Abstract
We present a new perspective on the concept of feed-forward compared to feedback mechanisms for motor control. We propose that conceptually all sensory information in real time provided to the brain and spinal cord can be viewed as a feed-forward phenomenon. We also propose that the spinal cord continually adapts to a broad array of ongoing sensory information that is used to adjust the probability of making timely and predictable decisions of selected networks that will execute a given response. One interpretation of the term feedback historically entails responses with short delays. We propose that feed-forward mechanisms, however, range in timeframes of milliseconds to an evolutionary perspective, that is, “evolutionary learning.” Continuously adapting events enable a high level of automaticity within the sensorimotor networks that mediate “planned” motor tasks. We emphasize that either a very small or a very large proportion of motor responses can be under some level of conscious vs automatic control. Furthermore, we make a case that a major component of automaticity of the neural control of movement in vertebrates is located within spinal cord networks. Even without brain input, the spinal cord routinely uses feed-forward processing of sensory information, particularly proprioceptive and cutaneous, to continuously make fundamental decisions that define motor responses. In effect, these spinal networks may be largely responsible for executing coordinated sensorimotor tasks, even those under normal “conscious” control.
Keywords: spinal automaticity, spinal cord injury, feed-forward control, central pattern generation, spinal learning
Introduction
The concepts of feed-forward and feedback mechanisms have been and continue to be a central part of neural strategies to control movement. The meaning of these terms, however, are evolving and becoming conceptually more sophisticated and more representative of how movements are controlled in in vivo systems (Manoonpong and others 2013; Potter and others 2014). Because human movements are performed in a dynamic environment, the central nervous system (CNS) must routinely adapt kinetically and kinematically to accommodate motor tasks ranging from predictable to unpredictable events. These accommodations have been proposed to take the form of feed-forward (proactive) and/or feedback (reactive) control strategies (Belen'kii and others 1967; Bouisset and Zattara 1987; Lacquaniti and Maioli 1989; Lyon and Day 1997; Massion 1992; Shiratori and Latash 2001; Toussaint and others 1997; Wolpert and Miall 1996).
Discussion of feedback versus feed-forward aspects of motor control, however, often lead to controversy mainly due to the lack of clarity of their presumed definitions. This ambiguity in large part has led to the difficulty in deriving a unifying theory of how these two concepts play a role in the translation of sensory input into motor events. For example, the term feed-forward control often is defined as a mechanism “that does not rely on feedback signals,” which, of course, is dependent on the definition of “feedback,” assuming that all sensory processing is either in a feed-forward or feedback mode. From the perspective of the brain as well as the spinal cord, if we assume that all neural control depending on sensory signals from multiple sensory systems provide continuous input under in vivo conditions, what differentiates feed-forward from feedback control? Clearly, in fast movements the role of anticipation prevails (Duysens and others 2000). If it is assumed that proactive mechanisms such as confronting unexpected obstacles that are recognized within a time window that allows adjustments to perturbations represent feed-forward control, then “feedback” logically is the control strategy used when one does not have sufficient time to accommodate to the environment successfully, that is, proceed without noticeable interruption. This time window for being able to modify the planned event, of course, must be a highly variable time, and the success of executing kinetic and kinematic responses will be a function of the time required for revising the movement planned.
Kuo (2002) proposed a hybrid of feedback and feedforward processes to be a more realistic model of locomotion. This hybrid model theoretically would allow a programmed process effected by central pattern generation to respond in an adaptive mode based on the ongoing sensory information (reflexes) it receives. In short, this means that the planned event can be modified based on the assumption that the “feedback” can induce changes as rapidly as needed to accommodate the unplanned (feedforward) disruption. This model of robotic control is one model of a “closed loop” control system, but it technically actually lacks the feed-forwardness that is evident even in insects (Potter and others 2014). An example of the conceptual limitations of this hybrid model with respect to the differences between planned feed-forward events (central pattern generation) and “feedback” (predictable reflex responses) is that both phenomena are in a constant adaptive state throughout all phases of a single step cycle. In this article, we examine the persistence of this automaticity in motor control from a perspective of the feed-forward mechanisms that are intrinsic to the spinal cord.
Thus, there is no definitive time delay from which the motor control strategy can be defined for a wide range of movement tasks as being a feedback or feed-forward phenomenon. We propose the following unifying concept in the translation of sensory input to motor output: technically all sensory input to neural networks intrinsic to the spinal cord, such as central pattern generators, are continuously biasing the neural control networks in a way that prepares for the upcoming events (Fig. 1). These intrinsic networks are continuously in some state of preparedness by their basic and by the sensory projections to these intrinsic networks, both of which have developed these feed-forward properties as a result of immediate, to decades, and even generations of experiences (Edgerton and others 2001a). Thus, all motor output is planned to a major degree based on experience, reflecting the automaticity that dominates our motor behavior.
Figure 1.
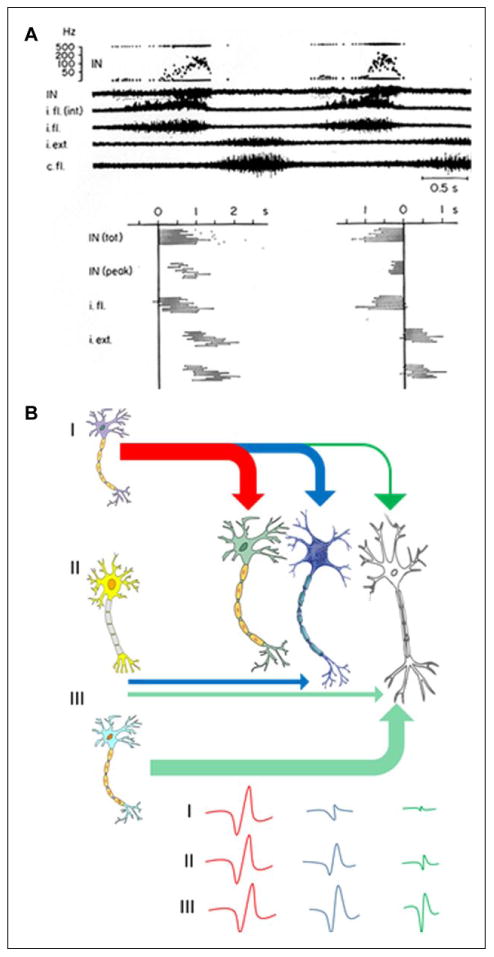
(A) Interneuron (IN) activity during central pattern generation. Lumbar (L7) interneuron activity as well as efferent activity in ipsilateral (i.) and contralateral (c.) flexor (fl.) and extensor (ext.) muscle filaments recorded in a spinal curarized cat injected with DOPA and Nialamide. The timing of activity within different muscle filaments for 10 consecutive cycles and the pattern of modulation of the interpulse intervals of an interneuron during these 10 cycles are shown. In the left graph the cycle starts with the onset of activity in the IN and in the right graph the zero point is moved to the end of the IN burst. It can be seen that the termination of activity in the IN is related tightly to the termination of the fl. burst (from Edgerton and others 1976). i.fl is the flexor motor filament recording and i.fl (int) is the signal rectified; IN (tot) and IN peak indicate the total duration of the activity and the peak frequency within a cycle, respectively. (B) A conceptual model as to how proprioception may function as a feed-forward system. This illustration depicts three INs that project to a series of other INs having a progressively less excitatory or inhibitory effect with multiple INs firing with a delay. The consequences of this chain of events and a smaller impact on neurons that project more distantly along the chain would result in a decreasing probability of that particular IN becoming activated at some subsequent time period. The ongoing ensembles of sensory input projecting to these networks will further define which combinations of INs are likely to be activated at any given time in a motor task is being performed. In the context of central pattern generation, spinal networks can generate highly predictable motor outputs in an unchanging environment, but they cannot adapt to a changing environment, and thus cannot control posture or locomotion in vivo.
