We show that the growth factor parathyroid hormone-like hormone (PTHLH) is expressed in acid-secreting parietal cells of the mouse stomach. We define the specific PTHLH mRNA isoforms expressed in human stomach and in human gastric cancer cell lines and show that gastrin induces PTHLH expression via transcription activation and mRNA stabilization. Our findings suggest that PTHLH is a gastrin-regulated growth factor that might contribute to gastric epithelial cell homeostasis.
Keywords: stomach, growth factor, parathyroid hormone-related protein, PTHrP, AU-rich element, mRNA stability
Abstract
Parietal cells play a fundamental role in stomach maintenance, not only by creating a pathogen-free environment through the production of gastric acid, but also by secreting growth factors important for homeostasis of the gastric epithelium. The gastrointestinal hormone gastrin is known to be a central regulator of both parietal cell function and gastric epithelial cell proliferation and differentiation. Our previous gene expression profiling studies of mouse stomach identified parathyroid hormone-like hormone (PTHLH) as a potential gastrin-regulated gastric growth factor. Although PTHLH is commonly overexpressed in gastric tumors, its normal expression, function, and regulation in the stomach are poorly understood. In this study we used pharmacologic and genetic mouse models as well as human gastric cancer cell lines to determine the cellular localization and regulation of this growth factor by the hormone gastrin. Analysis of PthlhLacZ/+ knock-in reporter mice localized Pthlh expression to parietal cells in the gastric corpus. Regulation by gastrin was demonstrated by increased Pthlh mRNA abundance after acute gastrin treatment in wild-type mice and reduced expression in gastrin-deficient mice. PTHLH transcripts were also observed in normal human stomach as well as in human gastric cancer cell lines. Gastrin treatment of AGS-E gastric cancer cells induced a rapid and robust increase in numerous PTHLH mRNA isoforms. This induction was largely due to increased transcriptional initiation, although analysis of mRNA half-life showed that gastrin treatment also extended the half-life of PTHLH mRNA, suggesting that gastrin regulates expression by both transcriptional and posttranscriptional mechanisms.
NEW & NOTEWORTHY We show that the growth factor parathyroid hormone-like hormone (PTHLH) is expressed in acid-secreting parietal cells of the mouse stomach. We define the specific PTHLH mRNA isoforms expressed in human stomach and in human gastric cancer cell lines and show that gastrin induces PTHLH expression via transcription activation and mRNA stabilization. Our findings suggest that PTHLH is a gastrin-regulated growth factor that might contribute to gastric epithelial cell homeostasis.
gastric parietal cells secrete acid into the stomach lumen in response to eating a meal. Acid secretion is highly regulated, with paracrine (histamine), neural (acetylcholine), and hormonal (gastrin) factors acting in concert to regulate parietal cell function (33). In particular, gastrin has been identified as a key inducer, with meal-induced gastrin targeting the oxyntic mucosa via cholecystokinin-B receptors (CCKBR; also known as CCK2R) expressed on histamine-secreting enterochromaffin-like (ECL) cells and acid-secreting parietal cells. Gastrin functions in a classic negative feedback loop to maintain homeostatic levels of gastric acid, with hormone secretion from gastrin-expressing endocrine cells (G cells) in the distal stomach regulated by luminal stomach pH (8).
In addition to regulation of gastric acid, gastrin acts as a growth factor to stimulate epithelial cell proliferation in the gastric corpus (18). Patients with gastrin-secreting neuroendocrine tumors in Zollinger-Ellison syndrome exhibit gastric mucosal hypertrophy in addition to high acid secretion. Several studies in the rat and mouse have also demonstrated that hypergastrinemia induces gastric hyperplasia and tissue hypertrophy, with expansion of parietal, ECL, and surface mucous cells (22, 32, 40). The mechanism for the gastrin growth effect is unknown. It has been suggested that gastrin receptors might be expressed on an oxyntic progenitor cell population and thereby gastrin could directly target proliferating progenitors to affect proliferation and/or differentiation (21). However, recent lineage tracing studies with a CCKBR-CreERT BAC transgenic mouse strain showed that in the gastric corpus only parietal and ECL cells were marked, suggesting that this receptor is not expressed in progenitor cells in this region of the stomach (11). As an alternative, several studies have suggested an indirect mechanism for gastrin induction of proliferation via gastrin-regulated growth factors produced in differentiated cell types that might target progenitor cells (18). Parietal cells are of particular interest because they are regulated by gastrin and are reported to be a rich source of growth factors, including Sonic hedgehog (41), Indian hedgehog (9), EGF ligands (amphiregulin and HB-EGF) (3, 26), vascular endothelial growth factor B, insulin-like growth factor-binding protein 2, and parathyroid hormone-like hormone [PTHLH; also known as parathyroid hormone-related protein (PTHrP)] (17, 19, 25).
PTHLH is broadly expressed in numerous tissues during development and adulthood, where it orchestrates key cellular events, such as proliferation and differentiation (35). In the stomach, PTHLH expression is poorly understood. Although several studies have reported gastric PTHLH, the cellular localization is debated, with conflicting studies suggesting expression in parietal cells (17, 25) or ECL cells (2, 23, 42). Importantly, expression appears to be regulated by gastrin as Pthlh mRNA abundance is decreased in mice with genetic deletion of gastrin or CCKBR (17, 19, 42) and increased after gastrin administration to gastrin-deficient mice (17). In addition to expression in the normal stomach, PTHLH is frequently highly expressed in gastric cancer (1, 16, 27, 28, 37). Thus PTHLH is of interest as a potential gastrin-regulated gastric growth factor.
In this study we examined the cellular expression pattern of PTHLH in the stomach, demonstrating specific localization to parietal cells and not to ECL cells. Mechanisms of gastrin regulation of PTHLH gene expression were examined in mouse models and in human gastric cancer cell lines. The findings suggest that gastric PTHLH is rapidly and transiently induced by gastrin simulation of CCKBR signaling in gastric parietal cells.
MATERIAL AND METHODS
Mice.
PthlhLacZ/+ (4) (provided by Dr. Arthur Broadus) and gastrin-deficient (10) mice were on a mixed CD-1;C57BL/6 and a C57BL/6 strain background, respectively. Mice were housed in ventilated and automated-watering cages under specific pathogen-free conditions. All animal procedures were approved by the University of Michigan Committee on Use and Care of Animals. For all experiments, male and female mice aged 1–4 mo were used. For gastrin treatment, mice were fasted overnight with free access to water before intraperitoneal injection with vehicle (150 mM NaCl, pH 7) or human gastrin I (250 µg/kg; Bachem).
Cells and pharmacologic inhibitor treatment.
