Abstract
Background
Metabolic syndrome (MetS) is a major risk factor for cardiovascular disease and diabetes. Previous studies in obese children demonstrating a positive association between serum uric acid (sUA) and components of MetS are confounded by lack of uniformity in age and pubertal status of children. Therefore, we have examined the role of sUA in MetS and its components in pre-pubertal children (Tanner Stage I, age ≤ 9 years).
Methods
Pre-pubertal obese children (32 boys, 27 girls, age 6–9 years) were recruited from Nuevo Leon, Mexico. For comparison, an equal number of children with normal body mass index (BMI) in the same age range (22 Boys, 39 girls, age 6–9 years) were also recruited from the same community. Presence of MetS and its components was defined according to the criteria of International Diabetes Federation. Fasting blood was analyzed for lipids, glucose, insulin, and uric acid.
Results
Among the obese children, sUA was positively associated with insulin resistance and hypertriglyceridemia and negatively associated with high density lipoprotein-cholesterol (HDLc). Subjects were three times more likely to have a MetS diagnosis per one unit (md/dL) difference in sUA. Of the 59 obese pre-pubertal children, 20 were classified as having MetS defined by the presence of abdominal obesity and two or more of other components described under methods. Of these, 57.1% (20/61) had sUA between 5.1 and 7.1 mg/dl.
Conclusions
The findings of this study clearly indicate a positive relationship between uric acid and MetS and its components in pre-pubertal obese children with Tanner stage I and ≤9 years of age.
Background
There has been a precipitous rise in the prevalence and magnitude of childhood obesity over the last few decades [1]. Unfortunately, it is difficult to determine the rate of prevalence of metabolic syndrome (MetS) in obese children due to complexity of definition, and differences such as ethnicity, gender and sexual maturity [2]. However, most studies support the notion that the prevalence of MetS is high among obese children, and it increases with degree of obesity [2].
Similar to adult population, there have been scores of epidemiologic and observational studies examining the role of serum uric acid (sUA) in MetS in children [3–11]. While in general results of these studies support a direct relationship between sUA and MetS, the data for analyses were pooled from pre-, peri- and post-pubertal children without any control for differences in their sexual maturity [3–11]. While the above studies [3–11] support the conclusion that the odds ratio of having metS or one or more its components is associated with sUA, seven of the studies [3, 4, 6, 7, 9–11] include data from pre-pubertal, post-pubrtal and post-pubertal children ranging in age between 4 and 18 years making it difficult to assess the role of sUA in MetS in just pre-pubertal children. The remaining two studies [5, 8] included peri-pubertal children ranging in age between 10 and 13 years.
Since sex steroids are known to control uricemia as well as sexual maturity [12–15], the lack of regard for sexual maturity in subject selection in these studies requires a re-examination of the relationship. Therefore, in the present study, we have examined the role of sUA in MetS and its components in obese pre-pubertal (Tanner stage I) elementary school children from Mexico and compared it with sexual maturity matched normal body mass index (BMI) children in the same age range.
Methods
Study population
In this cross-sectional study, pre-pubertal children were recruited through a summer health camp for childhood obesity prevention at Universidad Autónoma de Nuevo León [16]. For comparison, children with normal BMI were also recruited from the same community. The study subjects were from elementary schools in metropolitan area of Monterrey City and rural municipalities of Nuevo León, México. The summer camps are organized annually for obese children who come from low-income families living in poverty or extreme poverty and qualify for medical care through the public health system of México. The same socio-economic measures were applied to select the children with normal BMI.
Subject selection
The study was approved by the Research Ethical Committee of the Public Health and Nutrition School at Universidad Autónoma de Nuevo León, which is registered with the State Research Ethical Committee in concordance with the Health General Law of Mexico. All the children and their parents signed a letter of agreement and consent form.