The degree to which a planned event can be performed successfully will be inversely rated to the time required to reprogram the planned output. Thus, all motor responses in real time are determined by the preparedness or the physiological state of the nervous system (determined by both the most recent and long past experiences) to accommodate upcoming events. More specifically, the probability of a given response to even the most immediate potentially disruptive scenario has already been largely determined by the present physiological state.
A common thread in the evolution of the mechanisms that enable sensory information to be translated to successful motor acts seems to be the modulation of the probabilities of given movements to occur, including movements key to maximizing survival of the next generation of a specie. We will discuss several of these learning and memory-related phenomena that engage a range of cellular and molecular mechanisms to generate relatively “automatic” responses within a timeframe of milliseconds to years. Features of automaticity of the neural control of motor units in vertebrates are known to be within spinal cord circuitries (Bodine and others 1987). Thus, the spinal networks, even with normal descending input from the brain, can be the site at which the control of the coordination of motor pools to perform a sensorimotor task occurs (Grillner 1979).
Central Pattern Generation
A core component of this automaticity in motor control can be attributed to neural networks that can effect central pattern generation, that is, rhythmic, coordination of motor pools in the absence of rhythmic input. Beyond this coordinated rhythmicity of motor output, however, the ability of these same neural networks to process enormous amounts of sensory information in real time must be an essential component of the automaticity of motor behavior. The concept of central pattern generation is broadly recognized to play an important role in generating repetitive cyclic movements such as those occurring during walking, chewing, breathing, and so on. As related to posture and locomotion, technically central pattern generation is the generation of cyclic, highly coordinated efferent (motor) patterns driven by networks of interneurons in the lumbosacral spinal cord in the absence of any input from the brain or the peripheral sensory systems (Edgerton and others 1976). This highly coordinated motor output must result from some feed-forward functional connectivity built into the locomotor networks (Fig. 1). Although these networks have a remarkable capacity to generate a range of different cyclical motorlike outputs, they have no capability to respond to the immediate environment without receiving some sensory information. Therefore, they have no ability to control their output in a way that can sustain successful posture or locomotion, even in the most constant and simplest environment.
A crucial, but not generally emphasized or even recognized, property of the central pattern generation circuitry, however, is its ability to receive complex sensory patterns, recognize these as specific dynamic events that then can activate in real time a predictable and adaptive population of neurons in sequence to sustain successful stepping, even in the absence of input from the brain. We propose that this property is possible as a result of multiple feedforward mechanisms built on an evolving design over millions of years as well as the sensory experience within one's lifetime. What is the evidence that central pattern generators have the ability to execute a pattern of activation that will sustain a step cycle at any given stage while relying on sensory mechanisms? A number of experiments have demonstrated that although highly predictable activity patterns can be generated with central pattern generation that can execute locomotor-like efferent patterns, it cannot serve as a “controller” without sensory input (Shik and Orlovsky 1976). Herein we will present evidence supporting the concept that the peripheral sensory system can serve as a critical source of input to spinal “controllers” of locomotion and that all of these sensory inputs are processed via feed-forward mechanisms.
We propose that to produce a smooth, coordinated movement the dynamically generated ensembles of sensory input to the spinal networks project not only to the interneurons and motoneurons active at that instant, but also to the circuitry that will be activated subsequently. Thus, there is a robust feed-forward feature of the translation of sensory input to the central pattern generators (Fig. 1B). For example, feed-forward input in the lamprey, that is, networks that project excitatory and inhibitory input that modulate neuronal potentials to motoneurons that are up to 10 segments more caudal than the more rostral segments, has been reported (Parker and Grillner 1999). Effectively the sensorimotor circuitry is designed to predict with a high probability which neurons will have some depolarization event within the next few milliseconds.
Evidence for Spinal Automaticity
The sensory system can control locomotion even when there are unexpected events and when all supraspinal input to the lumbosacral spinal cord has been eliminated surgically via transection of the spinal cord of adult rats at a low- to mid-thoracic level (Gerasimenko and others 2007; Lavrov and others 2008). Experiments have been performed in which we implanted intramuscular electrodes to record the activity (electromyography [EMG]) of selected hindlimb muscles and electrodes placed epidurally to stimulate the lumbosacral region of the spinal cord. Delivering a tonic stimulus (∼40 Hz) at specific lumbosacral segments (i.e., L2 and S1) changed the properties of the spinal networks such that when sensory information associated with locomotor activity on a treadmill reached these networks, weight-supporting bipedal stepping was generated at a rate that matched the speed of the treadmill belt (Courtine and others 2009; Ichiyama and others 2008). Under the same conditions, but with the paws not touching the moving treadmill belt, there was little or no oscillatory movement of the limbs, highlighting the primary role of sensory input from the periphery. Evidence of the sensory system serving as the controller was demonstrated further by the observation that the kinematics of the stepping and the activation patterns of the relevant motor pools changed in a predictable and relatively normal way when the speed of the treadmill belt was changed. Changing the speed of locomotion is a very complex sensorimotor event that involves differential modulation of the amplitude and duration of activation of specific motor pools associated with the stance and swing phases of stepping (Gerasimenko and others 2007). It is significant that the more effective intensities of the epidural stimulation needed to recover complete motor paralysis do not in themselves induce stepping, but they enable weight-bearing stepping when the spinal cord receives the sensory cues associated with locomotion.
