Abstract
Group size is a fundamental component of sociality, and has important consequences for an individual's fitness as well as the collective and cooperative behaviours of the group as a whole. This review focuses on how the costs and benefits of group living vary in female primates as a function of group size, with a particular emphasis on how competition within and between groups affects an individual's energetic balance. Because the repercussions of chronic energetic stress can lower an animal's fitness, identifying the predictors of energetic stress has important implications for understanding variation in survivorship and reproductive success within and between populations. Notably, we extend previous literature on this topic by discussing three physiological measures of energetic balance—glucocorticoids, c-peptides and thyroid hormones. Because these hormones can provide clear signals of metabolic states and processes, they present an important complement to field studies of spatial and temporal changes in food availability. We anticipate that their further application will play a crucial role in elucidating the adaptive significance of group size in different social and ecological contexts.
This article is part of the themed issue ‘Physiological determinants of social behaviour in animals'.
Keywords: c-peptides, daily travel, ecological constraints model, glucocorticoids, group size, thyroid hormones
1. Introduction
Group size in many social animals, including most primates, can vary tremendously within and between populations. One of the most widely accepted hypotheses proposed to explain this large variation in group size is competition over food resources [1–4]. Competition influences the quality and quantity of food (energy) resources that are acquired from the environment, and these resources are then directed towards reproductive effort once an individual's basic metabolic requirements have been met [5]. Although the repercussions of short-term energy deficits may be mitigated by mobilizing energy reserves and/or decreasing metabolic rates, chronic energetic stress can have deleterious consequences, including immunosuppression, muscle wasting and reduced fertility [6,7]. Identifying how group-size variation affects an individual's energetic balance can thus have important implications for understanding fitness consequences for animals living in small versus large social groups. Given the energetic demands of pregnancy and lactation, factors affecting energetic status in mammals can arguably have a more significant effect on female relative to male fitness [8].
Here, we focus on the energetic consequences of group-size variation in female primates, emphasizing how group size affects resource competition within and between groups. Primates are an ideal taxon within which to examine this topic, given their remarkable diversity in social organization [9] and the fact that individuals in the majority of primate species form enduring social bonds with other group members [10]. For females in particular, the fitness advantages associated with these social bonds—and group living more broadly—have long been recognized (e.g. [11,12]), though a mechanistic understanding of how competition affects survival and reproduction via energetic pathways has remained elusive. Our overall objective is to guide future research on this topic by reviewing existing theories and methodologies. Following recent initiatives to integrate individual- and group-level processes in a landscape perspective [13], we emphasize the importance of predicting how interactions among individuals within groups affect interactions between groups, i.e. at the scale of populations, and vice versa.
(a). Scramble competition within groups
Increased within-group competition for food is predicted to be one of the major costs of group living (e.g. [1,14,15]). As group size increases, food patches are more rapidly depleted and/or each group member's encounter rate with food is diminished as the search fields of neighbouring individuals overlap. Two key strategies to compensate for the resulting declines in food availability are (i) to increase the time devoted to foraging activities and (ii) to increase daily travel length, so as to encounter more food patches [16–19]. The ecological constraints model emphasizes that the size, density and distribution of depletable food patches will influence the strength of this group-size effect. It predicts that travel costs will be highest in landscapes characterized by small, low density and/or sparsely distributed patches (reviewed in [20]). Numerous empirical studies on primates lend support to this model, particularly among species that compete for patchy, high-quality food resources (reviewed in [21,22]).
Beyond altering total foraging time and movement patterns, individuals in larger groups may mitigate the costs of intragroup competition by changing dietary preferences, foraging in lower-quality patches and/or increasing patch residence times (reviewed in [22]). It is important to note that individuals following these strategies may minimize energetic expenditure through decreased travel yet, in doing so, they may compromise energy intake by foraging on lower-quality resources. Lastly, individuals may also increase their distance to other group members, thereby reducing the extent of search path overlap between neighbouring animals [23,24]. If patch size is sufficiently large to accommodate the increased group spread, this strategy does not necessarily exclude group members from simultaneously foraging on equal-quality food items. It may, however, increase the vulnerability to predator attack for more isolated members positioned on the group's periphery (reviewed in [25]).
Given the importance of resource abundance and distribution for the relationship between group size and daily travel, the most applicable tests of the ecological constraints model have compared groups occupying the same habitat and/or how a single group responds to local habitat changes (reviewed in [22]). By contrast, major habitat differences between sites in comparative studies spanning broader (e.g. continental) scales can mask the effects of group size on ranging patterns. For example, cumulative monthly rainfall—an ecological variable capable of capturing substantial habitat differences—was the strongest predictor of daily travel path in two meta-analyses of baboon study populations ranging throughout East Africa [26,27]. In these same studies, group size was a relatively poor [27] or non-significant predictor of daily travel distance [26]. Johnson et al. [26] suggest that this lack of a group size effect may be explained by the stronger relative importance of ecological variables at broader spatial scale comparisons. These findings do not necessarily negate the importance of group size effects at the local scale.
