Abstract
RANK and CD40 activate NF-κB and MAPKs to similar levels via TRAF6. Even though overexpression of TRAF6 results in osteoclast formation, RANK but not CD40 promotes osteoclastogenesis. To understand the molecular basis for RANK-specific activity in osteoclastogenesis, we created an osteoclast formation system driven by anti-human CD40 antibody-mediated stimulation of a chimeric receptor, h40/mRK, which consists of the extracellular domain of human CD40 and the transmembrane and cytoplasmic domains of mouse RANK. By introducing mutations into three TRAF6-binding sites of RANK, we found that h40/mRK with a single TRAF6-binding site efficiently induced Ca2+ oscillation and expression of NFATc1, a master switch in osteoclastogenesis, whereas CD40 carrying a single TRAF6-binding site did not. However, expression of CD40 that was approximately 100 times greater than that of h40/mRK resulted in osteoclast formation, indicating that the RANK–TRAF6 signal is more potent than the CD40–TRAF6 signal in terms of NFATc1 activation and osteoclastogenesis. These results suggest that RANK may harbor a specific domain that amplifies TRAF6 signaling.
Keywords: bone remodeling, CD40, NFAT, NF-κB, RANK
Introduction
Interactions of ligands and receptors of the tumor necrosis factor (TNF) superfamily trigger critical intracellular signals that are essential for development, homeostasis and adaptive responses of the immune system (Bodmer et al, 2002). Among various sets of the TNF family ligand and its receptor, receptor activator of NF-κB (RANK, also known as TRANCE receptor) and RANK ligand (RANKL, also known as ODF, OPGL and TRANCE) play crucial roles in maintaining the homeostasis of bone remodeling, which is regulated by a balance of osteoblast-mediated bone formation and osteoclast-mediated bone resorption; stimulation of RANK in osteoclast progenitor cells is an essential step for osteoclast formation. RANKL- or RANK-deficient mice display osteopetrosis due to a lack of osteoclasts (Dougall et al, 1999; Kong et al, 1999). Excess formation or activity of osteoclasts in human leads to pathological bone resorption, as observed in postmenopausal osteoporosis, rheumatoid arthritis, Paget's disease and tumor bone metastases (Rodan and Martin, 2000; Takayanagi et al, 2000). Therefore, precise elucidation of the regulatory mechanisms of osteoclast formation, particularly the molecular mechanisms of RANK signaling, is essential for understanding the onset of skeletal diseases and for developing drugs to treat these diseases.
Intracellular signaling pathways of RANK are mediated by members of the TNF receptor-associated factor (TRAF) family (Galibert et al, 1998; Wong et al, 1998; Darnay et al, 1999; Hsu et al, 1999). To date, seven members of the TRAF family have been described (Inoue et al, 2000; Bouwmeester et al, 2004). Among them, TRAF2, TRAF3, TRAF5 and TRAF6 bind to the cytoplasmic tail of RANK in vitro. TRAF6 is the only TRAF involved in signaling from members of the Toll/IL-1R family by its interaction with IL-1R-associated kinase (IRAK) (Cao et al, 1996; Ishida et al, 1996a). Furthermore, TRAF2, TRAF3 and TRAF5 bind to the membrane-distal domain of the cytoplasmic tail of RANK, whereas TRAF6 interacts with the membrane-proximal domain). We and others (Lomaga et al, 1999; Naito et al, 1999) showed previously that TRAF6-deficient (TRAF6−/−) mice exhibit severe osteopetrosis and are defective in osteoclast formation due to defective signaling from RANK upon binding of RANKL. Furthermore, RANK-induced activation of NF-κB and MAPKs in osteoclast progenitor cells is abrogated in the absence of TRAF6 (Kobayashi et al, 2001), consistent with previous findings that NF-κB and MAPKs play crucial roles in osteoclastogenesis (Franzoso et al, 1997; Iotsova et al, 1997; Matsumoto et al, 2000; David et al, 2002; Yamamoto et al, 2002; Ikeda et al, 2004).
CD40, another member of the TNF receptor superfamily, transmits signals through TRAF family members, including TRAF2, TRAF3, TRAF5 and TRAF6 (Cheng et al, 1995; Ishida et al, 1996a, 1996b). As with RANK, TRAF2, TRAF3 and TRAF5 bind to the membrane-distal domain of the cytoplasmic tail of CD40, whereas TRAF6 interacts with the membrane-proximal domain (Tsukamoto et al, 1999).
Herein, we first show that TRAF6 plays a crucial role in CD40-mediated NF-κB and MAPK activation in osteoclast progenitor cells. Despite such similarities between roles of TRAF6 in RANK signaling and those in CD40 signaling, stimulation of CD40 in osteoclast progenitor cells does not result in osteoclast formation. Therefore, RANK, but not CD40, is able to transmit specific signals leading to osteoclastogenesis. Elucidation of the molecular mechanisms of this RANK-specific function in osteoclastogenesis is essential for developing drugs for the treatment of pathological bone resorption. Thus, we generated and expressed chimeric receptors in which the extracellular domain of human CD40 was fused to the transmembrane domain and cytoplasmic tail of mouse RANK, and we searched for specific structures in RANK critical for osteoclastogenesis.
Results
The RANK–TRAF6 pathway but not the CD40–TRAF6 pathway promotes osteoclastogenesis
When bone marrow cells were stimulated for 3 days with RANKL after 2 days of culture in the presence of M-CSF, multinucleated tartrate-resistant acid phosphatase-positive (TRAP+) osteoclasts were formed (Figure 1A). Osteoclast formation is dependent on RANK-mediated NF-κB activation, which requires TRAF6 (Kobayashi et al, 2001). To address whether activation of TRAF6 is sufficient to promote osteoclastogenesis, TRAF6 was overexpressed in osteoclast progenitor cells by retrovirus-mediated gene transfer. Multinucleated TRAP+ osteoclasts were formed, although these osteoclasts were smaller in number and size than those generated by RANKL stimulation (Figure 1A). These osteoclasts were able to resorb bone (data not shown), indicating that activation of TRAF6 by its overexpression is sufficient for the differentiation and activation of osteoclasts. In contrast, when TRAF2 was overexpressed by similar retrovirus-mediated transfection, no TRAP+ cells were observed (Figure 1A). Overexpression of TRAF5 also failed to induce osteoclast formation (data not shown). Thus, the ability to induce osteoclast formation is specific to TRAF6.
Figure 1.