Human gastric cancer cell lines AGS, NCI-N87, MKN-45, and 23132/87 were obtained from Dr. Juanita Merchant (University of Michigan). AGS cells stably transfected with a human CCKBR expression construct (AGS-E) were obtained from Dr. Timothy Wang (Columbia University) (15). AGS and AGS-E cells were cultured in DMEM containing 10% fetal calf serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, 0.292 mg/ml l-glutamine, and 2 mM sodium pyruvate. Puromycin (0.02 mg/ml) was added periodically during AGS-E culture to maintain the CCKBR expression construct. All other gastric cancer cell lines were cultured in RPMI-1640 containing fetal calf serum (10%, NCI-N87 and 23132/87; 20%, MKN-45), penicillin, streptomycin, l-glutamine, and sodium pyruvate (1 mM, NCI-N87 only).
For analysis of gastrin regulation of PTHLH mRNA, AGS or AGS-E cells were seeded in six-well plates (3 × 105 cells/well), cultured overnight, serum starved for 24 h, and then treated for 2–12 h with serum-free media containing vehicle or gastrin (10−7 M) before RNA isolation. For CCKBR signaling experiments, AGS-E cells were pretreated 30 min before gastrin treatment with pathway inhibitors: SB203580 (10−5 M), Ro-32–0432 (10−6 M), LY294002 (1.5 × 10−5 M), or AG 1478 (9 × 10−6 M; Calbiochem), or PD98059 (5 × 10−7 M; Cell Signaling) and RNA isolated 4 h after gastrin administration.
Whole mount β-galactosidase staining.
For analysis of β-galactosidase activity, stomachs from PthlhLacZ/+ mice were opened along the greater curvature, rinsed in PBS, pinned flat on dental wax, fixed for 1 h in 4% paraformaldehyde in PBS, and washed three times (15 min) in staining buffer (0.1 M sodium phosphate pH 7.3 containing 2 mM MgCl2 and 0.02% NP-40) before detection of β-galactosidase activity with 5-bromo-4-chloro-3-indyl-β-d-galactosidase (X-gal; Roche) staining solution [1 mg/ml X-gal in N,N-dimethylformamide, 5 mM K3Fe(CN)6, and 5 mM K4Fe(CN)6-3H2O in staining buffer] at 37°C for 5–16 h.
Immunohistochemistry.
Cryosections (8 µm) were incubated with rabbit anti-β-galactosidase antiserum (1:1,000; gift from Dr. J. Douglas Engel, University of Michigan) overnight at 4°C. Slides were rinsed in 0.01% TPBS and subsequently incubated for 1 h at room temperature with a mouse monoclonal antibody against H+-K+-ATPase α-subunit (1:500; Medical and Biological Laboratories, Nagoya, Japan) or a rabbit polyclonal antibody against chromogranin A (1:100; gift of Dr. Jens Rehfeld, University of Copenhagen). After being washed in 0.01% Triton X-100 in phosphate-buffered saline, samples were incubated with appropriate secondary antibodies conjugated to Cy2 or Cy3 (1:200 and 1:500, respectively; Jackson ImmunoResearch Laboratories). Sections were mounted with ProLong Gold anti-fade reagent DAPI (Invitrogen). Fluorescence images were captured with a Cool Snap digital camera mounted on a Nikon E800 upright fluorescence microscope.
RNA isolation and quantitative gene expression analysis.
RNA was isolated from mouse stomach or cultured cells using TRIzol (Invitrogen), DNase treated, and purified using the RNeasy Mini kit (Qiagen). RNA (1 µg) was reverse-transcribed using the iScript cDNA synthesis kit, as recommended by the manufacturer (Bio-Rad). mRNA abundance was measured by quantitative reverse transcription-PCR (qRT-PCR) analysis as described previously (17), using primers listed in Table 1. Expression levels were measured for individual samples (in triplicate) and normalized to the expression of glyceraldhyde 3-phosphate dehydrogenase (mouse Gapdh or human GAPDH) (n = 3–5 samples/group as detailed in figure legends). Basal expression of PTHLH in AGS cells was very low, and cycle threshold values were set at the assay sensitivity threshold of 34 cycles in some experiments.
Table 1.
Oligonucleotide primers for qRT-PCR measurement of transcript abundance
| Species/Gene | Forward (5′–3′) | Reverse (5′–3′) | Product, bp |
|---|---|---|---|
| Human | |||
| GAPDH | GAGTCCACTGGCGTCTTCACC | GAGGCATTGCTGATGATCTTGAGG | 164 |
| PTHLH | GGTGTTCCTGCTGAGCTACGC | TCGTCGCCGTAAATCTTGGATGG | 136 |
| TFF2 | TAACAGGACGAACTGCGGCTTC | GCACCAGGGCACTTCAAAGATG | 244 |
| Mouse | |||
| Gapdh | TCAAGAAGGTGGTGAAGCAGG | TATTATGGGGGTCTGGGATGG | 350 |
| Pthlh | GACGTACAAAGAACAGCCACTCA | TTTTTCTCCTGTTCTCTGCGTTT | 81 |
| Atp4a | TGTACACATGAGAGTCCCCTTG | GAGTCTTCTCGTTTTCCACACC | 157 |
| Gast | GGACCAGGGACCAATGAGG | CCAAAGTCCATCCATCCGTAGG | 173 |
qRT-PCR, quantitative reverse transcription polymerase chain; PTHLH, parathyroid hormone-like hormone.
Human PTHLH mRNA isoform expression.
The human PTHLH gene structure was drawn using GenePalette software (30). Normal human gastric RNA samples were purchased (Origene, Zyagen, and Cell Applications). Tissue or cell line RNA (1 µg) was reverse transcribed using the iScript cDNA synthesis kit, and specific mRNA isoforms were amplified using the Go Taq Flexi DNA Polymerase kit (Promega). Primer sequences, amplicon content, and product size for each PTHLH mRNA isoform are shown in Tables 2 and 3. Reaction mixtures (50 µl) contained 0.2 µM of each forward and reverse primer, 0.2 mM dNTPs, 1× reaction buffer, 2 mM MgCl2, and 0.05 units of Taq polymerase. Betaine (29) (1 M; Sigma) was used in PCR reactions that amplified the P3 PTHLH isoform. Amplification was performed with 35 cycles of denaturation at 95°C for 30 s, annealing at 55 or 60°C for 20 s, and extending at 72°C for 45 s. Amplicons were initially DNA sequenced to validate the specificity of the different assays for the various PTHLH mRNA isoforms. PCR reactions were analyzed on a 1.7% agarose gel stained with ethidium bromide to reveal PTHLH mRNA isoform expression.
Table 2.