(b). Contest competition between groups
Under some ecological conditions, the disadvantages of intragroup foraging competition in large groups may be offset by advantages associated with increased group size. In these cases, foraging time is expected to decrease as group size increases if larger groups outcompete smaller groups for food resources [1,28]. This concept, comparable to the principle of resource holding potential in individual-based contests [29], can have negative fitness consequences for individuals in relatively small groups. Extending the predictions of the ideal despotic distribution for conspecific interactions at the individual level [30], subordinate groups are expected to occupy poorer-quality habitats and, consequently, experience lower average reproductive success than dominant groups.
In many primate species, group size is a positive predictor of group-level dominance (reviewed in [31]). Despite findings that habitat quality influences individual fitness in primates [32,33], relatively few studies have explicitly evaluated whether intergroup differences in habitat quality result in differences between groups in the average individual fitness of group members. Some recent studies address this topic, however, with findings suggesting that larger (dominant) groups do indeed occupy higher-quality portions of the landscape [32,34,35]. The benefits individuals derive from such collective defence of resources can place a selective pressure on group members to form cooperative bonds with one another [1,36].
Foraging time may also decrease as group size increases if larger groups have advantages relative to smaller groups through information exchange about the quality and location of food [37,38]. Although information transfer regarding food resources has been studied in primates [39], the extent to which the rate of information transfer varies as a function of group size is largely unexplored [40] with the exception of research on food-associated calls. In several primate species, food availability and food quality/type influence the likelihood of call production (e.g. [41,42]). Long-distance calls in fission–fusion species may function, for example, to announce the caller's arrival at a food patch. Researchers have found that encountering food resources increases the likelihood that male chimpanzees will give a pant-hoot vocalization, which may attract allies and/or mates to the food patch and thereby augment subgroup size [43–46]. Similarly, food abundance, subgroup size and the caller's dominance rank influence the rate of food calling in spider monkeys [47]. The combined effect of these factors led researchers to suggest that callers share information contingent on how calling affects their competitive ability as group size and composition change.
(c). Contest competition within groups
For many group-living animals, group-size trade-offs are further nuanced by an individual's social status within the group. Across species, high rank typically confers reduced rates of received aggression and priority of access to food (reviewed in [48,49]). High-ranking individuals may also forage more efficiently due to fewer interruptions during feeding and reduced time required to meet caloric demands [50]. This pattern has been demonstrated in several primate species, such that dominants have higher food intake rates than subordinates [51–54].
Importantly, these patterns suggest that low-ranking individuals bear a disproportionate share of the costs of group living in that they may participate in collective behaviours (e.g. group vigilance and/or defence) yet have unequal access to food resources. The energetic consequences of this may be exacerbated if the distance travelled by the group as a whole is a major constituent of an individual's energetic expenditure. Specifically, low-ranking individuals travel at least as far as high-ranking individuals in cohesive groups yet may have lower energetic intake to offset these travel costs. Empirical evaluation of rank-based differences in energetic balance presents a novel direction for future research.
(d). A unifying framework: the concept of competitive regimes
For group-living animals, the concept of competitive regimes provides a framework for integrating the consequences of feeding competition within and between groups [53,55]. Three modes of competition are discussed in this framework: within-group scramble, within-group contest and between-group contest. The various combinations of these modes (e.g. the ‘competitive regime’) predict differences in female fitness within and between groups. For example, under intense between-group competition when resources are discrete and defensible, the model predicts that energy gain of females in smaller groups is less than that of females in larger groups. If this pattern is combined with within-group competition, energy gain declines linearly with female rank in each group. According to this holistic perspective, there should be an equilibrium point where a low-ranking female in a large (socially dominant) group has the equivalent energy gain to a high-ranking female in a small (socially subordinate) group. Janson [56, p. 54] notes that ‘differences among groups in the intensity of various forms of food competition should determine whether or not an individual would gain nutritionally by switching groups [57], and thus might help to regulate group size [58]’.
If the relative intensity of these intra- and intergroup pressures change over time, persistence of different-sized groups may be explained by fluctuating survival selection that alternatively favours small versus large groups. Although recent research on colonial cliff swallow provides novel evidence of how naturally occurring changes in the selection pressures can maintain a range of group sizes within a population [59], we are aware of no comparable studies to date on primates.
(e). Stable and optimal group sizes
Across and within mammalian species, there can be large variation in the extent to which group size and composition remain stable. At one extreme, groups are highly fluid in their social organization and subgroups of variable members form frequently; at the other extreme, groups are highly stable over long time periods and permanent group fissions or fusions occur infrequently (reviewed in [60]). This variation in the spatial and temporal cohesion of group members (fission–fusion dynamics) provides an opportunity to investigate how individuals balance group size trade-offs in socially complex settings. In particular, it presents a natural experiment for examining how optimal group size, defined as the group size ‘yielding maximum individual fitness' [61], varies in response to changing social and ecological pressures. The general consensus is that fissions reflect an imbalance between large groups and resource availability as mediated through intragroup competition for resources [62–65]. By contrast, fusions may reflect the inability of small groups to successfully compete against conspecific groups and/or avoid predation.
