor required priming to recruit PKCɛ signaling. Furthermore, while epinephrine-induced hyperalgesia requires intact cytoskeleton under normal conditions, PGE2-induced hyperalgesia required cytoskeleton only after carrageenan treatment.45
Epac-PKCɛ signaling
To determine the mechanisms underlying enhanced PGE2-induced hyperalgesia after inflammation, we studied the effects of PGE2 on ATP-induced activation of purinergic P2X3 receptor (P2X3R)-mediated currents in DRG neurons and α,β-meATP-induced behavioral responses under normal and inflammatory conditions.46,47 P2X3Rs were found to play a prominent role in transmission of sensory signals from the periphery to the spinal cord.48 Following inflammation or nerve injury, P2X3R-mediated ATP currents or α,β meATP induced nociceptive behavioral responses are enhanced, as a result of upregulation of total or membrane expression of P2X3Rs in DRG neurons.49–52 Under normal conditions, PGE2 increases P2X3R-mediated currents (IATP) in DRG neurons and P2X3R-mediated flinch responses through an activation of PKA.46,47 Following complete Freund’s adjuvant (CFA) injection to induce inflammation in the rat hindpaw, PGE2 produces a much larger increase in P2X3R responses in DRG neurons isolated from CFA-treated animals. The increase is mediated by both PKA and PKCɛ. PGE2 activates Epac only in those neurons isolated from CFA-treated rats. CPT occludes the PKC-mediated action but not PKA-mediated action of PGE2. There is no crosstalk between PKA- and PKC-dependent action of PGE2.47
Injury increases both Epacs 1 and 2 expression
We found that the expression of both Epac1 and 2 in rat DRGs is upregulated after inflammation. PGE2-induced hyperalgesia can be blocked by both Epac1 and 2 antagonists.53 Vasko et al.54 showed that only Epac2 is upregulated in rat DRGs after CFA-induced inflammation. In contrast, Epac1 expression in mice is upregulated after CFA treatment.55 Similar to our results, incision-induced inflammatory injury results in an increase in the expression of Epac1 and Epac2 in small and medium DRG neurons in rats.56 The percentages of neurons expressing Epac1 and Epac 2 are also significantly increased.
Filamentous-actin is downstream of Epac-PKCɛ signaling
It has been shown that in normal rats, cytoskeleton disruptors inhibit epinephrine-induced hyperalgesia, which depends on PKA and PKCɛ activity, but have no effect on PGE2-induced hyperalgesia, which depends on PKA activity alone.38,44,45 Following carrageenan-induced inflammation, PGE2-induced hyperalgesia becomes PKCɛ and ERK dependent and is sensitive to the cytoskeleton disruptor.45 Thus, cytoskeleton proteins are linked to the inflammation-induced PKCɛ-mediated nociception.
To find out the role of Epac in cytoskeleton modulation of PGE2-induced hyperalgesia in DRG neurons, we determined the effect of the filamentous-actin (F-actin) formation inhibitor, cytochalasin D (CD), on PGE2-elicited changes in IATP in DRG neurons isolated from normal and CFA-treated rats.57 CD was found to block PGE2-induced increase in IATP only in neurons isolated from CFA rats. The CD blocking effect is abolished in the presence of the PKC inhibitor, Bis. Behavioral studies also showed that CD had no effect on the basal and PGE2-induced α,β-meATP-mediated flinch responses in normal rats but effectively blocked the much enhanced PGE2-induced flinch responses in CFA rats.57 Thus, F-actin has a critical role in PKC signaling of IATP in DRG neurons of rats with inflammation, confirming the importance of cytoskeleton45 and suggesting the participation of P2X3Rs in producing hyperalgesic priming.
To determine the involvement of Epacs, we then used CPT, to activate Epac in normal DRG neurons and studied the effect of CD on IATP.57 CD was found to block CPT-induced enhancement of IATP, suggesting that F-actin plays a critical role in Epac-dependent PKCɛ signaling of IATP. To determine if F-actin directly affects PKCɛ activation in DRGs, we studied the effect of the F-actin formation inhibitor, latrunculin A (Lat-A), on the CPT-induced change in phosphorylated PKCɛ (pPKCɛ) expression and membrane expressed P2X3R (mP2X3R).57 Lat-A does not affect pPKCɛ expression but inhibits mP2X3R expression. Thus, F-actin is a downstream effector of Epac-PKCɛ signaling, i.e. Epac –> (PKCɛ signaling) –> F-actin –> (mP2X3R expression) (Figure 2). How F-actin affects P2X3R membrane distribution in DRG neurons has yet to be determined.