Further evidence of the controlling role of the sensory system is evident when the direction of the treadmill belt is reversed and spinal cats walk backwards (Smith and others 1998). The kinematics of backward stepping differed significantly from that with forward stepping as reflected in the differential patterns of activation of flexors and extensors of the proximal and distal muscles of the hindlimb. To further challenge this concept of sensory control, we tested whether spinal rats could step laterally when oriented in a gradually more sideward direction relative to the movement of the treadmill belt. The adaptation was immediate and finely tuned in real time to the kinematics approximated to what is known to occur in uninjured rats during sideward stepping, that is, the coordination and activation levels of the appropriate motor pools show a fundamentally different pattern compared to either forward or backward stepping (Courtine and others 2009; Shah and others 2012). Thus, spinal animals can adapt to different directions of stepping on a treadmill by relying only on the signature of the sensory input from the periphery, that is, proprioceptive and cutaneous input.
A similar conclusion regarding the controlling features of the sensory system was drawn when paralyzed humans were placed over a moving treadmill belt and allowed to bear different percentages of body weight on their lower limbs (Harkema and others 1997). As the level of load bearing was increased, the level of activation of the motor pools increased proportionately. These data demonstrate that the sensory system can perceive the level of loading on the lower limbs and can generate the appropriate motor pattern to accommodate the given load. It also is significant to note that a near-normal pattern of coordination of the motor pools was sustained at varying levels of loading. In fact, the level of coordination actually improved until the load became excessive, resulting in the activation pattern being disrupted and the lower limbs collapsing.
Translation of Physiological States to Predictable Motor Responses: A Prominent Feed-Forward Mechanism
It is commonly assumed that the sensory system is responsible for responses to immediate unanticipated motor tasks. Furthermore, these responses are viewed as a feedback mechanism, often referred to as a “reflex.” The corrective events that have been considered, however, have been very brief events, for example, a response to a quick stretch of a muscle group or a reflexive response to some pain sensation. These responses, however, cannot occur within the time frame necessary to continue a planned movement without disruption, such as stepping with the previously presumed trajectory. This common observation in itself demonstrates a feed-forward phenomenon in which the nervous system anticipates a requirement for a motor pool activation pattern similar to that generating the previous repetitive motion. There is insufficient time for the ongoing sensory information to reach the spinal cord and for supraspinal centers to “reprogram” so that an “unanticipated” movement can be accommodated sufficiently to avoid disruption of the movement. Another example of the feed-forwardness of the spinal cord is suggested by experiments in which a tripping stimulus is applied to the dorsum of the paw of spinal cats. Enhanced flexion ipsilaterally occurs if the stimulus was applied to the dorsum of the paw during the swing phase of the step cycle, whereas the opposite response occurred, that is, enhanced extension, if the same stimulus was applied during the stance phase of the step cycle (Forssberg and others 1975, 1977). These experiments clearly demonstrate that the spinal cord can interpret very complex signals in a highly dynamic situation and generate a highly predictable and useful motor output that increases the probability of sustaining the ongoing locomotion with minimal disruption. Is this simply a reflex? If so, then all of the motor tasks performed when there is no supraspinal input could by definition be viewed as a reflex.
The feed-forwardness of the tripping phenomenon in adult spinal cats is demonstrated when a tripping object is “anticipated” even after a single exposure to the tripping stimulus (Zhong and others 2012). Immediately after an initial tripped step, the activation pattern and kinematics of the hindlimbs change to a trajectory that minimizes the probability of contacting the object during the next step cycle. This cortico-centric interpretation of “anticipation” is perhaps best described biologically as “evolutionary-learning” (Edgerton and others 2001b) reflecting that the sensorimotor circuitry of the spinal cord has been designed to recognize and functionally respond positively to tripping perturbations. This “anticipation” of the tripping event clearly suggests that the sensory information induced by the tripping stimulus was processed in a manner that modified the subsequent step in a way that increased the probability of successfully continuing stepping, that is, elevating the paw to step over the obstacle. Thus, not only can the spinal networks, without any input from the brain, control locomotion in rather stereotypical situations, but these networks also can detect “unplanned” events and adapt the activation patterns of multiple and highly interactive motor pools accordingly within a matter of milliseconds. We suggest that this response demonstrates a feed-forward mechanism in the spinal circuitry that enables it to plan or anticipate encountering a constantly changing environment even when the change occurs irregularly or rarely.
Other studies have demonstrated that the spinal cord can adapt to perturbations involving changes in the force field during specific phases of a step cycle in spinal animals (Heng and de Leon 2007; Timoszyk and others 2002) and in spinal cord injured humans (Field-Fote and Dietz 2007; Gordon and others 2010; Lam and others 2006). For example, rats that were spinalized at a neonatal stage were trained to step bipedally with robotic arms attached at the ankle while supported in an upper body harness (Edgerton and others 2001a). Trials then were conducted during which the swing phase of the step cycle was perturbed by an upward force field designed to be proportional to the swing velocity. The trials were performed on two consecutive days and consisted of ∼180 steps, alternating bouts of 20 steps with and then 20 steps without the force field (Fig. 2A). The spinal rats responded immediately to the application of the force field by increasing step height and extending the step trajectory. The changes in the step trajectories for a sequence of steps with and without the force field were readily apparent (Fig. 2B). Both step height (Fig. 2B and C) and swing duration (Fig. 2D) were increased during the first bout, and then there was a gradual return to more normal step heights and swing durations with repeated bouts. The adaptation in step height was faster on subsequent days in the later trials compared to the earlier trials (Fig. 2E and F). The spinal cord thus exhibited a learning-like phenomenon to produce a corrective kinematics response to overcome perturbations encountered during the step cycle.
Figure 2.
