Abstract
Intermediate filament proteins (IFs), such as cytoplasmic keratins in epithelial cells and vimentin in mesenchymal cells and the nuclear lamins, make up one of the three major cytoskeletal protein families. Whether in digestive organs or other tissues, IFs share several unique features including stress-inducible overexpression, abundance, cell-selective and differentiation state expression, and association with >80 human diseases when mutated. Whereas most IF mutations cause disease, mutations in simple epithelial keratins 8, 18, or 19 or in lamin A/C predispose to liver disease with or without other tissue manifestations. Keratins serve major functions including protection from apoptosis, providing cellular and subcellular mechanical integrity, protein targeting to subcellular compartments, and scaffolding and regulation of cell-signaling processes. Keratins are essential for Mallory-Denk body aggregate formation that occurs in association with several liver diseases, whereas an alternate type of keratin and lamin aggregation occurs upon liver involvement in porphyria. IF-associated diseases have no known directed therapy, but high-throughput drug screening to identify potential therapies is an appealing ongoing approach. Despite the extensive current knowledge base, much remains to be discovered regarding IF physiology and pathophysiology in digestive and nondigestive organs.
Keywords: keratins, Mallory-Denk bodies, lamins, liver, intestine
intermediate filament proteins (IFs) make up the largest family among the three major cytoskeletal protein families in mammalian cells (25, 31). IFs include a large group of cytoplasmic and nuclear proteins, encoded by ~70 human genes, that are expressed cell selectively (12, 15, 27). For example, keratins are preferentially expressed in epithelial cells, whereas vimentin is found in mesenchymal cells, glial fibrillary acidic protein (GFAP) in glial and stellate cells, desmin in myocytes (smooth, cardiac, and skeletal), and lamins in the nucleus (Table 1). Of the IF gene family, keratins (K) are the largest subfamily and include 54 genes that encode keratin type I (K9–K28 and K31–K40) and II (K1–K8 and K71–K80) proteins (9, 63, 74). In digestive-type organs, the primary keratins consist of the simple epithelial (i.e., single-layered) keratins K7/K8/K18–K20/K23 (SEK; 52). These SEK, as other IFs, have preferential patterns of expression (Fig. 1A) within the intestinal epithelium (e.g., K20 is found primarily in the more differentiated cells), in ductal vs. nonductal epithelia (e.g., K19 is expressed in biliary epithelia but not in adult hepatocytes), and in metaplastic epithelia as in Barrett's esophagus (9, 51). In polarized epithelia, SEK are located below the apical terminal web region and are anchored to the apical junction complex (31, 60). All IFs are normally distributed in the cytoplasm except for the type V nuclear lamins (Table 1), but accumulating evidence indicates that in some contexts such as cancer, IFs may also be found at low levels in the nucleus (24).
Table 1.
Intermediate filament proteins
| Type | Proteins | Expression Cell Compartment | Examples of Associated Diseases |
|---|---|---|---|
| I | K9–K28; K31–K40 (hair and nails) | Epithelial tissues for K1–K28 | EBS (K5/K14); predisposition to acute or chronic liver disease (K8/K18/K19) |
| II | K1–K8; K71–K86 (hair and nails) | (obligate type I–II heteropolymers) | |
| III | Vimentin | Mesenchymal cells, including lens | Cataracts |
| GFAP | Glial cells | Alexander disease | |
| Desmin | Muscle cells | Desmin-related myopathy | |
| Syncoilin | Muscle cells | Unknown | |
| Peripherin | Peripheral neurons | Amyotrophic later sclerosis | |
| IV | Neurofilaments (light, medium, and heavy: NF-L, NF-M, and NF-H) | CNS neurons | CMT type 2; amyotrophic lateral sclerosis (predisposition) |
| α-Internexin | CNS neurons | Unknown | |
| Nestin | Stem and neuroepithelial cells | Unknown | |
| Synemin | Muscle cells | Unknown | |
| V | Lamins | Nuclei | FPLD2 (lamin A/C); APL (lamin B2) |
| VI | Bfsp1 (filensin); Bfsp2 (CP49) | Eye lens | Juvenile-onset cataracts |
For some of the IFs, only a few examples of associated diseases are listed (e.g., many other keratinopathies and laminopathies are not included, but none of these disorders are known to involve digestive organs).
APL, acquired partial lipodystrophy; Bfsp, beaded filament structural protein; CMT, Charcot-Marie-Tooth disease; CNS, central nervous system; CP49, cytoskeletal protein 49 kDa; EBS, epidermolysis bullosa simplex; FPLD2, Dunnigan familial partial lipodystrophy type 2; K, keratin.
Fig. 1.