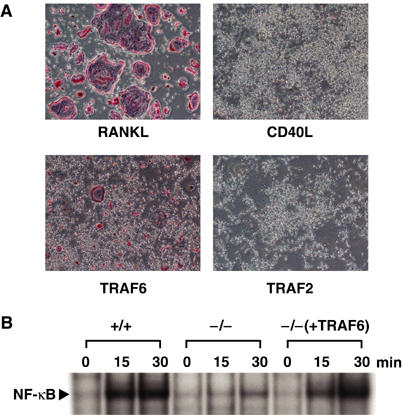
The CD40–TRAF6 pathway does not lead to osteoclastogenesis. (A) Ex vivo osteoclast formation in response to stimulation under various conditions. Bone marrow cells were cultured in the presence of M-CSF for 2 days, and nonadherent cells were discarded. Adherent cells were then stimulated with RANKL or CD40L (top) or infected with retrovirus expressing TRAF6 or TRAF2 (bottom). After an additional 3 days of culture, cells were fixed and stained for TRAP. (B) Requirement of TRAF6 in CD40-mediated NF-κB activation in osteoclast progenitor cells. Spleen cells derived from wild-type (+/+) or TRAF6−/− (−/−) mice were cultured for 3 days in the presence of M-CSF. A portion of the spleen cells derived from TRAF6−/− mice was infected with retrovirus expressing TRAF6 (pMX-TRAF6) (Kobayashi et al, 2001) after 1 day in culture and then cultured for 2 days with M-CSF. Cells were then stimulated with CD40L for indicated periods, and nuclear extracts were prepared. Electrophoretic mobility-shift assays were performed as described in Materials and methods.
CD40, another member of the TNF receptor superfamily, transmits signals through TRAF2, TRAF3, TRAF5 and TRAF6 (Ishida et al, 1996a, 1996b; Tsukamoto et al, 1999), as with RANK. To address whether TRAF6 plays a crucial role in CD40-mediated activation of NF-κB in osteoclast progenitor cells, splenocytes were prepared from either TRAF6−/− mice or littermate controls and cultured for 3 days in the presence of M-CSF, and stimulated with CD40L. Because the number of bone marrow cells was reduced significantly in TRAF6−/− mice due to severe osteopetrosis (Lomaga et al, 1999; Naito et al, 1999), splenocytes were used instead of bone marrow cells. CD40-mediated activation of NF-κB and MAPKs was observed in splenocytes from control mice but not in splenocytes from TRAF6−/− mice (Figure 1B, data not shown). Furthermore, when TRAF6 was complemented by retrovirus vector-mediated gene transfer, NF-κB and MAPKs activation was observed with CD40L stimulation, indicating that CD40-mediated NF-κB and MAPKs activation in osteoclast progenitor cells was TRAF6 dependent (Figure 1B, data not shown). In spite of significant NF-κB and MAPKs activation by the CD40–TRAF6 signal, stimulation of CD40 did not result in osteoclast formation (Figure 1A). These results led us to investigate why the RANK–TRAF6 pathway and TRAF6 overexpression but not the CD40–TRAF6 pathway promote osteoclast formation.
Establishment of an ex vivo osteoclast formation system driven by a chimeric receptor of CD40 and RANK
To elucidate the molecular mechanisms by which RANK signaling but not TRAF6 signaling mediated by other cytokine receptors such as CD40 and IL-1R induces osteoclastogenesis, we first compared the primary structures of the cytoplasmic tails of RANK and CD40 (Figure 2A). It has been reported that the cytoplasmic tail of RANK contains three TRAF6-binding sites and two critical sites for binding of other TRAF family members including TRAF2, TRAF3 and TRAF5 (Galibert et al, 1998; Wong et al, 1998; Darnay et al, 1999; Hsu et al, 1999; Ye et al, 2002). The cytoplasmic tail of CD40 possesses a single TRAF6-binding site as well as a binding site for TRAF2, TRAF3 and TRAF5 (Tsukamoto et al, 1999). Because binding sites for TRAF2, TRAF3 and TRAF5 in RANK are reported to be dispensable for osteoclast formation (Armstrong et al, 2002), we first focused on the difference in the number of TRAF6-binding sites between RANK and CD40. We hypothesized that three TRAF6-binding sites are required to transmit a signal strong enough to promote osteoclast formation, whereas a single TRAF6-binding site is not sufficient. To test this, we generated a retrovirus vector expressing a chimeric receptor in which the extracellular domain of human CD40 was fused to the transmembrane domain and cytoplasmic tail of mouse RANK (h40/Mrk; Figure 2A). After expressing h40/mRK in osteoclast progenitor cells by retrovirus-mediated gene transfer, the chimeric receptor can be stimulated with agonistic anti-human CD40 monoclonal antibody (G28-5) (Clark et al, 1988). Stimulation of osteoclast progenitor cells with G28-5 antibody did not induce activation of NF-κB and MAPKs (data not shown), whereas stimulation of these cells with CD40L resulted in marked activation of NF-κB (Figure 1B), indicating that G28-5 antibody does not stimulate mouse CD40. Therefore, the effect of signaling from endogenous mouse CD40 could be ruled out in our system. Furthermore, the activity of hCD40 and h40/mRK could be compared because the amount of each receptor expressed on the cell surface could be measured with the same anti-CD40 antibody. When h40/mRK was expressed in bone marrow cells cultured for 3 days with M-CSF and was stimulated with anti-CD40 antibody, multinucleated TRAP+ osteoclasts were generated efficiently. The number of multinucleated TRAP+ osteoclasts, number of nuclei per single osteoclast and intensity of TRAP staining were comparable to those observed in cells stimulated with RANKL (Figure 2B and C). In contrast, when hCD40 was expressed at levels similar to that of h40/mRK (Figure 4A) and was stimulated with anti-CD40 antibody, no osteoclasts were formed (Figure 2B and C). To exclude the possibility that the h40/mRK signal induces expression of RANKL, which then in turn stimulates RANK to trigger osteoclast formation, the effect of osteoprotegerin (OPG), which acts as a decoy receptor for RANKL, was examined. Whereas OPG completely blocked osteoclast formation induced by RANKL stimulation, the number of osteoclasts generated by h40/mRK signaling was not affected (Figure 2B and C), indicating elimination of RANKL induction in our system.
Figure 2.
