Abstract
Purpose
DOTA-AR, a bombesin-antagonist peptide, has potential clinical application for targeted imaging and therapy in Gastrin Releasing Peptide receptor (GRPr) positive malignancies when conjugated with a radioisotope such as 90Y. This therapeutic potential is limited by the fast washout of the conjugates from the target tumors. WST-11 (Weizmann STeba-11 drug; a negatively charged water-soluble palladium-bacteriochlorophyll derivative, Tookad® Soluble) Vascular targeted photodynamic therapy (VTP) is a local ablation approach recently approved for use in early stage prostate cancer. It generates reactive oxygen/nitrogen species within tumor blood vessels, resulting in their instantaneous destruction followed by rapid tumor necrosis. We hypothesize that the instantaneous arrest of tumor vasculature may provide a means to trap radiopharmaceuticals within the tumor thereby improving the efficacy of targeted radiotherapy.
Experimental design
GRPr positive prostate cancer xenografts (PC-3 and VCaP) were treated with 90Y-DOTA-AR with or without VTP. The uptake of radioisotopes was monitored by Cherenkov luminescence Imaging (CLI). The therapeutic efficacy of the combined VTP and 90Y-DOTA-AR in PC-3 xenografts was assessed.
Results
CLI of 90Y-DOTA-AR demonstrated longer retention of radiotracer within the VTP treated PC-3 xenografts compared with the non-VTP treated ones (p < 0.05) at all time points (24–144 hours) post 90Y-DOTA-AR injection. A similar pattern of retention was observed in VCaP xenografts. When 90Y-DOTA-AR administration was combined with VTP, tumor growth delay was significantly longer than for the control or the mono-therapy groups.
Conclusions
Tumor vascular arrest by VTP improves 90Y-DOTA-AR retention in the tumor microenvironment thereby enhancing therapeutic efficacy.
Keywords: 90Y-DOTA-AR, GRPr, WST-11 VTP, CLI, therapy
Introduction
Targeted imaging and radionuclide-based therapeutics have shown great promise for non-invasive clinical applications in cancers expressing specific biomarkers, especially those that are associated with key biologic functions such as mitogenesis, neoangiogenesis, and cell migration. Several radiolabeled monoclonal antibodies and bioactive small peptides have been developed to bind with high specificity to such biomarkers and tested for clinical utility. However, the therapeutic window is favorable only when the radiotracer remains in high concentrations within the environment of the targeted cancer cells and rapidly cleared from the circulation and normal organs. Radiolabeled peptides can be favorable in this regard as they can quickly diffuse into target cells and while clearing rapidly from circulation or nonspecific tissues with less immunogenicity compared to the radiolabeled monoclonal antibodies, resulting in high image contrast at early time points (1). However, uptake of radiopeptides in target cell populations is relatively “low” and the majority of administrated radiopeptides undergoes urinary excretion within 1 hour. Consequently, a large dose of radiopeptide is used to improve efficacy of radionuclide-based therapeutics (2, 3). The short dwell time of radiolabeled peptide within the target cells further limits the treatment efficacy of single dose regimens. These limitations have led to extensive efforts in looking for novel means to extend the residence of radioisotopes used as beta and alpha emitters in the tumor microenvironment.
While numerous studies aim at improving the tumor affinity of the radiotracer conjugates, we sought to provide a means for trapping radiotracers within the tumor microenvironment by utilizing WST-11 vascular targeted photodynamic therapy (VTP), a novel form of tissue treatment produced by a class of photosensitizing agents derived from bacteriochlorophyll.
VTP is a novel non-thermal ablation approach involving systemic administration of the photosensitizing agent WST-11 (Weizmann STeba-11 drug, Tookad Soluble®; Steba Biotech) followed by activation of this agent in the targeted tumor site by laser illumination with near infra-red light (753 nm). Conventional photodynamic therapy (PDT) agents are extravasated to the surrounding tissues mainly affecting the tumor cells (4), while WST-11 non-covalently binds to albumin and is sequestered within the circulation therefore mainly targeting the tumor vasculature until clearance (5, 6). Upon illumination, WST-11 generates reactive oxygen/nitrogen species such as superoxide, hydroxyl radicals and nitric oxide within the blood vessel, that mediate instantaneous arrest of the feeding arteries and draining veins producing an effect similar to profound, localized ischemia/reperfusion injury (6–8). Recent studies have demonstrated that permanent vascular arrest is limited to vessels smaller than 40 μm, which is well suited to the tumor microenvironment (9). We hypothesized that the ability of WST-11 VTP to permanently shut down the tumor circulation would reduce radiotracer washout from the tumor microenvironment and improve upon localized imaging and treatment effects.