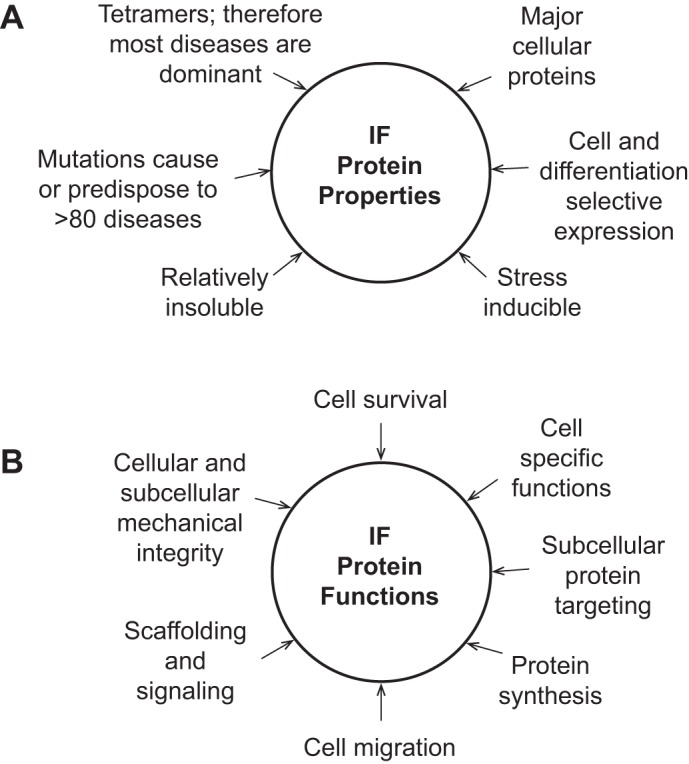
Properties and functions of intermediate filament proteins. The schematic lists well-defined properties (A) and functions (B) for IFs. Some examples of the IF-associated diseases are listed in Table 1.
IFs, as a group, share several unique properties (Fig. 1A). For example, despite the fact that keratins make up 0.2–0.5% of total cellular proteins in hepatocytes and enterocytes (87), they are stress inducible, and their protein and mRNA levels increase severalfold as noted during regenerative repair in the pancreas, toxin exposure and oxidative stress in the liver, and interleukin-6 simulation in the intestine (17, 75, 82, 87). Aside from induction of endogenous IFs during cell stress, expression of “new” IFs is also a hallmark of epithelial-to-mesenchymal transition during cancer progression in most cancers including gastrointestinal tumors (40, 61). IFs include dynamic and interchangeable filamentous and soluble pools, with the latter making up nearly 5% of the total K8/K18 fraction in cultured human colonic HT29 cells (52). Interchange between the soluble and insoluble IF pools is regulated by posttranslational modifications (PTM) such as phosphorylation and acetylation (66) and by interaction with a growing list of IF-associated proteins (67). IFs are obligate noncovalent tetramers that are either heterotetramers (e.g., 2 type I and 2 type II keratin molecules) or homotetramers (e.g., vimentin or desmin; 12, 63).
IFs also perform several important subcellular, cellular, and tissue functions that, in part, reflect their cell-selective expression (Fig. 1B). One critical IF function is to provide cellular and subcellular mechanical integrity, which at the cellular level is evident from the cell fragility disorders of the skin that are caused by epidermal keratin mutations (12, 27, 39). Similarly, hepatocytes are remarkably fragile when isolated by liver perfusion in the context of K18 mutation or absence of keratins (29, 38, 52). At the subcellular level, IFs play important roles in nuclear and mitochondrial shape and function (40, 52, 60, 72) and in the targeting of proteins to the apical and other subcellular compartments and maintenance of the epithelial barrier (52, 60). Another key function for IFs is to protect cells from apoptosis as has been clearly demonstrated for K8/K18 in the liver whereby keratin-null hepatocytes are markedly more susceptible to cell death compared with wild-type cells (9, 30, 43, 52). However, the importance of keratins as antiapoptotic proteins is context dependent since K8-null enterocytes are paradoxically more resistant to apoptosis compared with their wild-type counterparts and this resistance reverts to normal after treatment of the mice with antibiotics (20). Other important functions for IFs (Fig. 1B) include their role in cell migration (40, 61), modulation of protein synthesis (28), and serving as signaling scaffolds (9, 52, 57).
Intermediate Filament Genetic Variants in Digestive Disease
In contrast to most of the IF-associated diseases [IF-pathies (53)], whereby IF mutations have near-complete penetrance to cause disease, mutations in SEK predispose to disease, rather than precipitate disease per se (52, 54, 55). Among digestive organs, the liver is the primary involved organ, which is supported by multiple experimental genetic models, while other organs such as the intestine, pancreas, and stomach appear to be spared. The reason for this is that K8 and K18 are the only IFs expressed in adult hepatocytes whereas epithelia in other digestive organs express K7, K19, or K20 in addition to K8/K18 (52).
In terms of specific digestive organs, human K8 mutations appear not to be involved as either a cause or predisposition to pancreatitis or pancreatic cancer (62, 80), albeit a K8 transgenic overexpression model developed spontaneous pancreatitis and acinar cell dysplasia (5). However, the latter finding is likely due to massive keratin overexpression in the studied pancreata (5, 76). In addition, mice that lack K8 or K18, or overexpress mutant K18, are equally susceptible to experimental pancreatitis compared with wild-type mice (77, 78). Although genetic studies have not examined a potential role of K18 or K19 in pancreatitis, it is likely that the pancreas is not a major disease target for keratin mutations given the presence of other keratins in acinar and ductal cells and the likely protective induction of Reg2 (a member of the regenerating islet-derived stress-inducible family of proteins) in the context of keratin mutation (88). Redundancy in keratin expression and function is also a reason why keratin variants are unlikely to play a major direct role in intestinal human disease such as inflammatory bowel disease (IBD; 73). This is despite a profound ulcerative colitis-like phenotype in mice that lack K8 (2, 21). Even though potential keratin variant association with IBD has been described (56), the number of patients in that study was relatively small, and numerous genomewide association studies (44) and other large studies (73) have not strongly implicated keratin variants in IBD.
The strongest disease association of SEK, specifically K8/K18/K19, is predisposition to acute and chronic liver disease progression in carriers of select keratin variants (9, 52, 54, 68, 74, 81, 89). As such, K8, K18, and K19 variants have been associated with several acute and chronic liver diseases. Many of these variants are rare (<0.1% frequency) though some are more frequent (1–5%), and it appears that these variants are silent unless the liver is challenged by an underlying viral or autoimmune chronic liver disease or acutely with a hepatotoxin such as acetaminophen (68) or isoniazid (81).