The major goal of this study was to examine the association between sUA with MetS and its components in pre-pubertal children. Both sUA and the onset of puberty are affected by gender, age and adiposity [17]. Therefore, it was important that children selected for this study be not only comparable in age, but are also pre-pubertal. To this end, a pediatrician screened 200 consecutive obese children in the age group of 6–9 years for sexual maturity (Tanner Stages of puberty). Of these, 59 obese children were classified as Tanner Stage I (Table 1). A similar screening of normal BMI children yielded 61 children belonging to Tanner Stage I [16].
Table 1.
Characteristics of the study population
| Variables | Normal (n = 61) |
Obese (n = 59) |
p-value |
|---|---|---|---|
| Age, years | 7.3 ± 1.1 | 8.0 ± 1.0 | 0.001a |
| Tanner Stage | I | I | |
| Body Mass Index | 16.2 ± 1.2 | 26.2 ± 4.4 | 0.000a |
| Waist circumference, cm | 55.8 ± 3.9 | 83.8 ± 11.0 | 0.000b |
| Systolic blood pressure, mmHg | 83.0 ± 8.9 | 94.1 ± 10.9 | 0.000a |
| Diastolic blood pressure, mmHg | 59.6 ± 7.1 | 63.5 ± 9.6 | 0.056a |
| Fasting glucose, mg/dL | 80.6 ± 6.6 | 80.2 ± 6.7 | 0.741b |
| Fasting Insulin, UI/mL | 8.1 ± 3.2 | 26.8 ± 20.1 | 0.000 a |
| HOMA | 1.6 ± 0.7 | 5.3 ± 4.0 | 0.000a |
| Triglycerides, mg/dL | 104.5 ± 31.1 | 155.4 ± 88.9 | 0.001a |
| Total cholesterol, mg/dL | 160.7 ± 25.3 | 163.6 ± 34.0 | 0.598b |
| LDL-C, mg/dL | 87.3 ± 22.4 | 93.8 ± 28.5 | 0.165b |
| HDL-C, mg/dL | 52.9 ± 13.4 | 39.0 ± 8.2 | 0.000a |
| Uric Acid, mg/dL | 3.4 ± 0.6 | 4.4 ± 0.9 | 0.000b |
All data except Tanner Stage are presented as mean ± SD. aThe Mann-Whitney U test. bt-Test for independent samples
Anthropometric and blood pressure measurement
Weight was measured using digital scales (TanitaBC-533) while subjects were minimally clothed and without shoes, recorded to the nearest 100 g. Height was measured to the nearest 1 cm using a non-elastic tape meter while subjects were in a barefoot standing position, with their shoulders in a normal position. BMI was calculated as weight in kilograms divided by the square of height in meters. Presence of obesity was determined by BMI-for-age using WHO Reference [18]. Blood pressure (BP) was measured twice in the right arm of subjects who had been resting for at least 10 min in a seated position using a mercury sphygmomanometer.
Metabolic syndrome and its components
Presence of MetS was determined using the definition of the International Diabetes Federation (IDF) [19]. According to the IDF definition, someone has the metabolic syndrome if he or she has central adiposity (waist circumference (WC) ≥ 90th percentile) plus two or more of the following four factors [19]: a) systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, b) fasting triglycerides (TG) ≥150 mg/dL, c) high density lipoprotein (HDLc) < 40 mg/dL and d) fasting glucose ≥100 mg/dL).
Biochemical measurements
Blood was collected between 07:30 and 08:00 AM from the antecubital vein after an 8–12 h overnight fast and centrifuged within 2 h for separation of serum. Aliquoted samples were stored at −20 °C until analyses. Serum Total cholesterol and TG were determined enzymatically by an autoanalyzer using commercial kits available (Beckman Coulter, Inc., CA, USA). Serum HDLc was measured similarly after precipitation with magnesium phosphotungstate. Serum low density lipoprotein-cholesterol (LDLc) was calculated using Friedwald’s formula [20] as shown below.
