Abstract
ADHD is a complex condition with a heterogeneous presentation. Current diagnosis is primarily based on subjective experience and observer reports of behavioral symptoms – an approach that has significant limitations. Many studies show that individuals with ADHD exhibit poorer performance on cognitive tasks than neurotypical controls, and at least seven main functional domains appear implicated in ADHD. We discuss the underlying neural mechanisms of cognitive functions associated with ADHD with emphasis on the neural basis of selective attention, demonstrating the feasibility of basic research approaches for further understanding cognitive behavioral processes as they relate to human psychopathology. The study of circuit-level mechanisms underlying executive functions in nonhuman primates holds promise for advancing our understanding, and ultimately the treatment, of ADHD.
Keywords: Neuromodulator, Cognitive Domain, Prefrontal Cortex, Mental Illness
Bridging the Gap
Attention Deficit Hyperactivity Disorder (ADHD) is a complex condition affecting up to 10% of children in the US [1] and comprises a heterogeneous set of behavioral dysfunctions. While prior work demonstrates validity of the ADHD construct in general, ongoing debate regarding subtype validity demonstrates the need for a more coherent model. An etiological basis for ADHD remains elusive, as have been efforts to subtype ADHD using biological indicators, rather than solely relying on clinical assessments.
Decades of ADHD studies in humans have been conducted in parallel with research in animal models on the neural mechanisms and neural circuitry underlying attention and other cognitive functions. However, success in aligning these two bodies of evidence has been limited. In this review, we discuss the apparent gap between the clinical definition of ADHD and our current understanding of the neural circuits of cognition, with particular focus on selective attention as an example paradigm. We begin by describing the current challenges faced by clinicians diagnosing ADHD and the heterogeneity of cognitive dysfunction in the disorder. Next, we summarize current knowledge of the neural basis of selective attention in human and nonhuman primates. We then discuss how the neural mechanisms of selective attention might relate to mechanisms of other cognitive functions associated with ADHD. Finally, we discuss possible ways to move forward in mapping symptom phenotypes onto specific cognitive domains, and cognitive domains onto neural representations.
Diagnosis of ADHD
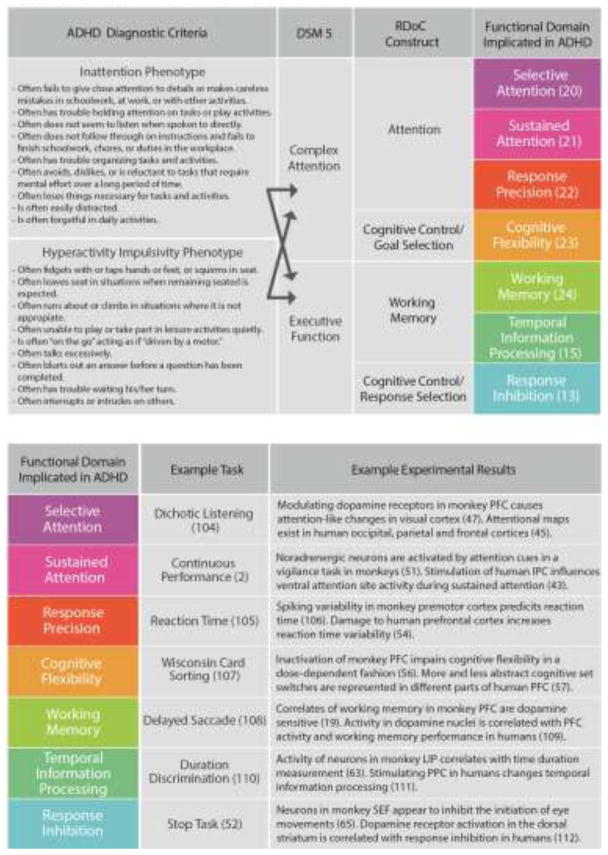
In current clinical practice, ADHD is diagnosed through observation and self-report of behavior. These are typically conducted through clinical interviews with the individual and family, and often use rating scales of ADHD symptoms. Consensus criteria for ADHD is defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which requires a persistent pattern of inattention and/or hyperactivity and impulsivity over a period greater than six months. A standard clinical encounter might unfold as follows: Parents present to a pediatrician’s office with concerns that their child has ADHD. The pediatrician reviews the family’s concerns, noting whether the child is struggling in the classroom, at home or in peer relationships. The clinician obtains a detailed developmental history, reviews recent behavior and interviews the child, carefully noting evidence for persistent patterns of disorganization, inattention, hyperactivity and/or impulsivity. The clinician also provides assessment forms for the family and teachers to fill out for collateral information. Review of the clinical assessment and standardized rating scales assists the clinician in determining whether the child’s current and past behavior demonstrates six or more features of inattention and/or six or more features of hyperactivity-impulsivity (see Figure 1, left), in order to establish a diagnosis of ADHD.
Fig. 1. ADHD Diagnostic Criteria and Functional Domains.
Relationship of functional domains implicated in ADHD to clinical diagnostic criteria and basic research findings. The central column lists functional domains implicated in ADHD, referenced with a representative study. Each functional domain has an associated example task, and example findings from human and nonhuman primate literature that yield insight into the underlying neural circuitry. To the left of the central column, we show how these functional domains would map onto RDoC constructs, DSM 5 cognitive domains; as well as the ADHD diagnostic criteria, which would fall under both complex attention and executive function. PFC = Prefrontal cortex, LIP = Lateral Intraparietal Cortex, PPC = posterior parital cortex, SEF = supplementary eye field.
Thus, current diagnosis of ADHD is primarily based on subjective experience and observer report of behavioral symptoms. This approach has significant limitations, particularly the difficulty of correlating these observed behaviors with underlying neurobiological processes. Attempts have been made to develop more quantitative assessments, such as Conners’ continuous performance task (CPT) [2]. However, these tools have only poor-to-fair predictive power (e.g. [3]). The CPT and similar instruments lack the specificity required to capture the broad heterogeneity in cognitive phenotypes that converge on the ADHD diagnosis (e.g. [4]), as such, neuropsychological tools are not routinely used in clinical practice. Furthermore, existing nosology of ADHD is restricted to three presentations -- inattentive, hyperactive/impulsive, and combined -- which also do not capture the full heterogeneity of the disorder (e.g. [5], [6]). These subtypes have been shown to be limited in their ability to predict treatment response to currently available interventions in ADHD [7], further reflecting the inadequacies of the existing diagnostic framework. It is increasingly accepted that the current conceptualization of ADHD reflects a constellation of related, but distinct, functional deficits (e.g. [8]). Therefore, a more granular classification of the disorder based on known neurobiological circuits would result in greater diagnostic accuracy and, most importantly, more targeted treatments. Genetic approaches also hold promise in providing insights into the heterogeneity of ADHD. Recent reviews [9,10] have identified numerous candidate genes in the dopaminergic, adrenergic, serotonergic and cholinergic pathways, demonstrating potentially dissociable pathways of risk. Intriguingly, they also suggest an association between ADHD and genes involved in general synaptic function (e.g. SNAP-25 and VAMP-2, members of the SNARE complex involved in endocytosis of synaptic vesicles). Future studies along these lines may ultimately lead to the identification of risk genes; again allowing targeted treatment for a subpopulation of ADHD individuals. A better suite of tools to identify and classify ADHD is clearly needed, which requires a better understanding of the neural mechanisms underlying this complex disorder. Drawing on advances in human and non-human primate research on the neural mechanisms of attention may provide important insights for updating the current ADHD clinical paradigm.