Aside from keratins, mutations in LMNA (the gene encoding the alternatively spliced lamin A and C proteins) result in a wide range of diseases that cause myopathy, neuropathy, premature aging, or lipodystrophy (4, 15, 85). Although lamin A/C is expressed in most differentiated tissues, its select organ disease manifestation is likely related to lamin mutation-related alteration in tissue-specific lamin-binding partners (85). Liver involvement occurs in lamin A/C mutations that cause partial lipodystrophy and metabolic syndrome including the Dunnigan familial partial lipodystrophy (FPLD2; typically heterozygous Arg482 substitutions with Trp, Gln, or Leu), with hepatosteatosis that progresses to nonalcoholic steatohepatitis in some patients (1, 16, 41). Liver involvement has also been described in a patient with acquired partial lipodystrophy (APL) harboring a mutation in LMNB2, which encodes lamin B2 (13). It is probable that liver involvement was present in the first association of lamin B2 mutations in four of nine patients with APL, described by Hegele et al., though liver involvement was not discussed (22).
Intermediate Filament Aggregation in the Context of Mallory-Denk Bodies and Porphyria
Keratins are critical for the formation of Mallory-Denk bodies (MDB), on the basis of extensive studies performed in genetic animal models (52, 69, 86). Formation of MDB aggregates occurs in the context of several liver diseases, particularly alcoholic and nonalcoholic liver disease (69, 86). K8 and K18 form the major protein components of MDB, and induction of a K8-to-K18 ratio >1 primarily in the context of steatohepatitis is essential for their formation (18, 52, 86). The significance of selective upregulation of K8 is that it serves as a substrate for transglutaminase 2, which, in turn, cross-links K8 via Lys-Gln isopeptide bond formation to several other proteins (32, 70). Keratin phosphorylation is also necessary for MDB formation since mice that overexpress the phosphomutant K8 S74A (or overexpress the natural human variant G62C that leads to a conformational change that blocks kinase access to K8 S74) have a markedly decreased ability to form MDB (32). Notably, K8 G62C is a natural human variant that predisposes to liver disease in humans (52, 68) and mice (19, 52).
Keratins and lamins also aggregate upon exposure to protoporphyrin IX and other porphyrins (11, 42, 65). Lamins A/C and B1 appear to be more sensitive than keratins to protoporphyrin IX-mediated aggregation in livers of mice harboring a ferrochelatase mutation or mice treated with the porphyrinogenic and MDB-inducing compound 3,5-diethoxycarbonyl-1,4-dihydrocollidine (65, 86). Most of the biochemically detectable aggregation is light induced and occurs upon exposure of cell or tissue lysates to light in the presence of porphyrins (11, 42). However, porphyrins can also cause end-stage liver disease in patients (64), clearly independent of light, and in this context there is also evidence of porphyrin-mediated light-independent protein aggregation (42). It remains to be determined whether porphyrins cause keratin and lamin aggregation (and likely other IFs such as GFAP in stellate cells) in liver by forming covalent adducts (which have not been described to date for any porphyrin) or via other mechanisms such as free radical-mediated protein alterations. The mode of porphyrin-mediated protein aggregation, the physiological consequences, and the involved proteins are different from what occurs in MDB (e.g., the nuclear changes that are associated with porphyria). It remains to be determined whether some of the porphyria manifestations, as found in the skin and internal organs (e.g., abdominal pain), are directly related to the observed porphyrin-induced protein aggregation.
Intermediate Filaments as Biomarkers of Cell Death and as Differentiation and Cancer Markers in Digestive Disorders
Most IFs, including type I but not type II keratins, are substrates for caspases during apoptosis-associated injury in experimental models or humans, with release of apoptotic fragments into cell culture media or the bloodstream (30, 43, 52). One likely consequence of caspase-mediated keratin cleavage during apoptosis is to allow the keratin filaments to reorganize with subsequent generation of apoptotic bodies. This is based on the inability of K8/K18 filaments to reorganize, and shunting of hepatocytes to necrosis rather than apoptosis, upon Fas ligand stimulation of transgenic mice (or hepatocytes ex vivo) that express caspase cleavage-resistant K18 (due to K18 mutation at its 2 caspase cut sites; 83).
SEK are also released upon necrosis and cell death in epithelial tumors (30). Of note, type II keratins (e.g., K7 and K8) are resistant to digestion by caspases whereas type I keratins (e.g., K18 and K19) generate readily detectable fragments due to the abundance of keratins (30). In acute and chronic liver injury alone, there have been >130 studies that assessed the association of keratin fragments with liver disease (30). Aside from pathological conditions, there is also extensive apoptotic activity and caspase digestion of K18 during normal fetal development (37). Although there are several limitations in the current assays that measure various serum K8/K18/K19 levels, including the lack of an assay that measures the product formed after cleavage at the second K18 caspase cut site (also conserved in K19), the utilization of such assays remains a useful adjunct for the assessment of liver injury and possibly other digestive organ damage (6, 30).
