Abstract
OBJECTIVE
Adverse early-life events are predisposing factors for Functional Neurological Disorder (FND) and Post-Traumatic Stress Disorder (PTSD). Cingulo-insular regions are implicated in the biology of both conditions, and are sites of stress-mediated neuroplasticity. We hypothesized that functional neurological symptoms and the magnitude of childhood abuse would be associated with overlapping anterior cingulate cortex (ACC) and insular volumetric reductions, and that FND and PTSD symptoms would map onto distinct cingulo-insular areas.
METHODS
This within-group Voxel-Based Morphometry study probes volumetric associations with self-report measures of functional neurological symptoms, adverse life events and PTSD symptoms in 23 mixed-gender FND patients. Separate secondary analyses were also performed in the subset of 18 women with FND to account for gender-specific effects.
RESULTS
Across the entire cohort, there were no statistically significant volumetric associations with self-report measures of functional neurological symptom severity or childhood abuse. In women with FND, however, parallel inverse associations were observed between left anterior insular volume and functional neurological symptoms as measured by the Patient Health Questionnaire-15 and the Screening for Somatoform Symptoms Conversion Disorder subscale. Similar inverse relationships were also appreciated between childhood abuse burden and left anterior insular volume. Across all subjects, PTSD symptom severity was inversely associated with dorsal ACC volume and the magnitude of lifetime adverse events was inversely associated with left hippocampal volume.
CONCLUSIONS
This study reveals distinct cingulo-insular alterations for FND and PTSD symptoms, and may advance our understanding of FND. Potential biological convergence between stress-related neuroplasticity, functional neurological symptoms and reduced insular volume was identified.
Keywords: Conversion Disorder, Functional Movement Disorders, Functional Weakness, Psychogenic Nonepileptic Seizures, Voxel-Based Morphometry
INTRODUCTION
While Functional Neurological Disorder (FND) (Conversion Disorder) is common and imposes significant healthcare costs[1–3], the neuropathophysiology of this condition remains poorly understood. Functional neurological symptoms comprise 16% of outpatient neurology referrals, second only to headache[4]. Patients with FND present with medically unexplained sensory and motor symptoms including fatigue, pain, non-dermatomal sensory deficits, limb weakness, gait difficulties, nonepileptic seizures and abnormal movements. In the United States, an estimated $256 billion is spent annually in healthcare for medically unexplained illness[5]. This public health problem is heightened by observations that FND is a neglected condition at the intersection between Neurology and Psychiatry. While neurologists are frustrated caring for this population and lack a neurobiological understanding of FND[6], psychiatrists are similarly uncomfortable treating patients whose chief complaint is physical.
Adverse life events are a risk factor for FND, and the magnitude of early-life maltreatment is associated with illness severity[7]. Studies suggest that between one-fourth and three-fourths of individuals with FND endorse early-life maltreatment[8–11]. Childhood abuse has been linked to increased FND severity, and individuals reporting several traumatic childhood events more often exhibit a multiplicity of FND symptoms[7]. In those with nonepileptic seizures, past sexual abuse is linked to earlier age of onset, more severe convulsions, intrusive traumatic recollections and disability[12]. Furthermore, the development of additional medically unexplained symptoms in FND is predicted by antecedent trauma[11]. These collective observations suggest that previously experienced adverse life events, particularly childhood abuse, are important predisposing factors for the development of functional neurological symptoms.
Childhood abuse is also linked to increased risk of developing affective disorders, and those with these conditions and past abuse exhibit greater illness severity, increased psychiatric comorbidities and less favorable prognoses[13]. Associations between FND and other trauma-related disorders including Post-Traumatic Stress Disorder (PTSD) are well documented[10,14]. Importantly, studies also suggest that gender may modulate the development of psychopathology following childhood abuse[15]. It is not yet known, however, if adverse life events affect developmentally vulnerable neural circuits in a disorder-specific manner or if aberrant neuroplastic changes following adverse life events facilitate a general increased predilection for psychopathology.
While FND is less well studied compared to other neuropsychiatric disorders, early neuroimaging studies suggest an important role for the cingulo-insular (salience) network in the pathophysiology of FND[16–19]. The anterior cingulate cortex (ACC) and the insula are two paralimbic convergent sites for the multimodal integration of affective, viscerosomatic/nociceptive and cognitive processing[20–22]. Compared to healthy controls, patients with nonepileptic seizures display structural alterations in the dorsal ACC[23] and insula[24]. Individuals with somatic symptom disorder with predominant pain compared to controls exhibit decreased cingulate and insular gray matter volumes[25]. In functional neuroimaging studies, patients with FND show heightened insular and ACC activations during motor tasks compared to controls[26,27]. Increased cingulate gyrus activations during affectively valenced face processing have also been observed in FND[28]. These convergent findings implicate cingulo-insular regions in the biology of FND, yet limited research has studied structural brain-symptom severity and brain-disease risk relationships in this population.
Neuroimaging studies also link cingulo-insular alterations to the pathophysiology of PTSD. Magnetic resonance imaging (MRI) studies show gray matter reductions in the ACC and hippocampus in patients with PTSD compared to controls[29,30], as well as inverse associations between ACC volume and PTSD severity[31]. To a lesser extent, gray matter reductions in the insula have also been characterized in PTSD[32]. While heightened insular activations may occur across anxiety disorders, reduced top-down rostral ACC and increased amygdala activations are well described in PTSD[30].
In this voxel-based morphometry (VBM) MRI study, we used a within-group design to dimensionally probe gray matter associations with self-report measures of functional neurological symptoms, adverse life events and PTSD symptoms in 23 patients with FND. Given the increased incidence of FND in women and observations that gender may modulate the development of psychopathology following childhood abuse[15], secondary analyses were also performed separately in the subgroup of 18 women to account for gender-specific effects. As detailed in several conceptual models by our group[16–18], we hypothesized that functional neurological symptom severity and the magnitude of childhood abuse would be associated with overlapping ACC and insular volumetric reductions. We also hypothesized that FND and PTSD symptom severity would map onto distinct cingulo-insular brain areas.