Cognitive Dysfunction in ADHD
Many studies show that individuals with ADHD exhibit poorer performance on cognitive tasks compared to neurotypical controls (reviewed in [11]). Meta-analyses indicate that populations of individuals with ADHD exhibit relatively consistent deficits in specific cognitive functions [12]. However, this is countered by substantial heterogeneity among the identified domains, leading to variable characterizations of ADHD as a predominant disorder of inhibition (e.g. [13]), delay aversion [14] or temporal processing [15], among others (e.g. [16]). The lack of a framework linking current DSM criteria to underlying neurobiological constructs reflects the complexity of the disorder. A better understanding of which specific cognitive domains are impaired in an individual increases the likelihood of identifying which specific neural circuits are compromised, which has substantial clinical implications. With this in mind, we surveyed the literature of cognitive behaviors implicated in the diagnostic criteria for ADHD, placing particular emphasis on studies that tested performance of individuals with ADHD on batteries of cognitive behaviors. (see Box 1: ADHD Cognitive Batteries). We identified seven functional domains implicated in ADHD (see Figure 1] that were described in the majority of several recent cognitive batteries [5,12,16,17], with an eye toward connecting cognitive domains to functions studied in animal models. Specific psychophysical tasks have been designed to measure an individual’s ability in each of the identified cognitive domains. Experiments in humans and monkeys, using variations on these tasks, have been informative in suggesting potential underlying circuits (see Figure 1, bottom). We define these functional domains as follows:
Box 1. ADHD Cognitive Batteries.
Several studies have attempted to find more sensitive measures than the Continuous Performance Test, which also have better predictive power. Attempts have been made to use cognitive batteries to ascertain what variables are the best predictors of ADHD (e.g. [12]). One study [16] performed a cognitive test battery on adults with ADHD and was ultimately able “to confirm that adult ADHD is neuropsychologically heterogeneous”. Another study [5] attempted to identify neuropsychological subtypes in individuals with and without ADHD and found several, related, groups that could be derived from typically-developing groups. Yet another study [17] used a cognitive test battery to predict which children would and would not respond to methylphenidate, and they identified several subgroups that might be good candidates. Notably there are also cognitive-function independent attempts to sub-categorize ADHD populations. For example, one study [90] proposes the use of temperament dimensions; which could be tested for using physiological measurements.
Selective Attention: the preferential processing of one stimulus in the presence of other stimuli (distractors). Sustained Attention: the ability to continuously perform a task over a prolonged period (e.g. minutes) without significant loss in performance. (Note that we distinguish this from ‘vigilance’, which can imply sustained attention that is specific to threats or dangers [18].) Response Precision: temporal and/or spatial precision in behavioral responses to stimuli or relevant events. Reaction time variability is a measurement of temporal response precision. Cognitive Flexibility: the ability to switch between tasks without a significant loss in performance. Working Memory: the ability to preserve a representation of information over time. Most neurophysiological or neuroimaging studies of working memory probe retention of information for periods on the order of seconds (e.g. [19]). Temporal Information Processing: the ability to accurately recognize or reproduce time intervals. Response Inhibition: the suppression of actions that are inappropriate for a given task.
Deficits in each of these domains have been described for individuals with ADHD (e.g. Selective Attention [20], Sustained Attention [21], Response Precision [22], Cognitive Flexibility [23], Working Memory [24], Temporal Information Processing [15], Response Inhibition [13]). While a review of the extensive literature on all of these cognitive domains is beyond the scope of this paper, we highlight recent research in one of these areas, selective attention, to better illustrate the potential alignment between human and animal research.
Neural Mechanisms of Selective Attention
Selective attention is one cognitive domain implicated in ADHD (see Box 2: Selective Attention Deficits in ADHD) for which there is significant insight into the underlying neural mechanisms. The term ‘attention’ is often used as shorthand for ‘selective attention’, which is the selective processing of certain stimuli over others (e.g. [25]). This is distinct from ‘sustained attention’, which refers more specifically to the maintenance of attention over a longer period of time, and may depend on the level of arousal [18]. Selective attention can be directed to a specific modality (e.g. auditory, visual) at the expense of others, to a specific region in space (spatial attention), or to a specific feature (feature-based attention). Attention also operates endogenously and/or exogenously. Figure 2 depicts example tasks that are specific to each of these categories. Endogenously driven attention, sometimes called top-down, involves selection based on current goals (e.g. search for lost keys). Exogenously driven attention, sometimes called bottom-up, causes a stimulus to be selected due to its greater physical salience (e.g. bright things and moving things). For a child with ADHD, an endogenous selective attention impairment might manifest as difficulty attending to the specific voice of a teacher among the background noise of other children chatting. Alternatively, that child might be more sensitive to salient, but irrelevant, sensory information, such as car horns honking outside of the classroom, due to heightened exogenous attention.
Box 2. Selective Attention Deficits in ADHD.
Numerous studies have compared selective attention in children diagnosed with ADHD to non-ADHD subjects (e.g [91]). Interestingly, these studies have produced conflicting results, either yielding no clear impairments (e.g. [92,93] ), or rather robust ones (e.g. [94–101] . However, it is thought that these contradictory results are in large part attributable to the immense diversity in the sensitivity and validity of behavioral assessments used in these studies [91]. Another potential source of the discrepancy of results may be variability in performance due to age [102]. Nevertheless, there appears to be ample evidence of an impairment in the filtering of distractors by children with ADHD compared to those without (e.g. [95,97,101] ).
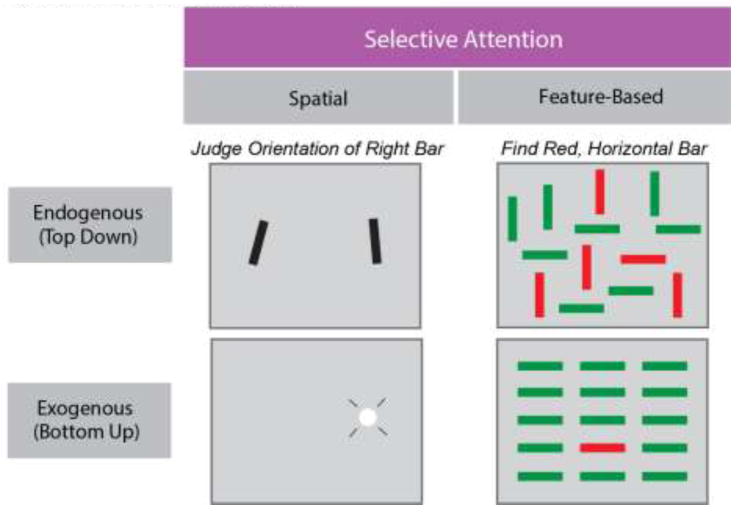
Fig. 2. Taxonomy of Selective Attention.
Taxonomy of selective attention. Varieties of selective attention in the visual modality. Selective processing of visual stimuli can occur either endogenously, in which particular goals, rules or motivations determine which of multiple stimuli is selectively processed, or exogenously, in which salient, external events determine selection (top and bottom rows). Selection can also occur across spatial and feature domains; stimuli can be selected based on their location or on their component visual features (e.g. color or shape). The examples shown depict visual displays that require a subject to attend to a particular location (top left) or to a particular object (top right) during endogenous attention, or in which attention is exogenously drawn to a location (flashed white circle, bottom left) or to a unique object (red bar among green, bottom right).
The use of specific tasks (Figure 2) that isolate different dimensions of selective attention (spatial vs feature-based; endogenous vs exogenous) has facilitated progress in identifying the distinct neural mechanisms and circuitry underlying those dimensions, as well as mechanisms they likely have in common (Figure 3). For example, in the visual modality, evidence from neurophysiological studies in nonhuman primates indicates that endogenous spatial attention appears to be controlled by a trio of structures including the frontal eye field (FEF) in prefrontal cortex (PFC) (e.g. [26]), the lateral intraparietal area (LIP) in parietal cortex [27], and the superior colliculus (SC) (e.g. [28]). Evidence to date suggests that one or more of these structures drives the selection of attended stimuli within posterior visual cortex (see [29] for review). However, much less is known about the mechanisms driving (endogenous) feature-based selective attention. Although there is a rich literature describing the modulation of neural activity during feature-based attention (e.g. [30]), only recently has evidence emerged of a causal role of distinct sub-regions of PFC in its control [31].