Research on species characterized by fluid social organization lends support to these predictions. For example, subgroup size was positively correlated with intragroup competition for food resources in fission–fusion species such as chimpanzees, bonobos and spider monkeys (e.g. [66–71]). In several studies, the explicit link between subgroup size and daily travel was examined and the expected positive correlation was found (e.g. [72,73]). Support has also come from studies of group fissioning in species typically characterized by stable social groups. For example, group fissions in yellow baboons are rare and are the only opportunity for females, the philopatric sex, to choose their group membership [74,75]. Analysis of female decisions during permanent fissions has shown that several factors—notably group size and the presence of matrilineal kin—are influential [76]. These findings are consistent with those observed for fission–fusion species in the extent to which they reveal the complexity of decision-making and party formation (e.g. [73,77]), and emphasize that food competition—likely mediated via group size—is one of several potential factors driving grouping patterns.
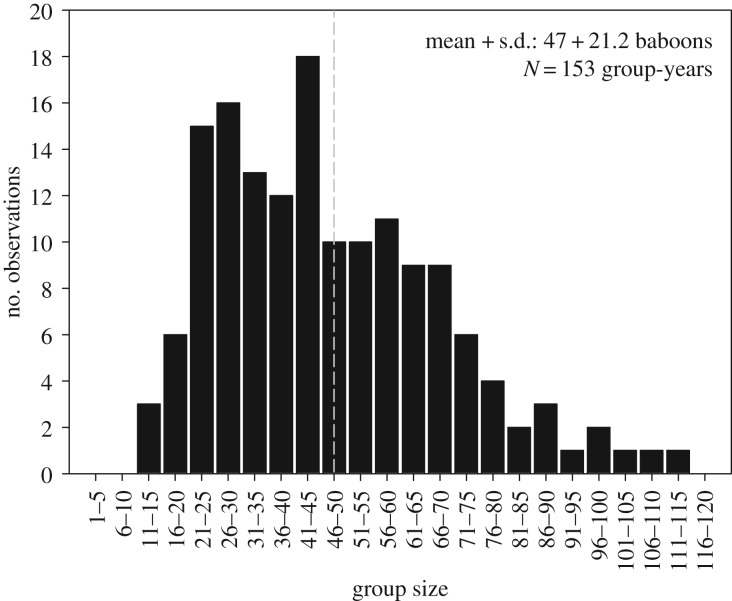
An intriguing question emerging from these results is why some primate groups persist above the optimal group size. For example, long-term monitoring of wild baboons by the Amboseli Baboon Research Project (ABRP) reveals a long tail in the upper end of the distribution of group size (figure 1), exceeding the optimal group size predicted by Markham et al. [78]. In the broader ecological literature, researchers assume that individuals move freely between groups as a reflection of an individual's own best interests. In this sense, a group at optimal size is extremely attractive to an immigrant, despite the fact that a new comer's immigration would cause the group size to increase above the optimum and therefore, the fitness of other group members to decline [61]. Considering the importance of not simply the number but the identity of other group members, fissioning in primates presents an important departure from these basic assumptions. Given the potential selective advantage individuals have in being able to adjust their grouping behaviour to current ecological conditions, factors potentially constraining plasticity and limiting the freedom of individuals to act independently—such as an individual's integration with other group members in a network of interconnected social relationships—require explanation. An additional argument is that group fissioning in some socially complex species is a collective action problem [79] that inhibits a rapid fission response to immediate conditions: fissions represent an opportunity to recalibrate the trade-offs individuals experience living in groups, but require a subset of the group members to form a new group. If too few individuals attempt to establish a new group, they may suffer more from between-group competition than formerly under intense within-group competition. This suggests that low-ranking animals living in large groups may tolerate the effects of a reduced net food intake via some benefits derived from group living (e.g. advantages in between-group competition and/or decreased predation risk).
Figure 1.
Distribution of total group size of the baboon social groups from 1971 to 2014. Group size was calculated as the total number of baboons present in each group on 1 January of each calendar year. Dashed line indicates the mean group size across all group-years. Data provided by ABRP.
(f). Beyond group size: group composition
Although many studies on group-size variation in primates focus exclusively on census counts of individuals, explicit consideration of the relative number of individuals in various age-sex classes (i.e. group composition) can have important implications for the energetic demands of the group as a whole and, therefore, the intensity of competition within and between groups.
At the individual level, estimates of an animal's metabolic rate—and, by extension, energetic needs and space-use requirements—have often been based on body mass and allometric scaling laws (e.g. [80–82]). Metabolic rate has also been calculated by a technique that uses isotopically labelled water to measure the rate of carbon dioxide production (‘doubly labelled water’ technique: [83]). While assessment using doubly labelled water provides a more accurate quantification of metabolic rate, the invasive nature of this approach (requiring animal capture at one or more time points) limits its implementation in many studies of wild primates (but see [84–87]). As such, scaling laws remain a valuable tool when researching species for which invasive measures are not readily obtained.
For species obligated to group living, the cumulative biomass and energetic demand of the group is expected to function comparably to relationships based on individuals in solitary species [88]. However, accurately quantifying biomass in wild populations can be difficult due to unknown ages and growth rates of the individuals observed in study groups. While larger groups should theoretically have larger energetic demands than smaller groups, empirical evaluation of how group size scales with group biomass and energetic demand has thus been lacking in primates (but see [89,90]). This shortcoming exists despite recognition that biomass is regarded as a more precise assessment of energetic demand than census counts of individuals [91,92].