Fasting plasma glucose was measured via colorimetric assay and insulin levels were determined using radioimmunoassay. Serum uric acid levels were determined colorimetrically using Uricase. Assays were done in triplicate and were performed at the General and Endocrinology Laboratories of the Hospital Universitario Dr. José Eleuterio González. The laboratory routinely monitors both inter- and intra-assay coefficients of variation for all assays with a goal to keep it at 5% or below. For example, in our insulin assay, inter- and intra-assay coefficient of variation was 2.9–3.8% and 2.5–4.4%, respectively. Insulin-resistance (IR) was evaluated with the aid of homeostasis model assessment (HOMA) and defined as HOMA >2.7 (HOMA-IR) [21].
Statistical analyses
Continuous data is presented as means ± SD. A post-hoc power analysis was performed to determine the statistical power to detect significant differences for the main comparison. Using a moderate effect size of 0.50, an alpha of 0.05, and a combined sample size n = 120; we calculated a power of 0.845, that meets the minimum suggested power for a study [22]. Data were analyzed using multivariate statistical software SPSS (version 22). Differences in the components of the MetS, age, Tanner stage, fasting insulin and HOMA among the different groups were analyzed by descriptive and exploratory statistical analyses. Mann-Whitney U test was applied to examine statistical significance among the variables such as age, BMI, systolic pressure, diastolic pressure, fasting insulin, HOMA, triglycerides and HDLc. A t-test for independent samples was used to examine differences in means between the obesity group and normal BMI control group based on identified factors: glucose, waist circumference, total cholesterol, and LDLc. To determine the statistical association between the presence of Met S and sUA, a binary logistic regression equation was utilized. The predictive variables were age, (continuous), the concentration of sUA (continuous), gender (dichotomous) and BMI (continuous). The scatter-plots were made with their respective linear correlation and equation of simple linear regression to examine the relationship between concentration of sUA to fasting insulin, HOMA, HDLc, and triglycerides.
Results
Distribution of sUA in obese and normal BMI pre-pubertal children in the age group 6–9 years is shown in Fig. 1. There were more children with high sUA in the obese group (43/59, Range: 2.5–7.5 mg/ml; 70% with sUA ≥4.0 mg/ml) than in the normal BMI group (9/61, Range: 2.2–4.8 mg/ml; 15% with sUA ≥4.0 mg/dl) (p = 0.001).
Fig. 1.
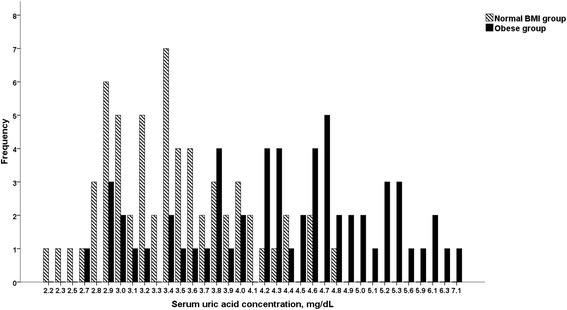
Distribution of the serum uric acid in obese and normal BMI pre-pubertal children (n = 120) aged 6–9 years. Frequency = Number of children
The baseline characteristics of children in the obese and normal BMI groups are shown in Table 1. Both normal BMI and obese groups were matched closely in sample size and age. Children in the obese group were only 8 months older, however, children in both groups were under 9 years of age. Furthermore, all obese and normal BMI children were pre-pubertal with Tanner stage I. Children in the obese group had significantly greater waist circumference (p = 0.001), increased systolic blood pressure (p = 0.001), elevated fasting insulin (p = 0.001), insulin resistance measured by HOMA (p = 0.001), higher TG (p = 0.002), higher sUA (p = 0.001), and lower HDLc (p = 0.001) compared to the normal BMI group. These data clearly show the presence of components of MetS in pre-pubertal obese children.
Of the 59 obese pre-pubertal children, 32 were boys and 27 were girls. We next analyzed gender differences between the prevalence of the components of MetS in obese children. Results presented in Table 2 show that obese girls were at a higher risk for insulin resistance than obese boys as shown by increased fasting insulin (p = 0.003) and HOMA values (p = 0.00). Also, obese girls had significantly lower HDLc than obese boys (p = 0.014). Interestingly, however, there was no difference in the levels of sUA between obese boys and obese girls (p = 0.543).