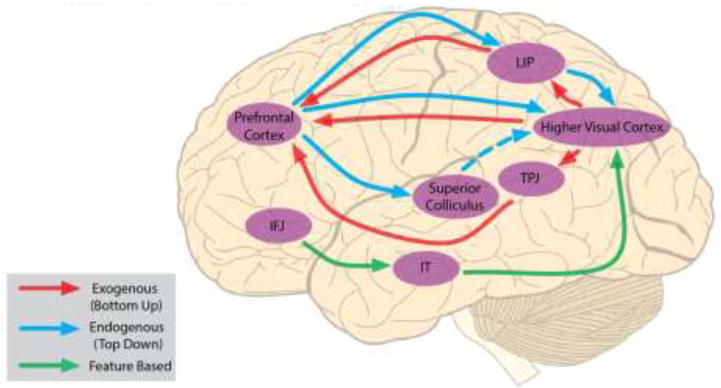
Fig. 3. Visual Selective Attention Networks in the Brain.
Selective attention networks in the primate brain. This combines results from human and nonhuman primates. Blue arrows indicate the flow of endogenous spatial information. Signals from the superior colliculus reach higher visual cortex by way of the thalamus (dashed line). Red arrows denote exogenous signals. Green arrows denote feature-based signals. LIP: Lateral Intraparietal Cortex (in humans this is more generally referred to as middle intraparietal sulcus) IT: Inferotemporal Cortex. TPJ: temporoparietal junction. IFJ: inferior frontal junction.
Compared to endogenous attention, considerably less is known about how attention is drawn to the physical, non-task-driven salience of particular stimuli. Although it is clear that salient stimuli preferentially activate neurons in the visual system (e.g. V1: [32]; V4: [33]), the basis of those effects remains unclear. It has been proposed that exogenous and endogenous attention mechanisms are combined in area LIP [34], the SC [35], and the FEF (e.g. [31]) to generate a saliency map [34]. This and other evidence (e.g. [36]) suggests that exogenous and endogenous attention are independent systems acting on the same substrate (i.e., visual cortex).
Parallel studies in humans also point to separable endogenous and exogenous attention systems [37]: specifically, the dorsal and ventral attention networks. Functional magnetic resonance imaging (fMRI) studies show that the human homologues of areas FEF and LIP (intraparietal sulcus in humans) are activated during endogenous spatial and feature-based attention tasks [37,38] and interference with these areas using transcranial magnetic stimulation impairs performance on said tasks (e.g. [39]). fMRI studies have also identified unique regions that are activated during exogenous attention tasks [40], specifically within the temporal-parietal junction (TPJ). Transcranial magnetic stimulation of the TPJ resulted in visuospatial neglect [41]. Monkey homologues of the human ventral (exogenous) attention network have not yet been established [42].
As in nonhuman primate experiments, there is also evidence that exogenous and endogenous signals are combined at some stages in the human brain. For example, one study [43] showed that transcranial magnetic stimulation of putative dorsal network parietal cortex caused changes in fMRI-recorded activations of a ventral network site. Further, a recent study of lesions in humans [44] provides evidence that the middle frontal gyrus is an area where endogenous and exogenous signals converge, specifically indicating it in switching attention from exogenous to endogenous control. Studies involving human subjects thus suggest that separate endogenous and exogenous attention networks exist, but that their signals are likely combined either in parietal cortex or the prefrontal cortex, perhaps as a priority map (reviewed in e.g. [45]).
Current evidence from animal studies is consistent with cholinergic signaling contributing preferentially to exogenous attention [46], whereas dopamine may preferentially contribute to endogenous attention [47]. Regarding the latter, changes in dopaminergic signaling have long been implicated in ADHD ( [48], but see [49]) and many of the medications currently prescribed act on dopamine release and re-uptake. A large body of research has explored the effect of stimulants, which act on catecholamine pathways, on ADHD symptoms (reviewed in e.g. [50]).
Selective attention circuits are complex, but the evidence described above demonstrates our ability to understand them using animal models and human imaging and stimulation techniques. This progress in identifying the components of exogenous and endogenous attention and the underlying circuit mechanisms, using both human and animal models, demonstrates the feasibility of such an approach for further understanding cognitive behavioral processes as they relate to human psychopathology. This has the potential to fill important gaps in current knowledge of these circuits in ADHD.
Neural Mechanisms of Other Cognitive Domains Associated with ADHD
Figure 4 summarizes the extensive literature describing the relationship between ADHD-related functional domains and the neural and neuromodulatory circuits that steer them. In addition to selective attention, described above, we provide a brief overview of current understanding of these other cognitive domains.
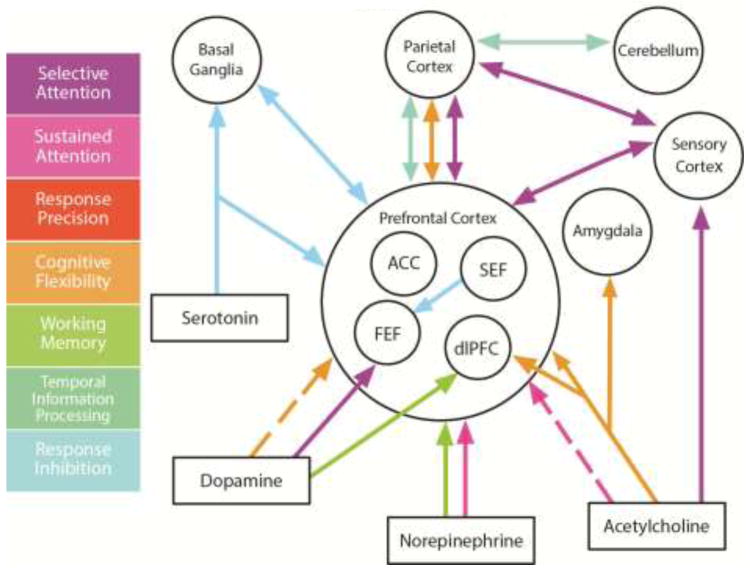
Fig. 4. Neural Circuits Underlying Cognitive Domains.
Summary of the interaction of neuromodulators and brain regions important for specific functional domains. The diagram shows neuromodulatory input to brain structures implicated in one or more functional domains as well as some of the major connections. The neuromodulators serotonin, dopamine, norepinephrine and acetylcholine are primarily released by specific subcortical nuclei: serotonergic neurons are located in the dorsal raphe nuclei, dopamine neurons that project to prefrontal cortex in the ventral tegmental area, norepinephrine-releasing neurons in the locus coeruleus, and cholinergic neurons in the nucleus basalis. FEF = frontal eye field, SEF = supplementary eye field, dlPFC = dorsolateral prefrontal cortex, ACC = anterior cingulate cortex.
Sustained Attention
Sustained attention may be at least partially mediated by the arousal system, and gated by norepinephrine neurons in the locus coeruleus (reviewed in [18]). One study [51] found that locus coeruleus neurons are selectively activated in a sustained attention task in monkeys. Another study [52] also showed that lesioning noradrenergic output from the locus coeruleus caused deficits in rats performing a sustained attention task. The cholinergic system has also been implicated in sustained attention, but the results are less conclusive (see [18] for review).
