Abstract
Cervical cancer is the leading cause of cancer-related mortality in the developing world, where HIV and Mycobacterium tuberculosis (TB) infection are also endemic. HIV infection is independently associated with increased morbidity and mortality among women with cervical cancer. TB is believed to increase the risk of malignancies and could cause chronic inflammation in the gynecologic tract. However, the relationship between cervical cancer and TB in settings hyperendemic for HIV is unknown. We found that 18 (10%) of a cohort of 180 women with cervical cancer in Botswana had a history of TB disease. Age and HIV infection were also associated with a history of TB disease. Our data show that prior TB disease is highly prevalent among patients with cervical cancer infected with HIV. The coexistence of cervical cancer, HIV infection, and prior TB infection might be higher than expected in the general population. Prospective studies are needed to better determine the impact of the collision of these three world health epidemics.
INTRODUCTION
Cervical cancer is the third most common malignancy in women worldwide and the leading cause of cancer-related death for women in developing countries. Globally, it is estimated that approximately half a million women are diagnosed with cervical cancer every year, and approximately 275,000 women die of the disease, 85% of whom live in low- and middle-income countries.1 In Botswana, cervical cancer is the most common gynecologic cancer and the leading cause of cancer death.2,3
HIV infection accelerates the progression toward cervical cancer and likely is associated with worse clinical outcomes.4 In Botswana, 60% of patients diagnosed with cervical cancer are also infected with HIV.2,5 With cervical cancer rates rising in developing countries where HIV is endemic, identifying and understanding the factors leading to increased morbidity and mortality in these populations should be a priority.6
The devastating impact of the colliding HIV and tuberculosis (TB) pandemics in resource-limited settings has been long recognized.7 HIV infection is one of the most important risk factors for progression to active TB disease. Furthermore, TB is the number one killer of people living with HIV in the world.5 Importantly, the impact of these pandemics is more severe in countries with more challenging health care systems, where TB and HIV are less controlled, and patients may have more advanced disease states compared with the developed world. Botswana has one of the highest TB prevalence rates in the world, and it is estimated that 60% to 75% of people diagnosed with TB are coinfected with HIV.8
An association between malignancies and TB was previously established.9 TB has been implicated in the pathogenesis of malignancies and may interfere with their diagnosis. TB and cancer frequently coexist, and the relative immunosuppression caused by cancer or its treatment may lead to reactivation of latent TB infections, leading to increased morbidity and mortality.9 However, data on the association between cervical cancer and TB are scarce.10
Despite the magnitude of the cervical cancer, HIV, and TB epidemics coexisting in many resource-limited settings, their associations and the potential interactions among all three pandemics have not been documented previously, although, as previously mentioned, those between HIV and cervical cancer as well as between HIV and TB have been explored. However, there is limited information on the impact of TB or the combination of TB and HIV on cervical carcinomas. In this study, we examined the association between cervical cancer and a prior history of TB by HIV serostatus in a prospective cohort of patients with cervical cancer in Botswana.
METHODS
Cervical cancer diagnosis and treatment in Botswana is centralized at Princess Marina Hospital, the largest referral hospital in the country, located in the capital, Gaborone. Approximately 95% of all patients diagnosed with any cancer in public hospitals from all over the country are referred there for confirmation of diagnosis and treatment. In this study, we enrolled consecutive patients referred to Princess Marina Hospital for the treatment of cervical cancer between July 2013 and January 2015. We collected demographic characteristics and cancer- and TB-specific information prospectively through patient interviews and medical record reviews. All data were collected before cancer treatment initiation. Prior TB disease was defined as an episode of clinically or microbiologically diagnosed TB requiring initiation of TB treatment at any point in the past. HIV testing was performed on all patients in accordance with Botswana’s national guidelines. Radiologic information was unavailable in most cases, and it was not analyzed in this study.
We first described the characteristics of patients with cervical cancer when stratified by history of active TB and cervical cancer. Associations were determined using χ2, t test, and Mann-Whitney testing as appropriate. A logistical regression model was developed to describe correlates of prior TB disease. Variables for the model were identified a priori on the basis of their conceptual importance and included age at diagnosis of cervical cancer, age of first sexual encounter, smoking history, and presence of HIV infection. A separate model was developed to determine the association of CD4 cell count categories using HIV-negative patients as referents. Odds ratios and 95% CI were calculated. Data processing and analyses were performed using Stata software (STATA, College Station, TX).This study was approved by the institutional review boards at the University of Pennsylvania, University of Botswana, and Botswana Ministry of Health. All patients provided informed consent.