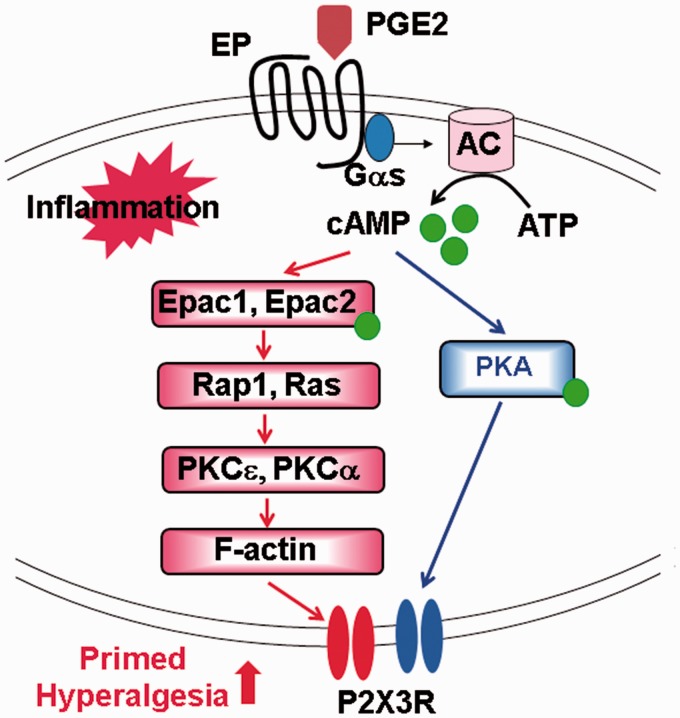
Figure 2.
Modeling signaling events elicited by PGE2 before and after inflammation. Activation of EP receptors by PGE2 promotes cAMP synthesis. Under normal conditions (blue), cAMP activates PKA and enhances the activity of P2X3Rs. After inflammation (red), cAMP also activates Epacs which leads to the activation of PKCɛ and PKCα to give rise to much enhanced P2X3R-mediated responses and exaggerated primed hyperalgesia.
PGE2: prostaglandin E2; AC: adenylyl cyclase; ATP: adenosine triphosphate; cAMP: cyclic adenosine 3′,5′-monophosphate; Epac: exchange protein activated by cAMP; PKA: protein kinase A; PKC: protein kinase C.
Epac-PKCα signaling
In addition, we found that PKCɛ is not the only PKC subtype activated in response to an increase in Epac expression in DRGs after inflammation. The activation of Epac also leads to PKCα activation.53 The role of PKCα has been largely ignored in the study of PGE2-induced hyperalgesia because, in an earlier report, PKCα was found to be minimally expressed in DRGs of neonatal rats.58 Subsequent studies have shown that PKCα is well expressed in DRGs prepared from embryonic,59 neonatal,53 adult,53,60 and aged rats.61 We found that an increase in P2X3R-mediated pain behaviors induced by CFA-induced inflammation can be inhibited by PKCα siRNA or PKCα inhibitors in adult rats.53 Furthermore, inflammation also increases phosphorylated PKCα (pPKCα) expression. Using CPT in normal rats to mimic the activation of Epac after inflammation, the same participation of PKCα in Epac signaling was also observed. These results strongly suggest that PKCα, in addition to PKCɛ, participates in Epac-mediated signaling of P2X3R-mediated nociception. Immunocytochemical analyses of the PKCα distribution in cultured DRGs and DRG slices showed that PKCα, like P2X3Rs, is expressed only in small and medium DRG neurons, while PKCɛ is expressed in both small–medium and large neurons.53 After inflammation, pPKCα is mostly associated with the cell membrane, while PKCɛ is found in the cytoplasm of DRG neurons. In addition, all of the P2X3R-containing cells express PKCα, while ∼75% of P2X3R-containing cells express PKCɛ. The contribution of Epac-induced PKCα activation on behavioral responses other than α,β, meATP-induced nocifensive hyperalgesia has yet to be determined.
Not all forms of PGE2-dependent hyperalgesic priming depend upon Epacs
Instead of inflammation-induced priming,8,38,44,62 a varying type of priming, termed type 2 hyperalgesic priming, has been recently observed. This hyperalgesic priming is induced by repeated applications of the mu-opioid receptor agonist, DAMGO,63 or an A1-adenosine receptor agonist.64 Type 2 hyperalgesia differs from inflammation-induced hyperalgesia in several significant ways. The primed hyperalgesia is PKA-, but not PKCɛ-mediated, is insensitive to translation inhibitor, occurs only in IB4 negative (IB4−) DRG cells, and is observed in both male and female rats.63 Although not tested, Epac is unlikely to be involved in type 2 hyperalgesia priming because of its sole dependence on PKA activity.