METHODS
Participants and psychometric assessments
Twenty-three subjects with FND (mean age=41.6±11.6 years; 18 women, 5 men; mean illness duration=3.7±4.5 years) were recruited from an integrated behavioral neurology-neuropsychiatry FND Clinic at the Massachusetts General Hospital[33]. All patients met diagnostic criteria for at least one FND subtype including clinically-established Functional Movement Disorders[34] (n=12), documented (n=6) or clinically-established (n=1) non-epileptic seizures[35] and/or exhibited positive examination findings for functional weakness (n=11)[36]. 7 out of 23 subjects also exhibited non-dermatomal sensory deficits and a distinct 7 individuals had mixed motor FND. Diagnoses were based on a clinical evaluation by a dual-trained and board certified neurologist and psychiatrist (D.L.P.). Exclusion criteria included: any significant major neurological disorder resulting in specific MRI abnormalities (i.e. stroke, severe traumatic brain injury), epileptic seizures, other movement disorders, poorly controlled major medical illnesses with known central nervous system consequences, ongoing alcohol dependence or illicit substance misuse, a history of mania or psychosis, and/or active suicidality. Additional current psychiatric diagnoses as measured by the Structured Clinical Interview (SCID) for DSM-IV-TR Axis I Disorders included major depressive disorder (n=8), dysthymia (n=2), panic disorder (n=10), generalized anxiety disorder (n=9), PTSD (n=5), other somatoform disorders (n=10), and alcohol abuse (n=1). 16 of the 23 subjects were taking psychoactive medications. Subjects provided signed informed consent and this study was approved by the Partners Human Research Committee. See Supplemental Table 1 for participants’ demographics and diagnoses.
Subjects participated in two closely timed research visits. In one visit, subjects completed the SCID and self-report measures including: the Patient Health Questionnaire-15 (PHQ-15)[37], Childhood Trauma Questionnaire (CTQ)[38], Life Events Checklist-5[39] (LEC-5), PTSD Checklist for DSM-5 (PCL-5)[40], Beck Depression Inventory-II (BDI)[41], and the Spielberger Trait Anxiety Inventory (STAI-T)[42]. On the day of scanning, subjects completed the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale (SOMS:CD)[43].
The PHQ-15 is a 15-item measure of somatic symptoms within the past four weeks; each item is scored on a 3-point scale ranging from “not bothered at all” to “bothered a lot”. The SOMS:CD is a 14-item measure of motor and sensory functional neurological symptoms within the past 7 days; each item is scored on a 5-point scale regarding the perceived degree of impairment ranging from “not at all” to “very severe”. To emphasize a trans-diagnostic, dimensional approach to brain-symptom relationships in this study, we refer to symptoms captured by both the PHQ-15 and the SOMS:CD as functional (psychogenic) neurological symptoms.
The CTQ is a 25-item measure of childhood/adolescent abuse and neglect; cumulative indices of abuse (sexual, physical and emotional (CTQ-Abuse)) and neglect (emotional and physical (CTQ-Neglect)) were calculated separately. The LEC-5 is a 17 category measure of lifetime adverse events, and the PCL-5 is a 20-item measure of PTSD symptoms that can be sub-divided into re-experiencing, avoidance, negative alterations in cognition and mood, and hyperarousal sub-domains.
MRI data acquisition
In the MRI session, subjects were placed in a Siemens 3 Tesla Trio scanner to acquire a 3D T1-weighted magnetization prepared rapid acquisition gradient echo sequence with the following parameters: orientation=sagittal; matrix size=256×256; voxel size=1×1×1mm; slice thickness=1mm, slices=160; repetition time=2300ms; echo time=2.98ms; field of view=256mm. Bi-temporal foam pads were used to restrict head motion.
MRI data preprocessing and analyses
Statistical Parametric Mapping 8 (SPM8; www.fil.ion.ucl.ac.uk/spm/) and the VBM8 toolbox were used to analyze data in Matlab. Each MRI sequence was visually inspected for quality and re-oriented along the anterior-to-posterior commissure. Thereafter, images were segmented into gray matter, white matter and cerebrospinal fluid components. The diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) was applied to the gray matter images for spatial normalization. To control for individual whole-brain volume differences, nonlinear modulation using the Jacobian determinants derived from the normalization process was implemented. The modulated gray matter images were smoothed with an 8mm full-width-at-half-maximum Gaussian kernel.
After preprocessing, SPM-based multiple regression was used to examine associations between covariates-of-interest and gray matter volumes with age and gender as nuisance variables. Separate regression analyses were performed for PHQ-15, SOMS:CD, CTQ-Total, CTQ-Abuse, CTQ-Neglect, LEC-5-“happened to me” score, PCL-5 total, and the 4 PCL-5 sub-scores. To consider gender effects, all analyses were also run for the 18 women controlling for age. Data were first reviewed at an uncorrected p≤0.001 and a cluster extent threshold of 50 voxels. Whole-brain corrections for multiple comparisons at the peak voxel-level used a family-wise error (FWE) rate of p<0.05. Given our specific a priori hypotheses for cingulo-insular cortices, we defined bilateral regions-of-interest (ROIs) in the ACC and insula using the WFU Pickatlas to perform small volume corrections (SVC). Since there is an extensive literature linking stress-related neuroplasticity to the hippocampus, bilateral hippocampii were also a ROI. A FWE rate of p<0.05 was used for SVCs at the peak-voxel level. To control for potential confounding effects of depression and anxiety, separate analyses were performed for statistically significant findings with BDI or STAI-T scores entered as nuisance variables.