Gastrin Releasing Peptide receptor (GRPr) (also known as bombesin receptor subtype 2) was shown in a large body of studies to be overexpressed in multiple human cancers at a high incidence rate (10). Specifically, an autoradiographic study of human prostate cancers demonstrated receptor specific binding of radiolabeled bombesin in all monitored tumors (n = 30) as well as in prostatic intraepithelial neoplasia (PIN). In contrast, normal prostate and benign prostate hyperplasia displayed only minimal binding of bombesin (11). As such, a number of radiolabeled bombesin analogs have been developed as targeting vectors for imaging and radionuclide therapy of tumors via specific binding to GRPr (12). Clinical studies with 99mTc- and 68Ga-labeled bombesin-based peptides have been reported for the imaging of metastasized prostate, breast and gastrointestinal stromal tumors with high contrast ratios (13–15). Among developed bombesin peptides, 111In-DOTA-AR (a potent bombesin antagonist, 111In-DOTA-PEG4-D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2), has demonstrated favorable binding affinities to GRPr and specific uptake in PC-3 human prostate cancer xenografts (16). The optimal uptake of 111In-DOTA-AR was reported to be at 1–4 hours post injection (p.i.) with high tumor-to-background ratios (16). DOTA-AR also allows labeling with therapeutic radioisotopes, such as 90Y (t1/2 = 64 hours) and 177Lu (t1/2 = 6.73 days) for radionuclide-based treatment with a rapid accumulation in prostate cancer xenografts. However, about 2/3 of delivered radioactivities of both 111In- and 90Y-DOTA-AR are washed out as early as 24 hours p.i. (16, 17), potentially diminishing the therapeutic efficacy. As 90Y is a high-energy beta emitter, which makes it suitable for non-invasive Cerenkov luminescence imaging (CLI), 90Y-DOTA-AR might be the right surrogate to investigate whether the arrest of tumor microvasculature in the target tissue enhances the radiopeptides retention. CLI is a low-cost modality to measure the radioactivity of beta or alpha emitter at multiple time points and our recent studies showed that there was a linear correlation between the active radiotracers concentrations and CLI signals for 90Y-DOTA-AR in prostate cancer tumor xenografts (17).
To test our hypothesis, we investigated the sequential treatment of prostate cancers with 90Y-DOTA-AR followed by VTP in human xenograft model(s). In this model, we utilized CLI to monitor the retention of the accumulated 90Y-DOTA-AR in the tumor tissues and measured the effects on subsequent tumor growth rate.
Materials and Methods
General
All chemicals were obtained from commercial sources and used without further purification. DOTA-AR, a bombesin antagonist, was synthesized using standard Fmoc strategy (16), and 90Y-DOTA-AR was prepared according to the previously published protocol from our laboratory (17). Radioactivity was measured using an appropriately calibrated dose calibrator (CAPINTEC CRC-30BC). Lyophilized WST-11 was obtained from Steba Biotech (Cedex, France). Prostate cancer cell lines, PC-3 and VCaP were purchased from ATCC. PC-3 cells were maintained in F-12K medium supplemented with 10% FBS, 1.5 g/L sodium bicarbonate and 2 mM L-glutamine and VCaP in DMEM with high-glucose,10% FBS and 2 mM L-glutamine.
Xenografts
All animal work was performed in accordance with a protocol approved by the IACUC of Memorial Sloan Kettering Cancer Center. To establish prostate tumor xenografts, 5 ×106 PC-3 or VCaP cells resuspended in 150 μl of 1:1 media/Matrigel (BD Biosciences, San Jose, CA) were inoculated into the shoulder area of athymic nude male mice (6–8 week old, NCI, Fredrick, MD). Tumor growth was monitored by caliper measurement twice a week and animal weight was followed as well. When the volume of tumors reached approximately 100 mm3, the animals were randomly assigned to different cohorts for further experiments.
VTP
WST-11, a photosensitizer, was reconstituted in sterile 5% dextran in water at 2 mg/mL under light protected condition and the aliquots were stored at −20°C. At the day of VTP treatment, an aliquot was thawed and filtered through 0.2 μm disc syringe filter (Sartorius Stedin Biotech North America, Bohemia, NY). The mice were intravenously injected (bolus for PC-3 xenograft study and infusion for VCaP xenograft study) with WST-11 (9 mg/kg) followed immediately by 10 minutes laser (Ceramoptec, Bonn, Germany) illumination (755 nm, 150 mW/cm) through a 1 mm frontal fiber (MedLight S.A., Ecublens, Switzerland). The light field was arranged to cover the entire tumor area plus 1 mm rim using red-light aiming beam.
CLI
After the mice were anesthetized with isoflurane (1%–4%), the uptake of 90Y-DOTA-AR in xenografts was followed by CLI using the IVIS Spectrum in vivo pre-clinical imaging system (Perkin-Elmer, Waltham, MA). Images for PC-3 xenografts were captured through 5 minute data acquisition at 3.8–4, 24, 48, 72, 96 and 144 hours post injection (p.i.) of 90Y-DOTA-AR (14.8 MBq) either with or without VTP application. For quantification of CLI signals, the areas of same size were manually outlined around tumors of in vivo images using Living Image® 2.60. CL intensity was expressed as average radiance, p/s/cm2/sr, where number of photons (p) per second (s) per unit surface area (cm2) per steradian (sr). To determine the effect of VTP on the retention of 90Y-DOTA-AR in another prostate cancer xenografts, mice bearing VCaP tumors were randomly assigned to 2 cohorts for the treatment with 90Y-DOTA-AR alone and 90Y-DOTA-AR/VTP combination. Radiotracer 90Y-DOTA-AR (14.8 MBq) was administered via retro orbital intravenous injection at 0 hour and the uptake of 90Y-DOTA-AR measured with CLI at 3.8 – 4 and 24 hours p.i.