RESULTS
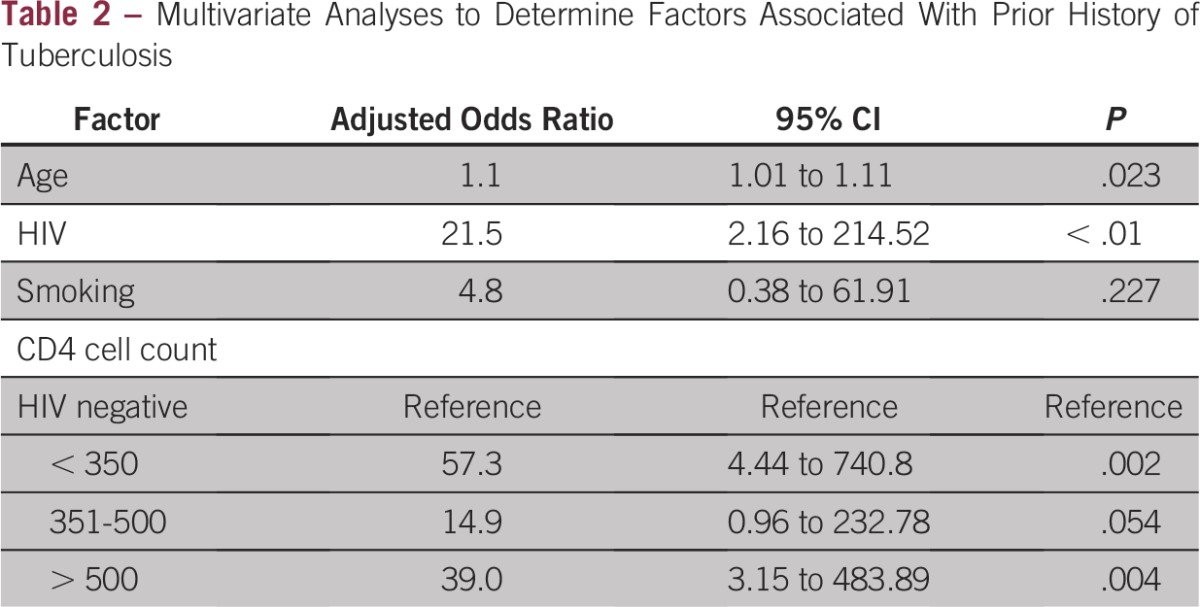
We enrolled 180 women during the study period, of whom 117 (65%) were infected with HIV at the time of their cervical cancer diagnosis. Overall, 18 (10%) patients with cervical cancer had a history of TB disease, and 16 (89%) of them were coinfected with HIV. All patients with TB were treated for their disease. All HIV-positive patients were either already receiving antiretroviral treatment or had started on antiretroviral treatment at the time of cancer diagnosis. The main demographic and clinical characteristics of the sample stratified by history of TB infection are listed in Table 1. Our bivariate analyses showed a significantly higher prevalence of HIV infection among patients with cervical cancer with a history of TB compared with those without a history of TB (16 [89.8%] of 18 patients v 101 [62.3%] of 162 patients; P = .02). As listed in Table 2, this difference remained significant in multivariable analysis (adjusted odds ratio [AOR], 21.5; 95% CI, 2.16 to 214.52; P < .01). Increasing age was also found to be significantly associated with prior TB disease after accounting for confounders (AOR, 1.1; 95% CI, 1.01 to 1.11; P = .02). In a separate multivariable model, we found that CD4 counts less than 350 cells/μL (AOR, 57.3; 95% CI, 4.44 to 740.8) and greater than 500 cells/mm3 (AOR, 39.0; 95% CI, 3.15 to 483.89) were associated with prior TB disease compared with no HIV infection.
Table 1.
