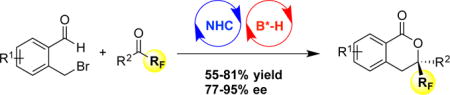
Abstract
A chiral NHC/Brønsted acid cooperative catalysis system has been developed for asymmetric annulation of functionalized benzaldehydes and activated ketones through dearomative generation of dienolate.
Keywords: NHC, Brønsted acid, cooperative, annulation
Graphical abstract
N-Heterocyclic Carbene (NHC) catalysis has been the focus of asymmetric organocatalysis for decades, featuring in umpolung transformations of aldehydes and effective activation of other carbonyls.1 One of the significant advantages of carbene catalysis is its incredible compatibility2 with other organocatalysts3–7 as well as metal catalysts.8–9 We have previously disclosed a productive carbene/Brønsted acid cooperative catalysis for highly enantioselective synthesis of trans-γ-lactams from α, β-unsaturated aldehydes and unactivated imines,5 a concept that has proven to be broad.6
Recently, a dienolate intermediate generated through NHC catalysis, either from linear α, β-unsaturated carbonyls with installed leaving groups (Scheme 1a, path I) or from aldehydes under oxidative conditions (Scheme 1a, path II), has been comprehensively studied by Ye,10 Chi11 and others.12 In 2013, Chi13 and coworkers reported a NHC-catalyzed oxidative formal [4+2] cycloaddition of heteroaryl aldehydes and activated ketones. The benzaldehyde analogue was nonproductive in Chi’s reaction. We hypothesized that a leaving group at the benzylic position would facilitate this process in a redox fashion (Scheme 1b).14 Herein, we report a highly enantioselective [4+2] annulation of 2-(bromomethyl)benzaldehydes and fluorinated ketones by the action of NHC/phosphoric acid cooperative catalysis.
Scheme 1.
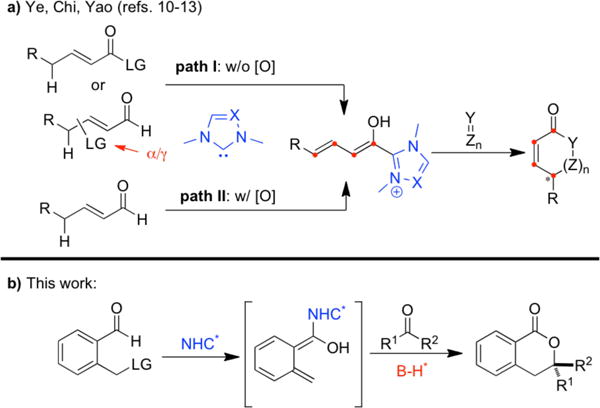
Pathways to dienolate intermediate through NHC catalysis
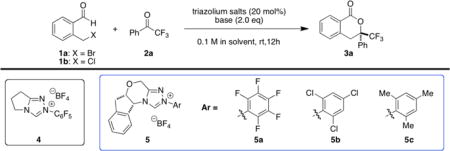
Starting with 2-(bromomethyl)benzaldehyde 1a and trifluoroacetophenone 2a as the substrates, use of achiral triazolium salt 4 gave desired product 3a in 57% yield (Table 1, entry 1). We then evaluated chiral carbene precursors (entries 2–4) and found that our aminoindanol-derived triazolium salt 5a with N-pentafluorophenyl group provided the best reactivity (entry 2). Further screening of solvents and bases disclosed that reaction with 5a and KOAc in cyclohexane was more efficient (61% yield and 76% ee, entry 6). Reaction of 2- (chloromethyl)benzaldehyde 1b and 2a led to the same product, but yield and enantioselectivity were both lower (entry 7).
Table 1.
NHC catalyst examination and reaction optimization
| |||||
|---|---|---|---|---|---|
| entry | Cat. | Solvent | Base | yield (%)a | ee |
| 1 | 4 | THF | K2CO3 | 57 | NA |
| 2 | 5a | THF | K2CO3 | 76 | 45 |
| 3 | 5b | THF | K2CO3 | 42 | 24 |
| 4 | 5c | THF | K2CO3 | N.R. | NA |
| 5 | 5a | n-hexane | KOAc | 64 | 63 |
| 6 | 5a | cyclohexane | KOAc | 61 | 76 |
| 7c | 5a | cyclohexane | KOAc | 21 | 59 |
Standard Conditions: 1a (0.10 mmol), 2a (0.20 mmol), 5a (20 mol%, 0.020 mmol) and base (0.20 mmol) in 1.0 mL of anhydrous solvent, under argon, 23° C, for 12h.