Response Precision
An increase in response time variability is a hallmark of ADHD (e.g. [22]), and many sources could contribute to it. An increase in variability is also apparent in the spatial domain: individuals with ADHD exhibit more variable movements (e.g. [53]). Imaging studies in humans most consistently link response precision with abnormalities in prefrontal cortical volumes (e.g. [54]) and activations (e.g. [55]).
Cognitive Flexibility
Goal-related information used in cognitive flexibility tasks is primarily represented in the PFC in monkeys (e.g. [56]) and humans [57]. Cognitive flexibility may also be tied to the neuromodulator acetylcholine. Cholinergic neurons may mediate the inhibition of the previously learned strategy encoded by PFC neurons (reviewed in [58]). Cholinergic signaling in the monkey amygdala may also play a role in this switch [59]. Alternatively, one study [60] proposes that, again, dopamine can steer cognitive flexibility, though in this case acting selectively through D2 dopamine receptors. It has also been shown that depleting serotonin from one region of marmoset PFC did not affect attentional set shifting [61].
Working Memory
Numerous studies, using a wide range of techniques, have demonstrated the importance of the prefrontal cortex for working memory (reviewed in [62]). As is the case with selective attention, dopamine D1 receptors also appear to modulate visuo-spatial working memory-related activity in the monkey PFC (e.g. [19]), suggesting that both functions may be mediated by similar neuromodulatory mechanisms in spite of non-overlapping networks controlling those functions.
Temporal Information Processing
Whether specific neuromodulator systems are involved in temporal information processing remains to be determined, but regions such as the prefrontal cortex, the cerebellum, and the basal ganglia all appear to be involved (reviewed in [15]). Interestingly, a recent study by [63] showed that neurons in monkey area LIP exhibit activity that is correlated with temporal information.
Response Inhibition
Signaling in the prefrontal cortex (particularly the inferior frontal cortex and the ventrolateral prefrontal cortex) and the basal ganglia is associated with response inhibition (reviewed in [64]). In monkeys, oculomotor response inhibition may be regulated by a specific prefrontal cortical area, the supplementary eye field, that can inhibit the initiation of eye movements [65]. Several studies in rodents have implicated serotonin in response inhibition (reviewed in [66]). Serotonergic neurons are active while rats wait for delayed rewards and blocking serotonin neuron activity results in premature responses [67].
This review has focused on high-level cognitive phenotypes of ADHD and their underlying circuits, but there are an increasing number of studies that suggest ADHD could be a disorder of motivation and the reward system (reviewed in e.g. [68]). One study [69] proposes that different sub-populations with ADHD exist, with distinguishable symptoms, cognitive and physiological profiles, which are caused by deficits in either the modulation of cortical control centers or reward circuits. Recent studies in nonhuman primates 1) identified the orbitofrontal cortex as a region that processes reward but not working memory-related information [70], 2) demonstrated reward signals integrate with action signals in at least one region of the prefrontal cortex (dorsal anterior cingulate cortex) [71] and 3) showed that administration of methylphenidate affected the temporal discounting of rewards [72].
Current DSM-5 criteria do not differentiate between cognitive domain phenotypes, nor is that its purpose as a diagnostic instrument. However, this inadvertently results in imprecise amalgamation of underlying mechanisms. For example, “Often fails to give close attention to details or makes careless mistakes in schoolwork, at work, or with other activities,” could represent a working memory, selective or sustained attention deficit. Our current limitations in identifying affected cognitive domains has significant real-world consequences, including increased exposure to medications without a biological basis for improving an affected domain (and associated behavioral symptoms), and increased duration of experiencing symptoms before treatment with an effective therapy. This problem has been somewhat elided in current practice, given that stimulants, the current cornerstone of ADHD therapy, act broadly and non-specifically on dopamine and norepinephrine reuptake throughout the brain [73], effectively reducing symptoms for a large proportion of individuals with ADHD. However, future development of drugs with more specific therapeutic targets could reduce side effects and curtail unrecognized negative consequences on neurodevelopment caused by the long-term non-specific increase in synaptic catecholamines. Data on long-term adverse effects for alternative ADHD medications (such as atomoxetine, clonidine and buproprion) is equally lacking, additionally highlighting the need for resolving the heterogeneity associated with ADHD. With adequate tools, we anticipate separation of ADHD cases into more meaningful groups, which may facilitate development of more selective treatments.
Bridging the Gap
Utility of Nonhuman Primate Models
A fuller understanding of the neural circuits and neuromodulatory systems that underlie the cognitive diversity of individuals with ADHD will be key to improving their diagnosis and treatment. Indeed, recent research has shown that these systems are highly complex, and the ADHD phenotype cannot adequately be characterized as a dopaminergic deficit as previously thought. Instead, much more sophisticated, direct interrogation of these neuromodulatory circuits is needed. Indeed it should be pointed out that ADHD is not unique in this regard. Many psychiatric disorders present with similar heterogeneity that arises from a complexity of neurological dysfunction not clearly directly related to current treatments (e.g. schizophrenia [74]).
While mechanistic hypotheses have been proposed for behavioral features of ADHD (e.g. [75]), empirical research validating these frameworks remains elusive. Experimental disruption of distinct neuropsychological circuits to determine the associated behavioral deficit may provide significant insights into ADHD taxonomy, particularly in the ways that primary deficits converge (or diverge) on downstream behavioral deficits. Unfortunately, our ability to tackle these questions in humans is limited, particularly in pediatric populations, where causal techniques such as TMS are not routinely used. At present, nonhuman primates represent excellent models for better understanding the neural basis of ADHD. With nonhuman primates we can, for example, interrogate how catecholaminergic circuits in the prefrontal cortex contribute to ADHD-linked behaviors (e.g. [19,47]), and explore the specific effect of ADHD medication on said circuits (e.g. [76]). The monkey’s perceptual system closely matches our own (reviewed in [77]). The same cognitive tasks employed in human psychophysical studies can be used in neurophysiological studies with NHPs (e.g. [78]). And finally, because we have greater access to the macaque brain than we do the human, we can study the neural mechanisms at play in greater detail. Although the majority of the nonhuman primate studies covered in this review were performed in macaque, the marmoset also represents an excellent model organism for interrogating visuo-cognitive neural circuits [79]. Like the macaque, its visual system closely corresponds to humans and it is capable of performing complex cognitive tasks (e.g. [61]). The marmoset also breeds quickly and can more readily be genetically manipulated [80].
Utility of a Common Framework
We urge clinical and basic researchers to use the same tasks in their subjects, allowing us to leverage what we learn in one species for understanding the other. It is critical to know how drugs that are currently being prescribed to treat ADHD affect the machinery that contributes to different cognitive behaviors. Unfortunately, experiments probing how ADHD drugs affect animals performing these behaviors have not been exhaustive (Figure 5). While the primary effects of first-line medications such as stimulants and atomoxetine are known, the specific mechanisms by which their up-regulation of catecholamines results in cognitive effects remain unclear. This critical knowledge gap also exists for other commonly prescribed ADHD medications, such as alpha-2 agonists and bupropion, and can be elucidated with greater precision in non-human animal models. Also, neurobiological mechanisms for other emerging treatment options, such as behavior therapy and cognitive training, have yet to be rigorously investigated. Behavior therapy, a program of behavioral correction usually guided by a clinician, has shown some promise in addressing ADHD symptoms [81], but there is very little longitudinal data on this approach’s effectiveness. Preliminary evidence in macaques shows that behavioral therapy using neurofeedback can modify cortical circuits and improve behavior [82]. Several studies show that cognitive training (regular practice of specific cognitive tasks) improves ADHD symptoms, but very few use quantitative measures of cognitive performance; and their results are inconclusive (reviewed in e.g. [83]). Training individuals with ADHD on one specific cognitive domain, e.g. working memory, has been found to result in improved performance on the trained and similar tasks [84–86]. However, while some studies [84,85] show subsequent improvement in ADHD symptoms, others do not (e.g. [86], reviewed in e.g. [87]). Two recent studies [88,89] showed that training in one cognitive domain does not transfer to other domains, therefore one possibility for the lack of general ADHD symptom improvement is that individuals with ADHD have deficits in more than one domain, or, again, that subgroups of individuals with ADHD who specifically exhibit working memory deficits should be targeted for this type of intervention. Finally, ADHD literature to date is largely limited by the exclusively symptom-based characterization of the disorder. Reduced reliance on the clinical diagnosis and more widespread use of well-established cognitive domains to characterize ADHD deficits in future research will allow greater capitalization on neurobiological advances and cross-species research.