Characteristics of Cervical Cancer Patients by History of Prior Tuberculosis in Botswana
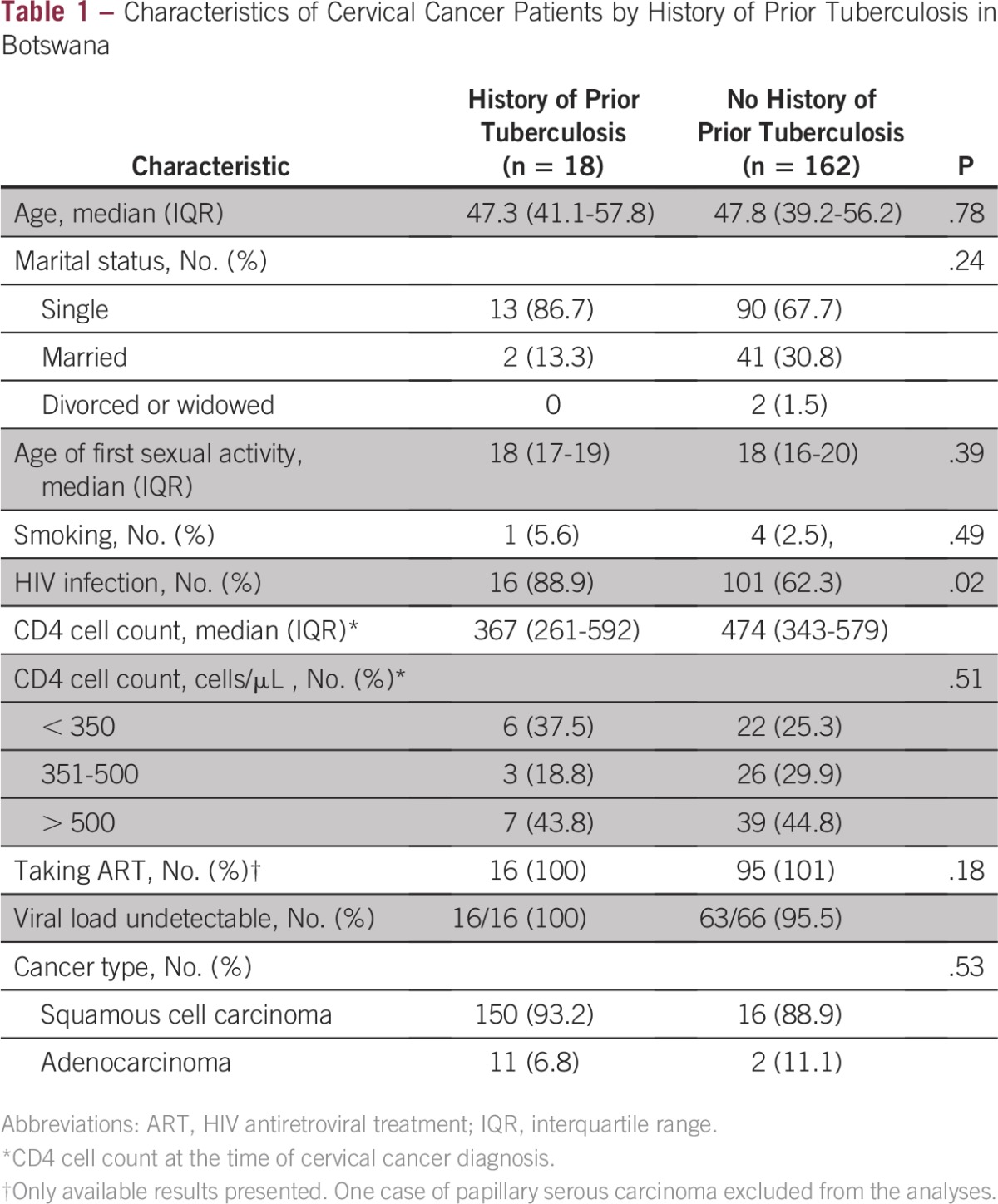
Table 2.
Multivariate Analyses to Determine Factors Associated With Prior History of Tuberculosis
DISCUSSION
In this study, we found that prior TB disease was common in our sample of patients with cervical cancer in Botswana. HIV infection was also highly prevalent among patients with cervical cancer who had TB disease before their diagnosis of cervical cancer. Our findings are not surprising, given the well-known association between HIV and TB. Nevertheless, they highlight an underappreciated overlap of three of the most devastating epidemics in developing countries: cervical cancer, HIV, and TB.
Our study design does not allow any inference regarding the role of TB in the causal pathway of cervical cancer, the temporal nature of events, or the presence of interactions between TB, HIV, and cervical cancer that may alter the natural course of any of those diseases. However, our results indicate that the coexistence of prior TB disease, HIV infection, and cervical cancer is common, suggesting that potential interactions between two or more of those conditions might be common as well. Disseminated (gynecologic) TB has the potential to lead to delays in cervical cancer diagnosis and an increase in morbidity and mortality during cancer treatment.11 Conversely, cervical cancer diagnosis may delay the diagnosis and treatment initiation of coexisting TB.11,12 Although TB has not been associated with the development of cervical cancer, chronic TB infection and inflammation in the gynecologic tract is an important cause of infertility in the developing world.13 Thus, the chronic inflammation due to chronic gynecologic TB infection might be a contributing factor in the progression toward cervical cancer.11,13,14 HIV infection is associated with a higher incidence, as well as increased morbidity and mortality, of both TB and cervical cancer, independently. Whether HIV infection modifies the relationship between TB and cervical cancer remains to be determined.