Here, we estimate biomass and energetic demand using detailed group composition data from a population of wild baboons in Amboseli, Kenya. Our primary objective in this analysis is to examine how group size and composition together influence group biomass and energetic demand. This population has been the subject of regular observation since 1971 by the ABRP. We focused our analyses on 1990–2014 hydrological years, a time period characterized by regular monthly censuses of two to six social groups. ABRP provides a valuable opportunity to examine the relationship between group size, biomass and energetic demand because all individuals in the study population are individually recognizable, births and deaths are typically accurate to within 2–3 days, and growth rates in this population have been established in previous work [93]. We estimated body mass for each individual in the group based on the individual's age and sex relative to growth equations for wild-feeding baboons in this study population [93]. For both sexes, we estimated birth weight as 0.775 kg (value used [94,95]). We used a maximum adult weight of 22 kg for males and 12 kg for females [93]. Once individual body mass was calculated, we applied Kleiber's Law, which estimates metabolic rate as body mass raised to ¾ [96]. Kleiber's Law has been supported in studies from a wide array of taxonomic groups [90,97,98], but see references [99,100].
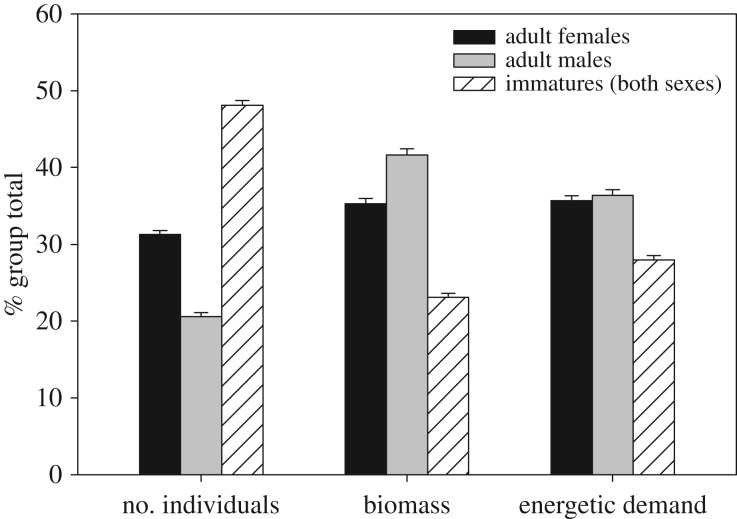
During our 25-year study period, we found that the average annual group size ranged from 19.9 to 115.0 individuals (N = 104 group-hydrological years, mean ± s.e.: 49.0 ± 2.12 individuals), average group biomass ranged from 161.0 to 1254.8 kg (mean ± s.e.: 519.5 ± 23.76 kg) and average group energetic demand estimated by Kleiber's Law ranged from 91.3 to 660.9 kg3/4 (mean ± s.e.: 275.5 ± 12.42 kg3/4). Average group biomass was strongly and positively correlated with group size (Spearman's correlation coefficient = 0.984, N = 104 group-hydrological years; p < 0.001), a pattern influenced by consistent per cent group compositions, despite variation in group size. A comparison of group composition by number of individuals, biomass and energetic demand (figure 2) suggests that various age–sex classes may have numerical superiority yet contribute minimally to the group's overall energetic requirements. This can have important implications for the group's collective actions, particularly in the light of recent work on ‘majority rule’ as a predictor of group-level movement decisions [101].
Figure 2.
Per cent contribution by age–sex class to group's total number of individuals, biomass (kg) and energetic demand (kg3/4) for 14 baboon social groups from 1990 to 2014 hydrological years (November–October). Analyses included only group-hydrological years in which the focal group was censused every calendar month during the hydrological year. Data provided by ABRP.
Group composition can affect the outcome of competition between groups. Age–sex biases in intergroup contest involvement have been documented in a wide range of primate species (e.g. [102,103]), and numerical superiority with regard to the age–sex class most likely to engage in intergroup attacks can be a stronger predictor of group-level dominance than an overall (total group size) advantage (reviewed in [31]). Group composition and biases in age–sex class involvement may be particularly important considerations when disentangling conflict related to food resources versus mating opportunities [104].
(g). Group size in the context of allostasis and the reactive scope model
Allostasis is the process of maintaining stability across the parameters essential for life (e.g. blood glucose, pH, body temperature) by altering levels of various physiological mediators such as glucocorticoids, catecholamines and cytokines [105]. Allostatic state refers to the mediator response required following predictable changes or events—such as daily and seasonal variations (e.g. photoperiod, mating), while allostatic load refers to the mediator response required following unpredictable changes or events (e.g. storms, predation). When the energetic demand essential for restoring homeostasis exceeds available energy stores or intake, an individual enters a state known as allostatic overload. The negative energy balance experienced during allostatic overload leads both to a decrease in body mass and a decline in reproductive performance [106,107], and prolonged duration of allostatic overload has adverse effects on the animal's health and survival [108].