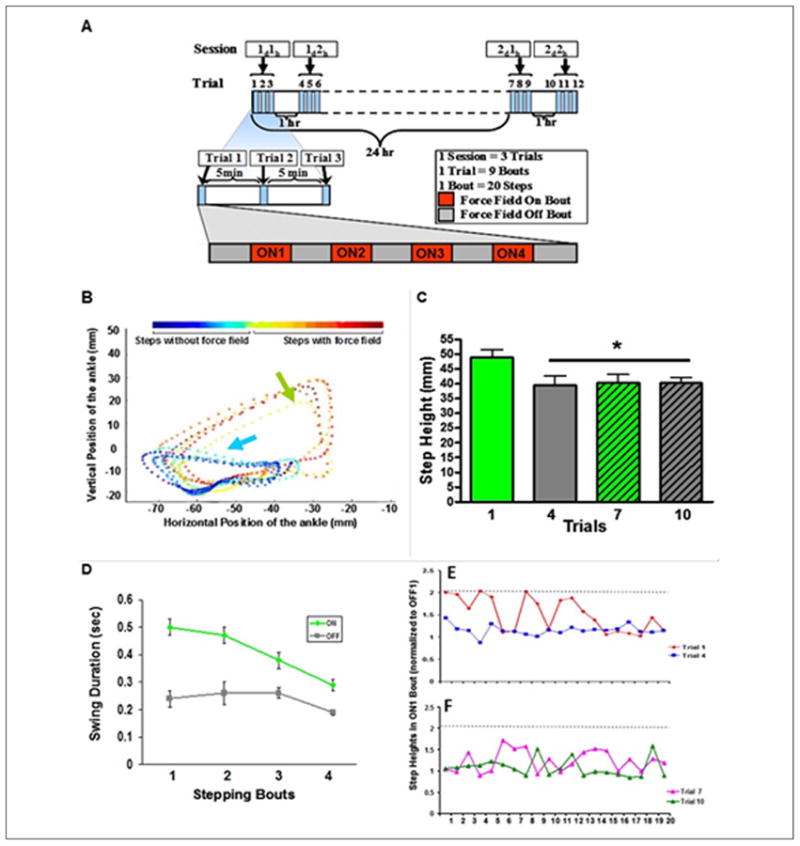
(A) An upward force was applied to one ankle of rats with a complete, mid-thoracic spinal cord transection. The hindlimb moved forward during assisted (rats suspended in a upper body harness) bipedal stepping on a treadmill during 20 steps (bout) without any force field applied, that is, the robotic arm acting passively (OFF), followed by 20 steps with the force field applied (ON). Each trial consisted of five OFF and four ON bouts, alternating every 20 steps (one Trial, ∼180 steps). Trials 1 to 3, 4 to 6, and 7 to 9 were performed 5 minutes apart, whereas trials 4 and 10 were performed 1 hour after trials 3 and 9, respectively. Trial 7 was performed 24 hours after trial 1. (B) An example of the foot trajectories in the x-y directions for a sequence of 10 steps without (blue arrow) and then with (green arrow) the force field applied. The force field was turned on after the fifth step. Color scale shows the temporal sequence of steps with and without the force field. (C) Mean (±SEM, n = 10 rats) step height of the first five steps of the ONI bout for trials 1, 4, 7, and 10. *Significantly different from trial 1. (D) The mean (±SEM) duration of the swing phase during four bouts with the force field ON and four bouts with the force field OFF during trial 1 for one rat. Step heights of all 20 steps for the ONI bout for trials 1 and 4 (E) and 7 and 10 (F) for one rat. The dashed horizontal line indicates the maximum step height in trial I. The step heights were normalized by dividing the individual step heights of each ON bout by the mean step height of the preceding OFF bout (C, D).
The feed-forward operational mode of the spinal circuitry for stepping also was demonstrated in paraplegic rats that were trained to step with robotic arms attached to the ankles to generate a fixed trajectory that mimicked the mean of the normal step cycle kinematics, thus eliminating step-to-step variability (Fig. 3A). In a second group of spinal rats the robotic arms controlled the ankle in an assistive mode using a force field to allow the step-to-step variation in the kinematics that occurs normally (Fig. 3B). The key point is the following. Imagine the step cycle being divided into very brief time bins and within each of these time bins the robot is programmed continuously to move from point A to point B (Fig. 3E). Given that variations in stepping trajectories and EMG patterns occur naturally, there was a high probability that the robot was programmed to reach point B within a time bin, but this point would differ from that which the spinal circuitry operating essentially on stochastic probabilities had planned. Therefore, within each time bin the robot would be correcting the output of the spinal circuitry. This occurred much less often in the assistas-needed mode. The result was a highly abnormal EMG pattern for an ankle flexor (tibialis anterior, TA) and extensor (soleus) marked by constant co-contractions for the fixed trajectory mode. Marked differences in the incidence of co-contraction between the two modes are obvious (Fig. 3C, D, and E).
Figure 3.
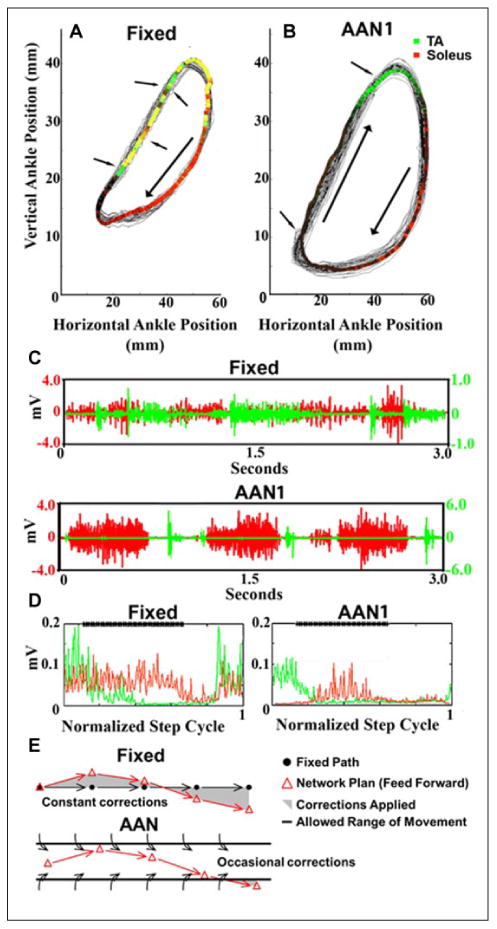
(A) (Fixed) and (B) (assist-as-needed, AANI) show the ankle trajectories recorded over 30 seconds of stepping with the fixed trajectory and the AANI modes, respectively. The colored areas represent the average EMG activity recorded from the soleus and tibialis anterior (TA): red, soleus activity; green, TA activity; and yellow, soleus and TA co-activation. The arrows point to examples of changes in direction due to the robotic arm guiding the ankle toward the trajectory. (C) Raw EMG activity of the soleus and TA during 3 seconds of movement for each paradigm (top, Fixed; bottom, AANI). (D) The average integrated EMG for the soleus (red) and TA (green) from over 30 continuous seconds of stepping for each paradigm (left, Fixed; right, AANI). The bold line at the top of the box “****” marks the stance phase of the step cycle. (E) Schematic illustrating that in the fixed mode the progression of the movement defined by the robot from one time bin to the next time bin is predetermined, whereas the spinal networks will generate an activation pattern reflecting some probability of where the next point will be for each time bin (feed-forward). The shaded area represents the variation from that hypothetical difference between the effects compared to the AAN mode. Thus, in the fixed mode, the spinal networks are in a constant mode of the planned kinematics were constantly being corrected. Unlike the fixed mode, the window of tolerance for each time bin without being corrected in the AAN mode is sufficient to avoid continuous corrections during each time bin because the “window” allowed the planned kinematics to occur without interruption resulting in more effective learning of the stochastic phenomena intrinsic to networks (modified from Ziegler and others 2010).