Upstream control and downstream actions of Epac signaling
G protein-coupled receptor kinase 2 modifies Epac activity
Recently, it was found that G protein-coupled receptor kinase 2 (GRK2) plays a prominent role in modulating hyperalgesia induced by tissue injury.65–67 GRK2 expression in DRGs is downregulated during a long-lasting inflammation induced by intraplantar application of a high-dose carrageenan.66 In heterozygous GRK2(±) mutant mice with a deletion of the GRK2 gene in Nav1.8+ sensory neurons, thus ∼50% reduction in GRK2 expression in nociceptors, intraplantar-injected PGE2 produces much enhanced and prolonged (72 h compared to 4–6 h in wild-type mice) hyperalgesia.66 The prolonged PGE2-induced hyperalgesia can be reproduced by the cAMP activator, 8-Br-cAMP, or by the Epac-activator, CPT, but fails to be blocked by the PKA inhibitor, H-89. Thus, in contrast to PGE2-induced hyperalgesia in wild-type mice which is PKA-mediated, the PGE2 action on the enhanced hyperalgesia in GKR2(±) mice is Epac1-mediated.66
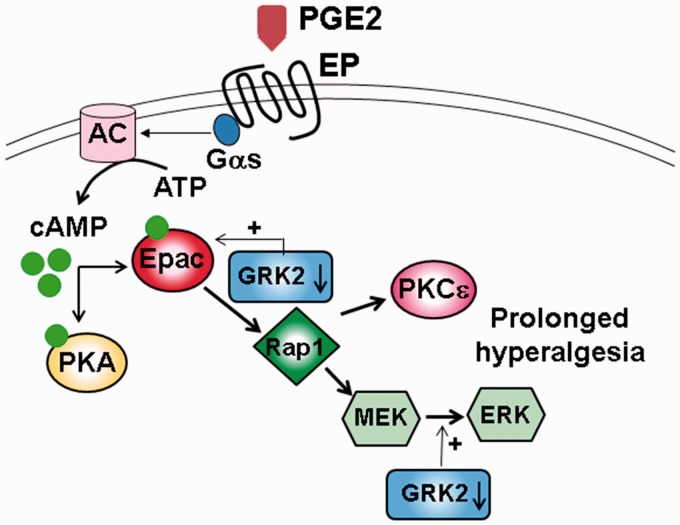
The observation that GRK2 co-immunoprecipitates with Epac1 suggests a direct association between GRK2 and Epac1 in DRGs.66 A detailed study of the influence of GRK2 on the Epac1 level found that phosphorylation of ser108 in Epac1 by GRK2 can lead to a reduction of Epac1 activation.68 Downstream consequence of a reduction of GRK2 was explored.66 Low GRK2 expression in GRK2(±) DRGs was found to facilitate CPT-induced Rap1 activation and increase the phosphorylated ERK1/2 level. PGE2- and CPT-induced hyperalgesia in GRK2(±) mice can be blocked by MEK1 or PKCɛ inhibitors. Thus, low GRK2 in DRGs promotes Epac1 activation in the Epac-Rap1 pathway which leads to the activation of MEK/ERK and PKCɛ to give rise to prolonged inflammatory hyperalgesia (Figure 3).
Figure 3.
Effect of the GRK2 expression on PGE2-induced hyperalgesia. GRK2 expression in DRGs is downregulated during inflammation resulting in the activation of Epac-Rap1-ERK/PKCɛ pathway and prolonged hyperalgesia.
PGE2: prostaglandin E2; GRK2: G protein-coupled receptor kinase 2; DRG: dorsal root ganglion; Epac: exchange protein activated by cAMP; PKC: protein kinase C; ERK: extracellular signal-regulated kinase; AC: adenylyl cyclase; ATP: adenosine triphosphate; cAMP: cyclic adenosine 3′,5′-monophosphate; PKA: protein kinase A.
To further determine the consequence of GRK2 level manipulation, Wang et al.55 found that upregulation of GRK2 expression using viral-based gene transfer in carrageenan-treated or PKCɛ-upregulated rats prevents the development of PGE2-induced hyperalgesia. Similarly, PGE2-induced chronic hyperalgesia failed to develop in Epac1± mice or in mice treated with Epac1 antisense oligodeoxynucleotides. None of the manipulations, e.g. upreregulation of GRK2 or reduction of Epac1 level, alters PGE2-induced hyperalgesia in normal rats. These observations suggest that maintaining proper levels of GRK2 and Epac1 is essential for preventing the development of chronic pain.
