Abstract
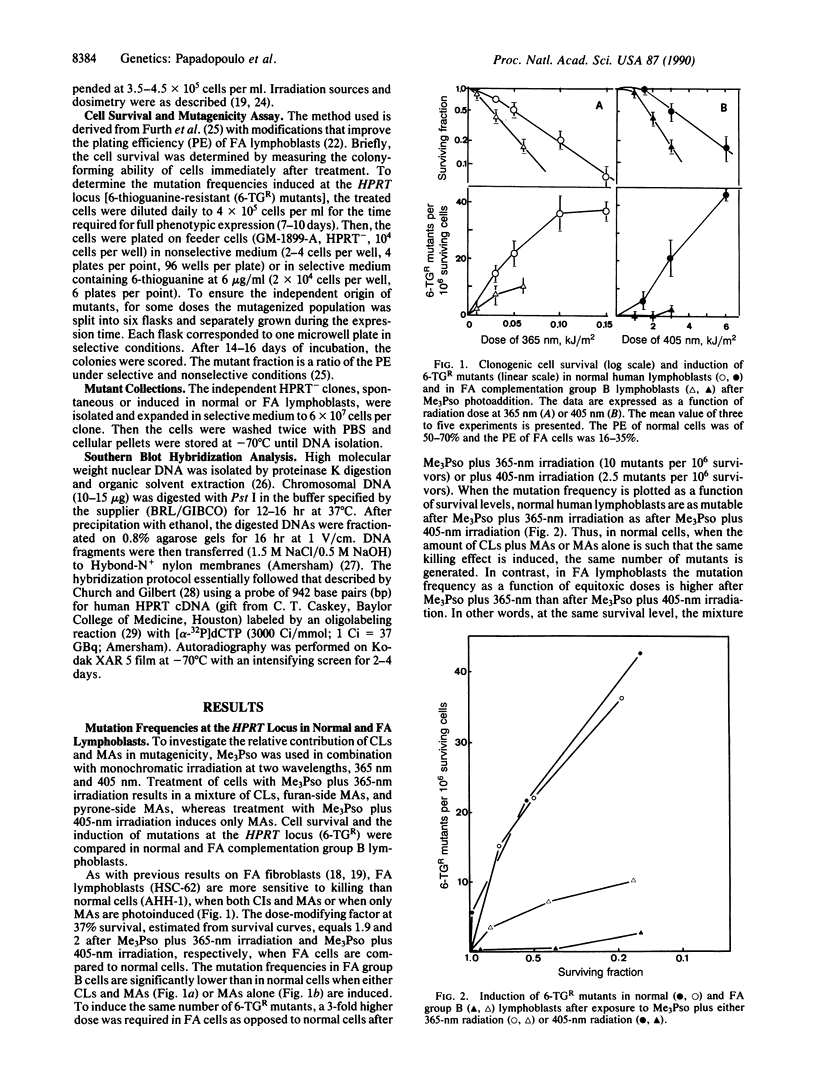
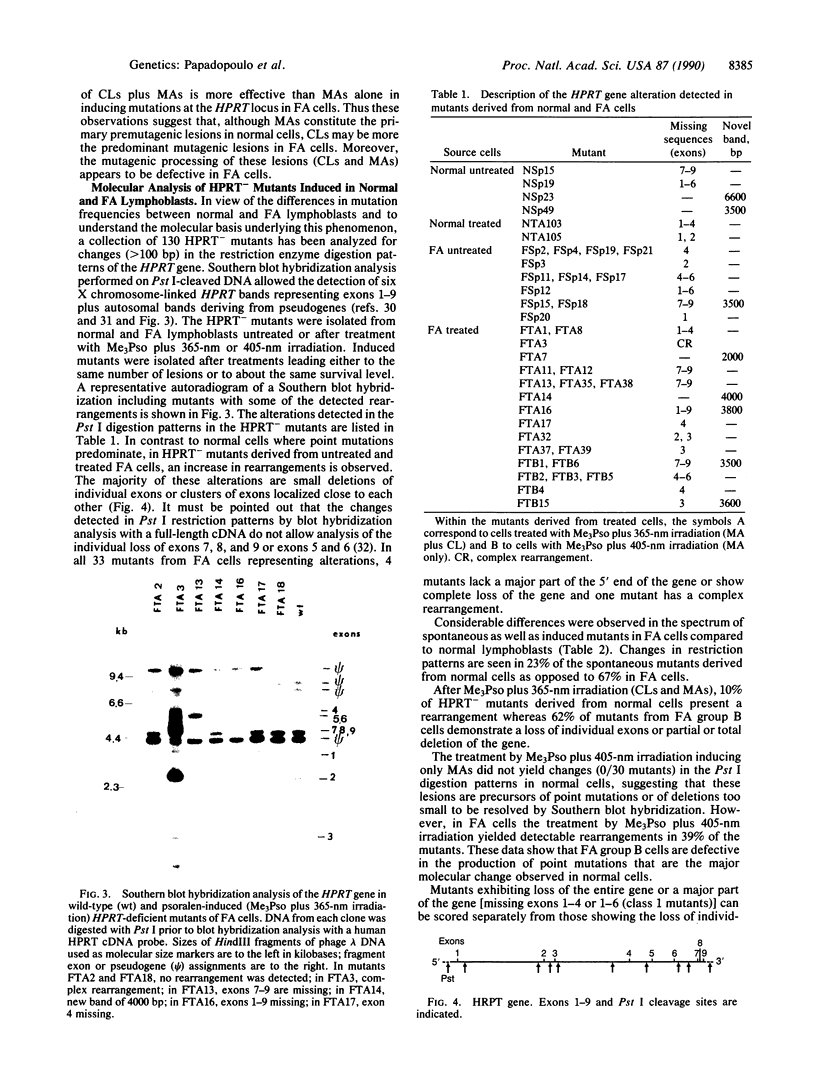
Fanconi anemia (FA) is an inherited human disorder associated with a predisposition to cancer and characterized by anomalies in the processing of DNA cross-links and certain monoadducts. We reported previously that the frequency of psoralen-photoinduced mutations at the HPRT locus is lower in FA cells than in normal cells. This hypomutability is shown here to be associated with an increased frequency of deletions in the HPRT gene when either a mixture of cross-links and monoadducts or monoadducts alone are induced. Molecular analysis of mutants in the HPRT gene was carried out. In normal cells the majority of spontaneous and induced mutants are point mutations whereas in FA deletion mutations predominate. In that case a majority of mutants were found to lack individual exons or small clusters of exons whereas in normal cells large (complete or major gene loss) and small deletions are almost equally represented. Thus we propose that the FA defect lies in a mutagenic pathway that, in normal cells, involves bypassing lesions and subsequent gap filling by a recombinational process during replication.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach A. D., Wolman S. R. Susceptibility of Fanconi's anaemia fibroblasts to chromosome damage by carcinogens. Nature. 1976 Jun 10;261(5560):494–496. doi: 10.1038/261494a0. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Papadopoulo D., Moustacchi E. Repair of 4,5',8-trimethylpsoralen plus light-induced DNA damage in normal and Fanconi's anemia cell lines. Cancer Res. 1988 Apr 15;48(8):2015–2020. [PubMed] [Google Scholar]
- Bredberg A., Nachmansson N. Psoralen adducts in a shuttle vector plasmid propagated in primate cells: high mutagenicity of DNA cross-links. Carcinogenesis. 1987 Dec;8(12):1923–1927. doi: 10.1093/carcin/8.12.1923. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Urlaub G., Steigerwalt R. W., Chasin L. A., Grunberger D. Characterization of mutations induced by 2-(N-acetoxy-N-acetyl)aminofluorene in the dihydrofolate reductase gene of cultured hamster cells. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6519–6523. doi: 10.1073/pnas.83.17.6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet R., Cassier C., Magaña-Schwencke N., Moustacchi E. Fate of photo-induced 8-methoxypsoralen mono-adducts in yeast. Evidence for bypass of these lesions in the absence of excision repair. Mutat Res. 1983 Aug;112(4):201–214. doi: 10.1016/0167-8817(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi C. L., Thilly W. G. Assay for gene mutation in a human lymphoblast line, AHH-1, competent for xenobiotic metabolism. Mutat Res. 1984 Sep;128(2):221–230. doi: 10.1016/0027-5107(84)90110-6. [DOI] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Glickman B. W. The specificity of UV-induced mutations at an endogenous locus in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth-Rysiecki G., Cornish K., Clarke C. A., Buchwald M. Identification of two complementation groups in Fanconi anemia. Somat Cell Mol Genet. 1985 Jan;11(1):35–41. doi: 10.1007/BF01534732. [DOI] [PubMed] [Google Scholar]
- Edwards A., Voss H., Rice P., Civitello A., Stegemann J., Schwager C., Zimmermann J., Erfle H., Caskey C. T., Ansorge W. Automated DNA sequencing of the human HPRT locus. Genomics. 1990 Apr;6(4):593–608. doi: 10.1016/0888-7543(90)90493-e. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Martin G. M., Monnat R. J., Jr Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5893–5897. doi: 10.1073/pnas.86.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth E. E., Thilly W. G., Penman B. W., Liber H. L., Rand W. M. Quantitative assay for mutation in diploid human lymphoblasts using microtiter plates. Anal Biochem. 1981 Jan 1;110(1):1–8. doi: 10.1016/0003-2697(81)90103-2. [DOI] [PubMed] [Google Scholar]
- Gennett I. N., Thilly W. G. Mapping large spontaneous deletion endpoints in the human HPRT gene. Mutat Res. 1988 Sep;201(1):149–160. doi: 10.1016/0027-5107(88)90121-2. [DOI] [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida R., Buchwald M. Susceptibility of Fanconi's anemia lymphoblasts to DNA-cross-linking and alkylating agents. Cancer Res. 1982 Oct;42(10):4000–4006. [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat Res. 1987 May;178(1):143–153. doi: 10.1016/0027-5107(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Vos J. M., Hanawalt P. C. Repair analysis of mitomycin C-induced DNA crosslinking in ribosomal RNA genes in lymphoblastoid cells from Fanconi's anemia patients. Mutat Res. 1989 May;217(3):185–192. doi: 10.1016/0921-8777(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Meuth M., Arrand J. E. Alterations of gene structure in ethyl methane sulfonate-induced mutants of mammalian cells. Mol Cell Biol. 1982 Nov;2(11):1459–1462. doi: 10.1128/mcb.2.11.