RESULTS
Associations between FND symptoms and childhood abuse
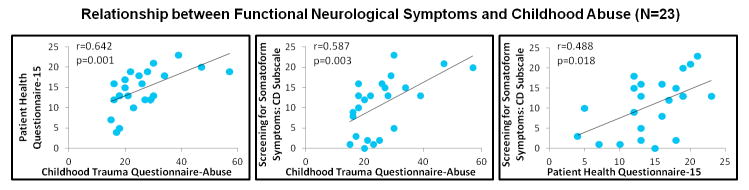
Statistically significant associations were observed between PHQ-15 and CTQ-Abuse scores (p=0.001; Spearman correlation coefficient=0.642), and between SOMS:CD and CTQ-Abuse scores across all patients (p=0.003; Spearman correlation coefficient=0.587) (Figure 1, Supplemental Figure 1). Positive associations were also appreciated between PHQ-15 scores and SOMS:CD scores (p=0.018; Spearman correlation coefficient=0.488). See Supplemental Table 2 for clinical scores.
FIGURE 1. Scatter plots of the positive associations between functional neurological symptoms and the magnitude of previously experienced childhood abuse.
Also shown, is the scatter plot of the positive correlation between the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale and the Patient Health Questionnaire-15. Spearman correlation coefficients are displayed.
Gray matter associations with FND symptoms and childhood abuse
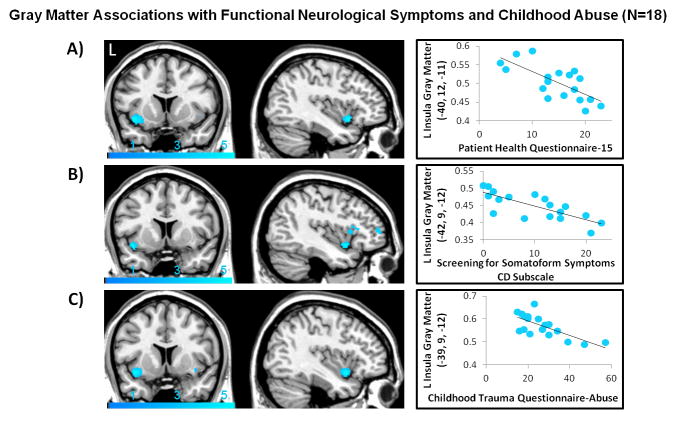
For the entire FND cohort, only a trend-level association was present between PHQ-15 scores and decreased left anterior insular volumes (puncorrected<0.001). A negative trend association was also observed between CTQ-abuse score and left anterior insular volumes (puncorrected<0.001) (Supplemental Figure 2). In women, these relationships showed separate statistically significant inverse relationships between PHQ-15 (psvc=0.032), SOMS:CD (psvc=0.038), CTQ-Abuse (psvc=0.021) and left anterior insular gray matter volumes (Figure 2, Table 1). Regression analyses secondarily controlling for STAI-T scores remained statistically significant, though these associations did not hold controlling for BDI scores (Supplemental Tables 3, 4).
FIGURE 2. Left anterior insula gray matter volume negatively correlated with functional neurological symptoms and previously experienced childhood abuse in women with Functional Neurological Disorder.
(A) Patient Health Questionnaire-15 scores were inversely associated with left anterior insular gray matter volume (psvc=0.032; peak z-score=−3.93). (B) Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale scores were also inversely correlated with left anterior insular gray matter volume (psvc=0.038; peak z-score=−3.87). (C) The magnitude of childhood abuse as measured by the Childhood Trauma Questionnaire showed a parallel negative association with left anterior insular gray matter volume (psvc=0.021; peak z-score=−4.04). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.
TABLE 1.
Statistically significant within-group gray matter volume associations with clinical measures of interest for the entire cohort (N=23) and for the subset of women with Functional Neurological Disorder (N=18).
| N | Measures of Interest | Cerebral Regions(Brodmann Area) | Peak Coordinates in MNI Space (mm) | Peak Voxel Z-Score | P Value(SVC) | Cluster Extent (mm3) | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| 23 | PTSD-Hyperarousal | Dorsal ACC (24) | 0 | 30 | 24 | −3.97 | <0.001 (0.014) | 229 |
| LEC-Happened To Me | L Hippocampus (20) | −33 | −27 | −12 | −4.01 | <0.001 (0.010) | 176 | |
| 18 | PHQ-15 | L Anterior Insula (48) | −40 | 12 | −11 | −3.93 | <0.001 (0.032) | 169 |
| SOMS:CD | L Anterior Insula (48) | −42 | 9 | −12 | −3.87 | <0.001 (0.038) | 53 | |
| CTQ-Abuse | L Anterior Insula (48) | −39 | 9 | −12 | −4.04 | <0.001 (0.021) | 156 | |
| PTSD-Avoidance | R dorsal/perigenual ACC (24) | 2 | 32 | 24 | −3.66 | <0.001 (0.046) | 86 | |
| LEC-Happened To Me | L Hippocampus (20) | −33 | −27 | −12 | −4.27 | <0.001 (0.005) | 230 | |
Small-volume corrected (SVC) findings are listed in parentheses. MNI, Montreal Neurological Institute; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; LEC, Life Events Checklist; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire.
Given the statistically significant parallel associations of functional neurological symptom severity and childhood abuse burden each associated with left anterior insular volume reductions in women, we conducted two separate post-hoc SPM-based regression analyses to investigate the potential relationship between these findings. When controlling for CTQ-Abuse scores and age in regression analyses, associations between PHQ-15 or SOMS:CD scores and left anterior insular gray matter volume did not remain statistically significant.