Oligonucleotide primers for RT-PCR analysis
| Gene | Primer | Primer Sequence (5′–3′) |
|---|---|---|
| PTHLH | F1 | AGGTACCTGCTTTCTAATA |
| PTHLH | F3 | TTCTCCGGCAGGTTTG |
| PTHLH | F4 | CCACGAACCCAGGAGAACTGC |
| PTHLH | F5 | GGAGACGATGCAGCGGAGAC |
| PTHLH | R6a | TTTCCTGCTCCTTGCGTTTCC |
| PTHLH | R6b | TTGCCCAGGTGTGAGAGTAAGG |
| PTHLH | R7 | GGCAATAAAGTAGGGTCC |
| PTHLH | R8 | CCAATGTGCAGTTTCATAGAGC |
| PTH1R | F | GGCGTCCCTCACCGTAGC |
| PTH1R | R | AGAGTAGAGCACAGCGTCCTTG |
F, forward; R, reverse with number indicating the PTHLH exon that contains the sequence (see Fig. 5).
Table 3.
qRT-PCR identification of PTHLH transcripts
| Transcript | Primers | Exons in Transcript | Product Size, bp |
|---|---|---|---|
| Promoter | |||
| All | F5, R6a | E5–E6a | 419 |
| P1 | F1, R6a | E1–E2–E3–E5–E6a | 915 |
| P1′ | F1, R6a | E1–E3–E5–E6a | 858 |
| P1″ | F1, R6a | E1–E5–E6a | 778 |
| P2 | F3, R6a | E3–E5–E6a | 677 |
| P3 | F4, R6a | E4–E5–E6a | 648 |
| 3′-UTR | |||
| 3′-UTRa | F5, R6b | E5–E6a–E6b | 901 |
| 3′-UTRb | F5, R7 | E5–E6a–E7 | 981 |
| 3′-UTRc | F5, R8 | E5–E6a–E8 | 691 |
Transcripts differ by promoter usage, alternative splicing, and differential 3′-untranslated region (3′-UTR) sequences. Promoter, exon, and 3′-UTR designations are as described in Fig. 5. Possible exonic sequences contained within amplified transcripts are listed for each primer set. Primer sequences are listed in Table 2. Promoter assays used an annealing temperature of 55°C, and 3′-UTR assays used an annealing temperature of 60°C.
Measurement of PTHLH transcription and mRNA half-life responses.
AGS-E cells (106) were treated with vehicle or gastrin (10−7 M), and PTHLH mRNA abundance was measured by qRT-PCR. Transcription initiation was blocked by actinomycin D (10 µg/ml in DMSO; Sigma) 30 min before gastrin treatment, and cells were harvested and RNA was isolated 4 h after gastrin induction. For measurement of mRNA half-life, actinomycin D was added 4 h after gastrin administration and RNA was isolated at various time points. PTHLH mRNA abundance was compared with values at time 0 when actinomycin D was added.
Statistics.
Quantitative data are presented as means ± SE and analyzed by Student’s t-test or one-way or two-way ANOVA followed by Dunnett’s or Tukey’s posttest (Graph Pad Prism) as detailed in figure legends. P < 0.05 was considered significant.
RESULTS
Gastric Pthlh expression is localized to parietal cells.
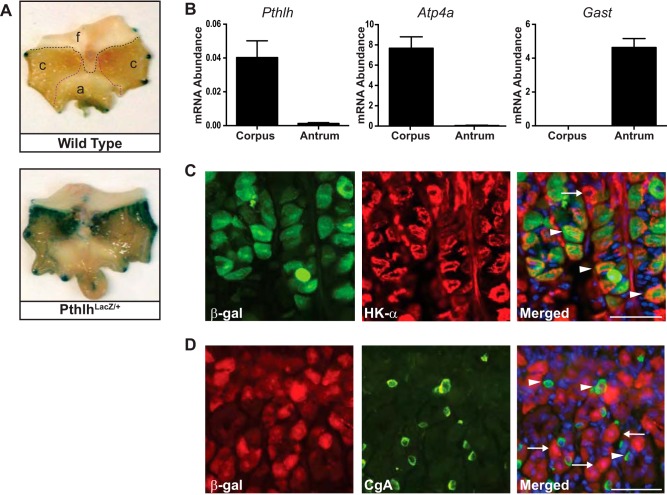
To determine the cellular localization of Pthlh, we measured LacZ reporter expression in PthlhLacZ/+ mice (4). Analysis of whole mount X-gal-stained stomach showed that β-galactosidase was mainly expressed in the gastric corpus, compared with forestomach or antrum (Fig. 1A). Accordingly, endogenous Pthlh mRNA was abundant in corpus, with little or no expression in antrum (Fig. 1B). Regional validity of these RNA samples was demonstrated by high levels of the parietal cell marker H+-K+-ATPase α-subunit transcript (Atp4a) in the corpus RNA and high levels of gastrin transcript (Gas) in the antral RNA. To identify which cells in the corpus express Pthlh, immunohistochemical costaining was performed in the PthlhLacZ/+ mouse stomach. This analysis demonstrated colocalization of antibodies for the β-galactosidase reporter and H+-K+-ATPase-α, suggesting that PTHLH is localized to parietal cells (Fig. 1C, arrowheads). In contrast, cells expressing β-galactosidase did not express the ECL cell marker chromogranin A (Fig. 1D).
Fig. 1.
Gastric parietal cells express parathyroid hormone-like hormone (Pthlh). A: whole mount 5-bromo-4-chloro-3-indyl-β-d-galactosidase (X-gal) staining in PthlhLacZ/+ (bottom) and wild-type (top) mouse stomach localized LacZ reporter expression to the corpus region (f, forestomach; c, corpus; a, antrum). The dark edge staining is an artifact due to pinning the tissue. B: quantitative (q)RT-PCR measurement of mRNA abundance for parathyroid hormone-like hormone (Pthlh), the corpus marker H+-K+-ATPase α-subunit (Atp4a), and the antral marker gastrin (Gast) in total RNA isolated from mouse gastric corpus and antrum. Values were normalized to Gapdh and are presented as means ± SE (n = 3–5). C: coimmunostaining PthlhLacZ/+ stomach cryosections with antibodies against the reporter β-galactosidase (β-gal; green) and the parietal cell marker H+-K+-ATPase α-subunit (HK-α; red) showed colocalization in a subset of parietal cells. Arrowheads, β-gal-expressing parietal cells; arrow, non-β-gal-expressing parietal cell. D: coimmunostaining of β-gal (red) and the enterochromaffin-like marker chromogranin A (CgA; green) showed no colocalization. Arrowheads, CgA-expressing cells; arrows, β-gal-expressing cells. Scale bars = 50 µm.