We acknowledge the limitations of our study. Multiple sources of bias could have confounded our findings. Our study was performed on a convenience sample of all consecutive patients with cervical cancer referred to the only cancer treatment center in Botswana, and we did not have a comparison group from the general population. Therefore, we are unable to determine whether the overall prevalence of prior TB infection was higher than what would be expected from the general population with or without stratifying it by HIV status. Our inability to prospectively determine the extent of active TB disease after cancer diagnosis severely limits any inference regarding the true burden of TB in our population. Prospective TB-attributable morbidity and mortality data are highly needed.
Despite these limitations, we believe that these results highlight the critical need to better understand the imminent collision of three of the most important pandemics of our time: HIV, TB, and cancer. Although data remain scant, HIV is a leading factor increasing the incidence of both AIDS-defining and non–AIDS-defining cancers in southern Africa. In Botswana, 43% of all cancers and an even greater proportion of cancer deaths are attributable to HIV. Future studies are needed to determine the extent of the interactions among these highly prevalent conditions worldwide.
ACKNOWLEDGEMENT
We thank Princess Marina Hospital, Gaborone Private Hospital, the Botswana Ministry of Health, and the Centers for Disease Control and Prevention for their constant support, and all our patients,, who made this study possible.
Footnotes
Authors’ disclosures of potential conflicts of interest and contributions are found at the end of this article.
Supported by the Center for AIDS Research, ASCO and Conquer Cancer Foundation, ASCO Young Investigator Award, and National Institutes of Health grant Nos. R01AI097045, K01AI118559, and U54CA190158-02. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
Financial support: Nicola M. Zetola, Surbhi Grover
Administrative support: Nicola M. Zetola, Surbhi Grover
Provision of study materials or patients: Nicola M. Zetola, Surbhi Grover
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Nicola M. Zetola
No relationship to disclose
Surbhi Grover
No relationship to disclose
Chawangwa Modongo
No relationship to disclose
Sebathu P. Chiyapo
No relationship to disclose
Memory Nsingo-Bvochora
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca
Mohan Narasimhamurthy
No relationship to disclose
Lilie L. Lin
Speakers' Bureau: Varian Medical Systems
Research Funding: Varian Medical Systems
Joseph Jarvis
Research Funding: Gilead Sciences
Sanghyuk S. Shin
Employment: Beckman Coulter
Erle Robertson
No relationship to disclose
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS One. 2015;10:e0135602. doi: 10.1371/journal.pone.0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramogola-Masire D, de Klerk R, Monare B, et al. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. J Acquir Immune Defic Syndr. 2012;59:308–313. doi: 10.1097/QAI.0b013e3182426227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: A systematic global review. Int J STD AIDS. 2014;25:163–177. doi: 10.1177/0956462413491735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botswana Ministry of Health: Botswana Aids Impact Survey (BAIS) IV Report 2013.
- 6.Chabner BA, Efstathiou J, Dryden-Peterson S. Cancer in Botswana: The second wave of AIDS in sub-Saharan Africa. Oncologist. 2013;18:777–778. doi: 10.1634/theoncologist.2013-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botswana Ministry of Finance and Development Planning . 2011 Population and Housing Census. Gaborone, Botswana: Central Statistics Office; 2011. [Google Scholar]
- 9.Cruz AT, Airewele G, Starke JR. Tuberculosis in pediatric oncology and bone marrow transplantation patients. Pediatr Blood Cancer. 2014;61:1484–1485. doi: 10.1002/pbc.24985. [DOI] [PubMed] [Google Scholar]
- 10.Divya A, Irfant A, Geetha P, et al. Cancer-like presentation of female genital tuberculosis. Indian J Tuberc. 2014;61:331–335. [PubMed] [Google Scholar]
- 11.Kumakech W, Zamblera D, Jolaoso A. The multifaceted presentation of tuberculosis in gynaecology: A masquerader as cervical cancer as well as a cause of primary infertility in the same patient. J Obstet Gynaecol. 2006;26:178–179. doi: 10.1080/01443610500475693. [DOI] [PubMed] [Google Scholar]
- 12.Bekele D, Bekuretsion Y. Tuberculosis of the cervix mimicking cervical cancer. Ethiop Med J. 2014;52:87–89. [PubMed] [Google Scholar]
- 13.Zhao FH, Varanasi AP, Cunningham CA, et al. Tuberculosis and oncogenic HPV: Potential co-infections in women at high-risk of cervical cancer in rural China. Asian Pac J Cancer Prev. 2011;12:1409–1415. [PubMed] [Google Scholar]
- 14.Singh N, Sumana G, Mittal S. Genital tuberculosis: A leading cause for infertility in women seeking assisted conception in North India. Arch Gynecol Obstet. 2008;278:325–327. doi: 10.1007/s00404-008-0590-y. [DOI] [PubMed] [Google Scholar]