Combination therapy
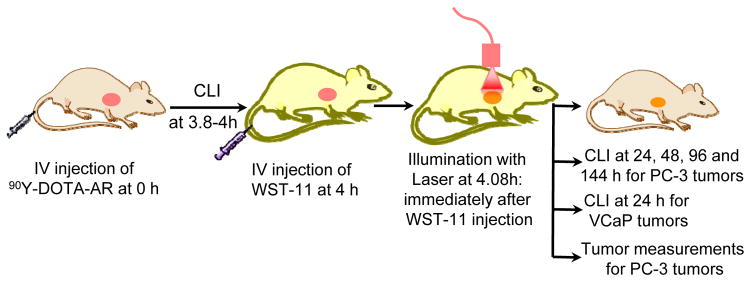
Mice bearing PC-3 or VCaP xenografts were randomly assigned to 4 different cohorts, including sham control (saline and illumination only), VTP, 90Y-DOTA-AR, and combination of 90Y-DOTA-AR and VTP. Radiotracer 90Y-DOTA-AR (14.8 MBq) was administered via retro orbital intravenous injection at 0 hour (Fig. 1). Since radiolabeled DOTA-AR displays an optimal tumor uptake at 1–4 hours p.i. in GRPr-expressing prostate cancer xenografts (16, 17), we performed VTP treatment at 4 hours p.i. of 90Y-DOTA-AR (Fig. 1). For VTP treatment, WST-11 was injected i.v. followed by laser illumination of tumor area to activate the photosensitizer in tumors. The radioactivities of 90Y-DOTA-AR in the xenografts were quantitatively measured via CLI at 3.8–4 hours p.i. of 90Y-DOTA-AR (for initial uptake prior to VTP) as well as at several later time points. The tumor volume and mouse weights for PC-3 xenografts were monitored one day prior to treatment followed by twice a week until tumor volume approached 1500 mm3. The diet and cage for the treated mice were changed every day in the first week of 90Y-DOTA-AR treatment. Organs of interest of sacrificed animals were collected for histological evaluation.
Figure 1. A workflow for sequential treatments with 90Y-DOTA-AR and VTP in mice with established tumor xenografts.
VTP was performed at 4 hours post injection (p.i.) of 90Y-DOTA-AR intended to trap maximum amount of radioactivity in tumors. The radioactivities of 90Y-DOTA-AR in the xenografts were quantitatively measured via CLI at 3.8–4 hours p.i. for initial uptake radioactivity prior to VTP as well as at 24, 48, 72, 96 and 144 hours p.i. of 90Y-DOTA-AR (PC-3 xenografts) or at 24 hours p.i. of 90Y-DOTA-AR (VCaP xenografts). PC-3 tumor growth was also monitored for anti-tumor effect of these therapies.
Radiation dosimetry
Based on the CLI data of 90Y-DOTA-AR uptake, the absorbed doses (Gy/MBq) by the PC-3 xenografts were calculated and compared between 90Y-DOTA-AR and 90Y-DOTA-AR/VTP combination treatment groups. CLI was performed at 3.8–4, 24, 48, 72, 96, and 144 hours post 90Y-DOTA-AR administration. The measured photon counts for CLI (p/s/cm2/sr) were converted to tumor activity using a previously established conversion factor (3.96 × 10−7 MBq/(p/s/cm2/sr)) (17) and subsequently, absorbed radiation dose-rate, r (t), was calculated using the equation.
In this equation, Δ is the equilibrium dose constant for 90Y (a fixed value of 0.54 g Gy/MBq h); φ is a size-dependent value representing the fraction of energy emitted by the tumor taken 90Y; A(t) is the 90Y activity present in the tumor (inferred from the CLI signal and the overall calibration factor); and m(t) is tumor mass (based on caliper measurements, ellipsoidal geometry and an assumed density of 1 g/ml). Absorbed fractions (φ) for 90Y were derived by interpolating data published by Bardiès and Chatal (18). Finally, the total absorbed radiation dose to tumor was calculated by numerical integration of dose-rate. The integration of dose-rate was stopped either at the last measurement time or at the time where the tumor appeared “ablated” (i.e., too small to be measured). Tumors that appeared “ablated” were assigned a nominal size (2 mm diameter sphere) for the purposes of terminal dose-rate calculation. Any possible terminal component of dose delivered after the end of integration was ignored.
Histology
Tumors, kidneys and pancreas were fixed in 10% buffered formalin (Fisher Scientific, Pittsburgh, PA), embedded in paraffin, sectioned at 5 micron thickness, and stained with hematoxylin-eosin (H&E). Histopathological analysis to assess tissue toxicity induced by different treatment regimens was performed by a board-certified veterinary pathologist. For immunohistochemistry (IHC) analysis of tumor, samples were stained with anti-mouse CD31 antibody (Dianova, Hamburg, Germany) to assess tumor vessel density and terminal deoxynucleotidyl transferease dUTP nick-end labeling (TUNEL) for cell death (19).
Statistical analysis
Data calculated using Microsoft Excel was expressed as mean ± SD. Student’s unpaired t test (GraphPad Prism 5, San Diego, CA, USA) was used to determine statistical significance at the 95 % confidence level for ROI analysis and two-way ANOVA test (GraphPad) for therapeutic efficacy in affecting tumor growth. Differences with p values < 0.05 were considered to be statistically significant.
Results
Work flow of sequential treatment of xenografts
Depiction of the experimental design is represented in Fig. 1. Tumor xenografts reached size criteria for treatment by 22 to 27 days following implantation. Mice bearing prostate cancer xenografts which express high level of GRPr (PC-3 and VCaP) (17) were used in this study.