Table 2.
Characteristics of the obese group by gender
| Obese | |||
|---|---|---|---|
| Variables | Boys (n = 32) |
Girls (n = 27) |
p-value |
| Age, years | 7.8 ± 1.0 | 8.2 ± 0.9 | 0.132a |
| Tanner Stage | I | I | |
| Body Mass Index | 26.3 ± 4.8 | 26.2 ± 3.9 | 0.999a |
| Waist circumference, cm | 84.4 ± 12.2 | 83.0 ± 9.5 | 0.646b |
| Systolic blood pressure, mmHg | 95.0 ± 13.1 | 92.9 ± 7.7 | 0.734a |
| Diastolic blood pressure, mmHg | 65.6 ± 11.3 | 61.1 ± 6.4 | 0.122a |
| Fasting glucose, mg/dL | 80.4 ± 6.4 | 79.9 ± 7.2 | 0.761b |
| Fasting Insulin, UI/mL | 20.5 ± 13.5 | 34.2 ± 24.1 | 0.002a |
| HOMA | 4.1 ± 2.7 | 6.7 ± 4.7 | 0.003a |
| Triglyceride, mg/dl | 144.3 ± 88.7 | 168.4 ± 89.0 | 0.191a |
| Total cholesterol, mg/dl | 167.5 ± 32.9 | 158.9 ± 35.4 | 0.337b |
| LDL-C, mg/dL | 98.0 ± 29.4 | 88.9 ± 27.2 | 0.226b |
| HDL-C, mg/dL | 41.3 ± 8.5 | 36.2 ± 7.0 | 0.017b |
| Uric Acid, mg/dL | 4.5 ± 1.0 | 4.3 ± 0.7 | 0.430b |
aThe Mann-Whitney U test. bt-Test for independent samples
We used a multivariate analysis to establish the association between sUA and components of MetS after adjusting for gender, age, and BMI (Table 3). The results show that with one unit difference (1 unit = 1 mg/dL) in sUA, there were 3.9 times more likely to have a MetS diagnosis as defined by IDF. Similarly, higher sUA levels are significantly associated with high waist circumference, high TG and low HDLc. Of the 59 obese pre-pubertal children, 20 were classified as having MetS defined by the presence of abdominal obesity and two or more of other components described under methods. Of these, 57.1% (20/61) had sUA between 5.1 and 7.1 mg/dl.
Table 3.
Adjusted odds ratios (95% CI)* for association 1 between the MetS and 2 its components with sUA
| Variables | Odds ratio | 95% CI | p-value | |
|---|---|---|---|---|
| Metabolic syndrome n = 59 | Age (yr) | 0.620 | [0.302, 1.273] | 0.193 |
| Uric Acid (mg/dL) | 3.942 | [1.589, 9.775] | 0.003 | |
| Gender | 0.208 | [0.048, 0.901] | 0.208 | |
| BMI (kg/m2) | 1.113 | [0.949, 1.304] | 0.187 | |
| Abdominal obesity (waist circumference > 90th percentile) n = 53 | Age (yr) | 0.677 | [0.328, 1.397] | 0.291 |
| Uric Acid (mg/dL) | 3.854 | [1.518, 9.787] | 0.005 | |
| Gender | 0.212 | [0.048, 0.930] | 0.040 | |
| BMI (kg/m2) | 1.074 | [0.907, 1.271] | 0.410 | |
| High triglyceride (≥150 mg/dL) n = 25 | Age (yr) | 0.504 | [0.124, 2.048] | 0.338 |
| Uric Acid (mg/dL) | 2.267 | [0.560, 9.180] | 0.251 | |
| Gender | 0.474 | [0.029, 7.801] | 0.601 | |
| BMI (kg/m2) | 1.406 | [0.835, 2.369] | 0.200 | |
| Low HDL-C (<40 mg/dL) n = 33 |
Age (yr) | 0.210 | [0.031, 1.008] | 0.110 |
| Uric Acid (mg/dL) | 65.751 | [2.509,1723.076] | 0.012 | |
| Gender | 0.040 | [0.001, 2.003] | 0.107 | |
| BMI (kg/m2) | 1.559 | [0.764, 3.182] | 0.223 |
All the models were adjusted by gender (men compared with women), age (continuous), body mass index (continuous) and concentration of uric acid (continuous)
*The unit of the exposures for the OR estimate was the presence or absence of Metabolic
Syndrome or its components- abdominal obesity, high triglycerides and low HDL-C
Data presented in Fig. 2 show the positive relationship (p < 0.05) between elevated sUA and measures of insulin sensitivity (insulin in Panel A and HOMA in Panel B). Similarly, hyperuricemia was positively associated (p < 0.05) with hypertriglyceridemia in Panel C and and negatively associated with HDLc in Panel D.