The concept of allostasis, with its focus on energetic demands, was broadened by Romero et al. [109] in the reactive scope model to incorporate additional components such as early developmental effects and short-term stressors (e.g. a predator attack) that do not affect energetic demand. In this reactive scope model, physiological mediators exist in four ranges: (i) predictive homeostasis (corresponds to allostatic state), the range of a mediator reflecting normal circadian and seasonal variation and allowing the animal to cope with predictable challenges; (ii) reactive homeostasis (corresponds to allostatic load), the range of a mediator needed to restore homeostasis after an unpredictable challenge; (iii) homeostatic overload (corresponds to allostatic overload), the range of a mediator when the mediator itself starts disrupting homeostasis, and (iv) homeostatic failure, the range of a mediator when the mediator is insufficient at maintaining homeostasis. See the following section for further discussion of these ranges with specific reference to glucocorticoid concentrations.
Allostasis and the reactive scope model are applicable to understanding group size effects on an individual's energetic balance. Because of increased intragroup competition for food, individuals in larger groups are expected to have higher allostatic load than individuals in smaller groups unless (i) larger groups outcompete smaller groups for food resources or (ii) individuals in larger groups increase their foraging time and/or efficiency. However, if there is a food shortage and the animal can no longer meet its energy requirement, it will enter a state of allostatic overload [105] or homeostatic overload [109]. Not all individuals in a group are equal in terms of allostatic load, and individual variation can affect the threshold between reactive homeostasis and homeostatic overload. For example, lactating females have higher energy expenditure than cycling females due to the cost of milk production. If energy intake cannot compensate for this increased demand, lactating females—in a state of negative energy balance—will deplete fat stores, which will lead to a decrease in their body mass and longer interbirth intervals [107,110]. If any individual in such a compromised state is subsequently exposed to additional challenges while still lacking energy reserves to decrease allostatic load, its probability of reaching allostatic overload is highly increased [105].
Dominance rank is another factor affecting an individual's allostatic load. In addition to reduced access to food resources, low-ranking animals are also subject to physical and psychological threats from dominants. These negative social interactions will lead to higher allostatic load in subordinate relative to dominant individuals, and will increase a low-ranking animal's susceptibility to stress pathologies. However, if obtaining and maintaining dominance rank are costly (obtained through aggression and not inherited), or if subordinate animals have good coping mechanisms (e.g. grooming), then high-ranking animals may have higher allostatic load than low-ranking ones [111].
(h). Physiological measures of energetic balance
As reviewed in the preceding sections, competition within and between groups proximately affects a female's energetic balance and ultimately impacts her reproduction and survivorship [1,49,112]. Despite theoretical interest in understanding the relative influence of these intra- and intergroup effects [53,55], there has been relatively little empirical evaluation of existing theories. This lack of research is due, in large part, to the challenge of accurately quantifying energetic balance in wild populations [53]. Several recent developments, however, provide researchers with non-invasive techniques to measure an animal's energetic balance. In the sections below, we discuss how three important biomarkers of energetic balance—glucocorticoid, c-peptide and thyroid hormone concentrations—can be applied to this topic.
(i). Glucocorticoids
Glucocorticoids are steroid hormones secreted by the adrenal glands in response to adverse stressors. According to the reactive scope model, glucocorticoid levels in the predictive homeostatic range regulate energetic metabolism linked to normal daily and seasonal demands by promoting foraging behaviour, energy intake and energy use [113,114]. In response to unpredictable events including environmental [115–118] and social stressors [7,119,120], glucocorticoid levels increase in the reactive homeostatic range. This elevation of glucocorticoid levels in the bloodstream leads to the rapid mobilization of glucose and contributes to the restoration of homeostasis by enhancing gluconeogenesis, increasing foraging behaviour and shutting down processes that are non-essential for immediate survival (i.e. growth, reproduction and immune function). As such, maintaining elevated glucocorticoid concentrations for extended periods of time entails ‘wear and tear’ costs as the animal concomitantly decreases basic self-maintenance and reproductive functions. Wear and tear costs are also experienced during homeostatic overload when glucocorticoids themselves begin having pathological consequences. Maintaining glucocorticoid secretion in homeostatic overload for long time periods (chronic stress) decreases an animal's ability to cope with stressors and leads to increased disease prevalence and decreased survival [108,121–123].
Glucocorticoid concentrations can be measured non-invasively in a variety of matrices (e.g. urine, faeces, saliva and hair), and therefore have been widely used to examine the effects of various stressors in wild animals [124–130]. Several studies have investigated how glucocorticoid levels vary as a function of group size in social animals. The majority of these studies found that individuals in larger groups have higher glucocorticoid concentrations relative to individuals in smaller groups (meadow voles [131]; African elephants [132]; cliff swallows [133]; American red squirrels [134]; rhesus monkeys [135]; king penguins [136]; see also review [137]). By contrast, other studies have found that individuals in smaller groups had higher glucocorticoid concentrations (sheep [138]; prairie voles [139]). Two other studies found that intermediate group size was optimal (yellow baboons [78]; ring-tailed lemurs [140]). In these studies, the authors hypothesized that the larger groups had elevated glucocorticoid levels due to the high cost of intra-group competition, while smaller groups had elevated glucocorticoid levels due to the high cost of inter-group competition and/or predation. Finally, some studies have found no correlation between glucocorticoid concentrations and group size, as each group size comes with its own costs and benefits [141,142]. The diversity of these patterns reveals the complexity of group size costs and benefits for different animal species.