In vivo accumulation of 90Y-DOTA-AR in prostate cancer xenografts
PC-3 xenografts
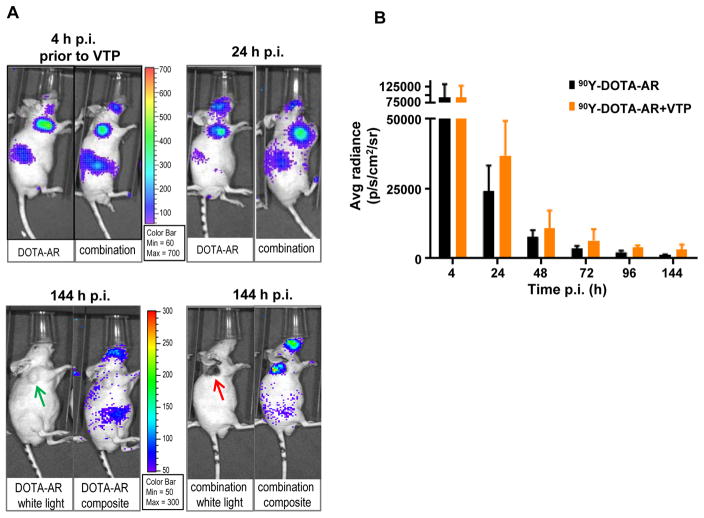
Fig. 2A shows a representative CLI of the mice bearing PC-3 xenografts treated with 90Y-DOTA-AR alone (DOTA-AR panel) and of the ones treated with 90Y-DOTA-AR/VTP combination (Combination panel). Tumors and kidneys are well visualized by CLI as reported by Lohrmann et al. (17). Due to the retro orbital injection of 90Y-DOTA-AR, facial signal was detected occasionally in some mice. After VTP treatment, tumor ablation (eschar formation observed at 144 hours post VTP) was clearly visible in both VTP (images not shown) and 90Y-DOTA-AR/VTP combination groups (Fig. 2A, 144 hour images in combination panel). High intensity of CLI signals in tumors was observed at 4 hours p.i. of 90Y-DOTA-AR (prior to VTP) and the intensities of signal decreased in a time-dependent manner for both groups. Significantly, CLI displayed enhanced retention of radioactivity for tumors treated with combination therapy compared to tumors treated with 90Y-DOTA-AR alone. Quantitative analysis of ROI with Living Image® 2.60 confirmed that PC-3 tumors treated with 90Y-DOTA-AR/VTP combination showed higher radioactivity retention at all time points measured after VTP treatment compared to 90Y-DOTA-AR alone such as 37,000 ± 12,400 and 24,200 ± 9,200 p/s/cm2/sr, respectively at 24 hours p.i (Fig. 2B, p < 0.05). At 144 hours p.i., there were low but detectable signals at an approximately 1.8 fold higher level in the combination treatment cohort (2,430 ± 426 vs 1320 ± 142 p/s/cm2/sr, p < 0.005). In contrast, the signal intensities at 4 hours p.i. in the two groups (prior to VTP treatment) were similar (92,600 ± 39,100 vs 91,300 ± 35,300 p/s/cm2/sr, p = 0.93). The half life of 90Y-DOTA-AR in PC-3 tumors (one phase decay) was 10.0 ± 0.6 hours in 90Y-DOTA-AR only group and 14.1 ± 0.7 hours in the combination group.
Figure 2. In vivo Cerenkov luminescence imaging (CLI) of 90Y-DOTA-AR in PC-3 tumor-bearing nude mice.
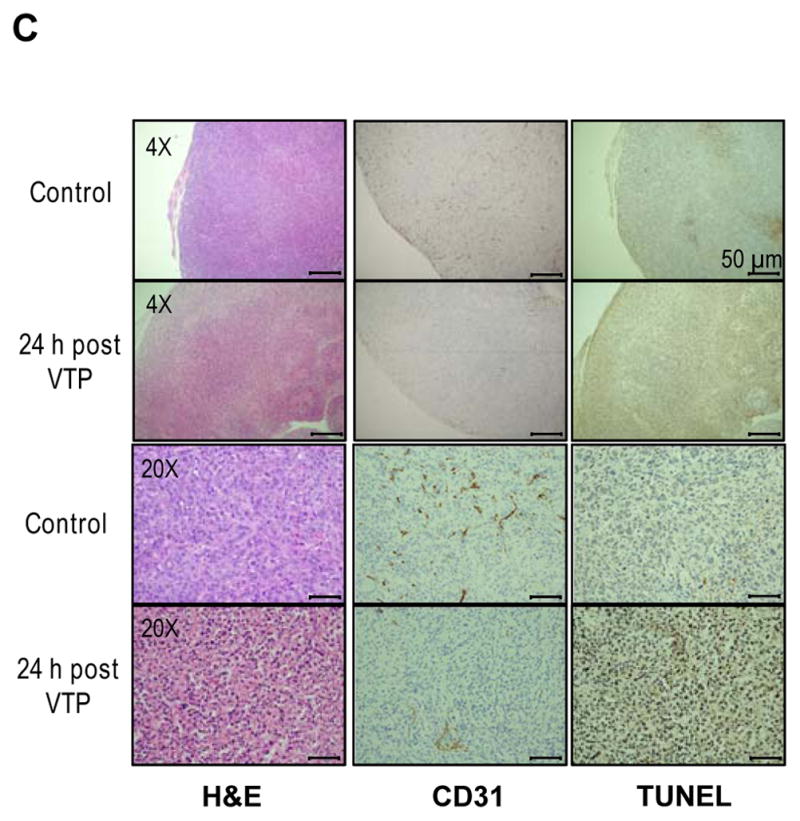
(A) Representative images of PC-3 xenografts treated with 90Y-DOTA-AR alone (DOTA-AR, n=12) or combination of 90Y-DOTA-AR and VTP (Combination, n=13) at 24 and 144 hours p.i. of 90Y-DOTA-AR. The images at 4 hours p.i. of 90Y-DOTA-AR show the uptake of 90Y-DOTA-AR prior to VTP treatment for both groups. After injection of 90Y-DOTA-AR, the radioactivity uptake within tumor was monitored using the IVIS Spectrum in vivo pre-clinical imaging system. Green arrows = tumor; red arrow = eschar formed post tumor ablation. (B) ROI analysis of 90Y-DOTA-AR CLI at each time point of imaging. VTP enhanced the retention of 90Y-DOTA-AR radioactivity within the tumors at all the time points measured after VTP treatment (p < 0.05). (C) PC-3 tumors were subjected to H&E staining and IHC of CD31 endothelial marker as well as TUNEL staining. Representative pictures of each group are shown. Tumor vasculature staining with anti-CD31 antibody was significantly reduced at 24 hours post VTP treatment alone compared to control specimen demonstrating VTP-induced tumor vessel destruction.