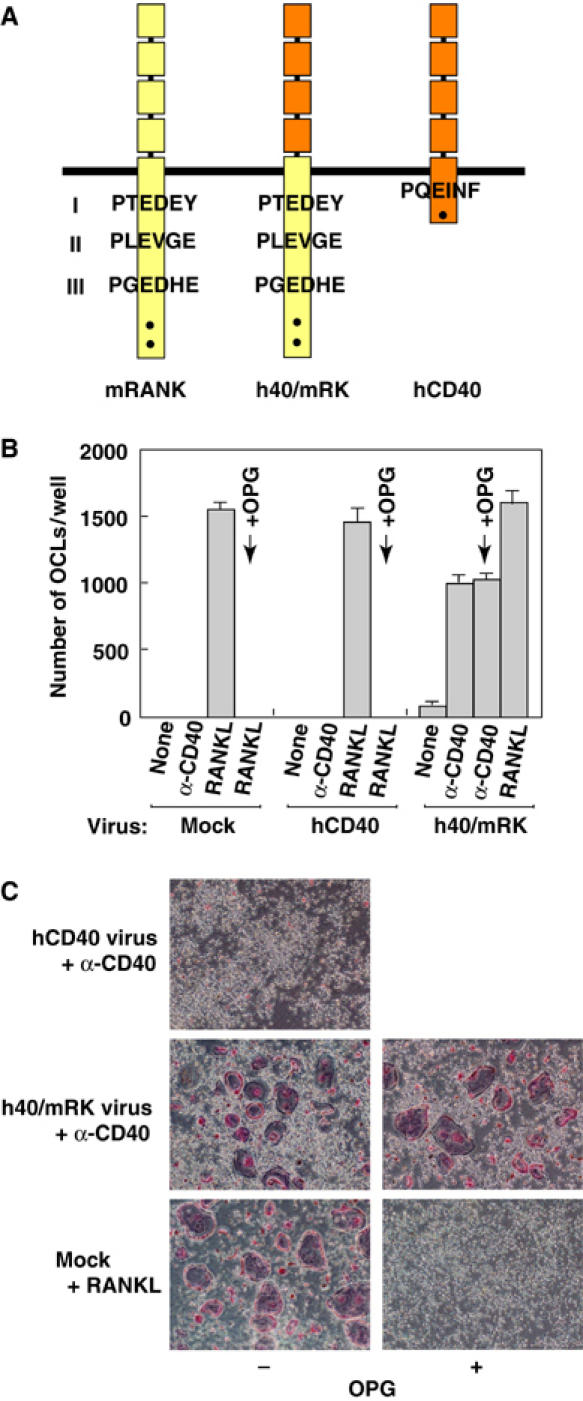
Establishment of an ex vivo assay system for osteoclast formation induced by a chimeric receptor of CD40 and RANK. (A) Schematic diagram of the chimeric receptor of human CD40 and mouse RANK. Yellow and orange boxes indicate mouse RANK and human CD40, respectively. Consensus TRAF6-binding sites are shown as Pro-X-Glu-X-X-(aromatic/acidic) and numbered (I, II, III) from the N-terminus. Dots denote binding sites for TRAF2, TRAF3 and TRAF5. (B, C) Effect of OPG on chimeric receptor-mediated osteoclastogenesis. Bone marrow cells were cultured for 1 day with M-CSF, and then mock-infected or infected with retrovirus expressing human CD40 or chimeric receptor h40/mRK followed by 2 days of culture with M-CSF. Cells were then unstimulated or stimulated with anti-CD40 antibody or RANKL as indicated in the presence or absence of OPG. At 3 days after stimulation, cells were fixed and stained for TRAP (C), and multinucleated TRAP+ cells were counted (B).
Figure 4.
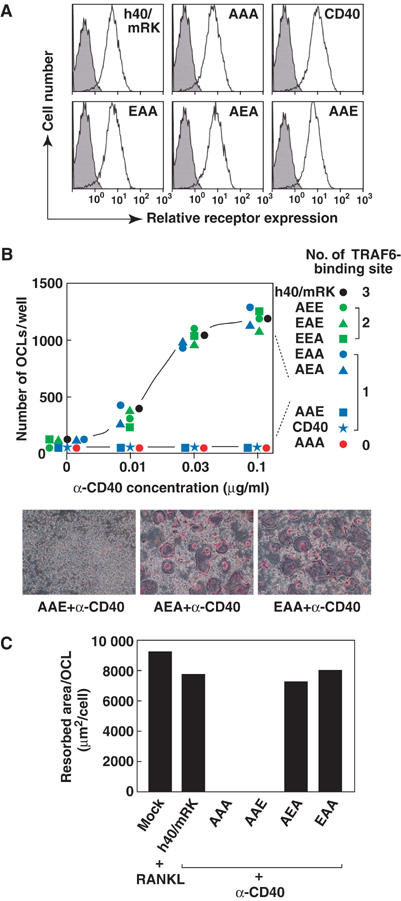
A single TRAF6-binding site is sufficient for formation of functional osteoclasts. (A) Cytometric analysis of surface expression of CD40, h40/mRK and its mutants. At 1 day after addition of puromycin, cells were harvested and incubated with phycoerythrin-conjugated anti-human CD40. Expression of CD40, h40/mRK and its mutants was analyzed by flow cytometry. (B) Ability of various chimeric receptor mutants to induce osteoclast formation. Bone marrow cells were cultured for 1 day with M-CSF and then infected with retrovirus expressing chimeric receptor h40/mRK or its various mutants. Infected cells were then cultured for 1 day with M-CSF and further cultured for 2 days with puromycin in addition to M-CSF to remove uninfected cells. Cells were then unstimulated or stimulated with three different concentrations of anti-human CD40 monoclonal antibody. At 3 days after stimulation, cells were fixed and stained for TRAP (bottom), and multinucleated TRAP+ cells were counted (top). (C) Bone-resorbing activity of osteoclasts generated by signals from h40/mRK or its mutants. Bone marrow cells were cultured on dentine slices for 1 day with M-CSF and then infected with retrovirus expressing chimeric receptor h40/mRK or its various mutants, followed by 2 days of culture with puromycin in addition to M-CSF. Cells were then stimulated with anti-CD40 antibody or RANKL for 3 days. Resorption pits on dentine slices were visualized by staining with 0.5% toluidine blue, and total pit area on dentine slices was measured. Osteoclasts formed under identical conditions were counted, and the resorption area per single osteoclast was calculated.
Specific binding of TRAF6 to the cytoplasmic tail of mouse RANK
Crystallographic study of TRAF6 in complex with TRAF6-binding peptides from CD40 and RANK led to the identification of a Pro-X-Glu-X-X-(aromatic/acidic residue) as a consensus TRAF6-binding motif (Ye et al, 2002). Three putative TRAF6-binding sites in RANK, which match the consensus TRAF6-binding motif, and a single binding site in CD40 have been reported. Substitution of Ala for Glu at the fourth amino-acid position of the TRAF6-binding motifs in CD40 and RANK abolishes binding to TRAF6 (Tsukamoto et al, 1999; Ye et al, 2002). Therefore, by introduction of such mutations into each TRAF6-binding motif of h40/mRK, we addressed whether the binding site plays a role in NF-κB activation. According to the results of transient transfection reporter assays, all three sites in RANK are required for full activation of NF-κB (data not shown) (Ye et al, 2002), and mutation of the binding site in CD40 results in a dramatic reduction of NF-κB activation (Tsukamoto et al, 1999). To evaluate the functional role of the three TRAF6-binding sites of RANK in osteoclastogenesis, we generated single, double and triple mutations at each of the Glu residues to disrupt one, two or three of the TRAF6-binding sites in h40/mRK (Figure 3A). Because of discrepancies in the identified TRAF6-binding sites of RANK among reports (Galibert et al, 1998; Wong et al, 1998; Darnay et al, 1999; Hsu et al, 1999; Ye et al, 2002), we first addressed whether the substitution of Ala for Glu in the three TRAF6-binding sites of RANK abolishes TRAF6 binding in our experimental system. The cytoplasmic tail of RANK, with double mutations (EAA, AEA or AAE) or with triple mutations (AAA) fused to GST, was coexpressed with FLAG-TRAF6 in 293T cells, and GST pull-down assays were performed. GST-EAA, -AEA and -AAE but not GST-AAA bound to TRAF6 (Figure 3B). To further confirm associations between the substitution mutations and the binding activity of each TRAF6-binding site, 17-mer peptides comprised of the wild-type or mutant TRAF6-binding site and adjacent amino acids derived from RANK were expressed as GST fusion proteins together with FLAG-TRAF6. GST pull-down assays revealed that TRAF6 associated with the 17-mer peptides and that single amino-acid substitution mutation of any of the three TRAF6-binding sites is sufficient to abolish TRAF6 binding. In both binding experiments, association of TRAF6 with binding site I (BS-I, EAA) was more efficient than that with binding site II or III (BS-II, AEA or BS-III, AAE), consistent with the finding of Ye et al (2002) that TRAF6 binds to BS-I with at least 10 times the affinity of binding to BS-II or BS-III. These clear associations between the mutations and the binding activity of each TRAF6-binding site of RANK allowed us to test our hypothesis that the number of TRAF6-binding sites is critical for osteoclastogenesis.