Feed-Forward Regulation of Equilibrium during Locomotor Behavior
Successful locomotion requires subtle adjustments in the coordination between trunk and limb movements to move the body forward (i.e., propulsion) while maintaining dynamic equilibrium (i.e., balance). During stepping, there are marked oscillations in the center of mass. Given the intrinsic variation in kinetics and kinematics from step-to-step during normal locomotion, what is the evidence that there can be a continuous feed-forward strategy built into the spinal networks that can accommodate this seemingly unpredictable variation in stepping?
We used an animal model of decerebration, that is, having no cortical involvement and functioning with a high level of automaticity, to address this issue. We observed that decerebrated cats can efficiently control equilibrium during locomotion facilitated by tonic epidural spinal cord stimulation using a feed-forward control strategy based solely on somatosensory input from the hindquarters (Musienko and others 2012). Activation of the treadmill immediately induced coordinated, full weight-bearing hindlimb locomotion with effective bilateral control of balance. Well-balanced stepping patterns were associated with left-right alternation of EMG activity in flexor and extensor hindlimb muscles, ground reaction forces (GRFs), and lateral pelvic displacements (Fig. 4). The pattern of GRFs generated by the left and right hindlimbs and the left-right displacements of the pelvis during consecutive step cycles was sufficient to sustain balance over the span of the 14 step cycles illustrated in spite of the wide ranges in left-right variations in GRFs from step to step (Fig. 4C). This range in GRFs is plotted in Figure 4D independent of the sequence of the steps that were generated: note that the sequence of steps with the larger GRF's are linked in red as consecutive steps showing that a larger GRF on one side is countered by a similarly large GRF on the other side in the subsequent step. This relationship means that the sensory signals from one step programmed the activation pattern required for the next step to sustain equilibrium during stepping. In fact, there is a very high correlation between the left versus right GRFs when plotted independent of order across steps (Fig. 4E) but no correlation when left versus right displacements are plotted in a random order (Fig. 4F). The cumulative left-right displacements for consecutive steps significantly (P < 0.001) fell outside the range of displacements that would be expected if the order of the cumulative displacements were randomized (Fig. 4G) The GRF amplitudes generated in the left hindlimb in the preceding step determined the GRF amplitudes produced by the right hindlimb in the next step (r = 0.91 ± 0.04). The same strong temporal pairings were detected when the correlation analysis was applied to the left-right maximal lateral displacements of the pelvis (r = 0.87 ± 0.09). The success of sustaining equilibrium does not depend on the proprioceptive and cutaneous input generated by the GRFs or displacements generated within any given single limb, but rather on the close match of proprioceptive and cutaneous input derived from the preceding step(s) of the contralateral limb. Thus, there is a continuous feed-forward process in a constantly updating mode by which the details of the activation of the spinal circuitry during a given step define the motor command that will be executed in the subsequent step.
Figure 4.
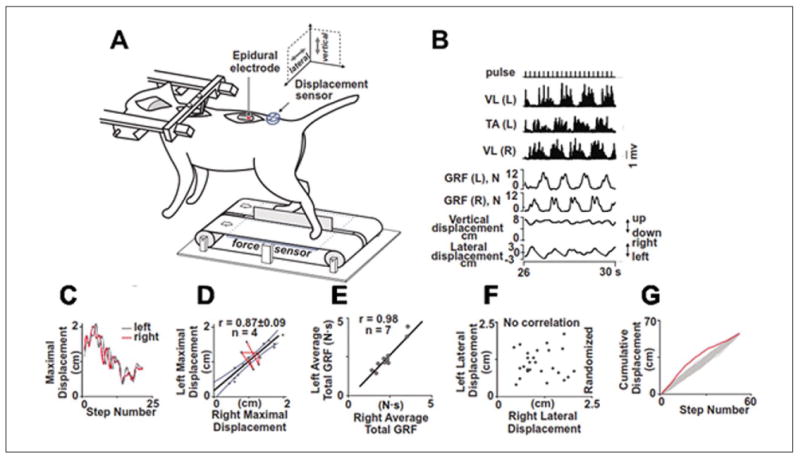
Feed-forward regulation of the balance in decerebrated cats facilitated by epidural spinal cord stimulation. (A) The cat is secured in a stereotaxic frame. A stimulating electrode is sutured epidurally at L5. An accelerometer is placed on the pelvis to record displacements and force sensors are placed beneath each belt to record ground reaction forces (GRFs) from the right (R) and left (L) hindlimbs. (B) Rectified EMGs from the R and L vastus lateralis (VL) and L tibialis anterior (TA) muscles, R and L GRFs, and vertical and lateral pelvis displacements are shown during stepping with epidural stimulation. (C) Sequences showing the maximal R-L pelvis displacements over 25 successive steps. (D) The range of L-R displacements exceeded 1 SD on several consecutive steps (red line), illustrating how an unusual displacement of one limb is followed by a proportionate displacement in the contralateral limb. (E) Correlation between L and R total GRFs during stepping for 10 experiments in 7 decerebrated cats (overall r = 0.98). The L-R variations in consecutive steps consistently fell out of the range for randomization. (F) There is no correlation when the order of the L-R lateral displacements was randomized. Cumulative R and L limb displacements plotted in order of occurrence (red line) or randomized (Monte Carlo 500 times, gray line) (G) (modified from Musienko and others 2012).