Isolated yield.
Determined by HPLC on a chiral stationary phase.
1b was used instead of 1a.
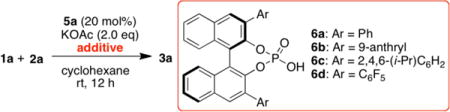
Various additives were then investigated under otherwise identical conditions (Table 2). Molecular sieves with different pore diameters generally lower the yields albeit with slightly higher ee (entries 1–3). Lewis acids compatible with chiral NHCs, such as LiCl and Sc(OTf)3, did not improve the reaction (entries 4–5). Speculating that the slightly improved selectivities observed with acetate as base were due to a potential role of the conjugate acid in the stereoselectivity determining event, we investigated the impact of BINOL-derived chiral phosphoric acid15 as co-catalyst. We were pleased to find that application of 6a (20 mol%) delivered 82% ee (entry 6). Steric and electronic variation of 3,3′-aryl substituents of chiral phosphoric acid continued to improve the enantioselectivity (entries 6–9) with 6d16 providing an optimized 95% ee (entry 9). Lower catalyst loading of 6d increased efficiency without erosion of enantioselectivity (entry 10). A combination of ent-5a with 6d was found to be less effective, indicating a match/mismatch situation between these two chiral catalysts (entry 11).
Table 2.
Additive Effect
| |||
|---|---|---|---|
| entry | additive | yield (%)a | ee (%)b |
| 1 | 3Å MS | 42 | 78 |
| 2 | 4Å MS | 58 | 77 |
| 3 | 5Å MS | 53 | 77 |
| 4 | LiCl (1.0 eq) | 52 | 77 |
| 5 | Sc(OTf)3 (10 mol%) | 55 | 71 |
| 6 | 6a (20 mol%) | 66 | 82 |
| 7 | 6b (20 mol%) | 63 | 84 |
| 8 | 6c (20 mol%) | 56 | 74 |
| 9 | 6d (20 mol%) | 51 | 95 |
| 10 | 6d (10 mol%) | 68 | 95 |
| 11c | 6d (20 mol%) | 64 | −84 |
Conditions: 1a (0.10 mmol), 2a (0.20 mmol), 5a (20 mol%, 0.020 mmol) and KOAc (0.20 mmol) in 1.0 mL of anhydrous cyclohexane, under argon, 23° C, for 12h.
See Table 1.
ent- 5a was used instead of 5a.
With optimal conditions in hand, we firstly explored the ketone scope (Scheme 2, part I). Electron-withdrawing substituents on the aryl ring of trifluoroacetophenones generally decrease the enantioselectivities. For example, reactions of para-fluoro, para-chloro and meta-fluoro analogs with 1a afford 5b, 5c and 5d in moderate yields and good selectivities. Electron rich trifluoroacetophenones and alkyl trifluoromethyl ketones fail to give any products presumably due to their lower reactivity. Annulations with ketones bearing longer-chain perfluoroalkyl units provide lactones 3e and 3f in good yields and enantioselectivities. Ethyl phenylgloxylate, on the other hand, does not participate in this reaction.
Scheme 2.