Figure 3.
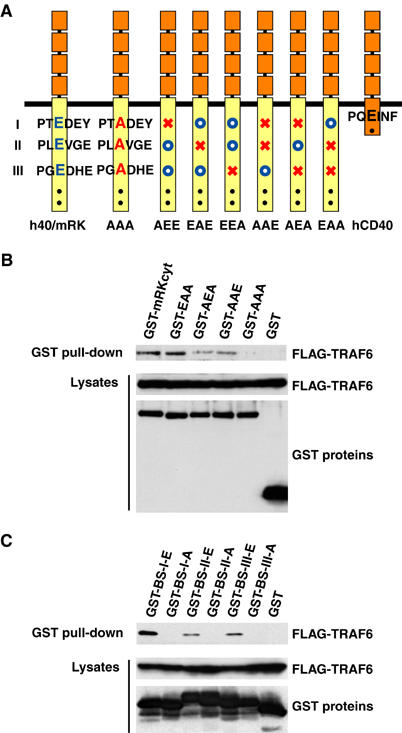
Specific binding of TRAF6 to the three TRAF6-binding sites of mouse RANK. (A) Schematic diagram of chimeric receptor h40/mRK and its mutants. Consensus TRAF6-binding sites are shown as Pro-X-Glu-X-X-(aromatic/acidic) and numbered (I, II, III) from the N-terminus. TRAF6-binding sites were altered by replacement of Glu (E, blue letters) with Ala (A, red letters). AAA denotes a mutant in which all three binding sites were mutated. In other mutants, open blue circles and red X's indicate intact and mutated binding sites, respectively. Dots denote binding sites for TRAF2, TRAF3 or TRAF5. (B) Specific binding of TRAF6 to the three TRAF6-binding sites located in the cytoplasmic tail of RANK. Cell extracts from 293T cells cotransfected with expression plasmids for FLAG-TRAF6 and GST-tagged RANK cytoplasmic tail (GST-mRKcyt) or its TRAF6-binding site mutants were prepared. The GST pull-down assay was performed, and TRAF6 bound to beads was then analyzed by Western blot with anti-FLAG antibody M2 (top). A portion of the lysate used for the GST pull-down assay was analyzed for the expression levels of FLAG-TRAF6 and various GST fusion proteins by Western blot with anti-FLAG antibody M2 (middle) or anti-GST antibody (bottom). (C) Specific binding of TRAF6 to the three TRAF6-binding sites isolated from the cytoplasmic tail of RANK. Cell extracts from 293T cells cotransfected with expression plasmids for FLAG-TRAF6 and GST-tagged 17-mer peptides containing each of the three putative TRAF6-binding sites of mouse RANK (BS-I: 336-SRKIPT(E/A)DEYTDRPSQP-352; BS-II: 369-PFQEPL(E/A)VGENDSLSQC-385; BS-III: 443-SGNTPG(E/A)DHEPFPGSLK-459) were prepared. The GST pull-down assay and Western blotting were performed as in (B).
A single TRAF6-binding site is sufficient for osteoclastogenesis
In our experiments, approximately 60% of bone marrow cells were infected with each of the recombinant retroviruses (data not shown). To compare the activities of various receptors without unexpected effects of uninfected cells in the assay system, cells were cultured in the presence of puromycin for 2 days after infection to remove uninfected cells. Nearly equal numbers of cells were selected among the different infected cultures. Therefore, we assumed that the densities of infected cells in each culture dish were similar among the different infected cultures when cells were stimulated with anti-CD40 antibody. After selection with puromycin, almost 100% of the cells expressed CD40, h40/mRK or h40/mRK mutants at similar levels (Figure 4A). Even upon stimulation by anti-CD40 antibody, the triple mutant (AAA) was unable to induce osteoclast formation, indicating that h40/mRK-mediated osteoclastogenesis is TRAF6 dependent (Figure 4B, top). Failure to induce the formation of osteoclasts from TRAF6-deficient splenocytes by h40/mRK signaling further supports this notion (data not shown). h40/mRK mutants containing two TRAF6-binding sites (AEE, EAE, EEA) induced osteoclast formation comparable to that by h40/mRK (Figure 4B, top). Among the double mutants, the AAE mutant, in which the first and second TRAF6-binding sites (I and II) were disrupted, failed to induce osteoclast formation. The other double mutants (AEA, EAA) induced osteoclast formation comparable to that by h40/mRK in terms of the number (Figure 4B, top) and size (Figure 4B, bottom) of osteoclasts. Furthermore, osteoclasts generated by double mutants showed bone-resorbing activity similar to that generated by h40/mRK or RANK (Figure 4C). These results indicate that a single TRAF6-binding site is sufficient to promote osteoclastogenesis.
Given that CD40, like the AEA and EAA mutants of h40/mRK, possesses a single TRAF6-binding site and that overexpression of TRAF6 results in osteoclast formation, we next looked for differences in downstream signals critical for osteoclastogenesis. Stimulation of h40/mRK and the AEA and EAA mutants by anti-CD40 antibody resulted in similar activation of NF-κB, JNK and p38 (Figure 5A). In contrast, the AAE and AAA mutants, which were unable to promote osteoclastogenesis, failed to activate NF-κB, JNK or p38 (Figure 5), strongly suggesting that activation of NF-κB, JNK and p38 is required for osteoclastogenesis, consistent with previously reported findings (Franzoso et al, 1997; Iotsova et al, 1997; Matsumoto et al, 2000; David et al, 2002; Yamamoto et al, 2002; Ikeda et al, 2004). However, stimulation of CD40 resulted in activation of NF-κB and MAPKs to similar levels and with kinetics similar to those induced by h40/mRK (Figure 5A), indicating that activation of NF-κB, JNK and p38 is not sufficient for osteoclastogenesis.
Figure 5.