Gray matter associations with PTSD symptoms and lifetime adverse events
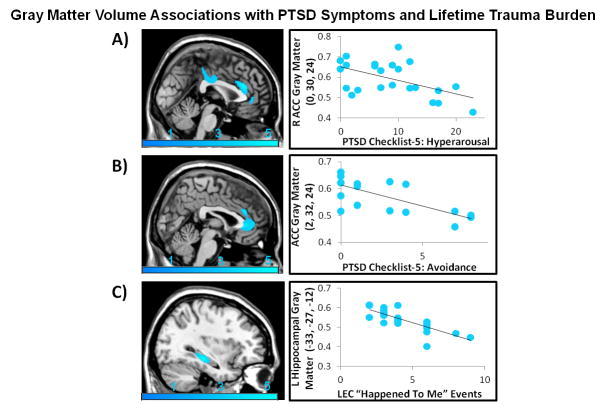
For the entire FND cohort, PCL-5 hyperarousal subscore was negatively associated with dorsal ACC (psvc=0.014) gray matter volumes. This finding remained negatively associated with hyperarousal controlling for BDI (psvc=0.001) and STAI-T (psvc=0.006). For women, PCL-5 avoidance subscore was negatively associated with dorsal/perigenual ACC (psvc=0.046) gray matter volumes. This inverse association also remained statistically significant when controlling for BDI (pSVC=0.012) and STAI-T (pSVC=0.016).
Analyses probing associations with cumulative lifetime adverse events across all FND subjects showed an inverse association between the LEC-5 “happened to me” score and left hippocampal volume (psvc=0.010). This association held controlling for BDI (psvc=0.017) and STAI-T (psvc=0.015). In women, a similar negative association between the LEC-5 “happened to me” score and left hippocampal volume was appreciated (psvc=0.005). This finding also held controlling for BDI (psvc=0.009) and STAI-T (psvc=0.006). See Supplemental Tables 3–5, Figure 3, and Supplemental Figure 3 for complete findings.
FIGURE 3. Across individuals with Functional Neurological Disorder (FND), PTSD symptom severity and lifetime trauma burden showed inverse associations with anterior cingulate cortex and hippocampal gray matter volumes respectively.
(A) Hyperarousal as measured by the PTSD Checklist for DSM-5 was negatively associated with dorsal anterior cingulate cortex (ACC) gray matter volume (psvc=0.014, peak z-score=−3.97). (B) In women with FND, avoidance as measured by the PTSD Checklist for DSM-5 was negatively associated with dorsal/perigenual ACC gray matter volume (psvc=0.046, peak z-score=−3.66). (C) Across all subjects, lifetime adverse events as measured by the “happened to me” item of the Life Events Checklist-5 (LEC) was negatively associated with left hippocampal gray matter volume (psvc=0.010, peak z-score=−4.01). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.
DISCUSSION
Consistent with our a priori hypotheses, women with FND showed significant associations between functional neurological symptoms and reduced left anterior insular gray matter volumes. In women, the magnitude of experienced childhood abuse was also associated with decreased left anterior insular volume. Post-hoc analyses showed that the relationship between functional neurological symptom severity and decreased insular volume was explained mainly by the magnitude of reported childhood abuse. These findings lend early support for potential childhood abuse related aberrant neuroplasticity in the pathophysiology of FND. In addition, while similar trend level associations were observed, there were no statistically significant associations between functional neurological symptoms, childhood abuse and left anterior insular volume in the mixed-gender FND cohort controlling for age and gender. These results suggest the potential for differential brain-trauma relationships across men and women. Also, in our entire mixed-gender FND cohort, PTSD symptom severity was associated with reduced ACC gray matter volumes. Lastly, consistent with the literature detailing associations between lifetime trauma burden and hippocampal volume, we observed an inverse relationship between the number of cumulative adverse events and left hippocampal volume across all FND subjects.
The insula, a paralimbic multimodal region implicated in viscerosomatic, visceromotor, homeostatic, affective and cognitive processes[21], is well positioned to play a central role in the multiplicity of symptoms experienced by patients with FND. Information from the lamina I spinothalomocortical pathway conveying physiological information from bodily tissues projects onto the posterior insula and ACC. Given this convergence of information, the posterior insula mediates the interoceptive representation of the physiological status of the body[17]. Affectively and motivationally valenced information from the amygdala, ACC and orbitofrontal cortex synapse onto the mid-insula, with the left mid insula mediating parasympathetic activity and the right mid insula mediating sympathetic tone[21]. Craig has suggested that a higher-order synthesis of this information occurs in the anterior insula, and this area may be critical for emotional and self awareness[21]. Given its structural and functional connectivity, the anterior insula is also implicated in the integration of internal feeling states, the detection and processing of differences between observed and expected bodily states, and the pathophysiology of mood and anxiety disorders[44].
While the insula is well-positioned to play a role in the pathophysiology of FND, the ACC is also a paralimbic region implicated in the integration of affective, viscerosomatic and cognitive processes. Meta-analyses have shown that the dorsal ACC is a convergent zone for negative emotional processing, pain processing and cognitive control[20]. A dorsal-ventral gradient exists in the ACC whereby dorsal regions are interconnected to lateral prefrontal and premotor regions and implicated in appraisal and behavioral expression of mood states. Perigenual and subgenual ACC subregions are reciprocally connected to the amygdala and facilitate top-down regulation of affective states. As detailed in our neurobiological framework for somatosensory amplification[17], the cognitive-affective neuroscience literature has demonstrated cingulo-insular involvement in mediating aberrant somatic experiences through negative expectation bias, alexithymia, pain catastrophizing and heightened visceral-somatic processing during negative mood states[17]. We have also postulated the construct of “neural functional unawareness” as a pathophysiological model for FND which implicates cingulo-insular abnormalities in mediating impaired emotional and interoceptive awareness. Our findings support selective left anterior insular volume reductions pertaining to functional neurological symptoms, while dorsal and perigenual ACC gray matter reductions related to PTSD symptom severity in patients with FND. We theorize that inverse associations between functional neurological symptoms and left anterior insular gray matter represent a neurobiological substrate for the impaired integration of affective, viscerosomatic and cognitive processing in patients with FND leading to deficits in emotional and bodily awareness.