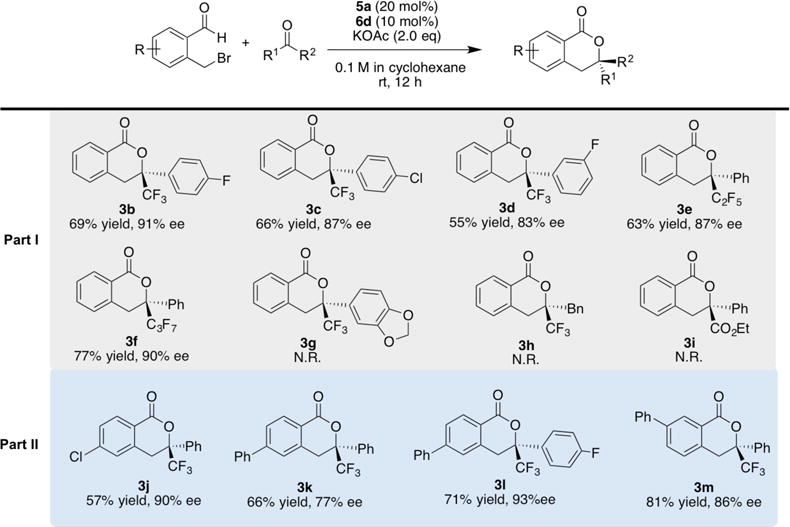
Substrate Scope
We next evaluated bromomethyl aryl aldehyde scope (Scheme 2, part II). Reaction of para-chlorosubstituted system with 2a provides 3j in 57% yield, with 90% ee. A phenyl substituent surprisingly had a huge impact on the reactivity. For example, 4-phenylbenzaldehyde delivers product in 66% yield and 77% ee (3j) while a 5-phenyl group gives much higher yield and enantioselectivity (3m). The use of para-fluorinated analog of 2a would offset the negative effect brought by 4-phenyl substituent on benzaldehyde, delivering 71% yield and 93% ee (3l).
We propose a catalytic cycle as shown in Scheme 3. There is an equilibrium between triazolium salt 5a and free carbene 5a′ in the presence of acetate.17 Activation of 1a by 5a′ generates a possible zwitterionic intermediate I, which undergoes proton transfer to form the Breslow intermediate II. Extrusion of the bromide within II gives dienolate intermediate III, which reacts with the carbonyl via a transition state analogous to IV. Formation of an ion pair between triazolium and chiral phosphate counterion would increase its solubility in cyclohexane, implying that the phosphoric acid catalyst may be acting as a chiral counterion although other mechanisms cannot be discounted. As mentioned above, a matched situation between the NHC and phosphoric acid backbone chirality is required for excellent stereocontrol. After C-C bond-forming, a new C-O bond forms through intermediate V and releases final product 3a, and free carbene 5a′ for the new catalytic circle.18
Scheme 3.
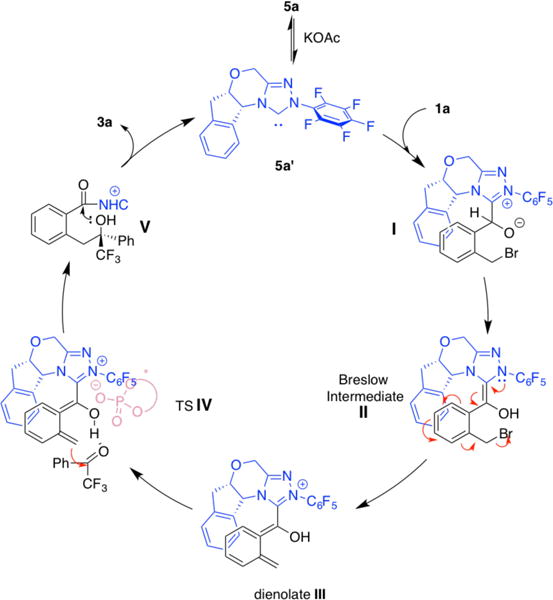
Proposed catalytic cycle
In conclusion, we have developed NHC/chiral phosphoric acid cooperative catalysis, enabling a highly enantioselective [4+2] annulation reaction of 2-(bromomethyl)benzaldehydes and fluorinated ketones. The phosphoric acid catalyst may be acting as a chiral Brønsted acid, a chiral counterion or phase transfer agent for the NHC catalyst. Cooperative activation between the NHC catalyst and phosphoric acid would provide new opportunities in asymmetric NHC catalysis and extension of the concept to other annulation modes may be possible.19
1H, 13C and 19F NMR spectra were recorded on a Bruker spectrometer (500 MHz, 125 MHz and 471 MHz for 1H, 13C and 19F NMR, respectively) at ambient temperature. High resolution mass spectra (HRMS) were obtained from Columbia University Mass Spectrometry Facility on a JOEL JMSHX110HF mass spectrometer using FAB+ ionization model. Infrared spectra were recorded on a Perkin Elmer Paragon 1000 FI-IR spectrometer. HPLC spectra were obtained on an Agilent 1100 series system. Optical rotation was obtained with an Autopol-III automatic polarimeter.