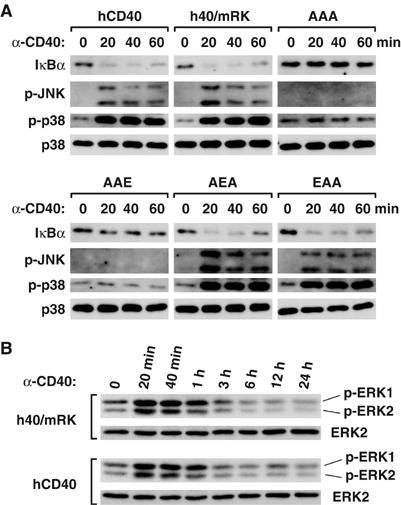
Activation of NF-κB and MAPKs by CD40 is similar to that by the chimeric receptor and its mutants that promote osteoclastogenesis. Bone marrow cells were cultured for 1 day with M-CSF and then infected with retrovirus expressing human CD40, chimeric receptor h40/mRK or its various mutants. Infected cells were cultured for 1 day with M-CSF and further cultured for 2 days with puromycin in addition to M-CSF to remove uninfected cells. Cells were then unstimulated or stimulated with anti-CD40 antibody for indicated periods. Cell lysates were prepared, and immunoblot analysis was performed with antibody reactive to IκBα, phosphorylated JNK (p-JNK), phosphorylated p38 (p-p38) or p38 (A). Lysates prepared from cells expressing h40/mRK or hCD40 were analyzed by Western blot with antibody reactive to phosphorylated ERK1, phosphorylated ERK2 or ERK2 (B).
RANK-specific amplification of TRAF6 signaling is required for osteoclastogenesis
It has been reported that the RANK–TRAF6 pathway induces expression of NFATc1 and evokes Ca2+ oscillations that lead to calcineurin-mediated activation of NFATc1, which in turn induces expression of osteoclast-specific genes (Ishida et al, 2002; Takayanagi et al, 2002). Furthermore, NFATc1-deficient embryonic stem cells fail to differentiate into osteoclasts upon RANKL stimulation, and ectopic expression of NFATc1 in precursor cells results in osteoclast formation (Takayanagi et al, 2002). Thus, NFATc1 is a critical transcription factor that is suggested to be a master switch for the differentiation and maturation of osteoclasts. Since NFAT cooperates with AP-1 by forming a complex (Macián et al, 2000) and RANKL-induced NFATc1 associates with c-Fos, a subunit of AP-1, to induce expression of osteoclast-specific genes (Takayanagi et al, 2002), we analyzed long-term activation of ERK, which is required for accumulation and activation of c-Fos (Murphy et al, 2002). Kinetics of ERK activation were not different between h40/mRK and CD40 (Figure 5B), suggesting that activity of AP-1 may be similarly induced by both receptors. We then analyzed Ca2+ oscillations induced by signals from CD40 and various chimeric receptors. Ca2+ oscillations were efficiently induced by h40/mRK and the EAA mutant, which are capable of promoting osteoclastogenesis, but not by CD40 or the AAE mutant, which are unable to induce osteoclast formation (Figure 6A). Therefore, we next analyzed the expression of NFATc1 induced by signals from CD40 and various chimeric receptors. Stimulation of h40/mRK resulted in strong induction of NFATc1, although the induction was weaker than that in response to RANKL (Figure 6B). The AEA and EAA mutants, which possess single TRAF6-binding sites and were capable of promoting osteoclastogenesis, also induced NFATc1 expression (Figure 6B). In contrast, the AAE and AAA mutants, which were unable to induce differentiation of osteoclasts or activate NF-κB or MAPKs, did not induce NFATc1 expression (Figure 6B). Interestingly, stimulation of CD40 under conditions in which both NF-κB and MAPKs were efficiently activated resulted in no induction of NFATc1, and osteoclasts were not observed (Figure 6B and C, CD40). These results indicate that RANK but not CD40 possesses a unique ability to induce and activate NFATc1 in addition to activation of NF-κB and MAPKs when the two receptors are equally expressed. To address whether high expression of CD40 results in osteoclastogenesis, we used either 20- or 100-fold concentrated virus stock solutions for infection. Although two populations of infected cells were generated by each concentration, higher peaks were seen in populations of cells expressing approximately 20 times (CD40(middle)) and 100 times (CD40(high)) the amount of surface CD40 in comparison to regular infection concentration (Figure 6C, left). Upon stimulation by anti-CD40 antibody, a significant amount of multinucleated TRAP+ osteoclasts was generated from CD40(high) cells, whereas osteoclasts were not generated from CD40(middle) cells (Figure 6C, right). Furthermore, a significant amount of NFATc1 was induced when CD40(high) cells were stimulated, while NFATc1 was not induced in CD40(middle) cells upon stimulation (Figure 6B). Therefore, the RANK–TRAF6 signal is theoretically approximately 100 times stronger than the CD40–TRAF6 signal in terms of NFATc1 induction and osteoclast formation.
Figure 6.
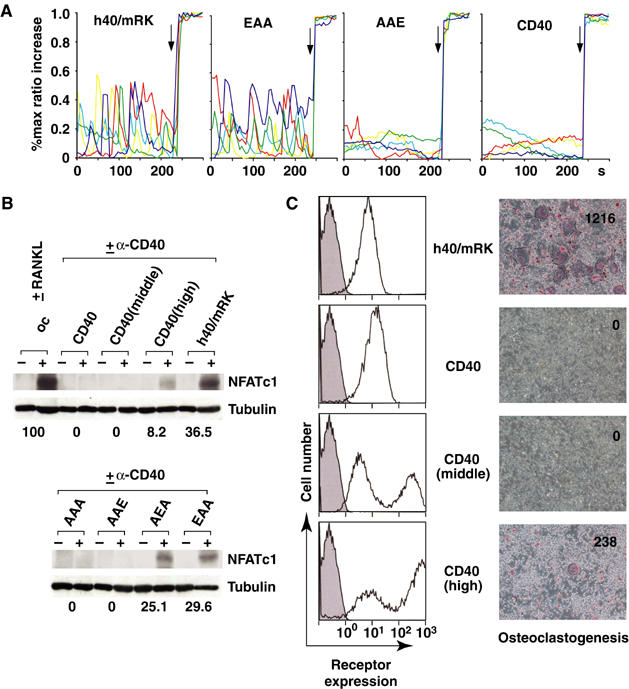
Amplification of the TRAF6-mediated signal by the cytoplasmic tail of RANK. Bone marrow cells were cultured for 1 day with M-CSF and then mock-infected or infected with retrovirus expressing human CD40, chimeric receptor h40/mRK or h40/mRK mutants. In the CD40(middle) and CD40(high) samples, 20- and 100-fold concentrated virus solution respectively, were used. Infected cells were then cultured for 1 day with M-CSF and further cultured for 2 days with puromycin in addition to M-CSF to remove uninfected cells. Mock-infected cells were cultured without puromycin. (A) Ca2+ oscillations induced by signaling from CD40, h40/mRK and h40/mRK mutants. Traces of [Ca2+]i changes in single cells expressing CD40, h40/mRK and h40/mRK mutants and stimulated with anti-CD40 antibody for 48 h are shown. [Ca2+]i changes in cells coloaded with fluo-4 and Fura Red were estimated as the ratio of fluorescence intensity of fluo-4 to Fura Red, and the maximum percent ratio increase from the baseline was plotted at intervals of 5 s. The maximum ratio increase was obtained by addition of 10 μM ionomycin at the end of each experiment (arrow). Each color indicates a different cell in the same field. (B) Induction of NFATc1 by signals from various receptors. Two days after infection, cells were left unstimulated or were stimulated with anti-CD40 antibody or RANKL for 2 days. Cell lysates were prepared, and Western blot analysis was performed with antibody to NFATc1. Relative amounts of NFATc1 normalized to that of tubulin are shown; the amount of NFATc1 induced by RANKL was set at 100. (C) Osteoclastogenesis in response to high expression of CD40. Surface expression of CD40 and h40/mRK was analyzed by flow cytometry (left). Two days after infection, cells were harvested and incubated with phycoerythrin-conjugated anti-human CD40. Ex vivo osteoclast formation by signals from h40/mRK and CD40 was analyzed (right). Two days after infection, cells were stimulated with anti-CD40 antibody for 3 days. Cells were then fixed and stained for TRAP. The number of multinucleated TRAP+ cells in each well was counted, and representative results of three independent experiments are shown in each photomicrograph.