Fig. 2.
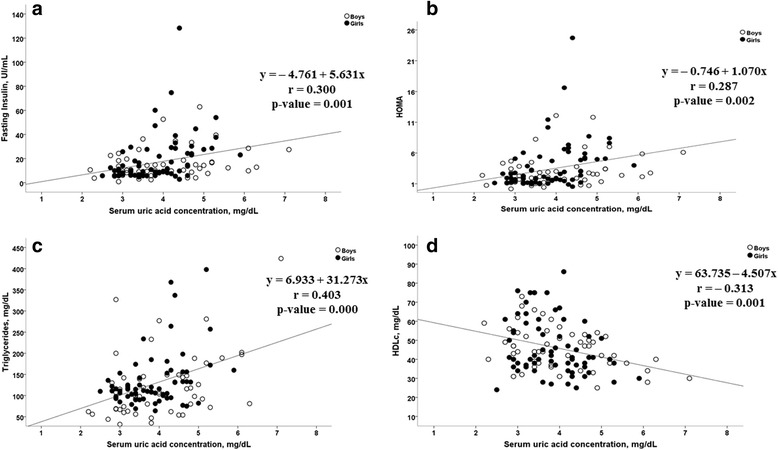
Association between serum serum uric acid concentration and fasting insulin, HOMA, trigycerides and HDLc. a Insulin (y = − 4.761 + 5.631×; r = 0.287; p-value = 0.001, two-tailed analysis; n = 119). b HOMA (y = − 0.746 + 1.070×; r = 0.300; p-value = 0.002, two-tailed analysis; n = 119). c Triglycerides (y = 6.933 + 31.273×; r = 0.403; p-value = 0.000, two-tailed analysis; n = 119). d HDLc (y = 63.735–4.507×; r = − 0.313; p-value = 0.001, two-tailed analysis; n = 119)
Discussion
The conclusion of the earlier studies examining relationship between sUA and higher odds ratio for MetS or its components in children are similar to our observation [3–11]. However, the conclusions reached in these studies were confounded because they did not control for race/ethnicity, gender, age and stages of sexual maturity in subject selection [3–11]. For example, Gill-Gampos et al. reported a positive association between the features of insulin resistance and hyperuricemia in prepubertal children ranging in ages between 6 and 12 years [23]. Similar to our results, Viazzi et al. reported that hyperuricemia was associated with increased blood pressure in children ranging in age between 6 and 18 years [24]. Multiple other studies have examined such associations and have suggested a role for hyperuricemia in MetS and its components in younger population [3–11]. The data presented in Table 3 are the first to show an association between risks for known components of MetS and sUA in obese prepubertal children (age: 6–9 years and Tanner Stage I) in Monterrey, Mexico. These data offer the possibility of use of sUA as a predictor of MetS in pre-pubertal children.