Ras instead of Rap1 mediates Epac-signaling in a subpopulation of DRG neurons
Aside from functioning as GEFs for the small GTPase Rap1,19,20 Epacs have also been shown to activate Ras in cardiac cells.69 To determine the downstream targets of Epac in rat DRGs, Shariati et al.70 studied the involvement of small G proteins Ras and Rap1 in Epac-mediated cell signaling. They found that the activation of Epac by CPT increases evoked action potential firing and promotes capsaicin-evoked calcitonin gene-related peptide (CGRP) release from rat DRG neurons grown in nerve growth factor (NGF)-supplemented culture medium. CPT activates both Rap1 and Ras in these cells. Inhibition of Ras through intracellular perfusion of Ras-neutralizing antibody or through an expression of a dominant negative Ras (DNA-Ras) can block Epac-induced increase in action potential firing or reduce capsaicin-induced CGRP release in DRG neurons. In contrast, inhibition of Rap1 is not effective. The observations have led to the conclusion that in small sensory neurons, Ras, instead of Rap1, is the small G protein mediating Epac-induced sensitization.70
Functional receptors controlled by Epac signaling
Besides P2X3R receptors, mechanosensitive Piezo2 channels have been shown to be modulated by Epac signaling.68,71 Eijkelkamp et al.71 found that activation of Epac1, not Epac2, sensitizes Piezo receptor responses in large (> 35 µm in diameter) DRG neurons and produces larger and long-lasting mechanical allodynia. Knocking down Epac1 reduces nerve injury-induced mechanical allodynia. The responses are both PKA and PKC independent. They further asserted that the Piezo2 receptors are not involved in inflammation-induced mechanical hyperalgesia. On the other hand, Singhmar et al.68 later showed that Piezo2 antisense oligodeoxynucleotide blocks prolonged mechanical hyperalgesia induced by CFA-induced inflammation. They proposed that Piezo2 receptors contribute to Epac1-mediated mechanical hyperalgesia in the late-phase CFA-induced hyperalgesia. It has yet to be shown if the late-phase hyperalgesia depends on PKA and/or PKC.
In addition to P2X3R and Piezo2 channels, Epacs have been found to enhance synaptic transmission at excitatory central synapses72 and act on ion and receptor channels to alter intracelluar Ca2+ concentration, exocytosis, and transporter activity in a variety of cells.73 In addition, Epacs were also found to regulate Schwann cell proliferation and differentiation in sciatic nerves74 and to affect the morphological change in cultured astrocytes75 and oligodendrocyte differentiation and myelination in brain.76 It would be interesting to determine if Epacs alter glial cell activity in DRGs, thus affecting glia-neuron interactions.
Compartmentalization of cAMP, PKA, and Epac in DRGs
It has been well documented that cAMP signals are spatially and temporally segregated in different subcellular regions.77 This compartmentalization allows downstream effectors of cAMP signaling, e.g. PKA and Epac, to target specific effectors. The level of cAMP is largely controlled by AC to synthesize cAMP from ATP and by cAMP-specific 3' 5' cyclic phosphodiesterases (PDEs) to hydrolyze cAMP to AMP. A-kinase anchor proteins (AKAPs) provide platforms to anchor PKA, AC, and PDEs to specific subcellular locations to regulate the concentration of cAMP, thus the local PKA activity.78,79 Accumulating evidence suggests that AKAPs also form complexes with Epacs to regulate cAMP-Epac signaling.80 Studies of Epac1 and muscle-specific AKAP (mAKAP) interactions in cardiac myocytes showed that Epac1 is not directly associated with mAKAPs. Instead, it forms complex with mAKAP through PDE4D3 at the nuclear membrane to inhibit extracellular signal-regulated kinase 5 (ERK5).81 In cortical neurons, PKA, Epac, and protein kinase B (PKB)/Akt form a multiprotein complex with AKAP150. PKA activation reduces while Epac activation increases PKB/Akt phosphorylation. AKAP150 therefore regulates neuronal functions by coordinating PKB/Akt activity through the opposite actions of PKA and Epac.82
AKAP79/150-PKA modulates TRPV1 function in DRGs
Many studies of compartmentalization of signaling molecules in DRGs have been conducted on transient receptor potential vanilloid receptor 1 (TRPV1) channels because they are well expressed in sensory neurons83,84 and participate in transmitting noxious stimulations, including heat and acids.85,86 TRPV1 are activated by capsaicin, and the capsaicin-induced responses are greatly sensitized by PGE2.87
AKAP150 was found to regulate PKA phosphorylation and promote activation of TRPV1 receptors.88,89 Studying AKAP150 expression in rat DRG slices, Brandao et al.90 found that 36% of DRG neurons express AKAP150. About 94% of AKAP150-positive cells are small unmyelinated C-fiber neurons, 5% of positive cells are medium-sized δ-fiber neurons, and ∼1% of cells are myelinated large neurons. AKAP150 was found to co-localize with TRPV1 and Cav1.2 in the soma and axon initial segment of small DRG neurons.