Because the precollicular post-mammillary decerebration essentially precluded visual input and the fixation of the head and spine at the thoracic level eliminated vestibular and proprioceptive head-neck-trunk reflexes as a source of ongoing dynamic control these balance-related adjustments relied entirely on the integration of somatosensory information arising from the moving hindquarters. Therefore, during locomotion spinal and/or brainstem circuits anticipate (feed-forward) the requirements to maintain balance during the subsequent steps, demonstrating “planned” locomotor commands to generate the necessary propulsion and balance in a highly automatic mode. Subsequent experiments suggested a similar phenomenon in mid-thoracic spinal cats (Musienko and others 2015).
In effect, there is a range and randomness in the variability of the stream of sensory ensembles during stepping that the spinal networks can readily accommodate sufficiently to sustain locomotion. Thus, the dynamics of such sensory cues are not “unexpected.” The probability of processing those dynamic sensory ensembles in real time via a mechanism that requires a process where the sensory ensembles from millisecond to millisecond can drive all of the neurons and muscles without a feed-forward mechanism seems unlikely.
Feed-Forward Control of Posture
Another example of how evolutionary design is built into our postural control is reflected in the robust anticipatory postural adjustment (APA) associated with the activation of muscles prior to an actual perturbation of balance (Massion 1992). The net effect of the APA minimizes the negative consequences of a predicted postural perturbation (Bouisset and Zattara 1987; Massion 1992). A rapid unilateral arm raise has been used extensively as a method to investigate the ability to control self-generated postural perturbations in humans. Belen'kii and colleagues (1967) were the first investigators to report that trunk and lower limb muscles are activated 50 to 100 ms before the initiation of arm motion in neurologically intact participants. It was reported that if the subjects see the oncoming perturbation during standing, they generate early postural adjustments (EPAs) and APAs, 400 to 500 ms and 100 to 150 ms prior to the impact, respectively. We have performed these tests in neurologically intact participants and showed that, in addition to the previously described APA phenomenon (Fig. 5A), there also is modulation in the excitability of the motoneurons that control the posteriorly located leg muscles preceding the initiation of arm movement (Fig. 5B).
Figure 5.
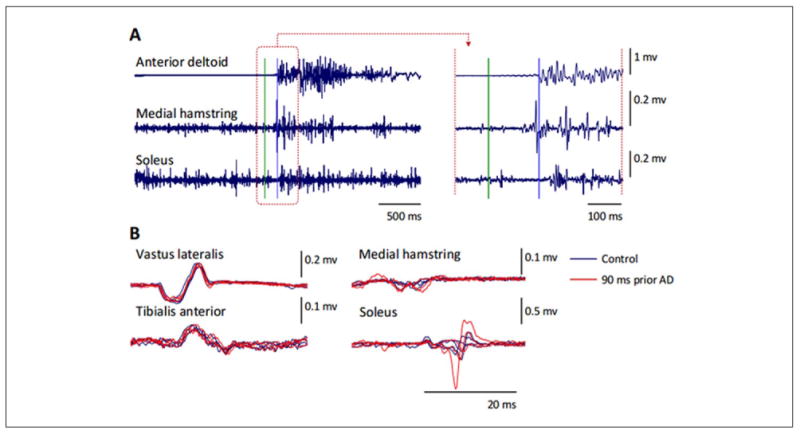
EMG activity in arm and leg muscles during a rapid unilateral arm raise in a neurologically intact individual when standing. (A) Time course of the EMG responses following the “Go!” command (indicated by the green horizontal line). Note the modulation of EMG activity in the soleus muscle prior to the onset of the response in the anterior deltoid muscles (indicated by the blue horizontal line). Right panels show a higher magnification of the responses for the time window (brown dashed square) in the left panel. (B) Spinally evoked motor potentials obtained under control conditions (blue traces, n = 4), and 90 ms prior to the anterior deltoid EMG response (“90 ms prior AD”, red traces, n = 3). Note the modulation of the evoked potentials in the posteriorly located leg muscles, that is, medial hamstring and soleus, whereas there was a lack of change in the response magnitude in the anteriorly located vastus lateralis and tibialis anterior.
It has been suggested that in this raising arm paradigm the neuronal circuits for voluntary movement are inhibited until the postural adjustment has reached a suitable level (Cordo and Nashner 1982). An alternative interpretation, and perhaps of greater fundamental importance, is that this order of activation reflects a fundamental evolutionary strategy of the different muscle groups to accommodate a 1G environment. Thus, this APA sequence is built into the command sequence of activation that occurs automatically, making any voluntary intent unnecessary. This interpretation is consistent with experiments done in microgravity. For example, the significance of the APA from an evolutionary perspective becomes patently evident when studied in a zero G environment where the APA is robust, that is, in the absence of any mechanical and perhaps even neural signal normally designed to prevent one from losing their balance at 1G. Alterations in the neuromuscular activation during APA in astronauts during weightlessness (Layne and Spooner 1990) and after returning from spaceflight (Layne and others 2001) suggest that the same network responses occur independently of gravitational forces. Since all living terrestrial organisms have evolved to accommodate 1G, this is another example of how the feed-forwardness and automaticity is designed to accommodate control of posture in a 1G environment.
To test the ability to generate an APA in individuals with a motor and sensory complete spinal cord injury, during assisted standing a participant was instructed to raise his right arm by flexing the shoulder as rapidly as possible until the arm was parallel to the floor in response to an auditory cue (Fig. 6).
Figure 6.