Even within simple epithelia, the somewhat selective expression of SEK renders them useful markers particularly for defining normal differentiation states as well as the cell origin of cancers and their metastases (since tumors generally maintain their original keratin expression patterns; 47). For example, K20 is expressed upon terminal differentiation and is found most prominently in small intestine villus-lining and colon surface-lining epithelial cells (aside from being found in other cell types such as gastric foveolar cells and the urothelium; 47). In addition, specific keratin staining using well-established antibodies is routinely done worldwide as a histopathologic diagnostic aide (45, 47), including use in assessing circulating tumor cells in patients with colorectal cancer (84). Furthermore, keratins may play a modulatory role in blunting the development of colon cancer though this possibility remains to be explored further. For example, K8-null mice, which develop spontaneous colitis and hyperproliferation (21), are predisposed to developing colonic tumors [compared with their K8(+/+) and K8(+/−) counterparts] upon treatment with azoxymethane or breeding with Apc(Min/+) mice (46).
Potential Therapeutic Approaches
Although major advances have been made in linking mutations in IF genes to >80 IF-pathies, there remains no specific directed current treatment for any IF-pathy, and in contrast with microfilaments and microtubules there are no known small molecules that interact directly with IFs. For digestive organ-associated IF-pathies, treatment can be envisioned and justified for severe acute liver failure whereby K8/K18 variants pose mainly a predisposition that manifests in a full-blown setting after a second hit such as exposure to a liver toxin. However, one potential challenge is that nearly 25 K8/K18 variants have been identified to date (52, 68), and it is possible that individualized targeted therapy will be needed depending on the pathogenesis of the mutation. In this context, the use of RNA therapeutics that specifically target the mutant allele is an attractive possibility (35). Our approach has been to focus on identifying compounds that can normalize the effect of K18 filament disrupting mutations as a proof of concept and a model keratin that could potentially extend to other IFs (71). Using this approach, the pan-kinase inhibitor PKC412 was identified as a compound that normalizes mutant K18 R90C disrupted filaments in cultured cells and in transgenic mice (33). This occurs by dephosphorylation of the keratin-binding protein nonmuscle myosin IIA, which, in turn, promotes its binding to K8/K18 and stabilizing the keratin filaments (33). One advantage of this unbiased drug-screening approach is that it has the potential to illuminate novel keratin and other IF signaling pathways or interactions that would otherwise be difficult to elucidate (33, 71).
One of the major challenges in identifying therapies for IF-pathies is that what works in cell culture and in animal models has not been effective in humans as noted in more than one drug trial for the Hutchinson-Gilford progeria syndrome that is caused by lamin A/C mutation (8). However, current efforts aimed at unbiased drug screening (36) or targeting aberrant signaling pathways that are triggered by lamin A/C mutation (48) are promising and may be of benefit in FPLD2 or APL that also involve the liver.
Closing Perspective
Tremendous gains have been made in the IF field since the first link of IFs to human disease was made for epidermal keratins in 1991 (12). The IF field remains exciting and wide open for many new important discoveries, and hopefully newcomers will join the field. Areas that remain to be explored span the spectrum of fundamental biophysical, structural, and biochemical understanding of IFs and their PTM (7, 23, 66) to disease association, diagnosis, and therapy (8, 33, 35, 36, 48, 71). For example, determining the crystal structure of IFs has been very challenging because of their inherent structure, and only subdomains of some of the IFs have been crystallized (7, 34). In terms of the quest for therapies for the IF-pathies and potential compound screening, the use of organism models that have a rapid generation time and genetic tools, such as Drosophila (3), is likely to be beneficial.
From the disease perspective, there are many gaps that remain to be filled including the following: 1) It would be relevant to define the disease association for several “orphan” IFs that have not been directly linked as a cause or predisposition to human disease (e.g., for SEK: K7, K20, and K23). 2) It would be germane to determine whether known or novel keratin variants that have been associated with a human disease (e.g., in K8, K18, K4, and K13) are involved as genetic modifiers of additional diseases. This includes gastric or intestinal disorders for K8/K18 and esophageal disorders for K4/K13 (among other keratins). In the liver, potential association of K8/K18 variants with steatohepatitis or with liver transplant failure is deserving of study. In the esophagus, K4 and K13 are the major keratins in the suprabasal layer (though they are also found in the oral mucosa; 14). K4/K13 mutation is only known as the cause of white sponge nevus syndrome (58, 59) yet the phenotype of K4-null mice in the esophagus is more extensive (49) and includes nuclear atypia. Therefore much more remains to be learned regarding keratin function and disease association in the esophagus. 3) It would be relevant to determine the pathophysiologic role of keratins that have been minimally studied such as the recent characterization of K23 during mouse and human biliary cell activation (17). 4) It would be important to define the digestive disease association of nonepithelial IFs such as vimentin, GFAP, and neurofilaments. For example, it is possible that variants of nonepithelial IFs could modulate gastrointestinal disorders in a noncanonical fashion. For example, GFAP is the major IF of glial cells, and it is increasingly evident that glial cells are likely to play pleiotropic roles in the enteric nervous system (50). Similarly, the functional roles of vimentin in epithelial tumor metastasis and epithelial-to-mesenchymal transition remain poorly understood (26, 61).
Other relevant areas of future study include a better understanding of the nature and utility of IF proteolytic fragments in different digestive disorders (30), the potential role of IFs in regulating the microbiome or regulation by the microbiome (20), the pathophysiologic role of IFs as stress proteins (75), which includes enhancing our limited understanding of their transcriptional control, and the subcellular and organellar functions of IFs (72, 79). Overall, there is plenty of room to expand on what is known regarding IF properties, functions, and disease association (Fig. 1 and Table 1). IFs remain exciting to study in both digestive and nondigestive organ contexts, with many to-be-made discoveries.