(S)-3-phenyl-3-(trifluoromethyl)isochroman-1-one (3a): Typical Procedure
To an oven-dried 5 mL vial with a magnetic stir bar, aldehyde 1a (0.10 mmol), ketone 2a (0.20 mmol), triazolium salt 5a (9.3 mg, 0.020 mmol), chiral phosphoric acid 6d (6.8 mg, 0.010 mmol), KOAc (19.6 mg, 0.40 mmol) were added before being transferred to an argon-filled glovebox. 1.0 mL of dry cyclohexane was added. The vial was tightly capped and removed from the glovebox. The reaction was vigorously stirred at room temperature. After 12h, the mixture was concentrated and the residue was subjected to flash silica gel chromatography (hexane: ether = 20:1) to yield lactone 3a (19.9 mg, 68% yield, 95% ee).
[α]20D = +87.8° (c = 0.014 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.98 (dd, J = 7.8, 1.3 Hz, 1H), 7.56 – 7.42 (m, 3H), 7.35 – 7.25 (m, 5H), 3.83 (d, J = 16.3 Hz, 1H), 3.69 (d, J = 16.3 Hz, 1H). Data matches literature report.14
(S)-3-(4-fluorophenyl)-3-(trifluoromethyl)isochroman-1-one (3b)
21.4 mg, 69% yield, 91% ee;
[α]20D = +90.9° (c = 0.017 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.97 (dd, J = 7.8, 1.3 Hz, 1H), 7.56 – 7.45 (m, 3H), 7.35 – 7.25 (m, 2H), 7.06 – 6.93 (m, 2H), 3.84 (d, J = 16.3 Hz, 1H), 3.64 (d, J = 16.4 Hz, 1H). Data matches literature report.14
(S)-3-(4-chlorophenyl)-3-(trifluoromethyl)isochroman-1-one (3c)
18.1 mg, 55% yield; 83% ee;
[α]20D = +75.6° (c = 0.016 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3): δ 7.26 (dd, J = 5.8, 2.9 Hz, 1H), 7.22–7.11 (m, 5H), 6.75–6.72 (m, 3H), 5.07 (s, 1H), 3.80 (dt, J = 11.2, 4.9 Hz, 1H), 3.34 (ddd, J = 11.1, 10.0, 4.1 Hz, 1H), 3.08 (ddd, J = 15.5, 10.1, 5.2 Hz, 1H), 2.91 (dt, J = 15.6, 4.3 Hz, 1H), 2.53–2.31 (m, 2H), 0.83 (t, J = 7.2 Hz, 3H). Data matches literature report.14
(S)-3-(3-fluorophenyl)-3-(trifluoromethyl)isochroman-1-one (3d)
21.1 mg, 68% yield; 87% ee;
[α]20D = +72.0° (c = 0.014 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.98 (dd, J = 7.9, 1.3 Hz, 1H), 7.52 (td, J = 7.6, 1.3 Hz, 1H), 7.35 – 7.22 (m, 5H), 7.01 (m, 1H), 3.84 (d, J = 16.3 Hz, 1H), 3.63 (d, J = 16.4 Hz, 1H);
13C NMR (125 MHz, CDCl3): δ 162.7 (d, J = 246 Hz), 161.9, 136.1 (d, J = 7.1 Hz), 134.9, 134.8, 130.5, 130.4 (d, J = 8.1 Hz), 128.3, 127.9, 124.2, 123.0 (q, J = 282 Hz), 122.8 (d, J = 2.6 Hz), 116.7 (d, J = 20.9 Hz), 114.6 (d, J = 23.8 Hz) 82.7 (q, J = 30.7 Hz), 31.1 (d, J = 1.5 Hz);
19F NMR (471 HZ, CDCl3) δ −78.6 (s), −110.3 (m);
IR (neat) 1742, 1594, 1445, 1280, 1185, 1115, 1073, 749, 729, 708 cm−1;
HRMS (ESI+) calcd for C16H11O2F4 as [M+H]+, 311.0695. Found 311.0696.