The efficacy of VTP in obliterating tumor vasculature of PC-3 xenografts was assessed via IHC using CD31 marker at 24 hours post VTP treatment (Fig. 2C). CD31 is a most sensitive and specific endothelial marker in paraffin sections. IHC results with anti-CD31antibody displayed markedly reduced vessel density in PC-3 tumors at 24 hours post VTP treatment compared to control tumors. In addition, TUNEL stained positive in VTP treated tumors displaying VTP-induced tumor cell death.
VCaP xenografts
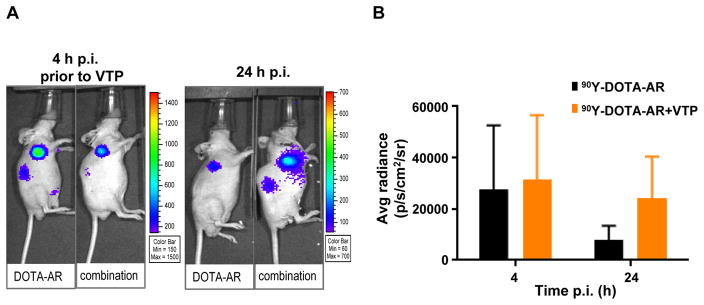
To confirm the general impact of VTP on the enhanced retention of radiopeptide within prostate cancer models, VCaP human prostate cancer xenografts were subjected to the same experimental work flow depicted in Fig. 1. VCaP tumors express high level of GRPr and displayed comparable uptake of 90Y-DOTA-AR to PC-3 xenografts (17). Radioactivity uptake within the VCaP tumors was monitored at 4 and 24 hours p.i. by CLI (Fig. 3). Signal intensities by quantitative ROI analysis for tumors treated with 90Y-DOTA-AR/VTP combination were 2.5-fold higher than for those treated with 90Y-DOTA-AR alone. The difference between groups was statistically significant (p < 0.05) at 24 hours p.i. These observations indicate that VTP treatment enhanced retention of delivered radioactivity in both androgen-independent (PC-3) and androgen dependent (VCaP) prostate cancer xenografts.
Figure 3. In vivo CLI of 90Y-DOTA-AR in VCaP tumor-bearing nude mice.
(A) Representative images of VCaP xenografts treated with 90Y-DOTA-AR alone (DOTA-AR) or combination of 90Y-DOTA-AR/VTP (Combination) at 4 and 24 hours p.i. (B) The graph demonstrates radiance for VCaP at 4 and 24 hours p.i. Tumor uptake of 90Y-DOTA-AR was not significantly different between two treatment groups at 4 hours p.i. (prior to VTP, p = 0.726, n = 13), but a 2.5-fold higher retention of the radioactivity was observed in the tumors of combination group at 24 hours p.i. (p < 0.0001, n = 10).
Dose estimation of 90Y-DOTA-AR in prostate cancer xenografts
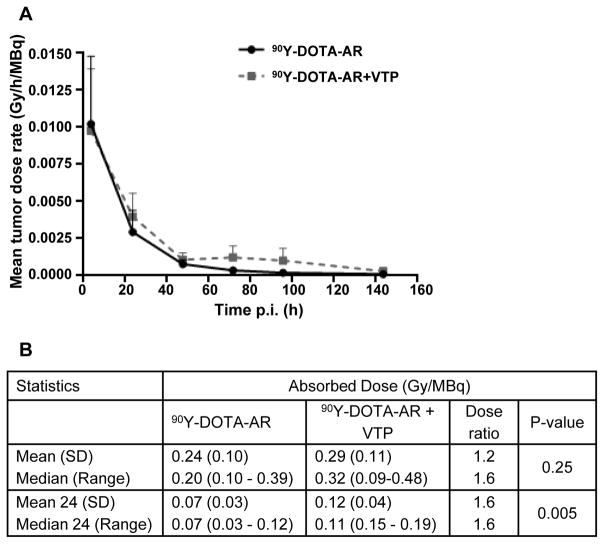
Fig. 4 shows the group mean (± SD) values of tumor dose-rate as a function of time for the two treatment groups. Absorbed doses estimated by integrating these curves were 0.24 (± 0.10) Gy/MBq for 90Y-DOTA-AR alone and 0.29 (± 0.11) Gy for combination of 90Y-DOTA-AR and VTP. The absorbed dose for the combination was approximately 20% greater and the trend towards higher dose-rates becomes clear at later times (≥ 24 hours p.i.). Absorbed doses (Gy/MBq) for individual PC-3 xenografts were calculated and compared on a group basis between 90Y-DOTA-AR and 90Y-DOTA-AR/VTP combination treatments. The table in Fig. 4B summarizes the statistical data, indicating that both mean and median radiation doses were higher for the combination and when only the radiation dose that was delivered after 24 hours p.i. was considered, the differences were highly significant (p < 0.01).
Figure 4. Estimated absorption dose of 90Y-DOTA-AR in PC-3 xenografts.
(A) The estimated dose ratio between 90Y-DOTA-AR and combination of 90Y-DOTA-AR/VTP is depicted. (B) The table shows the summary statistics for both treatment groups. Both mean and median radiation doses were higher for 90Y-DOTA-AR and VTP. Twenty four denotes radiation dose delivered ≥ 24 hours p.i.