In addition to quantifying differences between groups, glucocorticoids have been used to understand how individuals within the same group uniquely experience energetic trade-offs associated with group living. Much of this research has focused on the influences of dominance rank on glucocorticoid concentrations. The majority of studies have found that higher-ranking animals have lower glucocorticoid concentrations, a pattern reflecting priority of access to resources, and they can therefore usually meet their energetic demands more readily than low-ranking animals [119,120,123]. Lower-ranking animals are also subject to increased aggression and harassment leading to high levels of psychological stress. However, in some species, being a high-ranking animal can be energetically demanding due to the costs of mating and maintaining high ranks, and high-ranking animals can have higher glucocorticoid concentrations than lower-ranking animals [119,120,123,130].
Despite the frequent use of glucocorticoid concentrations as a reflection of energetic stress, there are several limitations with this interpretation. First, basal glucocorticoid levels regulate metabolism during normal activities, including conditions when the animal is not energetically limited [113,114]. Second, glucocorticoids are not linearly released during starvation. Rather, glucocorticoid levels have a transient increase in the first stage of starvation (glucose metabolism), then return to basal levels (fatty acid metabolism) and finally increase again in the last phase (protein breakdown) [109]. Finally, because glucocorticoid excretion can be stimulated by psychological stress as well as by energetic stress, interpreting the source of elevated glucocorticoid concentrations is challenging [7]. This challenge can be addressed by jointly assessing an individual's glucocorticoid concentrations with other physiological measures of energetic stress (see below), thereby providing insight into the relative contribution of psychological and energetic sources of stress.
(ii). C-peptides
Insulin, a peptide hormone secreted by the pancreas when blood glucose levels are high, regulates glucose metabolism by (i) facilitating the usage of glucose by cells when energy is needed or (ii) promoting the storage of glucose into the liver and the adipose tissues when glucose is in excess of the demands. C-peptides are a by-product of insulin production. In contrast with insulin, c-peptides are not metabolized by the liver and are excreted in urine in concentrations reflecting plasma levels, therefore providing a non-invasive measure of insulin production when urine collection is feasible.
Because of its role in glucose homeostasis, insulin is an important mediator of allostasis. Insulin is secreted when blood glucose is in excess and therefore high insulin levels—and high c-peptide levels—indicate a positive energetic balance (i.e. energy intake exceeds energy expenditure) and are usually associated with an increase in the body's fat store. Insulin can also inhibit energy intake and increases energy expenditure (e.g. increase thermogenesis) by its action on the central nervous system [143,144]. Finally, insulin plays an important role in reproduction, and low levels of insulin have been associated with decreases in spermatogenesis and ovarian function [107,110,143,145].
Studies quantifying c-peptide concentrations in wild populations have typically focused on the correlation between c-peptide levels and various factors affecting energetic balance, including food availability (orangutans [146,147]), reproductive state (rhesus monkeys [148]; chimpanzees [110]) and dominance rank (chimpanzees [149]). Two studies have looked specifically at the effect of group size on c-peptide concentrations. In chimpanzees, c-peptide concentrations were lower in larger parties as a consequence of the energetic costs of aggression [150]. By contrast, in bonobos, aggression frequency did not predict c-peptide concentrations and large groups were associated with high c-peptide concentrations, suggesting that the formation of large group size may be driven by food availability in bonobos [151]. Understanding the effects of group size on energy balance in stable societies for which fissions–fusions are rare events is similarly important as non-optimal group size in these societies is expected to be even more costly.
(iii). Thyroid hormones
Thyroid hormones, notably triiodothyronine and its prohormone thyroxine, are secreted by the thyroid gland and are essential in metabolic regulation. In periods of negative energetic balance, thyroid hormone concentrations decrease relative to concentrations during energetically favourable times. This decrease leads to the conservation of energy through a cessation of growth, a decrease in lipid mobilization, protein degradation and reduced metabolic rate [152,153].
Because of their key role in energy intake and expenditure, thyroid hormones are considered important biomarkers of an animal's energetic balance. For example, thyroid hormone concentrations increase during periods of high energetic demand and/or elevated basal metabolic rate such as times of moulting [154], somatic growth [155], testicular development [156], mating [157] and when animals were exposed to lower temperature [157]. Additionally, several studies have examined how thyroid hormone levels vary in response to changes in energetic input. In these studies, low thyroid hormone concentrations have been found in periods of food restriction, food shortage and/or low food quality [157–161].
Measuring thyroid hormone concentrations has traditionally relied on analyses of blood and urine samples (e.g. [155,162–164]). However, collection of blood and urine samples may disrupt the animal's behaviour and/or be impractical in many wild populations. Recent developments using faecal determination of thyroid hormone levels [159] can avoid these complications by providing a novel approach to non-invasively assessing energetic stress. Faecal determination of thyroid hormone levels has been used to non-invasively assess energetic balance in several diverse species including killer whales, monk seals, caribou and northern spotted owls [154,158,160,165]. Despite this record of success, this technique has only been validated in a few primate species to date [157,159,161]. To the best of our knowledge, no studies have specifically examined how group size affects thyroid hormone levels.