GRANTS
Our work has been supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants 47918 and 52951, Department of Veterans Affairs, and institutional NIDDK Grants 34933 to the University of Michigan and 56339 to Stanford University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.B.O. prepared figures, drafted manuscript, edited and revised manuscript, and approved final version of manuscript.
ACKNOWLEDGMENTS
This article was invited as part of a series highlighting Experimental Biology 2016: San Diego, CA. Dr. Omary was the 2016 recipient of the Gastrointestinal and Liver Physiology Section Horace W. Davenport Distinguished Lectureship.
I am very humbled by my selection to give the 2016 Horace W. Davenport Lecture, and I thank Dr. Nigel Bunnett for the opportunity to write this Mini-Review to commemorate the Davenport Lecture. The honor is extra special because Dr. Davenport was not only a giant in the field of gastrointestinal physiology but also chairman of the same department that I now have the privilege to serve. I have been fortunate to work with outstanding past and current trainees, many of whom now have their independent research programs or are equally successful pursuing other endeavors. I also thank the patients, their families, and other donors who have provided biospecimens, without which the disease-related aspects of studies described herein would have been impossible to uncover.
REFERENCES
- 1.Ajluni N, Meral R, Neidert AH, Brady GF, Buras E, McKenna B, DiPaola F, Chenevert TL, Horowitz JF, Buggs-Saxton C, Rupani AR, Thomas PE, Tayeh MK, Innis JW, Omary MB, Conjeevaram H, Oral EA. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf) 86: 698–707, 2017. doi: 10.1111/cen.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 8: 2964–2973, 1994. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- 3.Bohnekamp J, Cryderman DE, Thiemann DA, Magin TM, Wallrath LL. Using Drosophila for studies of intermediate filaments. Methods Enzymol 568: 707–726, 2016. doi: 10.1016/bs.mie.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet 28: 464–471, 2012. doi: 10.1016/j.tig.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova ML, Bravo A, Ramírez A, Morreale de Escobar G, Were F, Merlino G, Vidal M, Jorcano JL. Exocrine pancreatic disorders in transsgenic mice expressing human keratin 8. J Clin Invest 103: 1587–1595, 1999. doi: 10.1172/JCI5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castera L. Noninvasive evaluation of nonalcoholic fatty liver disease. Semin Liver Dis 35: 291–303, 2015. doi: 10.1055/s-0035-1562948. [DOI] [PubMed] [Google Scholar]
- 7.Chernyatina AA, Guzenko D, Strelkov SV. Intermediate filament structure: the bottom-up approach. Curr Opin Cell Biol 32: 65–72, 2015. doi: 10.1016/j.ceb.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Collins FS. Seeking a cure for one of the rarest diseases: progeria. Circulation 134: 126–129, 2016. doi: 10.1161/CIRCULATIONAHA.116.022965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol 14: 110–122, 2002. doi: 10.1016/S0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- 10.Davidson PM, Lammerding J. Broken nuclei: lamins, nuclear mechanics, and disease. Trends Cell Biol 24: 247–256, 2014. doi: 10.1016/j.tcb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elenbaas JS, Maitra D, Liu Y, Lentz SI, Nelson B, Hoenerhoff MJ, Shavit JA, Omary MB. A precursor-inducible zebrafish model of acute protoporphyria with hepatic protein aggregation and multiorganelle stress. FASEB J 30: 1798–1810, 2016. doi: 10.1096/fj.201500111R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem 63: 345–382, 1994. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 13.Gao J, Li Y, Fu X, Luo X. A Chinese patient with acquired partial lipodystrophy caused by a novel mutation with LMNB2 gene. J Pediatr Endocrinol Metab 25: 375–377, 2012. doi: 10.1515/jpem-2012-0007. [DOI] [PubMed] [Google Scholar]
- 14.Grace MP, Kim KH, True LD, Fuchs E. Keratin expression in normal esophageal epithelium and squamous cell carcinoma of the esophagus. Cancer Res 45: 841–846, 1985. [PubMed] [Google Scholar]
- 15.Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 84: 131–164, 2015. doi: 10.1146/annurev-biochem-060614-034115. [DOI] [PubMed] [Google Scholar]
- 16.Guénantin AC, Briand N, Bidault G, Afonso P, Béréziat V, Vatier C, Lascols O, Caron-Debarle M, Capeau J, Vigouroux C. Nuclear envelope-related lipodystrophies. Semin Cell Dev Biol 29: 148–157, 2014. doi: 10.1016/j.semcdb.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Guldiken N, Kobazi Ensari G, Lahiri P, Couchy G, Preisinger C, Liedtke C, Zimmermann HW, Ziol M, Boor P, Zucman-Rossi J, Trautwein C, Strnad P. Keratin 23 is a stress-inducible marker of mouse and human ductular reaction in liver disease. J Hepatol 65: 552–559, 2016. doi: 10.1016/j.jhep.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Guldiken N, Usachov V, Levada K, Trautwein C, Ziol M, Nahon P, Strnad P. Keratins 8 and 18 are type II acute-phase responsive genes overexpressed in human liver disease. Liver Int 35: 1203–1212, 2015. doi: 10.1111/liv.12608. [DOI] [PubMed] [Google Scholar]