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C., Meuth M. DNA sequence determination of gamma-radiation-induced mutations of the hamster aprt locus. Mutat Res. 1989 Oct;227(2):97–102. doi: 10.1016/0165-7992(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Jones I. M. Review of the molecular characteristics of gene mutations of the germline and somatic cells of the human. Mutat Res. 1990 Jul;231(1):87–108. doi: 10.1016/0027-5107(90)90179-8. [DOI] [PubMed] [Google Scholar]
- Moustacchi E., Papadopoulo D., Diatloff-Zito C., Buchwald M. Two complementation groups of Fanconi's anemia differ in their phenotypic response to a DNA-crosslinking treatment. Hum Genet. 1987 Jan;75(1):45–47. doi: 10.1007/BF00273837. [DOI] [PubMed] [Google Scholar]
- Nalbantoglu J., Phear G., Meuth M. DNA sequence analysis of spontaneous mutations at the aprt locus of hamster cells. Mol Cell Biol. 1987 Apr;7(4):1445–1449. doi: 10.1128/mcb.7.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas J. A., Hunter T. C., O'Neill J. P., Albertini R. J. Molecular analyses of in vivo hprt mutations in human T-lymphocytes. III. Longitudinal study of hprt gene structural alterations and T-cell clonal origins. Mutat Res. 1989 Dec;215(2):147–160. doi: 10.1016/0027-5107(89)90178-4. [DOI] [PubMed] [Google Scholar]
- Papadopoulo D., Averbeck D., Moustacchi E. High levels of 4,5',8-trimethylpsoralen photoinduced furan-side monoadducts can block cross-link removal in normal human cells. Photochem Photobiol. 1988 Mar;47(3):321–326. doi: 10.1111/j.1751-1097.1988.tb02733.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulo D., Averbeck D., Moustacchi E. The fate of 8-methoxypsoralen-photoinduced DNA interstrand crosslinks in Fanconi's anemia cells of defined genetic complementation groups. Mutat Res. 1987 Nov;184(3):271–280. doi: 10.1016/0167-8817(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Papadopoulo D., Porfirio B., Moustacchi E. Mutagenic response of Fanconi's anemia cells from a defined complementation group after treatment with photoactivated bifunctional psoralens. Cancer Res. 1990 Jun 1;50(11):3289–3294. [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romac S., Leong P., Sockett H., Hutchinson F. DNA base sequence changes induced by ultraviolet light mutagenesis of a gene on a chromosome in Chinese hamster ovary cells. J Mol Biol. 1989 Sep 20;209(2):195–204. doi: 10.1016/0022-2836(89)90272-6. [DOI] [PubMed] [Google Scholar]