We also observed an inverse relationship between childhood abuse burden and left anterior insular volume in women with FND. Post-hoc analyses showed that the relationship between insular volume and functional neurological symptoms in women could be explained by the magnitude of endorsed childhood abuse. This suggests that our parallel observations of functional neurological symptoms and childhood abuse each linked to reduced insular volume are inter-related. Given that the insula is also broadly implicated in the pathophysiology of mood and anxiety disorders, it is notable that the parallel associations between symptom severity and childhood abuse burden each associated with left anterior insular volume reductions remained significant when controlling for trait anxiety but not for depression scores. This implies a potential role for the left anterior insula in the convergence of functional neurological symptoms and negative mood in patients with FND. As shown in Figure 1 and Supplemental Figure 1, individuals with more childhood trauma reported increased functional symptoms as measured by both the PHQ-15 and the SOMS:CD scales. These observations are consistent with the literature detailing relationships between adverse early-life events and functional neurological symptom severity[7,11]. While requiring replication, our results suggest that aberrant neuroplastic changes in the left anterior insula potentially driven by childhood abuse may underlie aspects of the pathophysiology for FND.
Our findings can be contextualized based on basic science and human literature detailing maladaptive experience-dependent neuroplastic changes following chronic stress. Animal models of chronic stress have shown that the medial prefrontal cortex and the hippocampal CA3 region undergo dendritic spine density reductions following prolonged stress exposures[13]. In a VBM study of 130 healthy individuals, cumulative lifetime adverse events were associated with reduced medial prefrontal, insular and subgenual ACC volumes[45]. Adverse early-life events have been specifically associated with smaller ACC, insular, orbitofrontal cortex, caudate and hippocampal volumes[46,47]. A meta-analysis in psychiatric populations with childhood maltreatment also demonstrated reduced insular, orbitofrontal, parahippocampal, amygdalar, middle temporal, inferior frontal and post-central gyri gray matter in patients compared to controls[48]. Our finding of an association between cumulative adverse life events and reduced left hippocampal volume adds to the literature linking trauma burden to hippocampal volumes across several psychopathologies. Given that our findings also suggest specific gray matter-symptom severity relationships, the effects of trauma type, age of onset, trauma burden, gender effects and gene by experience relationships should be further studied in future investigations. These subsequent research efforts would help clarify the degree to which experience-dependent neuroplasticity increases a general vulnerability to the development of psychopathology, and the extent to which particular combinations of environmental and genetic factors contribute specifically to neural and phenotypic profiles.
There are a few potential limitations to this study. Our FND cohort had mixed symptoms including medically-unexplained abnormal movements, weakness, nonepileptic seizures, pain, fatigue and non-dermatomal sensory deficits. While we note that there have been increasing calls for greater research integration across FNDs[18], we acknowledge that this is debated in the field and this study is underpowered to include FND subtypes as nuisance variables. As is common in FND[14], our cohort also had significant mood and anxiety co-morbidities, as well as the majority were on psychoactive medications, which are potential confounds. It is possible that our FND cohort recruited across an integrated behavioral neurology-neuropsychiatry clinic could tend towards a population with greater psychopathology compared to FND patients recruited from general neurology settings. We note that patients with current major depressive disorder have been removed by some groups from study inclusion, however, it is our viewpoint that the frequent co-occurrence of depression and anxiety in FND likely reflects aspects of a shared pathophysiology. Comorbid psychiatric disorders are the rule, not the exception, in patients with FND. Inclusion of subjects with prominent psychiatric co-morbidities is more representative of the FND spectrum and increases the external validity of our sample to clinical practice. Furthermore, a strength of this study is our ability to secondarily control for depression or anxiety. It is also important to highlight that there are multiple risk factors for FND apart from adverse life events (and not all patients endorse past traumatic experiences), suggesting that more research is needed to investigate brain-disease risk relationships. Also, additional research is needed to clarify if the overlapping brain-symptom severity and brain-childhood abuse relationships appreciated in this study reflect gender-specific neural mechanisms of disease or rather, in-part, reflect the increased frequency of childhood abuse in women compared to men (See Supplemental Table 2)[12,15,49].
Our reliance on self-report measures of symptom severity (PHQ-15, SOMS:CD) may also not fully capture of the degree of physical disability in a given FND patient, and PHQ-15 scores may not reliably differentiate FND from other neurological populations[50]. The lack of a healthy control group could be seen as a limitation[51], however, this addition would not aid the study of functional neurological symptoms as continuous variables and the over-reliance on healthy subject group comparisons has recently been criticized in neuropsychiatric research.
The neurobiology of FND spans multiple networks, particularly given that right temporoparietal junction functional alterations in Functional Movement Disorders have been linked to deficits in motor intention awareness and self-agency[52,53]. In addition, it is likely that ACC alterations play important roles in predisposing factors such as alexithymia and dissociation[17,18]. More research is needed to investigate biological similarities and differences in the pathophysiology of FND across gender, as well as to clarify if the observed brain-symptom relationships represent disease-related or compensatory structural alterations.
In conclusion, we used a trans-diagnostic, symptom-based and disease-risk approach to characterize inverse associations between left anterior insular volume and indices of functional neurological symptoms and childhood abuse in patients with FND. We also observed differential cingulo-insular structural alterations, delineating inverse associations between PTSD symptoms and ACC volume. This study, while requiring replication, may advance our understanding of the neuropathophysiology of FND and suggests potential biological convergence between the magnitudes of experienced childhood abuse, functional neurological symptoms and reduced left anterior insular volume.