In mouse DRG neurons, PKA and AKAP150 were shown to co-immunoprecipitate with TRPV1.89 Forskolin/PGE2 application strongly reduces the capsaicin-induced desensitization of TRPV1-mediated Ca2+ influx or currents. In knock-in mutant AKAP150Δ36 mice in which a 36-residue PKA-binding domain at the C terminus of AKAP150 was removed, PGE2 was no longer effective in reducing TRPV1-mediated desensitization. Furthermore, PGE2-induced thermal hyperalgesia is significantly reduced in those mice.
Using immunoprecipitation analyses, Zhang et al.91 identified the TRPV1 C-terminal region bound to AKAP79/150. The sites on AKAP that interact with PKA, PKC, and calcineurin (CaN) were also determined. It was further identified that amino acids 736–745 in the TRPV1 C-terminal domain are critical residues bound to AKAP79/150.92 The corresponding region (amino acids 326–336) on AKAP79/150 that interacts with TRPV1 was later identified.93 A peptide designed to bind this region was synthesized and tested. The peptide blocked the sensitization of TRPV1 currents and inhibited inflammatory hyperalgesia in inflamed mice but had no effect in control mice.93 The results suggest that blocking TRPV1-AKAP79/150 interactions may be a superior way to targeted inhibition of TRPV1 activity.
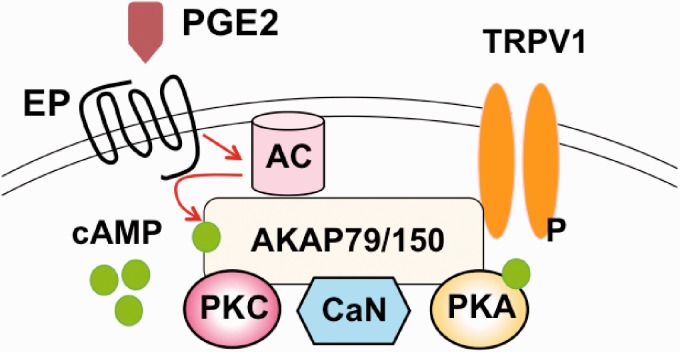
A recent study showed that anchoring AC to TRPV1-AKAP79/150- PKA complex is important for maintaining PGE2-dependent sensitization of TRPV1-activity in DRGs (Figure 4).94,95 On the other hand, activation of Epac by CPT has no effect on TRPV1 desensitization.89 Thus, Epac appears to have a limited role in modulating TRPV1 functions in DRGs.
Figure 4.
The AKAP150 complex facilitates PGE2-dependent modulation of TRPV1. The scaffolding protein AKAP controls the phosphorylation state of TRPV1 through its signaling complex containing AC, PKC, calcineurin (CaN), and PKA.
AKAP: A-kinase anchor protein; PGE2: prostaglandin E2; TRPV1: transient receptor potential vanilloid receptor 1; AC: adenylyl cyclase; PKC: protein kinase C; PKA: protein kinase A; cAMP: cyclic adenosine 3′,5′-monophosphate.
AC5/6-AKAP150-PKA complex is required for maintaining spinal cord injury-induced chronic pain
Another interesting example of signaling compartmentalization in DRGs is the requirement of scaffolding of AC, PKA, and AKAP150 in maintaining persistent spontaneous activity (SA) in nociceptors after spinal cord injury (SCI). Receiving a hit in the thoracic T10 spinal cord, rats displayed high sensitivity to mechanical and thermal stimulation. Nearly 60% of small (<25 µm) diameter DRG neurons at L4, 5 spinal cord levels showed long lasting (up to eight months) SA in or near their cell bodies.96 The SA in neurons requires persistent activity of AC, PKA, and the presence of scaffolding complex of AC5/6-AKAP150-PKA.97 Aside from a moderate increase in AC activity by Ca2+-calmodulin activation, the major change following SCI-induced hyperalgesia is a loss of sensitivity of AC5/6 to the inhibition by the inhibitory G protein alpha subunit (Gαi).97
Summary points and perspectives
Epacs play an essential role in producing enhanced PGE2-mediated hyperalgesia after inflammation. Inflammatory injury elicits a large increase in the expression of Epacs in sensory cells. Under normal conditions, PGE2 produces hyperalgesia by activating PKA alone. After inflammatory injury, PGE2 produces enhanced hyperalgesia by activating both PKA and Epac-PKC. Thus, P2X3R sensitization resulting from de novo Epac-PKC-dependent signaling after inflammation contributes to the hyperalgesic priming.
Epacs activate not only PKCɛ but also PKCα in DRG neurons after inflammation. Thus, Epac-induced activation of multiple PKC subtypes is likely to produce a complex modulation of DRG neuronal activity after tissue injury.