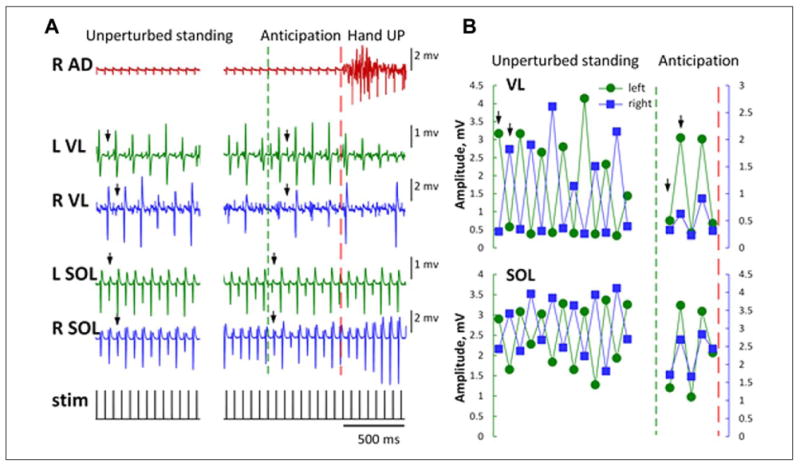
(A) EMG responses from leg muscles during assisted standing and a rapid unilateral arm raise in an individual with a complete motor and sensory spinal cord injury. A series of motor potentials with rhythmic amplitude modulation elicited by transcutaneous electrical spinal cord stimulation is shown. Arrows indicate reciprocal (during unperturbed standing) and synchronous (during the anticipation Hand UP phase) responses. (B) During unperturbed standing, the rhythmic amplitude modulation between the left and right vastus lateralis (VL) and soleus (SOL) muscles was reciprocal. Shortly before the right arm raise following the auditory command, the cyclic reciprocal amplitude modulation between the left and right muscles becomes synchronous. Arrows indicate reciprocal (during unperturbed standing) and synchronous (during the anticipation phase) responses. Red dashed line indicates the right arm raise; green dashed line indicates the onset of the anticipation phase. R and L, right and left. AD, anterior deltoid; stim, transcutaneous electrical stimulation.
It was observed that spinally evoked motor potentials in the vastus lateralis and soleus muscles were modulated during the anticipatory phase of the task following the auditory cue, that is, the relationship between the bilateral muscles changed from reciprocal to synchronous (Fig. 6A and B). Periodic modulation of repetitively elicited potentials recorded from leg muscles in humans during electrical stimulation of the lumbosacral spinal cord was recently described (Hofstoetter and others 2015). It was suggested to have resulted from the interaction of facilitatory and inhibitory mechanisms. Although our observations warrant systematic investigation, the revealed phenomenon of the anticipatory modulation of spinal automaticity in human after spinal cord injury may be another example of residual translesional descending control of the spinal neuronal networks (Angeli and others 2014; Harkema and others 2011). These observations are consistent with an immediate feed-forward neuromodulation during assisted standing even after a clinically complete spinal cord injury.
Feed-Forward versus Feedback Mode
Over time the concepts related to networks functioning in either a feed-forward or feedback mode have evolved toward a more comprehensive concept that more closely match sensorimotor functions as they might occur under in vivo conditions. We propose that a more conceptually correct view biologically is that all information (sensory) received from the external and internal environments is processed for its feed-forward effect. With this perspective, the issue is how forward in time and with what impact does that input have immediately and at some future time. For example, one of the most commonly referred to physiological “feedback” control systems within the spinal circuitry is one of the most rapid feedback responses, that is, the spinal Ia monosynaptic pathway. This quick response occurs in multiple synergistic and antagonist muscles within a short time (about 70 ms) after a mechanical perturbation of a given muscle. The exact delay being attributable to the conduction velocities is largely dependent on the length and size of the motor axons. The same mechanical stimulus, however, also induces more delayed responses (feed-forward with a longer time frame) through polysynaptic mechanisms. For example, this same pathway provides a feed-forward inhibitory response via Ia interneurons (thus a different time delay relative to some mechanical event) to multiple antagonistic muscles. Furthermore, even the simplest monosynaptic link to motoneurons is strongly state dependently modulated according to the phase of a step cycle (Capaday and Stein 1986, 1987; Crenna and Frigo 1987; Dyhre-Poulsen and others 1991; Edamura and others 1991). This emphasis of the proprioceptive system generating “reflex responses” reflects the view that the important features of proprioception is its role in correcting mistakes in the motor output generated as opposed to the role that it plays in real-time control of the motor events generated.
Concluding Remarks
The sensory information processed by spinal networks during postural, locomotor, and autonomic functions portrays a real-time systems-level update of the physical environment. The response to that systems-level update will be dependent on the physiological state of the networks at that instant. The multimodal sensory inputs generate an ensemble of mechanical and chemical events that provide the sense of instantaneous physiological states sufficient for spinal networks to make fundamental decisions that enable a continuing fine-tuning of motor and autonomic responses. We propose that there is constant updating in defining responses based on these sensory ensembles and the accommodation to this input influences movements over a timeframe of milliseconds to years. The precision derived from the proprioceptive and cutaneous input to spinal networks can serve as an elegant source of control of posture and locomotion without input from the brain. This level of fine control is based on multiple feed-forward mechanisms, even for the most immediate and seemingly unanticipated events. In response to all sensory input, the nervous system must “decide” not only the next immediate movement but also the subsequent ones predicted by the same sensory ensembles (Fig. 1B). In many if not most cases, particularly in those where the input represents a sudden change, this response will be automatically defined in a statedependent context, but not always with the desired precision and success.
We suggest that a more useful concept in efforts to understand the “biological logic” of the neuromuscular design for controlling motor and autonomic functions, at least as defined by spinal networks, is to consider the feed-forwardness rather than the “feed-backwardness” of the sensory, motor, and autonomic systems. If the proposed concepts of feed-forwardness are biologically sound, this raises the importance of identifying the different neural mechanisms by which the learning and memory of synaptic activity within a network that spans the time frames of milliseconds to minutes to hours to years and even to generations can occur.
Acknowledgments
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by NIH U01EB15521, R01EB007615, the Christopher & Dana Reeve Foundation, and Russian Foundation for Fundamental Research (Grant No. 16-29-08173-ofi-m). Partial support for data analysis and interpretation of the results was provided to YG, VRE, DS, PG, and IK from the Russian Science Foundation (Grans Nos. 14-45-00024 and 14-25-00167).