Inability of the TRAF6-binding site of RANK in the context of the cytoplasmic tail of CD40 to promote osteoclastogenesis
To address whether the TRAF6-binding site of RANK itself has the ability to promote osteoclastogenesis, CD40 carrying one of the TRAF6-binding sites of RANK in the form of 17-mer TRAF6-binding peptides instead of its own TRAF6-binding site (h40-BS-I, h40-BS-II, h40-BS-III; Figure 7A) was expressed in bone marrow cells. The expression level of these receptors was controlled to be similar to that of CD40 in CD40(middle) cells (Figure 7B, left) because this expression level was the highest expression level that did not result in osteoclast formation. Upon stimulation by anti-CD40 antibody, none of the TRAF6-binding site-substituted receptors promoted osteoclastogenesis (Figure 7, right), even though h40-BS-I, h40-BS-II and h40-BS-III activated NF-κB (Figure 7, middle). These results strongly suggest that the TRAF6-binding site of RANK itself is unable to promote osteoclastogenesis.
Figure 7.
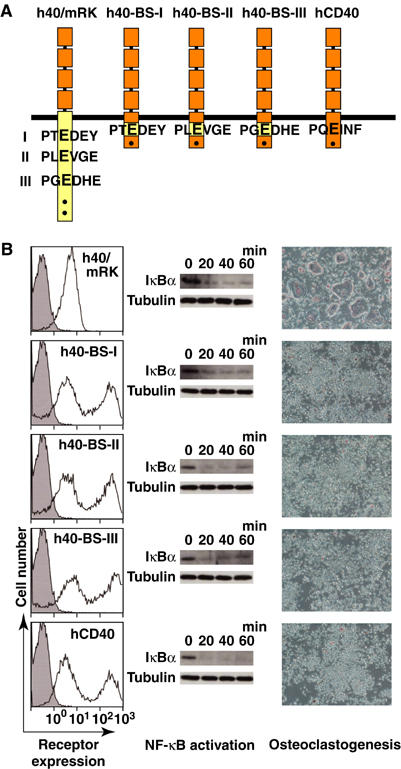
Inability of the TRAF6-binding site of RANK in the context of the cytoplasmic tail of CD40 to promote osteoclastogenesis. (A) Schematic diagram of chimeric receptors in which each TRAF6-binding site of RANK was substituted into CD40. (B) Expression levels and activities of various chimeric receptors and CD40. Chimeric receptors described in (A) were expressed in bone marrow cells, and the cells were then stimulated with anti-CD40 antibody as described in Materials and methods. Surface expression of various receptors was analyzed by flow cytometry (left). Degradation of IκBα upon stimulation was analyzed by Western blot (middle). Osteoclastogenesis was visualized by TRAP staining (right).
Discussion
In this study, we created an ex vivo osteoclast formation system driven by the signaling of a chimeric receptor, h40/mRK, which consists of the extracellular domain of human CD40 and the transmembrane and cytoplasmic domains of mouse RANK, to elucidate the molecular mechanisms by which RANK specifically promotes osteoclastogenesis. h40/mRK is stimulated by anti-CD40 monoclonal antibody that specifically stimulates human CD40 but not endogenous mouse CD40 present in progenitor cells. Therefore, expression levels and activities of hCD40, h40/mRK and mutant h40/mRK, into which mutations were introduced in the cytoplasmic region, could be compared. On the basis of data derived from this system, we conclude that two of the three putative TRAF6-binding sites of RANK (BS-I and BS-II in Figures 2A and 3A) can independently induce osteoclast formation. Therefore, the inability of CD40 to promote osteoclastogenesis is not caused by fewer TRAF6-binding sites in CD40 than in RANK but by the inability to induce sustained Ca2+ oscillations and subsequent induction of NFATc1, which is necessary for osteoclast formation (Ishida et al, 2002; Takayanagi et al, 2002). Because activation of NF-κB and MAPKs by the EAA or AEA mutant of h40/mRK was almost equal to that by CD40, RANK must have an additional ability to induce Ca2+-mediated expression and activation of NFATc1. Given that expression of NFATc1 and osteoclast formation can be induced by overexpression of TRAF6, RANK may amplify the TRAF6-mediated signal. In addition, approximately 100-fold expression of CD40 compared to that of h40/mRK resulted in low-level formation of functional osteoclasts with concomitant induction of NFATc1. This result also supports the idea that induction of sustained Ca2+ oscillations and NFATc1 requires amplification of TRAF6 activity. It has recently been reported that the Fc receptor common γ subunit (FcRγ) and DNAX-activating protein (DAP)12 activate calcium signaling through phospholipase Cγ during osteoclast formation (Koga et al, 2004). Therefore, the RANK–TRAF6 pathway but not the CD40–4TRAF6 pathway may activate signals through these immunoreceptor tyrosine-based activation motif (ITAM)-harboring adaptors, which may explain why stimulation of CD40 does not result in efficient induction of NFATc1 in osteoclast progenitor cells. Because the TRAF6-binding site of RANK in the context of the cytoplasmic tail of CD40 was unable to promote osteoclastogenesis, RANK may possess a unique domain that is separate from the TRAF6-binding site and acts to amplify TRAF6 activity by binding unidentified proteins or forming a specific conformation. Further studies are needed to elucidate the molecular mechanisms underlying RANK-specific amplification of TRAF6 function leading to calcium signaling and the induction of NFATc1.
Although the AAE mutant, in which only the TRAF6-binding site most distal to the transmembrane domain (BS-III in Figures 2A and 3A) is intact, activates NF-κB in transient transfection assays, this mutant did not activate NF-κB or MAPKs, nor did it promote osteoclastogenesis when expressed in progenitor cells and stimulated with anti-CD40 antibody. However, h40-BS-III, CD40 carrying the BS-III, activates NF-κB in a ligand stimulation-dependent manner, suggesting that BS-III may play a essential role in other functions of RANK including signaling leading to lymphotoxin secretion from fetal lymphotoxin-producing cells in lymph node organogenesis (Yoshida et al, 2002).