Treatment efficacy in PC-3 xenografts
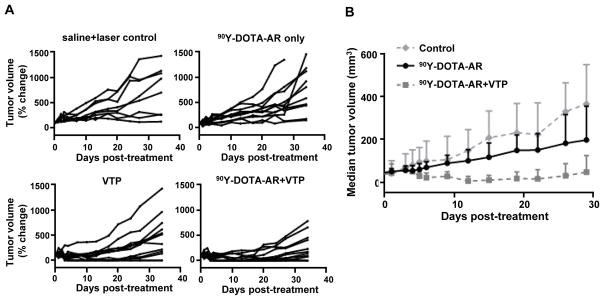
In vivo PC-3 tumor growth in nude mice was monitored to assess the anti-tumor effect of 90Y-DOTA-AR or VTP as mono-therapy, along with the 90Y-DOTA-AR and VTP combination. Tumor growth up to 34 days post treatment was followed twice a week and the individual tumor growth was plotted in Fig. 5A. The control group was sham-treated with laser illumination (without WST-11 administration) and saline injection (i.v.) as a vehicle. A single dose injection of 90Y-DOTA-AR did not demonstrate statistically significant anti-tumor effect on tumor growth compared to the control group and did not lead to any tumor regression (p = 0.97). This was expected as we selected a low dose of 90Y-DOTA-AR that had no significant effect on tumor growth on its own in order to determine any potential synergistic effect of VTP plus radiotherapy. VTP treatment alone had a modest though significant effect on tumor growth compared to control group (p < 0.01). However, combination treatment displayed superior efficacy in antitumor effect compared to 90Y-DOTA-AR group (p < 0.0001) or VTP group (p < 0.05), which indeed suggests a synergistic effect between 90Y-DOTA-AR and VTP. Three of the 12 PC-3 tumor bearing animals of combination therapy group and 2/12 of VTP alone treated group remained tumor free at 34 days post treatment. Fig. 5B depicts a superior effect of the combination treatment to 90Y-DOTA-AR single injection monotherapy.
Figure 5. Therapeutic efficacy of VTP, 90Y-DOTA-AR and 90Y-DOTA-AR/VTP combination on PC-3 xenografts.
(A) Individual tumor growth per each cohort is shown. Mice bearing PC-3 xenografts were randomly assigned to 4 different cohorts of sham control (saline and illumination only, n=8), VTP (n=12), 90Y-DOTA-AR (n=12), and combination of 90Y-DOTA-AR and VTP (n=13). 90Y-DOTA-AR alone did not demonstrate statistically significant anti-tumor effect on tumor growth (p = 0.97) while VTP treatment alone had a modest effect on tumor growth compared to control group (p< 0.01). Combination treatment displayed statistically significant antitumor effect compared to 90Y-DOTA-AR alone arm (p < 0.0001) or to VTP alone arm (p < 0.05) suggesting synergy between radiotherapy and VTP. Two-way ANOVA test was utilized for the statistical analysis. (B) The graph shows the median tumor volume as a function of time. This shows a clear demarcation between treatment groups with the combination of 90Y-DOTA-AR plus VTP having a much more pronounced therapeutic effect.
Assessment of potential toxic effects of combination therapy
Kidney and pancreas from mice bearing PC-3 tumors in 4 different treatment groups were collected at 41 days post treatment to investigate potential tissue toxicity caused by 90Y-DOTA-AR/VTP combination therapy. These are the main organs with high level of 90Y-DOTA-AR uptake along with tumors (17). No significant lesions and no evidence of treatment-induced lesions were observed in the kidney and pancreas of these mice (representative H&E pictures in Supplemental Fig. S1). Two mice had minimal focal tubular degeneration and necrosis in kidney: one control mouse and one 90Y-DOTA-AR/VTP treated mouse. This is a common naturally occurring lesion in experimentally naive mice.
Discussion
We hypothesized that the local destruction of tumor vessels by VTP may help trap radiotracers in the accumulated organs and thereby improve the clinical outcome of radionuclide-based treatment with beta or alpha emitter-labeled peptide. The current study describes proof-of-principle supporting this hypothesis by demonstrating accumulated 90Y-DOTA-AR in the tumor tissues was retained longer in the prostate cancer xenografts with resultant evidence of improved treatment efficacy from the combination of intratumoral vascular destruction following radiotracer delivery.
CLI as a non-invasive imaging modality
Cherenkov radiation generates visible light as a charged particle emitted from the decaying radioisotope travels through a dielectric medium faster than the speed of light. This visible light can be detected by an optical imaging platform. Several studies found correlations between the light intensities of Cerenkov radiation from different radioisotopes and the energy of particles (20, 21). Among those radionuclides, 90Y produces a high energy of pure beta particle with almost 100% incidence, which allows efficient measuring of the amount of radioactivity with CLI. Recently, CLI was utilized to quantitatively measure 90Y-DOTA-AR activities in prostate cancer xenografts and kidneys. A strong correlation between radioactivity concentration and in vivo CLI intensity (R2 = 0.94 – 0.98) were observed (17). Therefore, CLI is reliable, low-cost modality to monitor the retention of 90Y-DOTA-AR radioactivity in a non-invasive way. Our current study further demonstrates that 90Y-DOTA-AR radioactivity in PC-3 xenografts is detectable up to 144 hours p.i. of 90Y-DOTA-AR. Our observations also indicate that CLI could be used to measure the effect of VTP treatment for multiple time points after 90Y-DOTA-AR administration. In addition, most 90Y-DOTA-AR radioactivity was cleared from normal organs within 1 day p.i. as evident by the negligible irradiation dose. This finding is important for the clinical setting where 90Y-DOTA-AR-based CLI is utilized for guiding local therapy.