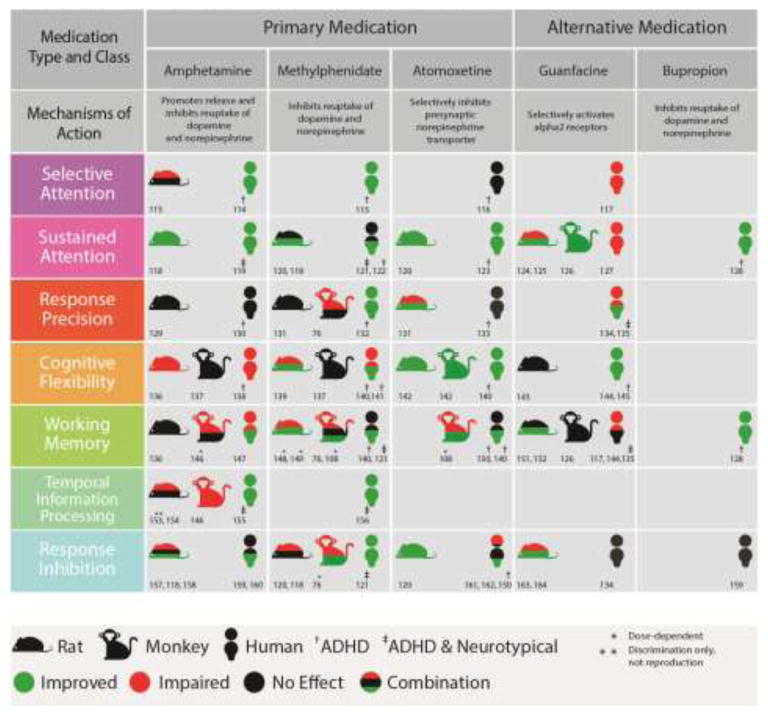
Fig. 5. Medication Effects on Functional Domains Implicated In ADHD.
Summary of effects of drugs on different functional domains implicated in ADHD across several different species: rodent, monkey and human. Drugs to treat ADHD come in different classes: typical examples of each of the types, as well as a superficial description of their method of action, are provided. Green indicates an improvement in behavior, red a worsening in behavior, and black no change in behavior. Split colors indicate cases where more than one effect was observed. In several cases different outcomes were observed to be caused by drug dosage -- e.g. a small dose might cause an improvement in working memory and large dose a deficit. These studies are denoted with a *. Note that clonidine and guanfacine are both alpha-2 receptor agonists, but can have very different effects [103]. Human studies were either performed in neurotypical populations, ADHD populations†, or mixed populations‡.
The results of the studies that have been performed so far lend support to the use of nonhuman primates as models for understanding this disorder. They appear to react to the drugs in similar ways to humans and in general there is very good correspondence in the behavioral response to drug manipulations across different species. But there are still many gaps in the literature that will be crucial to fill. First we must identify, where it is not yet known, whether these drugs affect specific behaviors. Then we can begin to explore the mechanisms through which medications affect cognition, so we can deploy them more strategically.
Concluding Remarks
Many challenges currently face research and clinical paradigms for quantifying and specifying symptoms and forms of ADHD. Use of more nuanced cognitive tests will help characterize ADHD deficits and the study of circuit-level mechanisms underlying executive functions in nonhuman primates holds promise for advancing our understanding, and ultimately the treatment, of ADHD.
Outstanding Questions Box.
To what extent are different populations of ADHD pathologies separable using psychophysical tasks? Are there psychophysical signatures of subtypes within this broadly-defined disorder?
Changes in which neural circuits contribute to the deficits in response precision that are a hallmark of ADHD?
How do non-standard medications affect temporal information processing in individuals with ADHD and neurotypical controls?
How do ADHD medications affect monkeys performing different cognitive tasks?
Relatedly, how do ADHD medications change the activity of neurons in regions of the monkey brain that are implicated in ADHD in humans?
Is it possible to determine the appropriate dosage for a medication, particularly those with known inverse-U effects on behavior, using read-out from simple psychophysical tests?
Many studies examining the effects of alpha-2 adrenergic receptor agonists in monkeys have used guanfacine, whereas clonidine is more typically prescribed for humans. Do both drugs have similar effects on behavior in the same individuals? If not, which one is superior at ameliorating ADHD symptoms?
How effective is behavioral therapy at alleviating different cognitive deficits in ADHD and what are the neural mechanisms underlying it?
Many of the cognitive domains implicated in ADHD are steered, at least in part, by the prefrontal cortex. How are PFC circuits activated by different tasks and how do ADHD medications affect neural signaling in this area during said tasks?
Trends Box.
Deficits in many different cognitive domains are associated with ADHD. Cognitive batteries that assess individuals with ADHD’s performance in these different cognitive domains show that the disorder is very heterogeneous.
ADHD medication that improves performance in one cognitive measure does not necessarily improve it in others.
Studies in human and nonhuman primates have revealed much about the underlying mechanisms of endogenous, exogenous, spatial- and feature-based selective attention.
The regions of the brain, and neuromodulators, that influence selective attention and other cognitive domains implicated in ADHD are non-overlapping.
Glossary
- Prefrontal Cortex
The prefrontal cortex is the front part of the brain’s frontal lobe and is comprised of many sub-regions including the frontal eye field, the supplementary eye field, dorsolateral prefrontal cortex, and anterior cingulate cortex.
- Catecholamines
Catecholamines are a class of neuromodulators that include dopamine and norepinephrine. Norepinephrine is a synonym for noradrenaline. Norepinephrine binds to noradrenergic receptors, and is released from noradrenergic neurons.
- Nosology
the branch of medical science dealing with the classification of diseases. The nosology of ADHD therefore deals with the clinical classification of ADHD.
- Conners’ performance task
This is a computer-based assessment of attention performance. It is not routinely used for diagnosis, but it is the most common test used in research on clinical populations.
- Disorder of Inhibition
This is the proposal that ADHD results from a failure in the suppression of actions that would interfere with goal-driven behavior.
- Disorder of Delay Aversion
This is the proposal that ADHD results from an altered motivational state and reward processing that penalizes delays. [14]
- Saliency Map
This is a neural representation of the environment that incorporates different types of information (e.g. color, contrast) into a global measure of conspicuity.