Pthlh mRNA abundance is induced by gastrin.
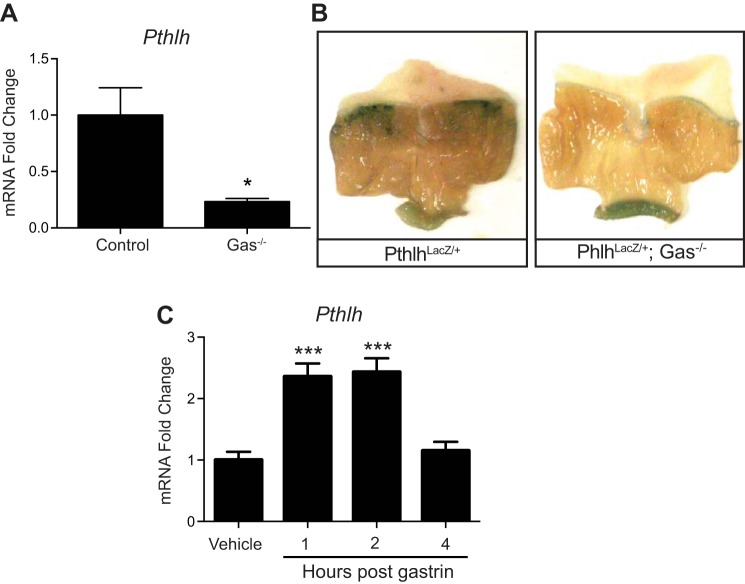
We next tested whether gastrin regulates gastric Pthlh gene expression in vivo. Analysis of gastrin-deficient mice showed a fourfold reduction in Pthlh mRNA abundance compared with wild-type mice (Fig. 2A). This regulation was also demonstrated by reduced PthlhLacZ/+ reporter gene expression observed by whole mount X-gal staining (Fig. 2B). In addition, gastrin treatment of wild-type mice demonstrated rapid and transient induction of Pthlh transcripts, which peaked at 1 and 2 h postinjection, returning to basal levels by 4 h (Fig. 2C). Together these results demonstrate that gastrin acutely regulates Pthlh mRNA abundance in vivo.
Fig. 2.
Gastrin stimulates Pthlh expression in vivo. A: Pthlh mRNA abundance was measured by qRT-PCR analysis of corpus RNA isolated from gastrin-deficient mice (Gas−/−) and Gas+/+ control littermates. *P < 0.05 vs. control by Student’s t-test. B: whole mount X-gal staining of PthlhLacZ/+ reporter mouse stomach on control or Gas−/− strain background. Staining at the pylorus is nonspecific. C: qRT-PCR measurement of Pthlh mRNA abundance in corpus isolated from wild-type mice at various times after gastrin injection (250 μg/kg). ***P < 0.001 vs. vehicle-injected group by a one-way ANOVA with Dunnett’s posttest. For A and C, data (means ± SE) are shown in reference to Gapdh expression measured in the same samples (n = 4 mice per group).
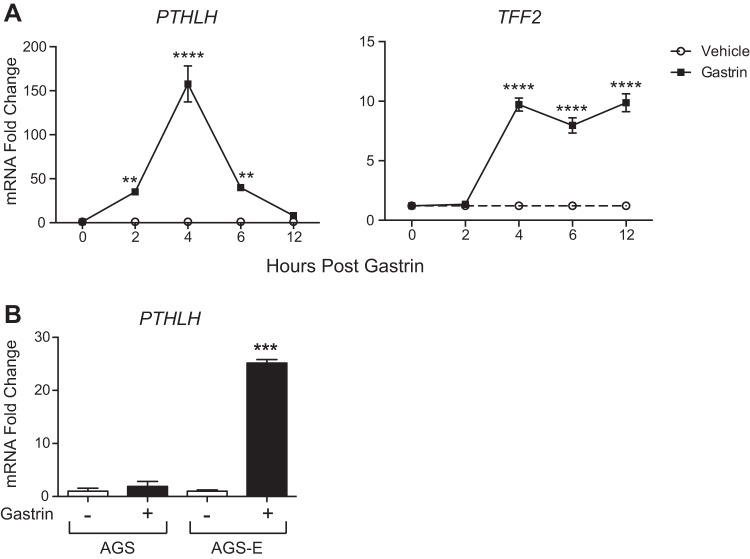
To uncover possible mechanisms for this induction, we examined gastrin induction in the human gastric cancer cell line AGS-E, which had been genetically engineered to express human CCKBR (15). Basal PTHLH expression was very low, close to the baseline level of detection in our assay system. Treatment with gastrin showed a robust induction of PTHLH mRNA in comparison with vehicle (Fig. 3A). Peak PTHLH expression (>150-fold induction) was observed at 4 h postgastrin treatment. We also examined the response of the TFF2 gene, which had previously been shown to be induced by gastrin (39). Induction of TFF2 mRNA expression was delayed and longer lasting than the pattern for PTHLH. These data suggest that gastrin can induce PTHLH expression rapidly and transiently and that gastrin has distinct mechanisms to regulate the expression of PTHLH and TFF2 gene expression.
Fig. 3.
Gastrin stimulates PTHLH expression in vitro. A: qRT-PCR measurement of PTHLH (left) or TFF2 (right) mRNA abundance in AGS-E cells treated with vehicle or human gastrin (10−7 M). **P < 0.005, ****P ≤ 0.0001 vs. time 0 for each treatment by two-way ANOVA with Dunnett’s posttest. B: qRT-PCR measurement of PTHLH mRNA abundance in AGS and AGS-E cells 6 h after gastrin administration. ***P ≤ 0.0005 vs. all groups by two-way ANOVA with Tukey’s posttest. All data (means ± SE) are shown in reference to GAPDH expression measured in the same samples (n = 3 independent RNA samples per group).
To study if PTHLH mRNA induction by gastrin is mediated by the gastrin receptor CCKBR, expression was measured in the parental AGS cell line, which does not express CCKBR, and compared with AGS-E cells. Gastrin treatment of AGS cells for 6 h was not different from vehicle-treated cells, while AGS-E cells exhibited a robust response to gastrin (Fig. 3B), suggesting that gastrin stimulates PTHLH expression via CCKBR signaling.
Signaling pathways mediating gastrin stimulation of PTHLH.