Supplementary Material
SUPPLEMENTAL TABLE 1. Demographic characteristics of patients with Functional Neurological Disorder (FND). †Indicates that these patients had splitting of the midline functional numbness on examination. *indicates that these patients also had functional voice symptoms. Abbreviations: M, male; F, female; PNES, Psychogenic Nonepileptic Seizures; FW, Functional Weakness; FMD, Functional Movement Disorder; NOS, not otherwise specified; MDD, Major Depressive Disorder; PDwAg, Panic Disorder with Agoraphobia; PDwoAg, Panic Disorder without Agoraphobia; GAD, Generalized Anxiety Disorder; Undiff somatoform, Undifferentiated Somatoform Disorder; PTSD, Post-Traumatic Stress Disorder; OCD, Obsessive Compulsive Disorder; Ag, Agoraphobia without Panic Disorder; LSD, Lysergic Acid Diethylamide; MIR, Mirtazapine; GBP, Gabapentin; SER, Sertraline; TZD, Trazodone; ZLP, Zolpidem; ECP, Escitalopram; DLX, Duloxetine; CLP, Clonazepam; DES, Desvenlafaxine; DOX, Doxepin; VEN, Venlafaxine; PGN, Pregabalin; FLX, Fluoxetine; DZP, Diazepam; NRT, Nortriptyline; QTP, Quetiapine; THP, Trihexyphenidyl; VPA, Valproic Acid; BUP, Bupropion; ROP, Ropirinole; DXAM, Dextroamphetamine; LRZ, Lorazepam; CLN, Clonidine; CTP, Citalopram.
SUPPLEMENTAL TABLE 2. Psychometric scores for the complete Functional Neurological Disorder cohort (N=23), and also for the 18 women and 5 men sub-groups. Using previously published cutoff scores for the presence of childhood abuse[49], 4 women and 0 men endorsed sexual abuse scores of above 8, 4 women and 1 man reported physical abuse scores above 8, and 10 women and 3 men endorsed emotional abuse scores above 10. FND, Functional Neurological Disorder; SD, standard deviation; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse; cumulative index of sexual, physical and emotional abuse; CTQ-Neglect, cumulative index of emotional and physical neglect, LEC-“Happened to Me”, Life Events Checklist-number of “happened to me” events; PCL-5, PTSD Checklist for DSM-5; PTSD, Post-Traumatic Stress Disorder; BDI, Beck Depression Inventory-II; STAI-T, Spielberger Trait Anxiety Inventory.
SUPPLEMENTAL TABLE 3. Within-group gray matter volume associations that remained statistically significant controlling for Beck Depression Inventory-II (BDI) score. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; LEC, Life Events Checklist; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse.
SUPPLEMENTAL TABLE 4. Within-group gray matter volume associations that remained statistically significant controlling for Spielberger Trait Anxiety Inventory (STAI-T) score. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; LEC, Life Events Checklist; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse.
SUPPLEMENTAL TABLE 5. Uncorrected within-group gray matter volume associations significant at a p≤0.001, cluster extent threshold of 50mm3. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; MCC, middle cingulate cortex; LEC, Life Events Checklist.
Also shown, is the scatter plot of the positive correlation between the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale and the Patient Health Questionnaire-15. Spearman correlation coefficients are displayed.
(A) The Patient Health Questionnaire-15 scores were inversely associated with left anterior insular gray matter volume (puncorrected<0.001; peak z-score=−3.37). (B) The magnitude of childhood abuse as measured by the Childhood Trauma Questionnaire also showed a trend negative association with left anterior insular gray matter volume (puncorrected<0.001; peak z-score=−3.71). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.
Lifetime adverse events as measured by the “happened to me” item of the Life Events Checklist-5 (LEC) was negatively associated with left hippocampal gray matter volume (psvc=0.005, peak z-score=−4.27). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.
Acknowledgments
We thank Bruce Rosen for providing pilot scans for this study and Benjamin Williams for his helpful comments on the manuscript.
STATEMENT OF FUNDING:
D.L.P. was funded by the NINDS R25NS065743-05S1, Dupont Warren Fellowship, Massachusetts General Hospital Physician-Scientist Development Award, and the Sidney R. Baer Jr. Foundation. This work was also supported by the shared instrumentation grant 1S10RR023401.
Footnotes
All authors report no conflicts of interest.
DISCLOSURES:
B.C.D., consultant at Merck, Med Learning Group and Haymarket; royalties from Oxford University Press and Cambridge University Press; on the editorial board of Neuroimage: Clinical, Cortex, Hippocampus, Neurodegenerative Disease Management. M.S.K., consultant at Forum Pharmaceuticals; editor for Schizophrenia Research. W.C.L., has served on the editorial boards of Epilepsia, Epilepsy & Behavior and Journal of Neuropsychiatry and Clinical Neurosciences; receives editor’s royalties from the publication of Gates and Rowan’s Nonepileptic Seizures, 3rd ed. (Cambridge University Press, 2010) and 4th ed. (2017); author’s royalties for Taking Control of Your Seizures: Workbook and Therapist Guide (Oxford University Press, 2015); has received research support from the NIH (NINDS 5K23NS45902 [PI]), Rhode Island Hospital, the American Epilepsy Society (AES), the Epilepsy Foundation (EF), Brown University and the Siravo Foundation; serves on the Epilepsy Foundation Professional Advisory Board; has received honoraria for the American Academy of Neurology Annual Meeting Annual Course; has served as a clinic development consultant at University of Colorado Denver, Cleveland Clinic, Spectrum Health and Emory University; and has provided medico legal expert testimony.