(S)-3-(perfluoroethyl)-3-phenylisochroman-1-one (3e)
21.6 mg, 63% yield; 87% ee;
[α]20D = +75.1° (c = 0.011 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.95 (dd, J = 7.8, 1.2 Hz, 1H), 7.50 – 7.44 (m, 3H), 7.33–7.24 (m, 4H), 7.22 (d, J = 7.6 Hz, 1H), 3.93 (d, J = 16.2 Hz, 1H), 3.66 (d, J = 16.2 Hz, 1H);
13C NMR (125 MHz, CDCl3): δ 162.1, 135.3, 134.6, 133.5, 130.2, 129.5, 128.7, 128.1, 127.9, 126.9, 124.4, 118.8 (qt, J = 287, 35.8 Hz), 111.3 (tq, J = 264, 35.6 Hz), 83.8 (t, J = 24.9 Hz), 31.4;
19F NMR (471 MHZ, CDCl3) δ −76.8 (s), −120.1, 121.5 (ABq, JAB = 280.0 Hz);
IR (neat) 1740, 1451, 1219, 1190, 1151, 1116, 1076, 745, 731, 716 cm−1;
HRMS (ESI+) calcd for C17H12O2F5 as [M+H]+, 343. 0575. Found 343. 0761.
(S)-3-(perfluoropropyl)-3-phenylisochroman-1-one (3f)
30.2 mg, 77% yield; 90% ee;
[α]20D = +107.3° (c = 0.015 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.96 (dd, J = 7.8, 1.3 Hz, 1H), 7.51 – 7.44 (m, 3H), 7.33 – 7.25 (m, 5H), 7.21 (d, J = 7.6 Hz, 1H), 3.95 (d, J = 16.2 Hz, 1H), 3.67 (d, J = 16.2 Hz, 1H);
13C NMR (125 MHz, CDCl3): δ 162.0, 135.2, 134.6, 133.5, 130.2, 129.4, 128.6, 128.1, 127.9, 127.1, 124.4, 118.8 (m), 116.3 (m), 113.9 (m), 111.9 (m), 109.9 (m), 107.8 (m), 84.7 (t, J = 25.3 Hz), 31.7;
19F NMR (471 MHz, CDCl3) δ −79.9 (t, J = 11.3 Hz, 3F), −116.1 – −118.4 (m, 2F), −120.5 (ddd, J = 289.6, 12.5, 4.7 Hz, 1F), −122.9 (ddd, J = 289.2, 12.5, 3.0 Hz, 1F);
IR (neat) 1741, 1461, 1450, 1340, 1226, 1125, 1074, 745, 730, 716, cm−1;
HRMS (ESI+) calcd for C18H12O2F7 as [M+H]+, 393. 0726. Found 393. 0718.
(S)-6-chloro-3-phenyl-3-(trifluoromethyl)isochroman-1-one (3j)
18.6 mg, 57% yield; 90% ee;
[α]20D = +112.9° (c = 0.018 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 1H), 7.54 – 7.50 (m, 3H), 7.40–7.33 (m, 3H), 7.32 – 7.29 (m, 2H), 3.83 (d, J = 16.4, 1H), 3.68 (d, J = 16.4 Hz, 1H);
13C NMR (125 MHz, CDCl3) δ 161.5, 141.1, 137.0, 133.1, 131.9, 129.7, 128.9, 128.7, 127.9, 126.9, 122.8, 123.1 (d, J = 282 Hz), 83.2 (d, J = 30.6 Hz), 31.0;
19F NMR (471 MHz, Chloroform-d) δ −78.7 (s);
IR (neat) 1736, 1600, 1270, 1185, 1168, 1095, 1074, 765, 720, 683 cm−1;
HRMS (ESI+) calcd for C16H11O2F3Cl as [M+H]+, 327. 0400. Found 327.0397.