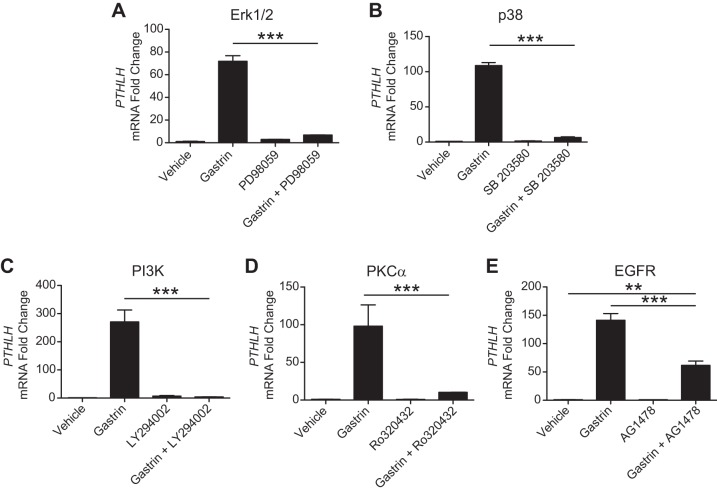
Activation of the gastrin receptor CCKBR is known to stimulate several downstream signaling pathways, including MAPK Erk1/2, MAPK p38, phosphatidylinositol 3-kinase, PKCα, and EGF receptor (EGFR) (31). We tested the importance of these signaling components by pretreatment of AGS-E cells with pharmacologic inhibitors. All of these compounds were observed to inhibit PTHLH induction (Fig. 4). While inhibition of Erk1/2, p38, phosphatidylinositol 3-kinase, or PKCα completely blocked gastrin-induced PTHLH expression (Fig. 4, A–D), the EGFR inhibitor only partially blocked the response (Fig. 4E).
Fig. 4.
Signaling pathways mediating gastrin induction of PTHLH. Pharmacological agents were used to inhibit various signaling pathways downstream of the gastrin receptor CCKBR. A: Erk1/2 inhibition with PD98059 (5 × 10−7 M). B: p38 inhibition with SB203580 (10−5 M). C: PI3K inhibition with LY294002 (1.5 × 10−5 M). D: PKCa inhibition with Ro320432 (10−6 M). E: EGFR inhibition with AG1478 (9 × 10−6 M). qRT-PCR measurement of PTHLH mRNA abundance in vehicle-treated, gastrin-treated, inhibitor-pretreated, or inhibitor-pretreated and gastrin-treated AGS-E cells 4 h after treatment. Values were normalized to GAPDH, and data are shown as means ± SE. **P < 0.01, ***P < 0.001, via one-way ANOVA with Tukey’s posttest (N = 3 independent RNA samples per group).
Gastrin induces multiple PTHLH mRNA isoforms.
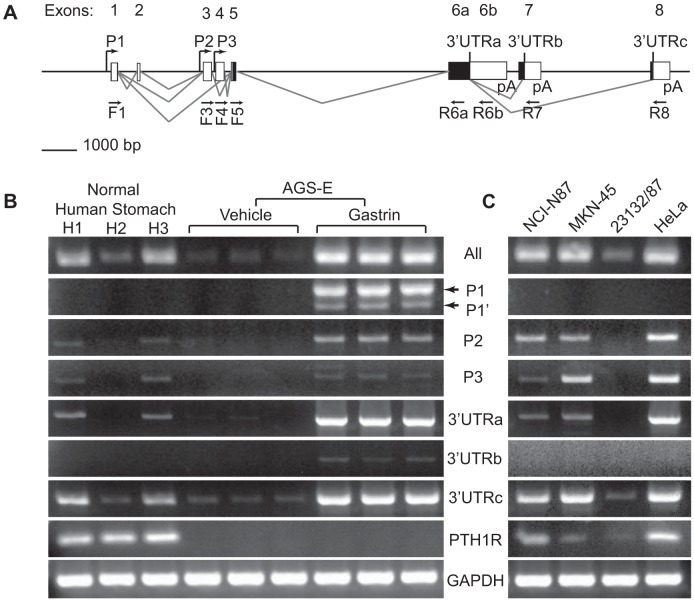
It is known that the human PTHLH gene expresses many different mRNA isoforms (24). Three different promoters and extensive alternative splicing give rise to numerous PTHLH mRNA species with differing 5′- and 3′-untranslated regions (UTRs); all isoforms include exons 5 and 6a (Fig. 5A). We designed multiple isoform-specific primers to measure PTHLH transcripts by RT-PCR analysis to determine which promoters (P1, P2, or P3) and 3′-UTRs (a, b, or c) were expressed in the normal human stomach and to identify which isoforms were induced by gastrin in AGS-E cells (Fig. 5B). Normal human stomach RNA contained PTHLH transcripts specific to promoters P2 and P3 but not P1 and 3′-UTRa and 3′-UTRc but not 3′-UTRb. Notably, basal PTHLH mRNA levels were very low in AGS-E cells. After gastrin treatment of AGS-E cells, there was a robust increase in PTHLH transcripts from all promoters (P1, P1’, P2, and P3) and containing all 3′-UTR regions (a, b, and c) (Fig. 5B). The observation of rapid induction of transcripts from different promoters to form multiple isoforms demonstrates that the gastrin induction is not due to activation of a specific transcriptional start site.
Fig. 5.
Gastrin induces multiple PTHLH mRNA isoforms. A: diagram of human PTHLH gene structure. The PTHLH gene consists of 8 exons (untranslated, white; translated, black) with 3 transcriptional starts (P1, P2, and P3). Alternative splicing generates isoforms with different 3′-untranslated regions (3′-UTRs: a, b, and c). Primer sets were designed to amplify all PTHLH mRNA species or specific isoforms from promoters P1, P2, and P3 and the 3′-UTRs (a, b, or c; see Tables 2 and 3 for primer sequences and amplicon information). The positions of the forward (F) and reverse (R) primers are indicated below the gene diagram. B: RT-PCR products generated from the RNA samples from normal human stomach (H1, H2, and H3) and RNAs from vehicle- or gastrin-treated AGS-E cells. RNAs were tested for the various indicated PTHLH isoforms and PTHLH receptor (PTH1R) expression; GAPDH was used as a reference for RNA quality and quantity. C: RT-PCR analysis of human gastric cancer cell lines as indicated. HeLa cells were used as a positive control.
Given that PTHLH expression is highly expressed in some gastric cancers, we also screened several additional human gastric cancer cell lines for PTHLH isoform expression. The human cervical cancer cell line HeLa was used as a positive control for most RT-PCR assays (Fig. 5C). NCI-N87, MKN-45, and 23132/87 gastric cancer cell lines expressed PTHLH, with low levels in 23132/87 cells. Analysis of PTHLH isoform expression showed a similar pattern as normal human stomach, with transcripts resulting from activation of P2, P3, and/or containing the regions from the 3′-UTRa and 3′-UTRc. Gastrin induction of PTHLH was not observed in these cell lines, presumably because they do not express CCKBR (data not shown).