- Visuospatial Neglect
This is a neurological condition which presents as a deficit in attention to one region of space, without an apparent deficit in sensation for that region.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polanczyk G, et al. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Conners CK, et al. Conners continuous performance test II (CPT II v. 5) Multi-Health Syst Inc. 2000;29:175–196. [Google Scholar]
- 3.Maoz H, et al. Association between continuous performance and response inhibition tests in adults with ADHD. J Atten Disord. 2015 doi: 10.1177/1087054715584056. [DOI] [PubMed] [Google Scholar]
- 4.Egeland J, Kovalik-Gran I. Measuring several aspects of attention in one test: The factor structure of conners's continuous performance test. J Atten Disord. 2010;13:339–346. doi: 10.1177/1087054708323019. [DOI] [PubMed] [Google Scholar]
- 5.Fair DA, et al. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109:6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willcutt EG, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. 2012;121:991–1010. doi: 10.1037/a0027347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Donk ML, et al. Predictors and moderators of treatment outcome in cognitive training for children with ADHD. J Atten Disord. 2016 doi: 10.1177/1087054716632876. [DOI] [PubMed] [Google Scholar]
- 8.Roberts BA, et al. Are there executive dysfunction subtypes within ADHD? J Atten Disord. 2013 doi: 10.1177/1087054713510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gizer IR, et al. Candidate gene studies of ADHD: A meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, et al. Molecular genetic studies of ADHD and its candidate genes: A review. Psychiatry Res. 2014;219:10–24. doi: 10.1016/j.psychres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Frazier TW, et al. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 12.Willcutt EG, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Barkley RA. Attention-deficit hyperactivity disorder and the nature of self-control. Guilford Press; 1997. [Google Scholar]
- 14.Sonuga-Barke EJ. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Toplak ME, et al. Temporal information processing in ADHD: Findings to date and new methods. J Neurosci Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Mostert JC, et al. Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: A systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol. 2015;25:2062–2074. doi: 10.1016/j.euroneuro.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott GR, et al. Cognitive testing to identify children with ADHD who do and do not respond to methylphenidate. J Atten Disord. 2014 doi: 10.1177/1087054714543924. [DOI] [PubMed] [Google Scholar]
- 18.Oken BS, et al. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 20.Mason DJ, et al. Insights into the control of attentional set in ADHD using the attentional blink paradigm. J Child Psychol Psychiatry. 2005;46:1345–1353. doi: 10.1111/j.1469-7610.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- 21.Egeland J, et al. Differentiating between ADHD sub-types on CCPT measures of sustained attention and vigilance. Scand J Psychol. 2009;50:347–354. doi: 10.1111/j.1467-9450.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 22.Kofler MJ, et al. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Borella E, et al. Beyond interference control impairment in ADHD: Evidence from increased intraindividual variability in the color-stroop test. Child Neuropsychol. 2013;19:495–515. doi: 10.1080/09297049.2012.696603. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy RJ, et al. Comparison of two measures of working memory impairments in 220 adolescents and adults with ADHD. J Atten Disord. 2016 doi: 10.1177/1087054716661232. [DOI] [PubMed] [Google Scholar]
- 25.Kastner S. A neural basis for human visual attention. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. MIT press; 2004. pp. 1514–1523. [Google Scholar]
- 26.Gregoriou GG, et al. Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat Neurosci. 2014 doi: 10.1038/nn.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wardak C, et al. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–9884. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Squire RF, et al. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- 30.Baldauf D, Desimone R. Neural mechanisms of object-based attention. Science. 2014;344:424–427. doi: 10.1126/science.1247003. [DOI] [PubMed] [Google Scholar]
- 31.Bichot NP, et al. A source for feature-based attention in the prefrontal cortex. Neuron. 2015;88:832–844. doi: 10.1016/j.neuron.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, et al. Modulation of neuronal responses by exogenous attention in macaque primary visual cortex. J Neurosci. 2015;35:13419–13429. doi: 10.1523/JNEUROSCI.0527-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burrows BE, Moore T. Influence and limitations of popout in the selection of salient visual stimuli by area V4 neurons. J Neurosci. 2009;29:15169–15177. doi: 10.1523/JNEUROSCI.3710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arcizet F, et al. A pure salience response in posterior parietal cortex. Cereb Cortex. 2011;21:2498–2506. doi: 10.1093/cercor/bhr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trappenberg TP, et al. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci. 2001;13:256–271. doi: 10.1162/089892901564306. [DOI] [PubMed] [Google Scholar]
- 36.Busse L, et al. Temporal dynamics of neuronal modulation during exogenous and endogenous shifts of visual attention in macaque area MT. Proceedings Of The National Academy Of Sciences. 2008;105:16380–16385. doi: 10.1073/pnas.0707369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vossel S, et al. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbetta M, et al. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- 39.Blankenburg F, et al. Studying the role of human parietal cortex in visuospatial attention with concurrent tms-fmri. Cereb Cortex. 2010;20:2702–2711. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kincade JM, et al. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meister IG, et al. Hemiextinction induced by transcranial magnetic stimulation over the right temporo-parietal junction. Neuroscience. 2006;142:119–123. doi: 10.1016/j.neuroscience.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 42.Patel GH, et al. Functional evolution of new and expanded attention networks in humans. Proc Natl Acad Sci U S A. 2015;112:9454–9459. doi: 10.1073/pnas.1420395112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leitão J, et al. Concurrent tms-fmri reveals interactions between dorsal and ventral attentional systems. J Neurosci. 2015;35:11445–11457. doi: 10.1523/JNEUROSCI.0939-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Japee S, et al. A role of right middle frontal gyrus in reorienting of attention: A case study. Front Syst Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprague TC, Serences JT. Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices. Nat Neurosci. 2013;16:1879–1887. doi: 10.1038/nn.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen EI. Control from below: The role of a midbrain network in spatial attention. Eur J Neurosci. 2011;33:1961–1972. doi: 10.1111/j.1460-9568.2011.07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson JM, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: Brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 49.Gonon F. The dopaminergic hypothesis of attention-deficit/hyperactivity disorder needs re-examining. Trends Neurosci. 2009;32:2–8. doi: 10.1016/j.tins.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 50.Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: Circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- 51.Aston-Jones G, et al. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carli M, et al. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 53.Papadopoulos N, et al. Is there a link between motor performance variability and social-communicative impairment in children with ADHD-CT: A kinematic study using an upper limb fitts' aiming task. J Atten Disord. 2015;19:72–77. doi: 10.1177/1087054712454569. [DOI] [PubMed] [Google Scholar]
- 54.Stuss DT, et al. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 55.Simmonds DJ, et al. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Kamigaki T, et al. Neurodynamics of cognitive set shifting in monkey frontal cortex and its causal impact on behavioral flexibility. J Cogn Neurosci. 2012;24:2171–2185. doi: 10.1162/jocn_a_00277. [DOI] [PubMed] [Google Scholar]