References
- 1.Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220–26. doi: 10.1136/jnnp-2013-305321. [DOI] [PubMed] [Google Scholar]
- 2.Perez DL, LaFrance WC., Jr Nonepileptic Seizures: An Updated Review. CNS Spectr. 2016;21:239–46. doi: 10.1017/S109285291600002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkler AE, Parikh NS, Chaudhry S, et al. Hospital revisit rate after a diagnosis of conversion disorder. J Neurol Neurosurg Psychiatry. 2016;87:363–66. doi: 10.1136/jnnp-2014-310181. [DOI] [PubMed] [Google Scholar]
- 4.Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics? --the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747–51. doi: 10.1016/j.clineuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry. 2005;62:903–10. doi: 10.1001/archpsyc.62.8.903. [DOI] [PubMed] [Google Scholar]
- 6.McMillan KK, Pugh MJ, Hamid H, et al. Providers’ perspectives on treating psychogenic nonepileptic seizures: frustration and hope. Epilepsy Behav. 2014;37:276–81. doi: 10.1016/j.yebeh.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Roelofs K, Keijsers GP, Hoogduin KA, et al. Childhood abuse in patients with conversion disorder. Am J Psychiatry. 2002;159:1908–13. doi: 10.1176/appi.ajp.159.11.1908. [DOI] [PubMed] [Google Scholar]
- 8.Stone J, Sharpe M, Binzer M. Motor conversion symptoms and pseudoseizures: a comparison of clinical characteristics. Psychosomatics. 2004;45:492–99. doi: 10.1176/appi.psy.45.6.492. [DOI] [PubMed] [Google Scholar]
- 9.Driver-Dunckley E, Stonnington CM, Locke DE, et al. Comparison of psychogenic movement disorders and psychogenic nonepileptic seizures: is phenotype clinically important? Psychosomatics. 2011;52:337–45. doi: 10.1016/j.psym.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Bowman ES, Markand ON. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am J Psychiatry. 1996;153:57–63. doi: 10.1176/ajp.153.1.57. [DOI] [PubMed] [Google Scholar]
- 11.Duncan R, Oto M. Predictors of antecedent factors in psychogenic nonepileptic attacks: multivariate analysis. Neurology. 2008;71:1000–05. doi: 10.1212/01.wnl.0000326593.50863.21. [DOI] [PubMed] [Google Scholar]
- 12.Selkirk M, Duncan R, Oto M, et al. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. 2008;49:1446–50. doi: 10.1111/j.1528-1167.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 13.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–33. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sar V, Akyuz G, Kundakci T, et al. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am J Psychiatry. 2004;161:2271–76. doi: 10.1176/appi.ajp.161.12.2271. [DOI] [PubMed] [Google Scholar]
- 15.MacMillan HL, Fleming JE, Streiner DL, et al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158:1878–83. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 16.Perez DL, Barsky AJ, Daffner K, et al. Motor and somatosensory conversion disorder: a functional unawareness syndrome? J Neuropsychiatry Clin Neurosci. 2012;24:141–51. doi: 10.1176/appi.neuropsych.11050110. [DOI] [PubMed] [Google Scholar]
- 17.Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci. 2015;27:e40–50. doi: 10.1176/appi.neuropsych.13070170. [DOI] [PubMed] [Google Scholar]
- 18.Perez DL, Dworetzky BA, Dickerson BC, et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci. 2015;46:4–15. doi: 10.1177/1550059414555905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voon V, Cavanna AE, Coburn K, et al. Functional Neuroanatomy and Neurophysiology of Functional Neurological Disorders (Conversion Disorder) J Neuropsychiatry Clin Neurosci. 2016;28:168–90. doi: 10.1176/appi.neuropsych.14090217. [DOI] [PubMed] [Google Scholar]
- 20.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Sepulcre J, Sabuncu MR, Yeo TB, et al. Stepwise connectivity of the modal cortex reveals the multimodal organization of the human brain. J Neurosci. 2012;32:10649–61. doi: 10.1523/JNEUROSCI.0759-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labate A, Cerasa A, Mula M, et al. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2011;53:377–85. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- 24.Ristic AJ, Dakovic M, Kerr M, et al. Cortical thickness, surface area and folding in patients with psychogenic nonepileptic seizures. Epilepsy Res. 2015;112:84–91. doi: 10.1016/j.eplepsyres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Valet M, Gundel H, Sprenger T, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- 26.Stone J, Zeman A, Simonotto E, et al. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom Med. 2007;69:961–69. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- 27.Voon V, Brezing C, Gallea C, et al. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. 2011;26:2396–403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aybek S, Nicholson TR, O’Daly O, et al. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10:e0123273. doi: 10.1371/journal.pone.0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Pitman RK, Rasmusson AM, Koenen KC, et al. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–87. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–43. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Xia W, Li L, et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Perez DL, Young SS, King JS, et al. Preliminary Predictors of Initial Attendance, Symptom Burden and Motor Subtype in a US Functional Neurological Disorders Clinic Population. Cogn Behav Neurol. 2016;29:197–205. doi: 10.1097/WNN.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 34.Williams DT, Ford B, Fahn S. Phenomenology and psychopathology related to psychogenic movement disorders. Adv Neurol. 1995;65:231–57. [PubMed] [Google Scholar]
- 35.LaFrance WC, Jr, Baker GA, Duncan R, et al. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–18. doi: 10.1111/epi.12356. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5) 5. American Psychiatric Pub; 2013. [Google Scholar]
- 37.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–36. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 39.Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The Life Events Checklist for DSM-5 (LEC-5) National Center for PTSD; 2013. wwwptsdvagov. [Google Scholar]