Reducing the GRK2 level in DRG neurons promotes Epac1 activation and leads to an activation of PKCɛ, thus prolonging inflammatory hyperalgesia. Upregulation of GRK2 levels in carrageenan-treated rats was found to inhibit PGE2-induced hyperalgesia. Thus, eliciting the control of Epac1 activity through adjusting GRK2 activity is likely an effective way to treat chronic pain.
AKAP interactions with AC, cAMP, PKA, and TRPV1 are essential for controlling neuronal activity in sensory neurons. Targeting the site on AKAP that is responsible for binding TRPV1 receptor can be a promising strategy to control the activity of TRPV1 receptor and PGE2-induced inflammatory thermal hyperalgesia.
Interactions among Epacs, scaffolding proteins, receptors, and ion channels in sensory neurons have not been studied extensively. Understanding the similarities and differences between PKA- and Epac-dependent molecular complexes and possible interactions among them will help us determine the basis underlying chronic pain and design strategies to avoid it.
Studying the mechanisms of Epac actions on nociceptors under disease states will provide new information on the roles of Epacs in pathogenesis and insights into effective treatment of diseases and chronic pain.
Author Contributions
LMH and YG wrote the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by grants from NINDS NS030045, NIDCR DE017813, National Institutes of Health.
References
- 1.Rathmell JP, Fields HL. Chapter 11. Pain: pathophysiology and management. In: Longo DL. (ed). Harrison's principles of internal medicine, 18e, 18th ed New York: McGraw-Hill, 2012, pp. 93–101. [Google Scholar]
- 2.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Q, Yaksh TL. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 2011; 24: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017; 3: 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron 2007; 55: 353–364. [DOI] [PubMed] [Google Scholar]
- 6.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 2015; 18: 24–32. [DOI] [PubMed] [Google Scholar]
- 7.Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem 2001; 276: 16083–16091. [DOI] [PubMed] [Google Scholar]
- 8.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 2007; 55: 365–376. [DOI] [PubMed] [Google Scholar]
- 9.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011; 31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meves H. The action of prostaglandins on ion channels. Curr Neuropharmacol 2006; 4: 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999; 79: 1193–1226. [DOI] [PubMed] [Google Scholar]
- 12.Bar KJ, Natura G, Telleria-Diaz A, et al. Changes in the effect of spinal prostaglandin E2 during inflammation: prostaglandin E (EP1-EP4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint. J Neurosci 2004; 24: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin CR, Amaya F, Barrett L, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther 2006; 319: 1096–1103. [DOI] [PubMed] [Google Scholar]
- 14.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem 2002; 131: 781–784. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama U, Iwatsubo K, Umemura M, et al. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev 2013; 65: 1010–1052. [DOI] [PubMed] [Google Scholar]
- 16.Walsh DA, Perkins JP, Krebs EG. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem 1968; 243: 3763–3765. [PubMed] [Google Scholar]
- 17.Beavo JA, Brunton LL. Cyclic nucleotide research—still expanding after half a century. Nat Rev Mol Cell Biol 2002; 3: 710–718. [DOI] [PubMed] [Google Scholar]
- 18.Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol 2003; 19: 23–44. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki H, Springett GM, Mochizuki N, et al. A family of cAMP-binding proteins that directly activate Rap1. Science 1998; 282: 2275–2279. [DOI] [PubMed] [Google Scholar]
- 20.de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998; 396: 474–477. [DOI] [PubMed] [Google Scholar]
- 21.de Rooij J, Rehmann H, van Triest M, et al. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem 2000; 275: 20829–20836. [DOI] [PubMed] [Google Scholar]
- 22.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol 2003; 4: 733–738. [DOI] [PubMed] [Google Scholar]
- 23.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 2010; 50: 355–375. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt M, Dekker FJ, Maarsingh H. Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 2013; 65: 670–709. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee U, Cheng X. Exchange protein directly activated by cAMP encoded by the mammalian rapgef3 gene: structure, function and therapeutics. Gene 2015; 570: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010; 11: 9–22. [DOI] [PubMed] [Google Scholar]
- 27.Rehmann H, Das J, Knipscheer P, et al. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 2006; 439: 625–628. [DOI] [PubMed] [Google Scholar]
- 28.Rehmann H, Arias-Palomo E, Hadders MA, et al. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 2008; 455: 124–127. [DOI] [PubMed] [Google Scholar]
- 29.Cheng X, Ji Z, Tsalkova T, et al. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008; 40: 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponsioen B, Gloerich M, Ritsma L, et al. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol 2009; 29: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consonni SV, Gloerich M, Spanjaard E, et al. cAMP regulates DEP domain-mediated binding of the guanine nucleotide exchange factor Epac1 to phosphatidic acid at the plasma membrane. Proc Natl Acad Sci U S A 2012; 109: 3814–3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khasar SG, Lin YH, Martin A, et al. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 1999; 24: 253–260. [DOI] [PubMed] [Google Scholar]