The ability of the double mutants EAA and AEA to induce ex vivo osteoclast formation and of the resulting osteoclasts to show bone-resorbing activity is similar to that of h40/mRK and the EEA mutant, which have two active TRAF6-binding sites. Why does RANK possess more than one TRAF6-binding site? Although the activation of NF-κB and MAPKs by EAA and AEA is similar to that induced by h40/mRK, the induction of NFATc1 by h40/mRK is slightly higher than that induced by the EAA and AEA mutants. Therefore, the several TRAF6-binding sites may cooperate to amplify TRAF6 activity more efficiently than a single binding site. Although the difference in ability of h40/mRK and that of single binding site mutants to induce NFATc1 does not correlate with their ability to promote osteoclastogenesis ex vivo, enhanced amplification of TRAF6 may be required for efficient osteoclast formation and for the establishment of normal bone-remodeling in vivo.
This study provides evidence that RANK-specific activation of TRAF6 leads to induction of NFATc1 and is critical for osteoclastogenesis. It has been reported that TRAF6 functions as a ubiquitin E3 ligase to catalyze lysine-63-linked ubiquitination of TRAF6 and IκB kinase (IKK)γ (also known as NEMO), which is triggered by oligomer formation of TRAF6 (Deng et al, 2000; Wang et al, 2001; Sun et al, 2004). Thus, RANK may facilitate oligomer formation of TRAF6 upon ligand stimulation much more efficiently than CD40. The requirement of TRAF6 E3 activity in NFATc1 induction remains to be elucidated. Identification of the specific structure of RANK that amplifies TRAF6 activity will be required to fully understand the molecular mechanisms of RANK-mediated osteoclast formation and to develop drugs to treat pathological bone resorption and bone metastasis of tumors.
Materials and methods
Construction of expression plasmids
Human CD40 cDNA was amplified by the polymerase chain reaction (PCR) and was inserted into the pMX-puro retrovirus vector (Kitamura, 1998) to generate pMX-hCD40. For expression of the h40/mRK chimeric receptor, PCR was used to amplify a cDNA fragment encoding the cytoplasmic tail and the transmembrane domain of mouse RANK (amino acids 199–625) and a cDNA fragment encoding the extracellular domain of human CD40 (amino acids 1–188). Both fragments were ligated into pMX-puro (Kitamura, 1998) to generate pMX-h40/mRK. Introduction of point mutations into the TRAF6-binding sites of hCD40/mRK was performed by the method of (Kunkel, 1985). For GST pull-down assays, cDNAs encoding the cytoplasmic tail of mouse RANK (amino acids 235–625) were isolated from pMX-h40/mRK and its TRAF6-binding site mutants and inserted into a mammalian expression vector, pME18S (Shiio et al, 1992), together with GST protein cDNA to express GST-tagged RANK cytoplasmic tail (GST-mRKcyt) and its mutant proteins. cDNAs encoding 17-mer peptides containing each of the three putative TRAF6-binding sites of mouse RANK (BS-I: 336-SRKIPT(E/A)DEYTDRPSQP-352; BS-II: 369-PFQEPL(E/A)VGENDSLSQC-385; BS-III: 443-SGNTPG(E/A)DHEPFPGSLK-459) were isolated by PCR with the use of pMX-h40/mRK and its TRAF6-binding site mutants as templates and were inserted into pME18S together with GST protein cDNA to express GST-BS-I, GST-BS-II and GST-BS-III. To express hCD40 carrying the TRAF6-binding site derived from mouse RANK, a portion of the hCD40 cDNA that encodes a 17-mer peptide containing the TRAF6-binding site (amino acids 229–245) was replaced with mRANK cDNA that encodes 17-mer peptides containing one of the TRAF6-binding sites (BS-I, BS-II or BS-III). The resulting chimeric cDNAs were inserted into pMX-puro to generate pMX-h40-BS-I, pMX-h40-BS-II and pMX-h40-BS-III.
Retrovirus-mediated gene transfer and ex vivo assays for differentiation and activation of osteoclasts
The packaging cell line Plat-E (2 × 106) cells were transfected with 2 μg of various retrovirus vectors. Virus stocks were prepared by collecting the cultured media 48 h after transfection. Bone marrow cells (1 × 106) derived from C57/BL6 mice were cultured in α-MEM/10% FBS with 10 ng/ml M-CSF (PeproTech) overnight in 24-well plates. Cells were subsequently incubated in virus stock medium containing 10 μg/ml polybrene for 4 h. Infected cells were then cultured for 1 day in the presence of M-CSF and were further cultured for 2 days with puromycin (2 μg/ml) in addition to M-CSF to remove uninfected cells. Cells were then unstimulated or stimulated with 0.1 μg/ml anti-human CD40 monoclonal antibody (G28-5: American Type Culture Collection (ATCC) HB9110) (Clark et al, 1988) or 100 ng/ml RANKL (Wako). Adherent cells were fixed in 10% formaldehyde, treated with ethanol:acetone (1:1) and stained for TRAP (Yasuda et al, 1998). TRAP+ multinucleated cells containing more than three nuclei were counted as osteoclasts. For pit formation assays, bone marrow cells were subjected to retroviral infection under identical conditions to those of the TRAP assay. Cells were recovered by addition of Cell Dissociation Solution Non Enzymatic (Sigma-Aldrich) and replated on serum-coated dentin slices (Wako). Anti-human CD40 antibody was added to the culture medium at 24 and 48 h after replating. Adherent cells were removed by ultrasonication after addition of 1 M NH4OH to the wells 6 days after the last anti-CD40 antibody administration. Resorption pits were visualized by staining with 0.5% toluidine blue, and the resorbed area was measured with the use of an image analysis system (System Supply) linked to a light microscope (Nikon). TRAP+ cells were counted in a matching set of cultures.
Cytometric analysis
Cells (1 × 106) were harvested with phosphate-buffered saline (PBS) containing 10 mM EDTA and incubated on ice for 1 h with phycoerythrin-conjugated anti-human CD40 antibody (Beckman-Coulter). Expression of h40/mRK or hCD40 was detected by flow cytometry (EPICS-XL; Beckman-Coulter).
GST pull-down assay and Western blot analysis
Human 293T cells were cotransfected with an expression vector for FLAG-tagged TRAF6 (pME-FLAG-TRAF6; Ishida et al, 1996a) and with an expression vector for GST-tagged RANK cytoplasmic tail or a 17-mer peptide containing a putative TRAF6-binding site. At 36 h after transfection, cells were harvested and lysed with TNE buffer (Ishida et al, 1996b) followed by centrifugation. The supernatant was incubated with glutathione-Sepharose beads (Amersham Biosciences) for 1 h at 4°C. After the beads were washed, the GST fusion protein complexes were separated on 10% polyacrylamide/SDS gels. For analysis of signal transduction, cells were washed with PBS and lysed in RIPA buffer (10 mM Tris–HCl (pH 7.2), 1% NP-40, 0.1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 1 mM EDTA). The lysates were separated on 10% polyacrylamide/SDS gels. For Western blot analysis, proteins separated on gels were transferred to PVDF membranes. The membranes were then incubated with anti-phospho-JNK, anti-phospho-p38, anti-phospho-p44/42 MAPK antibodies (Cell Signaling), anti-FLAG M2 monoclonal antibody (Sigma-Aldrich), anti-IκBα, anti-p38, anti-ERK2, anti-NFATc1 antibodies (Santa Cruz Biotechnology), anti-tubulin antibody (Oncogene) or anti-GST antibody prepared by injection of recombinant GST protein into rabbits. After three washes, the membranes were incubated for 1 h at room temperature with anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase (Amersham Biosciences). Immunoreactive proteins were visualized by the ECL detection system (Amersham Biosciences).