We also examined the expression of the PTHLH receptor by RT-PCR analysis of PTH1R transcripts in the same RNA samples. Interestingly, PTH1R mRNA was detected in normal human stomach RNA (Fig. 5B), as well as in mouse stomach RNA (data not shown). Although PTH1R transcripts were not detected in AGS-E cells (Fig. 5B), the other gastric cell lines studied as well as HeLa expressed this receptor, suggesting that PTHLH might function as an autocrine regulator in these cancer cell lines.
Mechanism of PTHLH mRNA induction by gastrin.
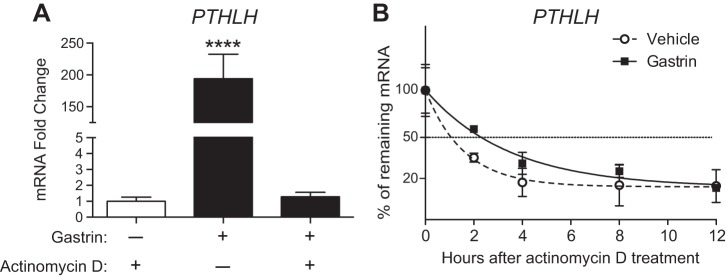
We determined whether the increase in PTHLH mRNA was due to transcriptional or posttranscriptional mechanisms. To test transcription we blocked RNA synthesis with actinomycin D before gastrin treatment in AGS-E cells. Analysis of PTHLH mRNA abundance at 4 h after gastrin or vehicle administration showed that the induction was prevented by actinomycin D, demonstrating that gastrin stimulates PTHLH gene transcription (Fig. 6A). We also tested posttranscriptional mechanisms. All of the PTHLH 3′-UTRs contain AU-rich destabilization elements (24), suggesting that gastrin might also act by stabilizing PTHLH mRNA. PTHLH mRNA half-life was measured in AGS-E cells by administration of actinomycin D at 4 h after gastrin or vehicle treatment, and mRNA abundance was measured at various time points after transcription was inhibited. We found that gastrin increased the half-life of PTHLH mRNA from 66 min in vehicle-treated cells to 144 min in gastrin-treated cells (Fig. 6B). This result suggests that gastrin also regulates PTHLH gene expression at the posttranscriptional level to stabilize the various isoforms.
Fig. 6.
Gastrin promotes PTHLH transcription and stabilizes PTHLH mRNA. A: analysis of PTHLH expression in AGS-E cells pretreated with actinomycin D 30 min before gastrin administration. mRNA abundance was measured 4 h postgastrin or vehicle administration. Values were normalized to GAPDH, and data are shown as means ± SE. ****P < 0.0001, via one-way ANOVA with Tukey’s posttest (n = 3 independent RNA samples per group). B: analysis of PTHLH mRNA half-life in AGS-E cells treated with vehicle or gastrin. Actinomycin D was added 4 h after gastrin induction and PTHLH mRNA abundance was measured at varying times by qRT-PCR analysis. Values were normalized to GAPDH and plotted to compare with values at time 0 (100%) when actinomycin D was applied.
DISCUSSION
This study examined the expression of the growth factor PTHLH in the mouse and human stomach. Analysis of PthlhLacZ/+ reporter mice localized expression to the corpus region of the stomach, specifically to acid-secreting parietal cells. This finding agrees with previous gene expression microarray studies that identified Pthlh transcripts in isolated mouse parietal cells purified by lectin-mediated cell panning (25) or by cell sorting (17). In contrast, our results do not support other studies in mouse and human that suggested PTHLH localized to ECL cells (2, 23, 42). Notably, those studies did not examine purified cell populations and localization was based on correlative inferences or low-resolution immunostaining. Thus, overall, the data support the conclusion that in the stomach PTHLH is primarily expressed in parietal cells.
The hormone gastrin directly targets parietal cells via CCKBR to regulate parietal cell function, including acid secretion and cellular maturation (13). We demonstrated that gastrin induces Pthlh mRNA in the mouse corpus. Acute gastrin treatment rapidly and transiently increased mRNA levels in wild-type mice, and gastrin-deficient mice exhibited reduced Pthlh mRNA and PthlhLacZ/+ reporter expression. These findings agree with earlier studies that showed decreased gastric Pthlh mRNA abundance in mice with genetic deletion of gastrin or its receptor CCKBR (17, 19, 42). We studied the mechanism for the response using a human gastric cancer cell line. Gastrin treatment of AGS-E cells similarly exhibited a rapid and transient induction in PTHLH mRNA, which was CCKBR dependent. Pharmacological inhibitors were used to identify the key receptor signal transduction pathways that regulate PTHLH mRNA abundance, revealing complex signaling inputs consistent with the known pathways downstream of CCKBR. Of interest is the EGFR arm of signal transduction as PTHLH expression is induced by EGFR signaling in keratinocytes (5, 12). Gastrin has been reported to induce expression of the EGF family members amphiregulin and HB-EGF (3, 34, 38). Whether gastrin induction of EGFR ligands in parietal cells contributes to PTHLH induction is an interesting future question.
The human PTHLH gene has three promoters (P1, P2, and P3) and three distinct 3′-ends (UTRa, UTRb, and UTRc) with different mRNA isoforms formed by alternative splicing (24). Our results show that the normal human stomach primarily expresses PTHLH isoforms from promoters P2 and P3, with 3′-UTRa and 3′-UTRc. Importantly, numerous PTHLH mRNA isoforms were rapidly induced by gastrin, including transcripts generated from all three PTHLH promoters and all three 3′UTRs, suggesting a global mechanism for the gastrin response.
Our previous gene expression profiling studies on gastrin-deficient and gastrin-replacement mice demonstrated coordinate regulation of a number of ECL cell and parietal cell genes involved in acid secretion (17, 19). In ECL cells, several of these gastrin-induced genes are linked to histamine synthesis, including histidine decarboxylase, vesicular monamine transporter 2, chromogranin A, and chromogranin B. Mechanistic studies in cultured cell models suggested that gastrin induces transcription of these ECL genes through a PKC-dependent MAP kinase signaling cascade to activate specific transcription factors binding to DNA elements in the promoter regions (6, 14). Fewer studies have been performed on mechanisms of gastrin regulation of parietal cell genes. Our observation that gastrin induces PTHLH transcription is consistent with the general observation that gastrin-CCKBR signaling regulates gene expression via activation of transcription factor binding.