With faecal determination, researchers can now also extend previous studies of stress physiology that have focused exclusively on glucocorticoids. These various triggers of glucocorticoid release (see above) have made it problematic to disentangle the source of elevated glucocorticoid concentrations, a difficulty that can now be addressed by assessing glucocorticoid and thyroid hormone concentrations simultaneously. Given that both energetic and social factors can impact fitness [166], the comparisons of a female's glucocorticoid and thyroid hormone concentrations will shed new insight into the pathways mediating inter-individual variation in health, survivorship and reproductive success.
2. Conclusion and future directions
Most primates are highly social and obligated to group living, yet primate groups can vary tremendously in size, composition and stability. Research addressing the causes and consequences of this variation date back to some of the earliest field studies in primatology, and remain a central topic of study today. In this review, we focused on the energetics of group-size variation in female primates, summarizing available literature on how group size affects the intensity and outcome of competition both within and between groups. From an energetic perspective, we highlighted several advantages to living in smaller groups (e.g. decreased within-group competition for food resources) and several advantages to living in larger groups (e.g. increased probability of winning between-group contests). In addition, we extended previous literature on this topic by discussing three physiological measures of energetic balance—glucocorticoids, c-peptides and thyroid hormones. Because these hormones can provide clear signals of metabolic states and processes, they present an important complement to field studies of spatial and temporal changes in food availability. We anticipate that their further application will play a crucial role in elucidating the adaptive significance of group size in different social and ecological contexts.
Acknowledgements
We thank Susan Alberts, Jeanne Altmann, Elizabeth Archie and Jenny Tung for permission to use data from the Amboseli baboon population. We are grateful to the government of the Republic of Kenya, to the Kenya Wildlife Services, the staff and wardens of Amboseli National Park and the local community of the Amboseli region. Our thanks also go to researchers with the Amboseli Baboon Research Project (ABRP) for their contributions to data collection and outstanding dedication in the field: R. Mututua, S. Sayialel and J. K. Warutere. Finally, we thank N. Learn and L. Roerish for their invaluable database assistance.
Ethics
All protocols adhered to the laws and guidelines of Kenya (Research Permit MOEST 13/001/C351 Vol. II) and were approved by the Princeton University Institutional Animal Care and Use Committee (IACUC 1547).
Data accessibility
Data underlying these analyses have been deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.r00n0) [167].
Authors' contributions
A.C.M. and L.R.G. participated in the design of the review and drafted the manuscript; A.C.M. analysed data and carried out statistical analyses. Both authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Financial support for the data presented from the ABRP was provided by NIA (R01AG034513-01 to Jeanne Altmann and Susan Alberts) and NSF (IBN-0322613 to Jeanne Altmann and Susan Alberts; IOS-0919200 to Susan Alberts).
References
- 1.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262–300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 2.Sterck EHM, Watts DP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 3.Snaith TV, Chapman CA. 2007. Primate group size and interpreting socioecological models: do folivores really play by different rules? Evol. Anthropol. Issues News Rev. 16, 94–106. ( 10.1002/evan.20132) [DOI] [Google Scholar]
- 4.Elgar MA. 1989. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biol. Rev. Camb. Philos. Soc. 64, 13–33. ( 10.1111/j.1469-185X.1989.tb00636.x) [DOI] [PubMed] [Google Scholar]
- 5.Brown JH, Marquet PA, Taper ML. 1993. Evolution of body size: consequences of an energetic definition of fitness. Am. Nat. 142, 573–584. ( 10.1086/285558) [DOI] [PubMed] [Google Scholar]
- 6.McAninch EA, Bianco AC. 2014. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann. NY Acad. Sci. 1311, 77–87. ( 10.1111/nyas.12374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapolsky RM. 2002. Endocrinology of the stress-response. In Behavioral endocrinology (eds Becker JB, Breedlove SM, Crews D, McCarthy MM), pp. 409–450. Cambridge, MA: MIT Press. [Google Scholar]
- 8.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine. [Google Scholar]
- 9.Janson CH. 1992. Evolutionary ecology of primate social structure. In Evolutionary ecology and human behavior (eds Smith EA, Winterhalder B), pp. 95–130. New York, NY: de Gruyter. [Google Scholar]
- 10.Van Schaik CP, Kappeler PM. 1997. Infanticide risk and the evolution of male–female association in primates. Proc. R. Soc. Lond. B 264, 1687–1694. ( 10.1098/rspb.1997.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 12.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 13.Arroyo-Rodriguez V, Fahrig L. 2014. Why is a landscape perspective important in studies of primates? Am. J. Primatol. 76, 901–909. ( 10.1002/ajp.22282) [DOI] [PubMed] [Google Scholar]