The results of these studies offer only a predictive relationship between sUA and MetS in obesity. The cause and effect relationship between sUA and obesity can only be speculated at this time. Approximately two thirds of total body uric acid is produced endogenously, while the remaining one third results from metabolism of dietary purines [25]. Hyperuricemia may occur because of increased production (overproducers), decreased excretion (underexcretors), or a combination of these two mechanisms. In a recent study, Tsushima et al. [26], demonstrated elevated uric acid secretion from whole adipose tissue in obese vs. lean mice, and from 3 T3-L1 adipocytes under hypoxia suggesting that purine catabolism to uric acid in adipose tissue could be enhanced in obesity. In support of decreased excretion, Yamashita et al. [27] reported marked reduction of renal uric acid excretion in obese subjects and its improvement by a low-calorie diet. Matsuura et al. [28] observed that while all obese subjects had higher sUA than normal-weight control subjects, subjects with visceral obesity were linked more closely to overproduction and under excretion of uric acid. Taken together, these studies support the thesis that both uric acid production and excretion play an active role in determining the state of sUA. However, since these foregoing observations came from animal, cell culture and adult human studies, their relevance to prepubertal children needs to be examined.
Limitations of this study
While the strength of this study lies in our demonstration of an association between higher sUA and higher odds ratio for components of MetS, this is a cross-sectional study and causality cannot be inferred. The relationship between pediatric obesity and MetS is complex because the risk for sequelae of both obesity and MetS vary among individuals based on ethnicity, socio-economic status and associated lifestyle practices [29].
Conclusions
Compared with normal weight children, obese children are more likely to experience hyperuricemia. Also there was a positive relationship between sUA and MetS and its components in pre-pubertal obese children with Tanner stage I and ≤9 years of age. Further studies are needed to understand role of uric acid in eliciting MetS and its components these children.
Acknowledgements
Authors thank Prof. Robertino Mera MD, PhD, an expert in epidemiology from Vanderbilt School of Medicine, Nashville, TN, USA for editing the manuscript.
Funding
This study was partially founded by the UANL Support Program of Scientific Research and Technology (PAICYT), the government of Nuevo León, México and the Endocrinology Service of UANL Hospital Dr. José Eleuterio González, Monterrey, Nuevo León, México.
Availability of data and materials
Due to ethics and consent agreements with parents of study subjects, the dataset cannot be deposited in publicly available repositories. Investigators wishing to access data can contact Prof. Elizabeth Solis Perez for a case by case determination.
Abbreviations
- BMI
Body mass index
- BP
Blood pressure
- HDLc
High density lipoprotein-cholesterol
- HOMA
Homeostasis model assessment
- IR
Insulin-resistance
- LDLc
Low density lipoprotein-cholesterol
- MetS
Metabolic syndrome
- OR
Odds ratio
- SD
Standard deviation
- sUA
Serum uric acid
- TG
Triglycerides
- WC
Waist circumference
Authors’ contributions
ESP contributed to the conception and organization of the study. She also worked contributed to manuscript writing. MAGM was responsible for data management and statistical multivariate analysis. ML-CL contributed to the conception of the study and revision of the manuscript. VLTG contributed to the concentration of database and supervised the laboratory reports. JZVP supervised the laboratory procedures and contributed to the methodology design. FJLG contributed to the interpretation and analysis of the results. VI, SJ, PV, and KB contributed to interpretation of the data and revisions of the manuscript. They reviewed and approved the final version of this manuscript. CP prepared the first draft of the manuscript. He also served as a liaison between all authors during the entire process of data analyses and manuscript writing. All authors read and approved the final manuscript.