- 19.Guldiken N, Zhou Q, Kucukoglu O, Rehm M, Levada K, Gross A, Kwan R, James LP, Trautwein C, Omary MB, Strnad P. Human keratin 8 variants promote mouse acetaminophen hepatotoxicity coupled with c-jun amino-terminal kinase activation and protein adduct formation. Hepatology 62: 876–886, 2015. doi: 10.1002/hep.27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habtezion A, Toivola DM, Asghar MN, Kronmal GS, Brooks JD, Butcher EC, Omary MB. Absence of keratin 8 confers a paradoxical microflora-dependent resistance to apoptosis in the colon. Proc Natl Acad Sci USA 108: 1445–1450, 2011. doi: 10.1073/pnas.1010833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci 118: 1971–1980, 2005. doi: 10.1242/jcs.02316. [DOI] [PubMed] [Google Scholar]
- 22.Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, Rodger NW, Durrington PN. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet 79: 383–389, 2006. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann H, Aebi U. Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol 8: a018242, 2016. doi: 10.1101/cshperspect.a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs RP, Jacob JT, Coulombe PA. Keratins are going nuclear. Dev Cell 38: 227–233, 2016. doi: 10.1016/j.devcel.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber F, Boire A, López MP, Koenderink GH. Cytoskeletal crosstalk: when three different personalities team up. Curr Opin Cell Biol 32: 39–47, 2015. doi: 10.1016/j.ceb.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50: 1–6, 2014. doi: 10.1165/rcmb.2013-0314TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev 21: 1581–1597, 2007. doi: 10.1101/gad.1552107. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, Coulombe PA. Emerging role for the cytoskeleton as an organizer and regulator of translation. Nat Rev Mol Cell Biol 11: 75–81, 2010. doi: 10.1038/nrm2818. [DOI] [PubMed] [Google Scholar]
- 29.Ku N-O, Michie S, Oshima RG, Omary MB. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol 131: 1303–1314, 1995. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku NO, Strnad P, Bantel H, Omary MB. Keratins: biomarkers and modulators of apoptotic and necrotic cell death in the liver. Hepatology 64: 966–976, 2016. doi: 10.1002/hep.28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ku N-O, Zhou X, Toivola DM, Omary MB. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol Gastrointest Liver Physiol 277: G1108–G1137, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kwan R, Hanada S, Harada M, Strnad P, Li DH, Omary MB. Keratin 8 phosphorylation regulates its transamidation and hepatocyte Mallory-Denk body formation. FASEB J 26: 2318–2326, 2012. doi: 10.1096/fj.11-198580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan R, Chen L, Looi K, Tao G-Z, Weerasinghe SV, Snider NT, Conti MA, Adelstein RS, Xie Q, Omary MB. PKC412 normalizes mutation-related keratin filament disruption and hepatic injury in mice by promoting keratin-myosin binding. Hepatology 62: 1858–1869, 2015. doi: 10.1002/hep.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CH, Kim MS, Chung BM, Leahy DJ, Coulombe PA. Structural basis for heteromeric assembly and perinuclear organization of keratin filaments. Nat Struct Mol Biol 19: 707–715, 2012. doi: 10.1038/nsmb.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JM, Nobumori C, Tu Y, Choi C, Yang SH, Jung HJ, Vickers TA, Rigo F, Bennett CF, Young SG, Fong LG. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest 126: 1592–1602, 2016. doi: 10.1172/JCI85908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Jung YS, Yoon MH, Kang SM, Oh AY, Lee JH, Jun SY, Woo TG, Chun HY, Kim SK, Chung KJ, Lee HY, Lee K, Jin G, Na MK, Ha NC, Bárcena C, Freije JM, López-Otín C, Song GY, Park BJ. Interruption of progerin-lamin A/C binding ameliorates Hutchinson-Gilford progeria syndrome phenotype. J Clin Invest 126: 3879–3893, 2016. doi: 10.1172/JCI84164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leers MP, Björklund V, Björklund B, Jörnvall H, Nap M. An immunohistochemical study of the clearance of apoptotic cellular fragments. Cell Mol Life Sci 59: 1358–1365, 2002. doi: 10.1007/s00018-002-8513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loranger A, Duclos S, Grenier A, Price J, Wilson-Heiner M, Baribault H, Marceau N. Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pathol 151: 1673–1683, 1997. [PMC free article] [PubMed] [Google Scholar]
- 39.Loschke F, Seltmann K, Bouameur JE, Magin TM. Regulation of keratin network organization. Curr Opin Cell Biol 32: 56–64, 2015. doi: 10.1016/j.ceb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem 290: 17145–17153, 2015. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lüdtke A, Genschel J, Brabant G, Bauditz J, Taupitz M, Koch M, Wermke W, Worman HJ, Schmidt HH. Hepatic steatosis in Dunnigan-type familial partial lipodystrophy. Am J Gastroenterol 100: 2218–2224, 2005. doi: 10.1111/j.1572-0241.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 42.Maitra D, Elenbaas JS, Whitesall SE, Basrur V, D'Alecy LG, Omary MB. Ambient light promotes selective subcellular proteotoxicity after endogenous and exogenous porphyrinogenic stress. J Biol Chem 290: 23711–23724, 2015. doi: 10.1074/jbc.M114.636001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marceau N, Schutte B, Gilbert S, Loranger A, Henfling ME, Broers JL, Mathew J, Ramaekers FC. Dual roles of intermediate filaments in apoptosis. Exp Cell Res 313: 2265–2281, 2007. doi: 10.1016/j.yexcr.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 44.McGovern DP, Kugathasan S, Cho JH. Genetics of inflammatory bowel diseases. Gastroenterology 149: 1163–1176.e2, 2015. doi: 10.1053/j.gastro.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miettinen M. Immunohistochemistry of soft tissue tumours - review with emphasis on 10 markers. Histopathology 64: 101–118, 2014. doi: 10.1111/his.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Misiorek JO, Lähdeniemi IA, Nyström JH, Paramonov VM, Gullmets JA, Saarento H, Rivero-Müller A, Husøy T, Taimen P, Toivola DM. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis 37: 777–786, 2016. doi: 10.1093/carcin/bgw063. [DOI] [PubMed] [Google Scholar]