- 57.Kim C, et al. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J Neurosci. 2011;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prado VF, et al. Cholinergic circuits in cognitive flexibility. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Saez A, et al. Abstract context representations in primate amygdala and prefrontal cortex. Neuron. 2015;87:869–881. doi: 10.1016/j.neuron.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puig MV, et al. Editorial: Neuromodulation of executive circuits. Front Neural Circuits. 2015;9:58. doi: 10.3389/fncir.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clarke HF, et al. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lara AH, Wallis JD. The role of prefrontal cortex in working memory: A mini review. Front Syst Neurosci. 2015:9. doi: 10.3389/fnsys.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jazayeri M, Shadlen MN. A neural mechanism for sensing and reproducing a time interval. Curr Biol. 2015;25:2599–2609. doi: 10.1016/j.cub.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aron AR, et al. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 66.Miyazaki K, et al. The role of serotonin in the regulation of patience and impulsivity. Mol Neurobiol. 2012;45:213–224. doi: 10.1007/s12035-012-8232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazaki KW, et al. Activation of dorsal raphe serotonin neurons is necessary for waiting for delayed rewards. J Neurosci. 2012;32:10451–10457. doi: 10.1523/JNEUROSCI.0915-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luman M, et al. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Sonuga-Barke EJ. Psychological heterogeneity in AD/hd--a dual pathway model of behaviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 70.Kennerley SW, Wallis JD. Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol. 2009;102:3352–3364. doi: 10.1152/jn.00273.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. J Neurosci. 2010;30:3339–3346. doi: 10.1523/JNEUROSCI.4874-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rajala AZ, et al. Decision-making: Effects of methylphenidate on temporal discounting in non-human primates. J Neurophysiol. 2015 doi: 10.1152/jn.00278.2015. jn.00278.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wood S, et al. Psychostimulants and cognition: A continuum of behavioral and cognitive activation. Pharmacol Rev. 2014;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miyamoto S, et al. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 75.Killeen PR, et al. A behavioral neuroenergetics theory of ADHD. Neurosci Biobehav Rev. 2013;37:625–657. doi: 10.1016/j.neubiorev.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Rajala AZ, et al. Dissociative effects of methylphenidate in nonhuman primates: Trade-offs between cognitive and behavioral performance. J Cogn Neurosci. 2012;24:1371–1381. doi: 10.1162/jocn_a_00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orban GA, et al. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Nakahara K, et al. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science. 2002;295:1532–1536. doi: 10.1126/science.1067653. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell JF, et al. Active vision in marmosets: A model system for visual neuroscience. J Neurosci. 2014;34:1183–1194. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sasaki E, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- 81.Safren SA, et al. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther. 2005;43:831–842. doi: 10.1016/j.brat.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Schafer RJ, Moore T. Selective attention from voluntary control of neurons in prefrontal cortex. Science. 2011;332:1568–1571. doi: 10.1126/science.1199892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rapport MD, et al. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. 2013;33:1237–1252. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 84.Klingberg T, et al. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 85.Gropper RJ, et al. Working memory training in college students with ADHD or LD. J Atten Disord. 2014;18:331–345. doi: 10.1177/1087054713516490. [DOI] [PubMed] [Google Scholar]
- 86.Cortese S, et al. Cognitive training for attention-deficit/hyperactivity disorder: Meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–174. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ansari S. The therapeutic potential of working memory training for treating mental disorders. Front Hum Neurosci. 2015;9:481. doi: 10.3389/fnhum.2015.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu ZX, et al. Effects of working memory training on neural correlates of go/nogo response control in adults with ADHD: A randomized controlled trial. Neuropsychologia. 2017;95:54–72. doi: 10.1016/j.neuropsychologia.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 89.Dovis S, et al. Improving executive functioning in children with ADHD: Training multiple executive functions within the context of a computer game. A randomized double-blind placebo controlled trial. PLoS One. 2015;10:e0121651. doi: 10.1371/journal.pone.0121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karalunas SL, et al. Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: Toward biologically based nosologic criteria. JAMA Psychiatry. 2014;71:1015–1024. doi: 10.1001/jamapsychiatry.2014.763. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Handbook of disruptive behavior disorders. Springer; 1999. [Google Scholar]
- 92.Hooks K, et al. Sustained and selective attention in boys with attention deficit hyperactivity disorder. Journal Of Clinical Child Psychology. 1994;23:69–77. [Google Scholar]
- 93.Dalebout SD, et al. Selective auditory attention and children with attention-deficit hyperactivity disordereffects of repeated measurement with and without methylphenidate. Language, Speech, And Hearing Services In Schools. 1991;22:219–227. [Google Scholar]
- 94.Ceci SJ, Tishman J. Hyperactivity and incidental memory: Evidence for attentional diffusion. Child Dev. 1984;55:2192–2203. [PubMed] [Google Scholar]
- 95.Prior M, et al. Auditory attentional abilities in hyperactive children. J Child Psychol Psychiatry. 1985;26:289–304. doi: 10.1111/j.1469-7610.1985.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 96.Satterfield JH, et al. Preferential neural processing of attended stimuli in attention-deficit hyperactivity disorder and normal boys. Psychophysiology. 1994;31:1–10. doi: 10.1111/j.1469-8986.1994.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 97.Carter CS, et al. Abnormal processing of irrelevant information in attention deficit hyperactivity disorder. Psychiatry Res. 1995;56:59–70. doi: 10.1016/0165-1781(94)02509-h. [DOI] [PubMed] [Google Scholar]
- 98.Pearson DA, et al. Comparison of sustained and selective attention in children who have mental retardation with and without attention deficit hyperactivity disorder. Am J Ment Retard. 1996;100:592–607. [PubMed] [Google Scholar]
- 99.Jonkman LM, et al. Event-related potentials and performance of attention-deficit hyperactivity disorder: Children and normal controls in auditory and visual selective attention tasks. Biol Psychiatry. 1997;41:595–611. doi: 10.1016/s0006-3223(96)00073-x. [DOI] [PubMed] [Google Scholar]
- 100.Friedman-Hill SR, et al. What does distractibility in ADHD reveal about mechanisms for top-down attentional control? Cognition. 2010;115:93–103. doi: 10.1016/j.cognition.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mason DJ, et al. Exploring selective attention in ADHD: Visual search through space and time. J Child Psychol Psychiatry. 2003;44:1158–1176. doi: 10.1111/1469-7610.00204. [DOI] [PubMed] [Google Scholar]
- 102.Brodeur DA, Pond M. The development of selective attention in children with attention deficit hyperactivity disorder. J Abnorm Child Psychol. 2001;29:229–239. doi: 10.1023/a:1010381731658. [DOI] [PubMed] [Google Scholar]
- 103.Jäkälä P, et al. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 104.Cherry EC. Some experiments on the recognition of speech, with one and with two ears. The Journal Of The Acoustical Society Of America. 1953;25:975–979. [Google Scholar]
- 105.TEICHNER WH. Recent studies of simple reaction time. Psychol Bull. 1954;51:128–149. doi: 10.1037/h0060900. [DOI] [PubMed] [Google Scholar]
- 106.Marcos E, et al. Neural variability in premotor cortex is modulated by trial history and predicts behavioral performance. Neuron. 2013;78:249–255. doi: 10.1016/j.neuron.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 107.BERG EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 108.Gamo NJ, et al. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.D'Ardenne K, et al. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proc Natl Acad Sci U S A. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Divenyi PL, Danner WF. Discrimination of time intervals marked by brief acoustic pulses of various intensities and spectra. Perception & Psychophysics. 1977;21:125–142. [Google Scholar]
- 111.Vicario CM, et al. Temporal accuracy and variability in the left and right posterior parietal cortex. Neuroscience. 2013;245:121–128. doi: 10.1016/j.neuroscience.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 112.Robertson CL, et al. Striatal D1- and d2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci. 2015;35:5990–5997. doi: 10.1523/JNEUROSCI.4850-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grilly DM, et al. Effects of cocaine and d-amphetamine on sustained and selective attention in rats. Pharmacol Biochem Behav. 1989;33:733–739. doi: 10.1016/0091-3057(89)90463-2. [DOI] [PubMed] [Google Scholar]
- 114.López J, et al. Effect of psychostimulants on distinct attentional parameters in attentional deficit/hyperactivity disorder. Biol Res. 2004;37:461–468. doi: 10.4067/s0716-97602004000300010. [DOI] [PubMed] [Google Scholar]
- 115.Balthazor MJ, et al. The specificity of the effects of stimulant medication on classroom learning-related measures of cognitive processing for attention deficit disorder children. J Abnorm Child Psychol. 1991;19:35–52. doi: 10.1007/BF00910563. [DOI] [PubMed] [Google Scholar]