Combination with VTP to increase tumor retention time of the accumulated radioactivity
This study demonstrates the potential for utilization of VTP as a means to augment the activity of radiotracers within tumors. The combinational strategy significantly increased intratumoral lifetime of radiopharmaceuticals in androgen-independent PC-3 human prostate cancer xenografts monitored by CLI (p < 0.05 in all time points post VTP). The radioactivity in combination cohort was approximately 1.5 fold higher in this model with approximately 40% increase of 90Y-DOTA-AR half life in tumors treated with VTP. Even higher retention of radioactivity in combination with VTP (2.5 fold) compared with radiotracer delivery alone was observed in androgen-dependent VCaP human prostate cancer xenograft model as well, indicating broad applicability of this approach. Our models also, however, suggested slight differences in the degree of radioactivity retention between tumor types, the cause for which is unclear. This could be variability within the experimental design or related to biologic differences in tumor microenvironment related to vascularity or radiotracer uptake. VTP targets tumor microvasculature exclusively affecting both small feeding arterioles and draining venules (8). Our preliminary histologic analysis did not identify obvious vascular differences yet more detailed studies could provide greater insight.
The longer retention of intratumoral radioactivity observed in combination cohorts nicely correlated to the treatment efficacy of PC-3 tumors (Fig. 5). This is especially striking when noting that, compared to controls, 90Y-DOTA-AR alone did not demonstrate any anti-tumor effect on tumor growth. However, the combination treatment displayed significant anti-tumor effects compared to both 90Y-DOTA-AR alone and VTP alone, suggesting a synergistic effect between two modalities.
Analysis of radiation dose-rates and total doses in the PC-3 tumors indicated that VTP produced an enhancement that was statistically significant for times ≥ 24 hours p.i. of radiotracer or approximately ≥ 20 hours after VTP treatment (Fig. 4). Radiotracer concentration was not significantly influenced before this time point although this phase of washout is fairly rapid with more variability seen in measurements over this interval likely reflected in differences in tracer clearance. Still, it is possible that adaptations in the approach might positively influence radioactivity accumulation in tumors at earlier time points. Multiple dosing of radiotracer and a shorter interval than 4 hour between the administration of radiotracer and VTP treatment are modifications that could be tried to achieve VTP-induced vascular breakdown while tumor uptake of radiotracer is less prone to washout. Alternatively, VTP treatment parameters could be altered to increase the degree of intratumoral vascular destruction or that of surrounding vessels that may reduce the amount of 90Y-DOTA-AR activity exiting from the tumors. Dose escalation studies of VTP parameters such as WST-11 concentration or/and laser fluence should provide better efficacy of VTP for the combination with 90Y-DOTA-AR. Preclinical large animal studies of VTP and multi-center phase 2 and 3 studies of localized prostate cancer have reported a dose dependent response rate that depends on the treatment conditions of WST-11 concentration and laser fluence (22–25). Further evaluation of these factors may be warranted.
Several clinical applications for this form of therapy are conceivable. Published trial data in localized prostate cancer has demonstrated excellent safety and tolerability for WST-11 VTP treatment within the pelvis without injury to surrounding, highly sensitive organs. Effective local tumor ablation is also impressive, with absent evidence of cancer in 80% of cases. Causes for the few local failures have not been elucidated however, combinatorial treatment with 90Y-DOTA-AR could be a means to improve upon these results, particularly in high-risk cancers. Other difficult-to-treat sites in soft tissue might also be amenable as in the case of local or regional recurrence after prior surgery or radiation. Studies in bone metastases remain to be performed although vascular dynamics in this compartment may influence the dynamics of treatment with this approach and establishing reliable pre-clinical models in this setting are a challenge requiring further refinement.
This study reports on the initial experience with combinatorial sequential therapy using VTP with radiopharmaceutical therapy demonstrating highly promising results that have potential to improve upon cytotoxic effects in prostate cancers. As VTP therapy doesn’t result in serious photo-toxicity and its interval between drug injection and laser illumination is short, it provides an attractive option to overcome limitations of fast washout of the accumulated radiopharmaceuticals from tumors. The experiments presented here constitute a proof-of-principle for the use of combined radionuclide/VTP strategies that may be further developed and eventually translated into clinical studies.
Supplementary Material
Translational Relevance.
The clinical utility of radionuclide conjugated bombesin antagonist, currently being investigated in a phase 1 trial for men with prostate cancer, can be hypothetically augmented by prolonging the radiotracer dwell time within the tumor microenvironment. Here we test the hypothesis that VTP application in concert with radioisotope delivery can achieve these goals. We show that indeed combination of the two treatment modalities improves imaging and delays progression of cancer xenografts compared to 90Y-DOTA-AR monotherapy. Since VTP is in use for localized prostate cancer, the proposed combination may undergo rapid translation to the clinical arena.
Acknowledgments
Financial support: This work was supported by NIH grant P50-CA84638 (HZ, WAW) and Thompson Family Foundation (KK, SLR, SJ, SK, AS, JAC). HZ was additionally supported by CURE Childhood Cancer Foundation.
Authors thank MSKCC Small Animal Imaging Core Facility (supported by NIH Small-Animal Imaging Research Program grant R24 CA83084 and NIH Center grant P30 CA08748).