- 47.Moll R, Divo M, Langbein L. The human keratins: biology and pathology. Histochem Cell Biol 129: 705–733, 2008. doi: 10.1007/s00418-008-0435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muchir A, Worman HJ. Targeting mitogen-activated protein kinase signaling in mouse models of cardiomyopathy caused by lamin A/C gene mutations. Methods Enzymol 568: 557–580, 2016. doi: 10.1016/bs.mie.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ness SL, Edelmann W, Jenkins TD, Liedtke W, Rustgi AK, Kucherlapati R. Mouse keratin 4 is necessary for internal epithelial integrity. J Biol Chem 273: 23904–23911, 1998. doi: 10.1074/jbc.273.37.23904. [DOI] [PubMed] [Google Scholar]
- 50.Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, De Giorgio R. Enteric glial cells: recent developments and future directions. Gastroenterology 147: 1230–1237, 2014. doi: 10.1053/j.gastro.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 51.Nurgalieva Z, Lowrey A, El-Serag HB. The use of cytokeratin stain to distinguish Barrett's esophagus from contiguous tissues: a systematic review. Dig Dis Sci 52: 1345–1354, 2007. doi: 10.1007/s10620-006-9399-3. [DOI] [PubMed] [Google Scholar]
- 52.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest 119: 1794–1805, 2009. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omary MB. ‘IF-pathies’: a broad spectrum of intermediate filament-associated diseases. J Clin Invest 119: 1756–1762, 2009. doi: 10.1172/JCI39894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omary MB, Coulombe PA, McLean WHI. Intermediate filament proteins and their associated diseases. N Engl J Med 351: 2087–2100, 2004. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 55.Owens DW, Lane EB. The quest for the function of simple epithelial keratins. BioEssays 25: 748–758, 2003. doi: 10.1002/bies.10316. [DOI] [PubMed] [Google Scholar]
- 56.Owens DW, Wilson NJ, Hill AJ, Rugg EL, Porter RM, Hutcheson AM, Quinlan RA, van Heel D, Parkes M, Jewell DP, Campbell SS, Ghosh S, Satsangi J, Lane EB. Human keratin 8 mutations that disturb filament assembly observed in inflammatory bowel disease patients. J Cell Sci 117: 1989–1999, 2004. doi: 10.1242/jcs.01043. [DOI] [PubMed] [Google Scholar]
- 57.Pallari HM, Eriksson JE. Intermediate filaments as signaling platforms. Sci STKE 2006: pe53, 2006. doi: 10.1126/stke.3662006pe53. [DOI] [PubMed] [Google Scholar]
- 58.Richard G, De Laurenzi V, Didona B, Bale SJ, Compton JG. Keratin 13 point mutation underlies the hereditary mucosal epithelial disorder white sponge nevus. Nat Genet 11: 453–455, 1995. doi: 10.1038/ng1295-453. [DOI] [PubMed] [Google Scholar]
- 59.Rugg EL, McLean WH, Allison WE, Lunny DP, Macleod RI, Felix DH, Lane EB, Munro CS. A mutation in the mucosal keratin K4 is associated with oral white sponge nevus. Nat Genet 11: 450–452, 1995. doi: 10.1038/ng1295-450. [DOI] [PubMed] [Google Scholar]
- 60.Salas PJ, Forteza R, Mashukova A. Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers 4: e1178368, 2016. doi: 10.1080/21688370.2016.1178368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68: 3033–3046, 2011. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider A, Lamb J, Barmada MM, Cuneo A, Money ME, Whitcomb DC. Keratin 8 mutations are not associated with familial, sporadic and alcoholic pancreatitis in a population from the United States. Pancreatology 6: 103–108, 2006. doi: 10.1159/000090029. [DOI] [PubMed] [Google Scholar]
- 63.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DAD, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol 174: 169–174, 2006. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singal AK, Parker C, Bowden C, Thapar M, Liu L, McGuire BM. Liver transplantation in the management of porphyria. Hepatology 60: 1082–1089, 2014. doi: 10.1002/hep.27086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singla A, Griggs NW, Kwan R, Snider NT, Maitra D, Ernst SA, Herrmann H, Omary MB. Lamin aggregation is an early sensor of porphyria-induced liver injury. J Cell Sci 126: 3105–3112, 2013. doi: 10.1242/jcs.123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol 15: 163–177, 2014. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonnenberg A, Wilson KL. Preface. Methods Enzymol 569: xix–xx, 2016. doi: 10.1016/S0076-6879(15)00666-7. [DOI] [PubMed] [Google Scholar]
- 68.Strnad P, Zhou Q, Hanada S, Lazzeroni LC, Zhong BH, So P, Davern TJ, Lee WM; Acute Liver Failure Study Group, Omary MB. Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology 139: 828–835, 2010. doi: 10.1053/j.gastro.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strnad P, Nuraldeen R, Guldiken N, Hartmann D, Mahajan V, Denk H, Haybaeck J. Broad spectrum of hepatocyte inclusions in humans, animals, and experimental models. Compr Physiol 3: 1393–1436, 2013. doi: 10.1002/cphy.c120032. [DOI] [PubMed] [Google Scholar]