Gastrin has also been shown to regulate gene expression through posttranscriptional mechanisms. PTHLH mRNA stability is regulated by AU-rich elements (ARE), which are present in all three 3′-UTRs (24). Analysis of gastrin effects on PTHLH mRNA half-life in AGS-E cells confirmed that gastrin stabilized PTHLH transcripts. Other studies have described ARE-binding proteins that can function to stabilize PTHLH mRNA, including HuR or AUF1 (7, 20, 36). The identity of ARE-binding proteins involved in gastrin stabilization was not determined in our study. However, a previous study demonstrated that gastrin stabilizes interleukin-8 and cyclooxygenase-2 mRNAs, which contain AREs, via the HuR-binding protein (36). Interestingly, similar to PTHLH, both interleukin-8 and cyclooxygenase-2 appear to be regulated by gastrin via both transcriptional and posttranscriptional mechanisms (36).
Overall, this study showed that PTHLH is expressed in gastric parietal cells and that gastrin induces this growth factor via both transcriptional and posttranscriptional means to increase abundance of several different PTHLH mRNA isoforms. Notably, PTHLH is highly expressed in some gastric cancers where it can incite humoral hypercalcemia of malignancy syndrome (1, 16, 27, 28, 37). Accordingly, our data demonstrate that PTHLH mRNA isoforms are expressed in several human gastric cancer cell lines. Furthermore, some gastric cancer cell lines also expressed mRNA for the PTHLH receptor PTH1R, suggesting that this growth factor might have an autocrine function in some gastric cancers.
GRANTS
A. Al Menhali was supported by a fellowship from the United Arab Emirates University. E. S. Demitrack was supported by an American Association for Cancer Research Debbie’s Dream Foundation Career Development Award. The research was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK-078926 and P01-DK-06041 (to L. C. Samuelson) and core support from the Michigan Gastrointestinal Research Center NIDDK Grant P30-DK-34933 and the University of Michigan Cancer Center Support National Cancer Institute Grant P30-CA-6592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A.M., T.M.K., and L.C.S. conceived and designed research; A.A.M., T.M.K., and E.SD performed experiments; A.A.M., T.M.K., E.S.D., and L.C.S. analyzed data; A.A.M., T.M.K., E.S.D., and L.C.S. interpreted results of experiments; A.A.M. and T.M.K. prepared figures; A.A.M. and L.C.S. drafted manuscript; A.A.M., T.M.K., E.S.D., and L.C.S. edited and revised manuscript; A.A.M., T.M.K., E.S.D., and L.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Arthur Broadus for the PthlhLacZ/+ mice, Timothy Wang for the AGS-E cell line, J. Douglas Engel and Jens Rehfeld for antibodies, and Sherif Karam for critical reading of the manuscript.
Present address of A. Al Menhali: Biology Dept., United Arab Emirates Univ., Al Ain, UAE.
REFERENCES
- 1.Alipov GK, Ito M, Nakashima M, Ikeda Y, Nakayama T, Ohtsuru A, Yamashita S, Sekine I. Expression of parathyroid hormone-related peptide (PTHrP) in gastric tumours. J Pathol 182: 174–179, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 2.Andersson N, Skrtic SM, Håkanson R, Ohlsson C. A gene expression fingerprint of mouse stomach ECL cells. Biochem Biophys Res Commun 332: 404–410, 2005. doi: 10.1016/j.bbrc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Beales IL. Gastrin and interleukin-1beta stimulate growth factor secretion from cultured rabbit gastric parietal cells. Life Sci 75: 2983–2995, 2004. doi: 10.1016/j.lfs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, Philbrick WM, Broadus AE. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res 21: 113–123, 2006. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- 5.Cho YM, Lewis DA, Koltz PF, Richard V, Gocken TA, Rosol TJ, Konger RL, Spandau DF, Foley J. Regulation of parathyroid hormone-related protein gene expression by epidermal growth factor-family ligands in primary human keratinocytes. J Endocrinol 181: 179–190, 2004. doi: 10.1677/joe.0.1810179. [DOI] [PubMed] [Google Scholar]
- 6.Cramer T, Jüttner S, Plath T, Mergler S, Seufferlein T, Wang TC, Merchant J, Höcker M. Gastrin transactivates the chromogranin A gene through MEK-1/ERK- and PKC-dependent phosphorylation of Sp1 and CREB. Cell Signal 20: 60–72, 2008. doi: 10.1016/j.cellsig.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Danilin S, Sourbier C, Thomas L, Rothhut S, Lindner V, Helwig JJ, Jacqmin D, Lang H, Massfelder T. von Hippel-Lindau tumor suppressor gene-dependent mRNA stabilization of the survival factor parathyroid hormone-related protein in human renal cell carcinoma by the RNA-binding protein HuR. Carcinogenesis 30: 387–396, 2009. doi: 10.1093/carcin/bgn275. [DOI] [PubMed] [Google Scholar]
- 8.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol 63: 119–139, 2001. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Feng R, Aihara E, Kenny S, Yang L, Li J, Varro A, Montrose MH, Shroyer NF, Wang TC, Shivdasani RA, Zavros Y. Indian Hedgehog mediates gastrin-induced proliferation in stomach of adult mice. Gastroenterology 147: 655–666.e9, 2014. doi: 10.1053/j.gastro.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 274: G561–G568, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa Y, Jin G, Wang H, Chen X, Westphalen CB, Asfaha S, Renz BW, Ariyama H, Dubeykovskaya ZA, Takemoto Y, Lee Y, Muley A, Tailor Y, Chen D, Muthupalani S, Fox JG, Shulkes A, Worthley DL, Takaishi S, Wang TC. CCK2R identifies and regulates gastric antral stem cell states and carcinogenesis. Gut 64: 544–553, 2015. doi: 10.1136/gutjnl-2014-307190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heath JK, Southby J, Fukumoto S, O’Keeffe LM, Martin TJ, Gillespie MT. Epidermal growth factor-stimulated parathyroid hormone-related protein expression involves increased gene transcription and mRNA stability. Biochem J 307: 159–167, 1995. doi: 10.1042/bj3070159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinkle KL, Samuelson LC. Lessons from genetically engineered animal models. III. Lessons learned from gastrin gene deletion in mice. Am J Physiol Gastrointest Liver Physiol 277: G500–G505, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Hocker M. Molecular mechanisms of gastrin-dependent gene regulation. Ann N Y Acad Sci 1014: 97–109, 2004. doi: 10.1196/annals.1294.010. [DOI] [PubMed] [Google Scholar]