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocrine reviews. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 2.Ambrosini V, Fani M, Fanti S, Forrer F, Maecke HR. Radiopeptide imaging and therapy in Europe. J Nucl Med. 2011;52:42S–55S. doi: 10.2967/jnumed.110.085753. [DOI] [PubMed] [Google Scholar]
- 3.Graham MM, Menda Y. Radiopeptide imaging and therapy in the United States. J Nucl Med. 2011;52:56S–63S. doi: 10.2967/jnumed.110.085746. [DOI] [PubMed] [Google Scholar]
- 4.Juarranz A, Jaén P, Sanz-Rodríguez F, Cuevas J, González S. Photodynamic therapy of cancer. Basic principles and applications. Clin Transl Oncol. 2008;10:148–54. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 5.Vakrat-Haglili Y, Weiner L, Brumfeld V, Brandis A, Salomon Y, McLlroy B, et al. The microenvironment effect on the generation of reactive oxygen species by Pd-bacteriopheophorbide. J Am Chem Soc. 2005;127:6487–97. doi: 10.1021/ja046210j. [DOI] [PubMed] [Google Scholar]
- 6.Mazor O, Brandis A, Plaks V, Neumark E, Rosenbach-Belkin V, Salomon Y, et al. WST11, a novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution and vascular-targeted photodynamic activity using melanoma tumors as a model. Photochem Photobiol. 2005;81:342–51. doi: 10.1562/2004-06-14-RA-199. [DOI] [PubMed] [Google Scholar]
- 7.Gross S, Gilead A, Scherz A, Neeman M, Salomon Y. Monitoring photodynamic therapy of solid tumors online by BOLD-contrast MRI. Nat Med. 2003;9:1327–31. doi: 10.1038/nm940. [DOI] [PubMed] [Google Scholar]
- 8.Madar-Balakirski N, Tempel-Brami C, Kalchenko V, Brenner O, Varon D, Scherz A, et al. Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad. PLoS One. 2010;5:e10282. doi: 10.1371/journal.pone.0010282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimm SY, Tarin TV, Monette S, Srimathveeravalli G, Gerber D, Durack JC, et al. Nonthermal ablation by using intravascular oxygen radical generation with WST11: dynamic tissue effects and implications for focal therapy. Radiology. 2016;281:109–18. doi: 10.1148/radiol.2016141571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–46. [PubMed] [Google Scholar]
- 11.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res. 1999;59:1152–9. [PubMed] [Google Scholar]
- 12.Sturzu A, Sheikh S, Echner H, Nagele T, Deeg M, Amin B, et al. Rhodamine-marked bombesin: a novel means for prostate cancer fluorescence imaging. Invest New Drugs. 2014;32:37–46. doi: 10.1007/s10637-013-9975-2. [DOI] [PubMed] [Google Scholar]
- 13.Mansi R, Fleischmann A, Macke HR, Reubi JC. Targeting GRPR in urological cancers-from basic research to clinical application. Nat Rev Urol. 2013;10:235–44. doi: 10.1038/nrurol.2013.42. [DOI] [PubMed] [Google Scholar]
- 14.Mather SJ, Nock BA, Maina T, Gibson V, Ellison D, Murray I, et al. GRP receptor imaging of prostate cancer using [(99m)Tc]Demobesin 4: a first-in-man study. Mol Imaging Biol. 2014;16:888–95. doi: 10.1007/s11307-014-0754-z. [DOI] [PubMed] [Google Scholar]
- 15.Kahkonen E, Jambor I, Kemppainen J, Lehtio K, Gronroos TJ, Kuisma A, et al. In vivo imaging of prostate cancer using [Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res. 2013;19:5434–43. doi: 10.1158/1078-0432.CCR-12-3490. [DOI] [PubMed] [Google Scholar]
- 16.Abiraj K, Mansi R, Tamma ML, Fani M, Forrer F, Nicolas G, et al. Bombesin antagonist-based radioligands for translational nuclear imaging of gastrin-releasing peptide receptor-positive tumors. J Nucl Med. 2011;52:1970–78. doi: 10.2967/jnumed.111.094375. [DOI] [PubMed] [Google Scholar]
- 17.Lohrmann C, Zhang H, Thorek DL, Desai P, Zanzonico PB, O’Donoghue J, et al. Cerenkov Luminescence Imaging for Radiation Dose Calculation of a 90Y-Labeled Gastrin-Releasing Peptide Receptor Antagonist. J Nucl Med. 2015;56:805–11. doi: 10.2967/jnumed.114.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardies M, Chatal JF. Absorbed doses for internal radiotherapy from 22 beta-emitting radionuclides: beta dosimetry of small spheres. Phys Med Biol. 1994;39:961–81. doi: 10.1088/0031-9155/39/6/004. [DOI] [PubMed] [Google Scholar]
- 19.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorek D, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, et al. Cerenkov imaging- anew modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2:163–73. [PMC free article] [PubMed] [Google Scholar]
- 21.Das S, Thorek DL, Grimm J. Cerenkov imaging. Adv Cancer Res. 2014;124:213–34. doi: 10.1016/B978-0-12-411638-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimm SY, Tarin TV, Monette S, Srimathveeravalli G, Gerber D, Durack JC, et al. Nonthermal ablation by using intravascular oxygen radical generation with WST11: dynamic tissue effects and implications for focal therapy. Radiology. 2016;281:109–18. doi: 10.1148/radiol.2016141571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray KS, Winter AG, Corradi RB, LaRosa S, Jebiwott S, Somma A, et al. Treatment effects of WST11 vascular targeted photodynamic therapy for urothelial cell carcinoma in swine. J Urol. 2016;196:236–43. doi: 10.1016/j.juro.2016.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azzouzi AR, Barret E, Bennet J, Moore C, Taneja S, Muir G, et al. TOOKAD® Soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol. 2015;33:945–53. doi: 10.1007/s00345-015-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzouzi AR, Barret E, Moore CM, Villers A, Allen C, Scherz A, et al. TOOKAD® Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013;112:766–74. doi: 10.1111/bju.12265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.