- 70.Strnad P, Harada M, Siegel M, Terkeltaub RA, Graham RM, Khosla C, Omary MB. Transglutaminase 2 regulates Mallory body inclusion formation and injury-associated liver enlargement. Gastroenterology 132: 1515–1526, 2007. doi: 10.1053/j.gastro.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Sun J, Groppi VE, Gui H, Chen L, Xie Q, Liu L, Omary MB. High-throughput screening for drugs that modulate intermediate filament proteins. Methods Enzymol 568: 163–185, 2016. doi: 10.1016/bs.mie.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tao GZ, Looi KS, Toivola DM, Strnad P, Zhou Q, Liao J, Wei Y, Habtezion A, Omary MB. Keratins modulate the shape and function of hepatocyte mitochondria: a mechanism for protection from apoptosis. J Cell Sci 122: 3851–3855, 2009. doi: 10.1242/jcs.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tao G-Z, Strnad P, Zhou Q, Kamal A, Zhang L, Madani ND, Kugathasan S, Brant SR, Cho JH, Omary MB, Duerr RH. Analysis of keratin polypeptides 8 and 19 variants in inflammatory bowel disease. Clin Gastroenterol Hepatol 5: 857–864, 2007. doi: 10.1016/j.cgh.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr Opin Cell Biol 32: 73–81, 2015. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Toivola DM, Strnad P, Habtezion A, Omary MB. Intermediate filaments take the heat as stress proteins. Trends Cell Biol 20: 79–91, 2010. doi: 10.1016/j.tcb.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toivola DM, Nakamichi I, Strnad P, Michie SA, Ghori N, Harada M, Zeh K, Oshima RG, Baribault H, Omary MB. Keratin overexpression levels correlate with the extent of spontaneous pancreatic injury. Am J Pathol 172: 882–892, 2008. doi: 10.2353/ajpath.2008.070830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Toivola DM, Baribault H, Magin T, Michie SA, Omary MB. Simple epithelial keratins are dispensable for cytoprotection in two pancreatitis models. Am J Physiol Gastrointest Liver Physiol 279: G1343–G1354, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Toivola DM, Ku N-O, Ghori N, Lowe AW, Michie SA, Omary MB. Effects of keratin filament disruption on exocrine pancreas-stimulated secretion and susceptibility to injury. Exp Cell Res 255: 156–170, 2000. doi: 10.1006/excr.1999.4787. [DOI] [PubMed] [Google Scholar]
- 79.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol 164: 911–921, 2004. doi: 10.1083/jcb.200308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Treiber M, Schulz HU, Landt O, Drenth JP, Castellani C, Real FX, Akar N, Ammann RW, Bargetzi M, Bhatia E, Demaine AG, Battagia C, Kingsnorth A, O'Reilly D, Truninger K, Koudova M, Spicak J, Cerny M, Menzel HJ, Moral P, Pignatti PF, Romanelli MG, Rickards O, De Stefano GF, Zarnescu NO, Choudhuri G, Sikora SS, Jansen JB, Weiss FU, Pietschmann M, Teich N, Gress TM, Ockenga J, Schmidt H, Kage A, Halangk J, Rosendahl J, Groneberg DA, Nickel R, Witt H. Keratin 8 sequence variants in patients with pancreatitis and pancreatic cancer. J Mol Med (Berl) 84: 1015–1022, 2006. doi: 10.1007/s00109-006-0096-7. [DOI] [PubMed] [Google Scholar]
- 81.Usachov V, Urban TJ, Fontana RJ, Gross A, Iyer S, Omary MB, Strnad P; Drug-Induced Liver Injury Network . Prevalence of genetic variants of keratins 8 and 18 in patients with drug-induced liver injury. BMC Med 13: 196, 2015. doi: 10.1186/s12916-015-0418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem 282: 8219–8227, 2007. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- 83.Weerasinghe SVW, Ku N-O, Altshuler PJ, Kwan R, Omary MB. Mutation of caspase-digestion sites in keratin 18 interferes with filament reorganization, and predisposes to hepatocyte necrosis and loss of membrane integrity. J Cell Sci 127: 1464–1475, 2014. doi: 10.1242/jcs.138479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welinder C, Jansson B, Lindell G, Wenner J. Cytokeratin 20 improves the detection of circulating tumor cells in patients with colorectal cancer. Cancer Lett 358: 43–46, 2015. doi: 10.1016/j.canlet.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 85.Worman HJ, Schirmer EC. Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol 34: 101–112, 2015. doi: 10.1016/j.ceb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res 313: 2033–2049, 2007. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 87.Zhong B, Zhou Q, Toivola DM, Tao G-Z, Resurreccion EZ, Omary MB. Organ-specific stress induces mouse pancreatic keratin overexpression in association with NF-κB activation. J Cell Sci 117: 1709–1719, 2004. doi: 10.1242/jcs.01016. [DOI] [PubMed] [Google Scholar]
- 88.Zhong B, Strnad P, Toivola DM, Tao GZ, Ji X, Greenberg HB, Omary MB. Reg-II is an exocrine pancreas injury-response product that is up-regulated by keratin absence or mutation. Mol Biol Cell 18: 4969–4978, 2007. doi: 10.1091/mbc.E07-02-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhong B, Strnad P, Selmi C, Invernizzi P, Tao G-Z, Caleffi A, Chen M, Bianchi I, Podda M, Pietrangelo A, Gershwin ME, Omary MB. Keratin variants are overrepresented in primary biliary cirrhosis and associate with disease severity. Hepatology 50: 546–554, 2009. doi: 10.1002/hep.23041. [DOI] [PMC free article] [PubMed] [Google Scholar]