- 40.Blevins CA, Weathers FW, Davis MT, et al. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J Trauma Stress. 2015;28:489–98. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 41.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio, TX: 1996. p. 78204-2498. [Google Scholar]
- 42.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; 1970. [Google Scholar]
- 43.Rief W, Hiller W. A new approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics. 2003;44:492–98. doi: 10.1176/appi.psy.44.6.492. [DOI] [PubMed] [Google Scholar]
- 44.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–87. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 45.Ansell EB, Rando K, Tuit K, et al. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2011;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 47.Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171:854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 49.Bevilacqua L, Carli V, Sarchiapone M, et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carson AJ, Stone J, Hansen CH, et al. Somatic symptom count scores do not identify patients with symptoms unexplained by disease: a prospective cohort study of neurology outpatients. J Neurol Neurosurg Psychiatry. 2015;86:295–301. doi: 10.1136/jnnp-2014-308234. [DOI] [PubMed] [Google Scholar]
- 51.Nicholson TR, Aybek S, Kempton MJ, et al. A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J Neurol Neurosurg Psychiatry. 2014;85:227–29. doi: 10.1136/jnnp-2013-305012. [DOI] [PubMed] [Google Scholar]
- 52.Maurer CW, LaFaver K, Ameli R, et al. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology. 2016;87:564–70. doi: 10.1212/WNL.0000000000002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voon V, Gallea C, Hattori N, et al. The involuntary nature of conversion disorder. Neurology. 2010;74:223–8. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE 1. Demographic characteristics of patients with Functional Neurological Disorder (FND). †Indicates that these patients had splitting of the midline functional numbness on examination. *indicates that these patients also had functional voice symptoms. Abbreviations: M, male; F, female; PNES, Psychogenic Nonepileptic Seizures; FW, Functional Weakness; FMD, Functional Movement Disorder; NOS, not otherwise specified; MDD, Major Depressive Disorder; PDwAg, Panic Disorder with Agoraphobia; PDwoAg, Panic Disorder without Agoraphobia; GAD, Generalized Anxiety Disorder; Undiff somatoform, Undifferentiated Somatoform Disorder; PTSD, Post-Traumatic Stress Disorder; OCD, Obsessive Compulsive Disorder; Ag, Agoraphobia without Panic Disorder; LSD, Lysergic Acid Diethylamide; MIR, Mirtazapine; GBP, Gabapentin; SER, Sertraline; TZD, Trazodone; ZLP, Zolpidem; ECP, Escitalopram; DLX, Duloxetine; CLP, Clonazepam; DES, Desvenlafaxine; DOX, Doxepin; VEN, Venlafaxine; PGN, Pregabalin; FLX, Fluoxetine; DZP, Diazepam; NRT, Nortriptyline; QTP, Quetiapine; THP, Trihexyphenidyl; VPA, Valproic Acid; BUP, Bupropion; ROP, Ropirinole; DXAM, Dextroamphetamine; LRZ, Lorazepam; CLN, Clonidine; CTP, Citalopram.
SUPPLEMENTAL TABLE 2. Psychometric scores for the complete Functional Neurological Disorder cohort (N=23), and also for the 18 women and 5 men sub-groups. Using previously published cutoff scores for the presence of childhood abuse[49], 4 women and 0 men endorsed sexual abuse scores of above 8, 4 women and 1 man reported physical abuse scores above 8, and 10 women and 3 men endorsed emotional abuse scores above 10. FND, Functional Neurological Disorder; SD, standard deviation; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse; cumulative index of sexual, physical and emotional abuse; CTQ-Neglect, cumulative index of emotional and physical neglect, LEC-“Happened to Me”, Life Events Checklist-number of “happened to me” events; PCL-5, PTSD Checklist for DSM-5; PTSD, Post-Traumatic Stress Disorder; BDI, Beck Depression Inventory-II; STAI-T, Spielberger Trait Anxiety Inventory.
SUPPLEMENTAL TABLE 3. Within-group gray matter volume associations that remained statistically significant controlling for Beck Depression Inventory-II (BDI) score. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; LEC, Life Events Checklist; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse.
SUPPLEMENTAL TABLE 4. Within-group gray matter volume associations that remained statistically significant controlling for Spielberger Trait Anxiety Inventory (STAI-T) score. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; LEC, Life Events Checklist; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse.
SUPPLEMENTAL TABLE 5. Uncorrected within-group gray matter volume associations significant at a p≤0.001, cluster extent threshold of 50mm3. SVC, Small Volume Corrected; MNI, Montreal Neurological Institute; PHQ-15, Patient Health Questionnaire-15; SOMS:CD, Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale; CTQ, Childhood Trauma Questionnaire; CTQ-Abuse, cumulative indices of sexual, physical and emotional abuse; PTSD, Post-Traumatic Stress Disorder; ACC, anterior cingulate cortex; MCC, middle cingulate cortex; LEC, Life Events Checklist.
Also shown, is the scatter plot of the positive correlation between the Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale and the Patient Health Questionnaire-15. Spearman correlation coefficients are displayed.
(A) The Patient Health Questionnaire-15 scores were inversely associated with left anterior insular gray matter volume (puncorrected<0.001; peak z-score=−3.37). (B) The magnitude of childhood abuse as measured by the Childhood Trauma Questionnaire also showed a trend negative association with left anterior insular gray matter volume (puncorrected<0.001; peak z-score=−3.71). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.
Lifetime adverse events as measured by the “happened to me” item of the Life Events Checklist-5 (LEC) was negatively associated with left hippocampal gray matter volume (psvc=0.005, peak z-score=−4.27). Images are thresholded at a voxel-wise uncorrected p-value of 0.005 for visualization purposes. To display in the scatter plots a measure of gray matter volume for visualization purposes only, the MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean gray matter volumes for each subject at a 5mm sphere centered at the peak coordinate of statistically significant findings.