- 15.Höcker M, Zhang Z, Merchant JL, Wang TC. Gastrin regulates the human histidine decarboxylase promoter through an AP-1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 272: G822–G830, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Ito M, Nakashima M, Alipov GK, Matsuzaki S, Ohtsuru A, Yano H, Yamashita S, Sekine I. Gastric cancer associated with overexpression of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor in relation to tumor progression. J Gastroenterol 32: 396–400, 1997. doi: 10.1007/BF02934499. [DOI] [PubMed] [Google Scholar]
- 17.Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics 24: 124–132, 2006. doi: 10.1152/physiolgenomics.00133.2005. [DOI] [PubMed] [Google Scholar]
- 18.Jain RN, Samuelson LC. Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol 291: G762–G765, 2006. doi: 10.1152/ajpgi.00172.2006. [DOI] [PubMed] [Google Scholar]
- 19.Jain RN, Samuelson LC. Transcriptional profiling of gastrin-regulated genes in mouse stomach. Physiol Genomics 29: 1–12, 2007. doi: 10.1152/physiolgenomics.00176.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS, Lee KY, Kim HJ, Chi SG. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology 135: 2030–2042, 2008. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Kazumori H, Ishihara S, Kawashima K, Fukuda R, Chiba T, Kinoshita Y. Analysis of gastrin receptor gene expression in proliferating cells in the neck zone of gastric fundic glands using laser capture microdissection. FEBS Lett 489: 208–214, 2001. doi: 10.1016/S0014-5793(01)02084-1. [DOI] [PubMed] [Google Scholar]
- 22.Konda Y, Kamimura H, Yokota H, Hayashi N, Sugano K, Takeuchi T. Gastrin stimulates the growth of gastric pit with less-differentiated features. Am J Physiol Gastrointest Liver Physiol 277: G773–G784, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Chen J, Guo Y, Yang L, Zhao C, Bai L. The expression of PTHLH in human gastric mucosa enterochromaffin-like cells. Dig Dis Sci 56: 993–998, 2011. doi: 10.1007/s10620-010-1375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin TJ. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol Rev 96: 831–871, 2016. doi: 10.1152/physrev.00031.2015. [DOI] [PubMed] [Google Scholar]
- 25.Mills JC, Syder AJ, Hong CV, Guruge JL, Raaii F, Gordon JI. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci USA 98: 13687–13692, 2001. doi: 10.1073/pnas.231332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Isozaki K, Kayanoki Y, Kanayama S, Shinomura Y, Taniguchi N, Matsuzawa Y. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology 109: 1051–1059, 1995. doi: 10.1016/0016-5085(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima T, Konda Y, Kanai M, Izumi Y, Kanda N, Nanakin A, Kitazawa S, Chiba T. Prohormone convertase furin has a role in gastric cancer cell proliferation with parathyroid hormone-related peptide in a reciprocal manner. Dig Dis Sci 47: 2729–2737, 2002. doi: 10.1023/A:1021005221934. [DOI] [PubMed] [Google Scholar]
- 28.Nozu T, Takahashi A, Uehara A, Kohgo Y, Suzuki T. Undifferentiated carcinoma in the cardioesophageal junction which produces parathyroid hormone related protein. Intern Med 34: 695–699, 1995. doi: 10.2169/internalmedicine.34.695. [DOI] [PubMed] [Google Scholar]
- 29.Prasov L, Brown NL, Glaser T. A critical analysis of Atoh7 (Math5) mRNA splicing in the developing mouse retina. PLoS One 5: e12315, 2010. doi: 10.1371/journal.pone.0012315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebeiz M, Posakony JW. GenePalette: a universal software tool for genome sequence visualization and analysis. Dev Biol 271: 431–438, 2004. doi: 10.1016/j.ydbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Rozengurt E, Walsh JH. Gastrin, CCK, signaling, and cancer. Annu Rev Physiol 63: 49–76, 2001. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 32.Ryberg B, Tielemans Y, Axelson J, Carlsson E, Håkanson R, Mattson H, Sundler F, Willems G. Gastrin stimulates the self-replication rate of enterochromaffinlike cells in the rat stomach. Effects of omeprazole, ranitidine, and gastrin-17 in intact and antrectomized rats. Gastroenterology 99: 935–942, 1990. doi: 10.1016/0016-5085(90)90610-D. [DOI] [PubMed] [Google Scholar]
- 33.Samuelson LC, Hinkle KL. Insights into the regulation of gastric acid secretion through analysis of genetically engineered mice. Annu Rev Physiol 65: 383–400, 2003. doi: 10.1146/annurev.physiol.65.092101.142213. [DOI] [PubMed] [Google Scholar]
- 34.Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, McLaughlin JT. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 286: G992–G999, 2004. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- 35.Strewler GJ. The physiology of parathyroid hormone-related protein. N Engl J Med 342: 177–185, 2000. doi: 10.1056/NEJM200001203420306. [DOI] [PubMed] [Google Scholar]
- 36.Subramaniam D, Ramalingam S, May R, Dieckgraefe BK, Berg DE, Pothoulakis C, Houchen CW, Wang TC, Anant S. Gastrin-mediated interleukin-8 and cyclooxygenase-2 gene expression: differential transcriptional and posttranscriptional mechanisms. Gastroenterology 134: 1070–1082, 2008. doi: 10.1053/j.gastro.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 37.Sugishita K, Tanno M, Kijima M, Kamoshita H, Mitamura T, Hayakawa K. Malignant hypercalcemia due to gastric endocrine cell carcinoma. Intern Med 34: 104–107, 1995. doi: 10.2169/internalmedicine.34.104. [DOI] [PubMed] [Google Scholar]
- 38.Tsutsui S, Shinomura Y, Higashiyama S, Higashimoto Y, Miyazaki Y, Kanayama S, Hiraoka S, Minami T, Kitamura S, Murayama Y, Miyagawa J, Taniguchi N, Matsuzawa Y. Induction of heparin binding epidermal growth factor-like growth factor and amphiregulin mRNAs by gastrin in the rat stomach. Biochem Biophys Res Commun 235: 520–523, 1997. doi: 10.1006/bbrc.1997.6824. [DOI] [PubMed] [Google Scholar]
- 39.Tu S, Chi AL, Lim S, Cui G, Dubeykovskaya Z, Ai W, Fleming JV, Takaishi S, Wang TC. Gastrin regulates the TFF2 promoter through gastrin-responsive cis-acting elements and multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol 292: G1726–G1737, 2007. doi: 10.1152/ajpgi.00348.2006. [DOI] [PubMed] [Google Scholar]
- 40.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918–1929, 1996. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, L Gumucio D, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274, 2007. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 42.Zhao CM, Kodama Y, Flatberg A, Beisvag V, Kulseng B, Sandvik AK, Rehfeld JF, Chen D. Gene expression profiling of gastric mucosa in mice lacking CCK and gastrin receptors. Regul Pept 192-193: 35–44, 2014. doi: 10.1016/j.regpep.2014.08.002. [DOI] [PubMed] [Google Scholar]