(S)-3,6-diphenyl-3-(trifluoromethyl)isochroman-1-one(3k)
24.3 mg, 66% yield; 77% ee;
[α]20D = +93.1° (c = 0.019 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.0 Hz, 1H), 7.58 – 7.50 (m, 4H), 7.48 – 7.40 (m, 4H), 7.36 – 7.28 (d, J = 7.4 Hz, 3H), 3.89 (d, J = 16.2 Hz, 1H), 3.75 (d, J = 16.3 Hz, 1H);
13C NMR (125 MHz, CDCl3): 162.3, 147.4, 139.1, 135.8, 133.5, 130.9, 129.6, 129.1, 128.8, 128.7, 127.2, 127.0, 126.9, 126.3, 123.3 (d, J = 282 Hz), 123.0, 83.2 (d, J = 30.4 Hz), 31.3;
19F NMR (471 MHz, CDCl3) δ −78.7 (s);
IR (neat) 2923, 1737, 1611, 1450, 1271, 1238, 1169, 1130, 1073, 758, 740, 721, 695 cm−1;
HRMS (ESI+) calcd for C22H16O2F3 as [M+H]+, 369.1102. Found 369.1100.
(S)-3-(4-fluorophenyl)-6-phenyl-3-(trifluoromethyl)isochroman-1-one (3l)
27.5 mg, 71% yield; 93% ee;
[α]20D = +103.5° (c = 0.014 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 8.1 Hz, 1H), 7.58 – 7.51 (m, 5H), 7.49 – 7.39 (m, 4H), 7.02 (t, J = 8.6 Hz, 2H), 3.89 (d, J = 16.3 Hz, 1H), 3.70 (d, J = 16.3 Hz, 1H);
13C NMR (125 MHz, CDCl3): δ 163.2 (d, J = 249 Hz), 162.1, 147.6, 139.0, 135.6, 131.0, 129.4 (d, J = 3.3 Hz), 129.2 (d, J = 8.7 Hz), 129.1, 128.8, 127.3, 127.0, 126.3, 123.1 (d, J = 282 Hz), 122.8, 116.0 (d, J = 21.7 Hz), 82.8 (d, J = 30.5 Hz), 31.3;
19F NMR (471 MHz, CDCl3) δ −78.9 (s), −110.5 (tt, J = 8.4, 5.0 Hz);
IR (neat) 1739, 1611, 1512, 1239, 1171, 1073, 988, 836, 745, 697 cm−1;
HRMS (ESI+) calcd for C22H15O2F4 as [M+H]+, 387.1008. Found 387.1002.
(S)-3,7-diphenyl-3-(trifluoromethyl)isochroman-1-one (3m)
29.8 mg, 81% yield; 86% ee;
[α]20D = +118.0° (c = 0.028 g/ml, CHCl3);
1H NMR (500 MHz, CDCl3) δ 8.21 (d, J = 1.9 Hz, 1H), 7.72 (dd, J = 7.9, 2.0 Hz, 1H), 7.58 – 7.48 (m, 4H), 7.41 (t, J = 7.5 Hz, 2H), 7.38 – 7.28 (m, 5H), 3.86 (d, J = 16.3 Hz, 1H), 3.73 (d, J = 16.3 Hz, 1H);
13C NMR (125 MHz, CDCl3) δ 162.4, 141.3, 138.9, 134.0, 133.5, 133.1, 129.6, 129.0, 128.8, 128.7, 128.4, 128.1, 127.1, 126.9, 124.7, 123.3 (d, J = 282 Hz), 83.0 (d, J = 30.4 Hz), 30.8;
19F NMR (471 MHz, CDCl3) δ −78.8 (s);
IR (neat) 2924, 1742, 1451, 1306, 1221, 1169, 1073, 760, 745, 702 cm−1;
HRMS (ESI+) calcd for C22H16O2F3 as [M+H]+, 369.1102. Found 369.1100.
Supplementary Material
Acknowledgments
We thank NIGMS (GM72586) for support of this work.
Footnotes
Supporting Information
Is there Supporting Information to be published? Click here, then the arrow, and choose YES or NO.
Primary Data
Is there Primary Data to be associated with this manuscript? Click here, then the arrow, and choose YES or NO.