- 116.Nagashima M, et al. Neuropharmacological effect of atomoxetine on attention network in children with attention deficit hyperactivity disorder during oddball paradigms as assessed using functional near-infrared spectroscopy. Neurophotonics. 2014;1:025007. doi: 10.1117/1.NPh.1.2.025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tiplady B, et al. Selective effects of clonidine and temazepam on attention and memory. Journal Of Psychopharmacology. 2005;19:259–265. doi: 10.1177/0269881105051529. [DOI] [PubMed] [Google Scholar]
- 118.Andrzejewski ME, et al. The effects of clinically relevant doses of amphetamine and methylphenidate on signal detection and DRL in rats. Neuropharmacology. 2014;79:634–641. doi: 10.1016/j.neuropharm.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sostek AJ, et al. Effects of amphetamine on vigilance performance in normal and hyperactive children. J Abnorm Child Psychol. 1980;8:491–500. doi: 10.1007/BF00916502. [DOI] [PubMed] [Google Scholar]
- 120.Navarra R, et al. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Progress In Neuro-Psychopharmacology And Biological Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 121.Tamminga HG, et al. Effects of methylphenidate on executive functioning in attention-deficit/hyperactivity disorder across the lifespan: A meta-regression analysis. Psychol Med. 2016;46:1791–1807. doi: 10.1017/S0033291716000350. [DOI] [PubMed] [Google Scholar]
- 122.Lufi D, et al. The effect of methylphenidate on sustained attention among adolescents with attention-deficit hyperactivity disorder. Neurocase. 2015;21:802–808. doi: 10.1080/13554794.2015.1023317. [DOI] [PubMed] [Google Scholar]
- 123.Ni HC, et al. Atomoxetine could improve intra-individual variability in drug-naïve adults with attention-deficit/hyperactivity disorder comparably with methylphenidate: A head-to-head randomized clinical trial. J Psychopharmacol. 2016;30:459–467. doi: 10.1177/0269881116632377. [DOI] [PubMed] [Google Scholar]
- 124.Bushnell PJ, et al. Detection of visual signals by rats: Effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology (Berl) 1997;134:230–241. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- 125.Sagvolden T. The alpha-2a adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Funct. 2006;2:41. doi: 10.1186/1744-9081-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Decamp E, et al. Effects of the alpha-2 adrenoceptor agonist guanfacine on attention and working memory in aged non-human primates. European Journal Of Neuroscience. 2011;34:1018–1022. doi: 10.1111/j.1460-9568.2011.07815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coull JT, et al. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology (Berl) 1995;120:322–332. doi: 10.1007/BF02311180. [DOI] [PubMed] [Google Scholar]
- 128.Conners CK, et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996;35:1314–1321. doi: 10.1097/00004583-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 129.Loos M, et al. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a go/no-go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 130.Aggarwal A, Lillystone D. A follow-up pilot study of objective measures in children with attention deficit hyperactivity disorder. J Paediatr Child Health. 2000;36:134–138. doi: 10.1046/j.1440-1754.2000.00464.x. [DOI] [PubMed] [Google Scholar]
- 131.Jentsch JD, et al. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- 132.Coghill DR, et al. Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: Evidence from a systematic review and a meta-analysis. Biol Psychiatry. 2014;76:603–615. doi: 10.1016/j.biopsych.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 133.Bédard AC, et al. Differential impact of methylphenidate and atomoxetine on sustained attention in youth with attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2015;56:40–48. doi: 10.1111/jcpp.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Logemann HN, et al. The effect of noradrenergic attenuation by clonidine on inhibition in the stop signal task. Pharmacol Biochem Behav. 2013;110:104–111. doi: 10.1016/j.pbb.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Bilder RM, et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. Journal Of The American Academy Of Child & Adolescent Psychiatry. 2016 doi: 10.1016/j.jaac.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Featherstone RE, et al. A sensitizing regimen of amphetamine that disrupts attentional set-shifting does not disrupt working or long-term memory. Behav Brain Res. 2008;189:170–179. doi: 10.1016/j.bbr.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 137.Soto PL, et al. Long-term exposure to oral methylphenidate or dl-amphetamine mixture in peri-adolescent rhesus monkeys: Effects on physiology, behavior, and dopamine system development. Neuropsychopharmacology. 2012;37:2566–2579. doi: 10.1038/npp.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Advokat C. What are the cognitive effects of stimulant medications? Emphasis on adults with attention-deficit/hyperactivity disorder (ADHD) Neuroscience & Biobehavioral Reviews. 2010;34:1256–1266. doi: 10.1016/j.neubiorev.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 139.Chu R, et al. Differentiation of rodent behavioral phenotypes and methylphenidate action in sustained and flexible attention tasks. Brain Res. 2016;1641:306–319. doi: 10.1016/j.brainres.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang L, et al. Comparative study of OROS-MPH and atomoxetine on executive function improvement in ADHD: A randomized controlled trial. Int J Neuropsychopharmacol. 2012;15:15–26. doi: 10.1017/S1461145711001490. [DOI] [PubMed] [Google Scholar]
- 141.Dyme IZ, et al. Perseveration induced by methylphenidate in children: Preliminary findings. Progress In Neuro-Psychopharmacology And Biological Psychiatry. 1982;6:269–273. doi: 10.1016/s0278-5846(82)80177-2. [DOI] [PubMed] [Google Scholar]
- 142.Seu E, et al. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 144.Choi Y, et al. The effect of α-2 adrenergic agonists on memory and cognitive flexibility. Cognitive And Behavioral Neurology. 2006;19:204–207. doi: 10.1097/01.wnn.0000213919.95266.0d. [DOI] [PubMed] [Google Scholar]
- 145.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 146.Schulze GE, Paule MG. Acute effects of d-amphetamine in a monkey operant behavioral test battery. Pharmacol Biochem Behav. 1990;35:759–765. doi: 10.1016/0091-3057(90)90355-l. [DOI] [PubMed] [Google Scholar]
- 147.Mattay VS, et al. Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage. 2000;12:268–275. doi: 10.1006/nimg.2000.0610. [DOI] [PubMed] [Google Scholar]
- 148.Berridge CW, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 149.Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in attention deficit hyperactivity disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chamberlain SR, et al. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Marrs W, et al. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- 152.Mair RD, et al. Effects of clonidine in the locus coeruleus on prefrontal-and hippocampal-dependent measures of attention and memory in the rat. Psychopharmacology (Berl) 2005;181:280–288. doi: 10.1007/s00213-005-2263-x. [DOI] [PubMed] [Google Scholar]
- 153.Hampson CL, et al. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and d-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behav Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- 154.Sanchez-Castillo H, et al. Subjective and real time: Coding under different drug states. International Journal Of Comparative Psychology/ISCP; Sponsored By The International Society For Comparative Psychology And The University Of Calabria. 2015:28. [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson TW, et al. Estimating the passage of minutes: Deviant oscillatory frontal activity in medicated and unmedicated ADHD. Neuropsychology. 2013;27:654–665. doi: 10.1037/a0034032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luman M, et al. The unique and combined effects of reinforcement and methylphenidate on temporal information processing in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2015;35:414–421. doi: 10.1097/JCP.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 157.van Gaalen MM, et al. Amphetamine decreases behavioral inhibition by stimulation of dopamine D2, but not D3, receptors. Behav Pharmacol. 2009;20:484–491. doi: 10.1097/FBP.0b013e3283305e3b. [DOI] [PubMed] [Google Scholar]
- 158.Sagvolden T, Xu T. L-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of attention-deficit/hyperactivity disorder (ADHD) Behav Brain Funct. 2008;4:3. doi: 10.1186/1744-9081-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Experimental And Clinical Psychopharmacology. 2008;16:113. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allman AA, et al. Effect of d-amphetamine on inhibition and motor planning as a function of baseline performance. Psychopharmacology (Berl) 2010;211:423–433. doi: 10.1007/s00213-010-1912-x. [DOI] [PubMed] [Google Scholar]
- 161.Graf H, et al. Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biol Psychiatry. 2011;69:890–897. doi: 10.1016/j.biopsych.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 162.Nandam LS, et al. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2011;69:902–904. doi: 10.1016/j.biopsych.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 163.Effect of clonidine in the 5-tiral inhibitory avoidance test using juvenile spontaneously hypertensive rats. J Pharmacol Sci. 2014 [Google Scholar]
- 164.Bari A, et al. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]