- Rousset S., Nocentini S., Revet B., Moustacchi E. Molecular analysis by electron microscopy of the removal of psoralen-photoinduced DNA cross-links in normal and Fanconi's anemia fibroblasts. Cancer Res. 1990 Apr 15;50(8):2443–2448. [PubMed] [Google Scholar]
- Sabatier L., Dutrillaux B. Effect of caffeine in Fanconi anemia. I. Restoration of a normal duration of G2 phase. Hum Genet. 1988 Jul;79(3):242–244. doi: 10.1007/BF00366244. [DOI] [PubMed] [Google Scholar]
- Sarasin A. Shuttle vectors for studying mutagenesis in mammalian cells. J Photochem Photobiol B. 1989 Apr;3(2):143–155. doi: 10.1016/1011-1344(89)80057-0. [DOI] [PubMed] [Google Scholar]
- Sasaki M. S., Tonomura A. A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Res. 1973 Aug;33(8):1829–1836. [PubMed] [Google Scholar]
- Schroeder T. M., Anschütz F., Knopp A. Spontane Chromosomenaberrationen bei familiärer Panmyelopathie. Humangenetik. 1964;1(2):194–196. doi: 10.1007/BF00389636. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Hinkle L., Collins A. R., Waldren C. A. Chinese hamster ovary mutant UV-1 is hypomutable and defective in a postreplication recovery process. Somatic Cell Genet. 1981 May;7(3):307–320. doi: 10.1007/BF01538856. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Thacker J. The molecular nature of mutations in cultured mammalian cells: a review. Mutat Res. 1985 Jun-Jul;150(1-2):431–442. doi: 10.1016/0027-5107(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Stankowski L. F., Jr, Machanoff R., Hsie A. W. Detection of deletion mutations in pSV2gpt-transformed cells. Mol Cell Biol. 1984 Jul;4(7):1411–1415. doi: 10.1128/mcb.4.7.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Simons J. W., Arwert F., Natarajan A. T., van Zeeland A. A. Mutations induced by X-rays at the HPRT locus in cultured Chinese hamster cells are mostly large deletions. Mutat Res. 1985 Dec;144(4):281–286. doi: 10.1016/0165-7992(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]
- Yatagai F., Glickman B. W. Mutagenesis by 8-methoxypsoralen plus near-UV treatment: analysis of specificity in the lacI gene of Escherichia coli. Mutat Res. 1986 Dec;163(3):209–224. doi: 10.1016/0027-5107(86)90019-9. [DOI] [PubMed] [Google Scholar]
- de Jong P. J., Grosovsky A. J., Glickman B. W. Spectrum of spontaneous mutation at the APRT locus of Chinese hamster ovary cells: an analysis at the DNA sequence level. Proc Natl Acad Sci U S A. 1988 May;85(10):3499–3503. doi: 10.1073/pnas.85.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]