Authors’ information
Elizabeth Solís Pérez is a Full Time Professor and Chair of the Childhood Obesity Program, in the Public Health and Nutrition School at the Universidad Autónoma de Nuevo León, Monterrey, NL, México (UANL). Her interest and expertise areas lies in nutrition during childhood obesity and related diseases. Mario Alberto González Medina is a Professor in the Public Health and Nutrition School at UANL. His research expertise lies in the multivariate data analysis. Manuel López-Cabanillas Lomelí is a Full Time Professor at Public Health and Nutrition School at UANL. His area of interest is on nutrition intervention programs and his expertise lies in food sciences. Verónica Lissett Tijerina González is a clinical research assistant at Endocrinology Service, University Hospital at UANL. Her area of interest is in nutrition research and childhood Obesity. Jesús Zacarías Villarreal Pérez is a Professor of Medicine at UANL. Member of the National System of Investigators (SNI) in México, Head of the Endocrinology Service at the University Hospital. His area of interest is in research of metabolic diseases, obesity, diabetes mellitus and metabolic syndrome. Fernando J Lavalle-Gonzalez is a Professor of Medicine at UANL, Member of the National System of Investigators (SNI) in México, Head of the Clinic of Diabetes at the University Hospital, President of the Mexican Board of Endocrinology. His area of interest is in research of Diabetes Mellitus, Obesity and MetS. Victorine Imrhan is Professor in the Department of Nutrition and Food Sciences at Texas Woman’s University (TWU). Her research expertise is associated with nutrition education in obese children. Shanil Juma is Associate Professor in the Department of Nutrition and Food Sciences at TWU. His expertise is associated with age related conditions such as osteoarthritis and osteoporosis and the anti-inflammatory and anti-oxidative action of dietary polyphenols. Parakat Vijayagopal is Professor in the Department of Nutrition and Food Sciences at TWU. His expertise is in nutrition and obesity. Kittipong Boonme is Assistant Professor in the School of Management at TWU. His research expertise lies in applied statistics. Chandan Prasad is a Professor in the Department of Nutrition and Food Sciences at the TWU, Denton, TX and Professor (Emeritus), Department of Internal Medicine (Endocrinology) at the Louisiana State University Health Sciences Center, New Orleans, LA. His research expertise lies in the exploration of inflammatory biomarkers in human as well as animal models of obesity.
Ethics approval and consent to participate
The study was approved by the Research Ethical Committee of the Public Health and Nutrition School at Universidad Autónoma de Nuevo León, which is registered with the State Research Ethical Committee in concordance with the Health General Law of Mexico. All the children and their parents signed a letter of agreement and consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elizabeth Solis Perez, Email: elizabeth.solis@uanl.mx.
Mario Alberto González Medina, Email: mario.gonzalez@iiiepe.edu.mx.
Manuel Lopez-Cabanillas Lomeli, Email: lolm27@hotmail.com.
Verónica Tijerina González, Email: veronica.tijerinag@uanl.mx.
Jesús Zacarías Villarreal Pérez, Email: zacvilla@yahoo.com.mx.
Fernando J. Lavalle González, Email: drfernandolavalle@hotmail.com
Victorine Imrhan, Email: VImrhan@mail.twu.edu.
Shanil Juma, Email: SJuma@twu.edu.
Parakat Vijayagopal, Email: PVijayagopal@mail.twu.edu.
Kittipong Boonme, Email: kboonme@mail.twu.edu.
Chandan Prasad, Phone: +1 904-898-2652, Email: CPrasad@mail.twu.edu.