References and Notes
- 1.Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Chem Rev. 2015;115:9307. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For reviews about compatible catalysts in one system, see:; (a) Allen AE, MacMillan DWC. Chem Sci. 2012;3:633. doi: 10.1039/C2SC00907B. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shao Z, Zhang H. Chem Soc Rev. 2009;38:2745. doi: 10.1039/b901258n. [DOI] [PubMed] [Google Scholar]; (c) Du Z, Shao Z. Chem Soc Rev. 2013;42:1337. doi: 10.1039/c2cs35258c. [DOI] [PubMed] [Google Scholar]; (d) Chen DF, Han ZY, Zhou XL, Gong LZ. Acc Chem Res. 2014;47:2365. doi: 10.1021/ar500101a. [DOI] [PubMed] [Google Scholar]
- 3.For carbene/amine catalysis, see:; (a) Lathrop SP, Rovis T. J Am Chem Soc. 2009;131:13628. doi: 10.1021/ja905342e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ozboya KE, Rovis T. Chem Sci. 2011;2:1835. doi: 10.1039/C1SC00175B. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jacobsen CB, Jensen KL, Udmark J, Jørgensen KA. Org Lett. 2011;13:4790. doi: 10.1021/ol2018104. [DOI] [PubMed] [Google Scholar]
- 4.For carbene/base catalysis, see:; (a) Filloux CM, Lathrop SP, Rovis T. Proc Natl Acad Sci USA. 2010;107:20666. doi: 10.1073/pnas.1002830107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Padmanaban M, Biju AT, Glorius F. Org Lett. 2011;13:5624. doi: 10.1021/ol2023483. [DOI] [PubMed] [Google Scholar]; (c) Izquierdo J, Orue A, Scheidt KA. J Am Chem Soc. 2013;135:10634. doi: 10.1021/ja405833m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kuwano S, Harada S, Kang B, Oriez R, Yamaoka Y, Takasu K, Yamada K. J Am Chem Soc. 2013;135:11485. doi: 10.1021/ja4055838. [DOI] [PubMed] [Google Scholar]; (e) Youn SW, Song HS, Park JH. Org Lett. 2014;18:1028. doi: 10.1021/ol5000617. [DOI] [PubMed] [Google Scholar]; (f) Reddi Y, Sunoj RB. ACS Catal. 2015;5:1956. [Google Scholar]
- 5.Zhao X, DiRocco DA, Rovis T. J Am Chem Soc. 2011;133:12466. doi: 10.1021/ja205714g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Liu G, Wilkerson PD, Toth CA, Xu H. Org Lett. 2012;14:858. doi: 10.1021/ol203375y. [DOI] [PubMed] [Google Scholar]; (b) Fu Z, Sun H, Chen S, Tiwari B, Li G, Chi YR. Chem Commun. 2013;49:261. doi: 10.1039/c2cc36564b. [DOI] [PubMed] [Google Scholar]; (c) Li JL, Sahoo B, Daniliuc CG, Glorius F. Angew Chem Int Ed. 2014;53:10515. doi: 10.1002/anie.201405178. [DOI] [PubMed] [Google Scholar]; (d) Xu J, Chen X, Wang M, Zheng P, Song B-A, Chi Y R. Angew Chem Int Ed. 2015;54:5161. doi: 10.1002/anie.201412132. [DOI] [PubMed] [Google Scholar]; (e) Pareek M, Sunoj RB. ACS Catal. 2016;6:3118. [Google Scholar]; (f) Wang Y, Tang M, Wang Y, Wei D. J Org Chem. 2016;81:5370. doi: 10.1021/acs.joc.6b00656. [DOI] [PubMed] [Google Scholar]
- 7.For a combination of carbene with two distinct organocatalysts, see:; Wang MH, Cohen DT, Schwamb B, Mishra RK, Scheidt KA. J Am Chem Soc. 2015;137:5891. doi: 10.1021/jacs.5b02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a review on carbene/Lewis acid catalysis, see:; Cohen DT, Scheidt KA. Chem Sci. 2012;3:53. doi: 10.1039/C1SC00621E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For carbene/transition metal catalysis, see:; (a) DiRocco DA, Rovis T. J Am Chem Soc. 2012;134:8094. doi: 10.1021/ja3030164. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Namitharan K, Zhu T, Chen J, Zheng P, Li X, Yang S, Song BA, Chi YR. Nat Commun. 2014;5:3982. doi: 10.1038/ncomms4982. [DOI] [PubMed] [Google Scholar]; (c) Liu K, Hovey MT, Scheidt KA. Chem Sci. 2014;5:4026. doi: 10.1039/C4SC01536C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Guo C, Fleige M, Janssen-Müller D, Daniliuc CG, Glorius F. J Am Chem Soc. 2016;138:7840. doi: 10.1021/jacs.6b04364. [DOI] [PubMed] [Google Scholar]