- 33.Aley KO, Messing RO, Mochly-Rosen D, et al. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci 2000; 20: 4680–4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Numazaki M, Tominaga T, Toyooka H, et al. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 2002; 277: 13375–13378. [DOI] [PubMed] [Google Scholar]
- 35.Parada CA, Yeh JJ, Joseph EK, et al. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci 2003; 17: 1847–1852. [DOI] [PubMed] [Google Scholar]
- 36.Sweitzer SM, Wong SM, Peters MC, et al. Protein kinase C epsilon and gamma: involvement in formalin-induced nociception in neonatal rats. J Pharmacol Exp Ther 2004; 309: 616–625. [DOI] [PubMed] [Google Scholar]
- 37.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 2005; 25: 6119–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain 2005; 113: 185–190. [DOI] [PubMed] [Google Scholar]
- 39.Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain 2003; 105: 143–150. [DOI] [PubMed] [Google Scholar]
- 40.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience 2010; 169: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araldi D, Ferrari LF, Levine JD. Gi-protein-coupled 5-HT1B/D receptor agonist sumatriptan induces type I hyperalgesic priming. Pain 2016; 157: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aley KO, Martin A, McMahon T, et al. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 2001; 21: 6933–6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dina OA, Aley KO, Isenberg W, et al. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci 2001; 13: 2227–2233. [DOI] [PubMed] [Google Scholar]
- 44.Parada CA, Yeh JJ, Reichling DB, et al. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience 2003; 120: 219–226. [DOI] [PubMed] [Google Scholar]
- 45.Dina OA, McCarter GC, de Coupade C, et al. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron 2003; 39: 613–624. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Li GW, Huang LY. Prostaglandin E2 potentiation of P2X3 receptor mediated currents in dorsal root ganglion neurons. Mol Pain 2007; 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Gu Y, Li GW, et al. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol 2007; 584: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu JG. P2X receptor-mediated modulation of sensory transmission to the spinal cord dorsal horn. Neuroscientist 2003; 9: 370–378. [DOI] [PubMed] [Google Scholar]
- 49.Xu GY, Huang LY. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci 2002; 22: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol 2004; 554: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu GY, Huang LY. Ca2+/calmodulin-dependent protein kinase II potentiates ATP responses by promoting trafficking of P2X receptors. Proc Natl Acad Sci USA 2004; 101: 11868–11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, Li GW, Wang C, et al. Mechanisms underlying enhanced P2X receptor-mediated responses in the neuropathic pain state. Pain 2005; 119: 38–48. [DOI] [PubMed] [Google Scholar]
- 53.Gu Y, Li G, Chen Y, et al. Epac-protein kinase C alpha signaling in purinergic P2X3R-mediated hyperalgesia after inflammation. Pain 2016; 157: 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasko MR, Habashy Malty R, Guo C, et al. Nerve growth factor mediates a switch in intracellular signaling for PGE2-induced sensitization of sensory neurons from protein kinase A to Epac. PLoS One 2014; 9: e104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Heijnen CJ, van Velthoven CT, et al. Balancing GRK2 and EPAC1 levels prevents and relieves chronic pain. J Clin Invest 2013; 123: 5023–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuda M, Oh-Hashi K, Yokota I, et al. Acquired exchange protein directly activated by cyclic adenosine monophosphate activity induced by p38 mitogen-activated protein kinase in primary afferent neurons contributes to sustaining postincisional nociception. Anesthesiology 2017; 126: 150–162. [DOI] [PubMed] [Google Scholar]
- 57.Gu Y, Wang C, Li G, et al. EXPRESS: F-actin links Epac-PKC signaling to purinergic P2X3 receptors sensitization in dorsal root ganglia following inflammation. Mol Pain 2016; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cesare P, Dekker LV, Sardini A, et al. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron 1999; 23: 617–624. [DOI] [PubMed] [Google Scholar]
- 59.Olah Z, Karai L, Iadarola MJ. Protein kinase C(alpha) is required for vanilloid receptor 1 activation. Evidence for multiple signaling pathways. J Biol Chem 2002; 277: 35752–35759. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Wang P, Zou X, et al. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol 2010; 222: 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma W, Zheng WH, Belanger S, et al. Effects of amyloid peptides on cell viability and expression of neuropeptides in cultured rat dorsal root ganglion neurons: a role for free radicals and protein kinase C. Eur J Neurosci 2001; 13: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 62.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009; 32: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Araldi D, Ferrari LF, Levine JD. Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci 2015; 35: 12502–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Araldi D, Ferrari LF, Levine JD. Adenosine-A1 receptor agonist induced hyperalgesic priming type II. Pain 2016; 157: 698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eijkelkamp N, Heijnen CJ, Willemen HL, et al. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci 2010; 30: 2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eijkelkamp N, Wang H, Garza-Carbajal A, et al. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci 2010; 30: 12806–12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrari LF, Bogen O, Alessandri-Haber N, et al. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience 2012; 222: 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singhmar P, Huo X, Eijkelkamp N, et al. Critical role for Epac1 in inflammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proc Natl Acad Sci U S A 2016; 113: 3036–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez De Jesus M, Stope MB, Oude Weernink PA, et al. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J Biol Chem 2006; 281: 21837–21847. [DOI] [PubMed] [Google Scholar]
- 70.Shariati B, Thompson EL, Nicol GD, et al. Epac activation sensitizes rat sensory neurons through activation of Ras. Mol Cell Neurosci 2016; 70: 54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eijkelkamp N, Linley JE, Torres JM, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat Commun 2013; 4: 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci 2008; 28: 7991–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holz GG, Kang G, Harbeck M, et al. Cell physiology of cAMP sensor Epac. J Physiol 2006; 577: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bacallao K, Monje PV. Opposing roles of PKA and EPAC in the cAMP-dependent regulation of Schwann cell proliferation and differentiation. PLoS One 2013; 8: e82354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vardjan N, Kreft M, Zorec R. Dynamics of beta-adrenergic/cAMP signaling and morphological changes in cultured astrocytes. Glia 2014; 62: 566–579. [DOI] [PubMed] [Google Scholar]
- 76.Yang HJ, Vainshtein A, Maik-Rachline G, et al. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun 2016; 7: 10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willoughby D, Wong W, Schaack J, et al. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. Embo J 2006; 25: 2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dodge-Kafka KL, Kapiloff MS. The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways. Eur J Cell Biol 2006; 85: 593–602. [DOI] [PubMed] [Google Scholar]
- 79.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol 2007; 19: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Breckler M, Berthouze M, Laurent AC, et al. Rap-linked cAMP signaling Epac proteins: compartmentation, functioning and disease implications. Cell Signal 2011; 23: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 81.Dodge-Kafka KL, Soughayer J, Pare GC, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 2005; 437: 574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nijholt IM, Dolga AM, Ostroveanu A, et al. Neuronal AKAP150 coordinates PKA and Epac-mediated PKB/Akt phosphorylation. Cell Signal 2008; 20: 1715–1724. [DOI] [PubMed] [Google Scholar]
- 83.Alessandri-Haber N, Dina OA, Joseph EK, et al. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci 2006; 26: 3864–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smani T, Dionisio N, Lopez JJ, et al. Cytoskeletal and scaffolding proteins as structural and functional determinants of TRP channels. Biochim Biophys Acta 2014; 1838: 658–664. [DOI] [PubMed] [Google Scholar]
- 85.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 2001; 24: 487–517. [DOI] [PubMed] [Google Scholar]
- 86.Szabo A, Helyes Z, Sandor K, et al. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: in vivo study using gene-deficient mice. J Pharmacol Exp Ther 2005; 314: 111–119. [DOI] [PubMed] [Google Scholar]
- 87.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 1998; 18: 6081–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeske NA, Diogenes A, Ruparel NB, et al. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain 2008; 138: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schnizler K, Shutov LP, Van Kanegan MJ, et al. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 2008; 28: 4904–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brandao KE, Dell'Acqua ML, Levinson SR. A-kinase anchoring protein 150 expression in a specific subset of TRPV1- and CaV 1.2-positive nociceptive rat dorsal root ganglion neurons. J Comp Neurol 2012; 520: 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 2008; 59: 450–461. [DOI] [PubMed] [Google Scholar]
- 92.Fischer MJ, Btesh J, McNaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci 2013; 33: 7407–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Btesh J, Fischer MJ, Stott K, et al. Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. J Neurosci 2013; 33: 9184–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Efendiev R, Bavencoffe A, Hu H, et al. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem 2013; 288: 3929–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esseltine JL, Scott JD. AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends Pharmacol Sci 2013; 34: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bedi SS, Yang Q, Crook RJ, et al. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J Neurosci 2010; 30: 14870–14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bavencoffe A, Li Y, Wu Z, et al. Persistent electrical activity in primary nociceptors after spinal cord injury is maintained by scaffolded adenylyl cyclase and protein kinase a and is associated with altered adenylyl cyclase regulation. J Neurosci 2016; 36: 1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]