References
- 1.Von Hippel PT, Nahhas RW. Extending the history of child obesity in the United States: the fels longitudinal study, birth years 1930 to 1993. Obesity (Silver Spring, Md) 2013;21(10):2153–2156. doi: 10.1002/oby.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R. Childhood Metabolic Syndrome: Must we define it to deal with it? Diabetes Care. 2011;34(Suppl 2):S171–S176. doi: 10.2337/dc11-s214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Civantos Modino S, Guijarro de Armas MG, Monereo Mejías S, et al. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol Nutr. 2012;59:533–538. doi: 10.1016/j.endonu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Cardoso AS, Gonzaga NC, Medeiros CC, et al. Association of uric acid levels with components of metabolic syndrome and non-alcoholic fatty liver disease in overweight or obese children and adolescents. Pediatr (Rio J) 2013;89:412–418. doi: 10.1016/j.jped.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Pacifico L, Cantisani V, Anania C, et al. Serum uric acid and its association with metabolic syndrome and carotid atherosclerosis in obese children. Eur J Endocrinol. 2009;160(1):45–52. doi: 10.1530/EJE-08-0618. [DOI] [PubMed] [Google Scholar]
- 6.Sun D, Li S, Zhang X, et al. Uric acid is associated with metabolic syndrome in children and adults in a community: The Bogalusa Heart Study. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0089696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115(19):2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 8.Ságodi L, Fehér V, Kiss-Tóth E, et al. Metabolic complications of obesity during adolescence, particularly regarding elevated uric acid levels. Orv Hetil. 2015;156:888–895. doi: 10.1556/650.2015.30140. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Jiang R, Li L, et al. Prevalence and risk factors of metabolic syndrome in school adolescents of northeast China. Pediatr Endocrinol Metab. 2014;27:525–532. doi: 10.1515/jpem-2013-0336. [DOI] [PubMed] [Google Scholar]
- 10.Ishiro M, Takaya R, Mori Y, et al. Association of uric acid with obesity and endothelial dysfunction in children and early adolescents. Ann Nutr Metab. 2013;62:169–176. doi: 10.1159/000346227. [DOI] [PubMed] [Google Scholar]
- 11.Koborová I, Gurecká R, Hlavatá A, et al. Association between asymptomatic hyperuricaemia and metabolic syndrome in the adolescents. Vnitr Lek. 2015;61:42–49. [PubMed] [Google Scholar]
- 12.Mumford SL, Dasharathy SS, Pollack AZ, et al. Serum uric acid in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle study. Hum Reprod. 2013;28(7):1853–1862. doi: 10.1093/humrep/det085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamopoulos D, Vlassopoulos C, Seitanides B, et al. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol. 1977;85:198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- 14.Ducharme JR, Forest MG, De Peretti E, et al. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42:468–476. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- 15.Garbagnati E, Boschetti M. Uric acid homeostasis in lean and obese girls during pubertal development. Metabolism. 1994;43:819–21. doi:10.1016/0026-0495(94)90260-7. [DOI] [PubMed]
- 16.Gutiérrez JP, Rivera-Dommarco J, Shamah-Levy T, Villalpando-Hernández S, Franco A, Cuevas-Nasu L, Romero-Martínez M, Hernández-Ávila M. Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública (MX); 2012. [Google Scholar]
- 17.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999-2006. Metabolism. 2012;61:554–561. doi: 10.1016/j.metabol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onis MD, Onyango AW, Borghi E, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome. Available from: https://www.idf.org.
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Turner RC, Holman RR, Matthews D, et al. Insulin deficiency and insulin resistance interaction in diabetes: estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism. 1979;28:1086–1096. doi: 10.1016/0026-0495(79)90146-X. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioural sciences. Hillside. NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 23.Gil-Campos M, Aguilera CM, Cañete R, et al. Uric acid is associated with features of insulin resistance syndrome in obese children at prepubertal stage. Nutr Hosp. 2009;24(5):607–613. [PubMed] [Google Scholar]
- 24.Viazzi F, Antolini L, Giussani M, et al. Serum uric acid and blood pressure in children at cardiovascular risk. Pediatrics. 2013;132(1):e93–e99. doi: 10.1542/peds.2013-0047. [DOI] [PubMed] [Google Scholar]
- 25.Bobulescu IA, Moe OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, Shirakura T, Kato K, Imaizumi K, Takahashi H, Tamura M, Maeda N, Funahashi T, Shimomura I. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita S, Matsuzawa Y, Tokunaga K, Fujioka S, Tarui S. Studies on the impaired metabolism of uric acid in obese subjects: marked reduction of renal urate excretion and its improvement by a low-calorie diet. Int J Obesity. 1986;10:255–264. [PubMed] [Google Scholar]
- 28.Matsuura E, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. 1998;47:929–933. doi: 10.1016/S0026-0495(98)90346-8. [DOI] [PubMed] [Google Scholar]
- 29.DeBoer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279–289. doi: 10.1586/eem.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethics and consent agreements with parents of study subjects, the dataset cannot be deposited in publicly available repositories. Investigators wishing to access data can contact Prof. Elizabeth Solis Perez for a case by case determination.