Footnotes
Reprints and permissions: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VRE, YG, RRR, and PG are researchers on the study team who hold shareholder interest in NeuroRecovery Technologies. VRE is president and chair of company's board of directors. VRE, YG, RRR, and PG hold certain inventor ship rights on intellectual property licensed by the regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(Pt 5):1394–409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belen'kii VE, Gurfinkel VS, Pal'tsev EI. Control elements of voluntary movements. Biofizika. 1967;12(1):135–41. [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. J Neurophysiol. 1987;57(6):1730–45. doi: 10.1152/jn.1987.57.6.1730. [DOI] [PubMed] [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech. 1987;20(8):735–42. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude-modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6(5):1308–13. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol (Lond) 1987;392:513–22. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47(2):287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):1333–42. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenna P, Frigo C. Excitability of the soleus H-reflex arc during walking and stepping in man. Exp Brain Res. 1987;66(1):49–60. doi: 10.1007/BF00236201. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80(1):83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Dyhre-Poulsen P, Simonsen EB, Voigt M. Dynamic control of muscle stiffness and H reflex modulation during hopping and jumping in man. J Physiol. 1991;437:287–304. doi: 10.1113/jphysiol.1991.sp018596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edamura M, Yang JF, Stein RB. Factors that determine the magnitude and time course of human H-reflexes in locomotion. J Neurosci. 1991;11(2):420–7. doi: 10.1523/JNEUROSCI.11-02-00420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton R, Grillner S, Sjostrom A, Zangger P. Central generation of locomotion in vertebrates In: Stein PS, Stuart DG, editors Neural control of locomotion. New York: Plenum; 1976. pp. 439–64. [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, et al. Retraining the injured spinal cord. J Physiol. 2001a;533(Pt 1):15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, de Leon RD. Neural Darwinism in the mammalian spinal cord In: Patterson MM, Grau JW, editors Spinal cord plasticity: alterations in reflex function. Berlin: Springer; 2001b. pp. 185–206. [Google Scholar]
- Field-Fote EC, Dietz V. Single joint perturbation during gait: preserved compensatory response pattern in spinal cord injured subjects. Clin Neurophysiol. 2007;118(7):1607–16. doi: 10.1016/j.clinph.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85(1):103–7. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977;132(1):121–39. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, et al. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98(5):2525–36. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Gordon KE, Wu M, Kahn JH, Schmit BD. Feedback and feedforward locomotor adaptations to ankle-foot load in people with incomplete spinal cord injury. J Neurophysiol. 2010;104(3):1325–38. doi: 10.1152/jn.00604.2009. [DOI] [PubMed] [Google Scholar]
- Grillner S. Interaction between central and peripheral mechanisms in the control of locomotion. Prog Brain Res. 1979;50:227–35. doi: 10.1016/S0079-6123(08)60823-7. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77(2):797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. J Neurosci. 2007;27(32):8558–62. doi: 10.1523/JNEUROSCI.1635-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstoetter US, Danner SM, Freundl B, Binder H, Mayr W, Rattay F, et al. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J Neurophysiol. 2015;114(1):400–10. doi: 10.1152/jn.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, et al. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28(29):7370–5. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control. 2002;6(2):129–45. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Maioli C. The role of preparation in tuning anticipatory and reflex responses during catching. J Neurosci. 1989;9(1):134–48. doi: 10.1523/JNEUROSCI.09-01-00134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Anderschitz M, Dietz V. Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol. 2006;95(2):766–73. doi: 10.1152/jn.00473.2005. [DOI] [PubMed] [Google Scholar]
- Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, et al. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J Neurosci. 2008;28(31):7774–80. doi: 10.1523/JNEUROSCI.1069-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne CS, Mulavara AP, McDonald PV, Pruett CJ, Kozlovskaya IB, Bloomberg JJ. Effect of long-duration spaceflight on postural control during self-generated perturbations. J Appl Physiol (1985) 2001;90(3):997–1006. doi: 10.1152/jappl.2001.90.3.997. [DOI] [PubMed] [Google Scholar]
- Layne CS, Spooner BS. EMG analysis of human postural responses during parabolic flight microgravity episodes. Aviat Space Environ Med. 1990;61(11):994–8. [PubMed] [Google Scholar]
- Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115(2):345–56. doi: 10.1007/pl00005703. [DOI] [PubMed] [Google Scholar]
- Manoonpong P, Parlitz U, Worgotter F. Neural control and adaptive neural forward models for insect-like, energyefficient, and adaptable locomotion of walking machines. Front Neural Circuits. 2013;7:12. doi: 10.3389/fncir.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, et al. Somatosensory control of balance during locomotion in decerebrated cat. J Neurophysiol. 2012;107(8):2072–82. doi: 10.1152/jn.00730.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Gorskii OV, Kilimnik VA, Kozlovskaya IB, Courtine G, Edgerton VR, et al. Regulation of posture and locomotion in decerebrate and spinal animals. Neurosci Behav Physiol. 2015;45(2):229–37. [Google Scholar]
- Parker D, Grillner S. Activity-dependent metaplasticity of inhibitory and excitatory synaptic transmission in the lamprey spinal cord locomotor network. J Neurosci. 1999;19(5):1647–56. doi: 10.1523/JNEUROSCI.19-05-01647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter SM, El Hady A, Fetz EE. Closed-loop neuroscience and neuroengineering. Front Neural Circuits. 2014;8:115. doi: 10.3389/fncir.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Gerasimenko Y, Shyu A, Lavrov I, Zhong H, Roy RR, et al. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur J Neurosci. 2012;36(1):2054–62. doi: 10.1111/j.1460-9568.2012.08106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56(3):465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. Anticipatory postural adjustments during load catching by standing subjects. Clin Neurophysiol. 2001;112(7):1250–65. doi: 10.1016/s1388-2457(01)00553-3. [DOI] [PubMed] [Google Scholar]
- Smith JL, Carlson-Kuhta P, Trank TV. Motor patterns for different forms of walking: cues for the locomotor central pattern generator. Ann N Y Acad Sci. 1998;860:452–5. doi: 10.1111/j.1749-6632.1998.tb09073.x. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol. 2002;88(6):3108–17. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- Toussaint HM, Commissaris DA, Beek PJ. Anticipatory postural adjustments in the back and leg lift. Med Sci Sports Exerc. 1997;29(9):1216–24. doi: 10.1097/00005768-199709000-00015. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9(8):1265–79. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Ziegler MD, Zhong H, Roy RR, Edgerton VR. Why variability facilitates spinal learning. J Neurosci. 2010;30(32):10720–26. doi: 10.1523/JNEUROSCI.1938-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Roy RR, Nakada KK, Zdunowski S, Khalili N, de Leon RD, et al. Accommodation of the spinal cat to a tripping perturbation. Front Physiol. 2012;3:112. doi: 10.3389/fphys.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]