- 10.(a) Shen LT, Shao PL, Ye S. Adv Synth Catal. 2011;353:1943. [Google Scholar]; (b) Chen X-Y, Xia F, Cheng J-T, Ye S. Angew Chem Int Ed. 2013;52:10644. doi: 10.1002/anie.201305571. [DOI] [PubMed] [Google Scholar]; (c) Cheng JT, Chen XY, Gao ZH, Ye S. Eur J Org Chem. 2015;1047 [Google Scholar]; (d) Jia WQ, Zhang HM, Zhang CL, Gao ZH, Ye S. Org Chem Front. 2016;3:77. [Google Scholar]
- 11.(a) Mo J, Chen X, Chi YR. J Am Chem Soc. 2012;134:8810. doi: 10.1021/ja303618z. [DOI] [PubMed] [Google Scholar]; (b) Xu J, Jin Z, Chi YR. Org Lett. 2013;15:5028. doi: 10.1021/ol402358k. [DOI] [PubMed] [Google Scholar]; (c) Wang M, Huang Z, Xu J, Chi YR. J Am Chem Soc. 2014;136:1214. doi: 10.1021/ja411110f. [DOI] [PubMed] [Google Scholar]; (d) Li BS, Wang Y, Jin Z, Zheng P, Ganguly R, Chi YR. Nat Commun. 2015;6:6207. doi: 10.1038/ncomms7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Yao C, Xiao Z, Liu R, Li T, Jiao W, Yu C. Chem - Eur J. 2013;19:456. doi: 10.1002/chem.201202655. [DOI] [PubMed] [Google Scholar]; (b) Xiao Z, Yu C, Li T, Wang XS, Yao C. Org Lett. 2014;16:3632. doi: 10.1021/ol501224p. [DOI] [PubMed] [Google Scholar]; (c) Liu R, Yu C, Xiao Z, Li T, Wang X, Xie Y, Yao C. Org Biomol Chem. 2014;12:1885. doi: 10.1039/c3ob42008f. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Yang S, Song BA, Chi YR. Angew Chem Int Ed. 2013;52:11134. doi: 10.1002/anie.201305861. [DOI] [PubMed] [Google Scholar]
- 14.During the course of these investigations, Glorius et al. reported a similar but racemic reaction. One example involving a chiral NHC catalyst provided product in 48% ee; see:; Janssen-Müller D, Singha S, Olyschläger T, Daniliuc CG, Glorius F. Org Lett. 2016;18:4444. doi: 10.1021/acs.orglett.6b02335. [DOI] [PubMed] [Google Scholar]
- 15.For reviews, see:; (a) Akiyama T. Chem Rev. 2007;107:5744. doi: 10.1021/cr068374j. [DOI] [PubMed] [Google Scholar]; (b) Akiyama T, Mori T. Chem Rev. 2015;115:9277. doi: 10.1021/acs.chemrev.5b00041. [DOI] [PubMed] [Google Scholar]; (c) Terada M. Chem Commun. 2008;4097 doi: 10.1039/b807577h. [DOI] [PubMed] [Google Scholar]; For pioneering work, see:; (d) Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem Int Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; (e) Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 16.(a) Momiyama N, Nishimoto H, Terada M. Org Lett. 2011;13:2126. doi: 10.1021/ol200595b. [DOI] [PubMed] [Google Scholar]; (b) Terada M, Li F, Toda Y. Angew Chem Int Ed. 2014;53:235. doi: 10.1002/anie.201307371. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Glover GS, Oberg KM, Dalton DM, Rovis T. Synlett. 2013;1229 doi: 10.1055/s-0033-1338842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A concerted [4+2] cycloaddition may also be operative in this step.
- 19.Ni Q, Xiong J, Song X, Raabe G, Enders D. Synlett. 2015;26:1465. doi: 10.1055/s-0034-1381004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