Electrophoretic mobility-shift assays
Nuclear extracts were prepared as described (Andrews and Faller, 1991) from bone marrow cells that were either unstimulated or stimulated with CD40 ligand (CD40L, R&D Systems). Equal amounts of extract (5 μg protein) were incubated with 32P-labeled double-stranded oligonucleotide containing a κB site from the mouse κ light-chain enhancer. The DNA–protein complexes were analyzed as described previously (Kobayashi et al, 2001).
Ca2+ measurement
For Ca2+ measurement, cells were incubated in the presence of 5 μM fluo-4 AM, 5 μM Fura Red AM and 0.05% pluronic F127 for 30 min in serum-free α-MEM. Cells were then washed twice with α-MEM and incubated in the presence of α-MEM with 10% FBS and M-CSF for 20 min. Cells loaded with these dyes were washed three times with Hanks' balanced salt solution and mounted on the inverted stage of a confocal microscope (Leica). At an excitation wavelength of 488 nm, emission at 505–530 nm for fluo-4 and 600–680 nm for Fura Red was analyzed simultaneously at 5-s intervals. To estimate the intracellular Ca2+ concentration ([Ca2+]i) in single cells, the ratio of the fluorescence intensity under baseline conditions was divided by the maximum ratio increase obtained by adding 10 μM ionomycin and was expressed as the percent maximum ratio increase.
Acknowledgments
We thank Drs T Matsumura, T Akiyama and K Semba for helpful discussions, S Yamane, S Azuma, S Maeda and M Ogino for technical assistance and Y Tanaka for secretarial assistance. This work was supported by Grants-in-Aid for Special Coordination Funds for Promoting Science and Technology and Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government, and by grants from the Cosmetology Research Foundation and the Mitsubishi Foundation.
References
- Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19: 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC (2002) A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem 277: 44347–44356 [DOI] [PubMed] [Google Scholar]
- Bodmer JL, Schneider P, Tschopp J (2002) The molecular architecture of the TNF superfamily. Trends Biochem Sci 27: 19–26 [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G (2004) A physical and functional map of the human TNF-α/NF-κB signal transduction pathway. Nat Cell Biol 6: 97–105 [DOI] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV (1996) TRAF6 is a signal transducer for interleukin-1. Nature 383: 443–446 [DOI] [PubMed] [Google Scholar]
- Cheng G, Cleary AM, Ye ZS, Hong DI, Lederman S, Baltimore D (1995) Involvement of CRAF1, a relative of TRAF, in CD40 signaling. Science 267: 1494–1498 [DOI] [PubMed] [Google Scholar]
- Clark EA, Yip TC, Ledbetter JA, Yukawa H, Kikutani H, Kishimoto T, Ng MH (1988) CDw40 and BLCα-specific monoclonal antibodies detect two distinct molecules which transmit progression signals to human B lymphocytes. Eur J Immunol 18: 451–457 [DOI] [PubMed] [Google Scholar]
- Darnay BG, Ni J, Moore PA, Aggarwal BB (1999) Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-κB-inducing kinase. J Biol Chem 274: 7724–7731 [DOI] [PubMed] [Google Scholar]
- David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF (2002) JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci 115: 4317–4325 [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361 [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11: 3482–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert L, Tometsko ME, Anderson DM, Cosman D, Dougall WC (1998) The involvement of multiple tumor necrosis factor receptor (TNFR)-associated factors in the signaling mechanisms of receptor activator of NF-κB, a member of the TNFR superfamily. J Biol Chem 273: 34120–34127 [DOI] [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96: 3540–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Nishimura R, Matsubara T, Tanaka S, Inoue J, Reddy SV, Hata K, Yamashita K, Hiraga T, Watanabe T, Kukita T, Yoshioka K, Rao A, Yoneda T (2004) Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest 114: 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T (2000) Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp Cell Res 254: 14–24 [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3: 1285–1289 [DOI] [PubMed] [Google Scholar]
- Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277: 41147–41156 [DOI] [PubMed] [Google Scholar]
- Ishida T, Mizushima S, Azuma S, Kobayashi N, Tojo T, Suzuki K, Aizawa S, Watanabe T, Mosialos G, Kieff E, Yamamoto T, Inoue J (1996a) Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem 271: 28745–28748 [DOI] [PubMed] [Google Scholar]
- Ishida T, Tojo T, Aoki T, Kobayashi N, Ohishi T, Watanabe T, Yamamoto T, Inoue J (1996b) TRAF5, a novel tumor necrosis factor receptor-associated factor family protein, mediates CD40 signaling. Proc Natl Acad Sci USA 93: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T (1998) New experimental approaches in retrovirus-mediated expression screening. Int J Hematol 67: 351–359 [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J (2001) Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20: 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428: 758–763 [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323 [DOI] [PubMed] [Google Scholar]
- Kunkel TA (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82: 488–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macián F, Garcia-Rodriguez C, Rao A (2000) Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J 19: 4783–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Sudo T, Saito T, Osada H, Tsujimoto M (2000) Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J Biol Chem 275: 31155–31161 [DOI] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J (2002) Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4: 556–564 [DOI] [PubMed] [Google Scholar]
- Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, Inoue J (1999) Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4: 353–362 [DOI] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289: 1508–1514 [DOI] [PubMed] [Google Scholar]
- Shiio Y, Yamamoto T, Yamaguchi N (1992) Negative regulation of Rb expression by the p53 gene product. Proc Natl Acad Sci USA 89: 5206–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ (2004) The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell 14: 289–301 [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S (2000) Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43: 259–269 [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901 [DOI] [PubMed] [Google Scholar]
- Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J (1999) Two differently regulated nuclear factor κB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci USA 96: 1234–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ (2001) TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351 [DOI] [PubMed] [Google Scholar]
- Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y (1998) The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem 273: 28355–28359 [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Miyazaki T, Kadono Y, Takayanagi H, Miura T, Nishina H, Katada T, Wakabayashi K, Oda H, Nakamura K, Tanaka S (2002) Possible involvement of IκB kinase 2 and MKK7 in osteoclastogenesis induced by receptor activator of nuclear factor κB ligand. J Bone Miner Res 17: 612–621 [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H (2002) Distinct molecular mechanism for initiating TRAF6 signalling. Nature 418: 443–447 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S (2002) Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity 17: 823–833 [DOI] [PubMed] [Google Scholar]