- 14.Terborgh J. 1983. Five New World primates: a study in comparative ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 15.Janson CH. 1985. Aggressive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav. Ecol. Sociobiol. 18, 125–138. ( 10.1007/BF00299041) [DOI] [Google Scholar]
- 16.Blumstein DT, Daniel JC, Evans CS. 2001. Yellow-footed rock-wallaby group size effects reflect a trade-off. Ethology 107, 655–664. ( 10.1046/j.1439-0310.2001.00699.x) [DOI] [Google Scholar]
- 17.Caraco T. 1979. Time budgeting and group-size: theory. Ecology 60, 611–617. ( 10.2307/1936081) [DOI] [Google Scholar]
- 18.Korstjens AH, Verhoeckx IL, Dunbar RIM. 2006. Time as a constraint on group size in spider monkeys. Behav. Ecol. Sociobiol. 60, 683–694. ( 10.1007/s00265-006-0212-2) [DOI] [Google Scholar]
- 19.Pollard KA, Blumstein DT. 2008. Time allocation and the evolution of group size. Anim. Behav. 76, 1683–1699. ( 10.1016/j.anbehav.2008.08.006) [DOI] [Google Scholar]
- 20.Chapman CA, Chapman LJ. 2000. Determinants of group size in primates: the importance of travel costs. In On the move: how and why animals travel in groups (eds Boinski S, Garber PA), pp. 24–42. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Majolo B, de Bortoli Vizioli A, Schino G. 2008. Costs and benefits of group living in primates: group size effects on behaviour and demography. Anim. Behav. 76, 1235–1247. ( 10.1016/j.anbehav.2008.06.008) [DOI] [Google Scholar]
- 22.Schulke O, Ostner J. 2012. Ecological and social influences on sociality. In The evolution of primate societies (eds Mitani JC, Call J, Kappeler PM, Palombit RA, Silke JB), pp. 195–219. Chicago, IL: University of Chicago Press. [Google Scholar]
- 23.Koenig A. 2000. Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus). Behav. Ecol. Sociobiol. 48, 93–109. ( 10.1007/s002650000198) [DOI] [Google Scholar]
- 24.Janson CH. 1990. Ecological consequences of individual spatial choice in foraging groups of brown capuchin monkeys, Cebus apella. Anim. Behav. 40, 922–934. ( 10.1016/S0003-3472(05)80994-7) [DOI] [Google Scholar]
- 25.Pulliam HR, Caraco T. 1984. Living in groups: is there an optimal group size? In Behavioural ecology: an evolutionary approach, 2nd edn (eds Krebs JR, Davies NB), pp. 122–147. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 26.Johnson C, Piel AK, Stewart FA, King AJ. 2015. The ecological determinants of baboon troop movements at local and continental scales. Mov. Ecol. 3, 14 ( 10.1186/s40462-015-0040-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunbar RIM. 1992. Time: a hidden constraint on the behavioural ecology of baboons. Behav. Ecol. Sociobiol. 31, 35–49. ( 10.1007/BF00167814) [DOI] [Google Scholar]
- 28.Schoener TW. 1971. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404. ( 10.1146/annurev.es.02.110171.002101) [DOI] [Google Scholar]
- 29.Parker GA. 1974. Assessment strategy and the evolution of fighting behavior. J. Theor. Biol. 47, 223–243. ( 10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 30.Fretwell SD. 1972. Populations in a seasonal environment. Monographs in population biology 5. Princeton, NJ: Princeton University. [PubMed] [Google Scholar]
- 31.Cheney DL. 1987. Interactions and relationships between groups. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT), pp. 267–281. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 32.Marshall AJ. 2010. Effect of habitat quality on primate populations in Kalimantan: gibbons and leaf monkeys as case Studies. In Indonesian primates (eds Gursky S, Supriatna J), pp. 157–177. New York, NY: Springer. [Google Scholar]
- 33.Emery Thompson M, Kahlenberg SM, Gilby IC, Wrangham RW. 2007. Core area quality is associated with variance in reproductive success among female chimpanzees at Kibale National Park. Anim. Behav. 73, 501–512. ( 10.1016/j.anbehav.2006.09.007) [DOI] [Google Scholar]
- 34.Crofoot MC, Gilby IC, Wikelski MC, Kays RW. 2008. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA 105, 577–581. ( 10.1073/pnas.0707749105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris TR. 2006. Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav. Ecol. Sociobiol. 61, 317–329. ( 10.1007/s00265-006-0261-6) [DOI] [Google Scholar]
- 36.Willems EP, Hellriegel B, van Schaik CP. 2013. The collective action problem in primate territory economics. Proc. R. Soc. B 280, 20130081 ( 10.1098/rspb.2013.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberg JF, Muckenhirn NA, Rudran R. 1972. The relation between ecology and social structure in primates. Science 176, 863–874. ( 10.1126/science.176.4037.863) [DOI] [PubMed] [Google Scholar]
- 38.Clutton-Brock TH. 1974. Primate social organisation and ecology. Nature 250, 539–542. ( 10.1038/250539a0) [DOI] [Google Scholar]
- 39.King B. 1991. Social information transfer in monkeys, apes, and hominids. Yearb. Phys. Anthropol. 34, 97–115. ( 10.1002/ajpa.1330340607) [DOI] [Google Scholar]
- 40.Rodman PA. 1988. Resources and group sizes in primates. In The ecology of social behavior (ed. Slobodchikoff), pp. 83–108. New York, NY: Academic Press. [Google Scholar]
- 41.Hauser MD, Marler P. 1993. Food-associated calls in rhesus macaques (Macaca mulatta): I. Socioecological factors. Behav. Ecol. 4, 194–205. ( 10.1093/beheco/4.3.194) [DOI] [Google Scholar]
- 42.Elowson AM, Tannenbaum PL, Snowdon CT. 1991. Food associated calls correlate with food preferences in cottontop tamarins. Anim. Behav. 42, 931–937. ( 10.1016/S0003-3472(05)80145-9) [DOI] [Google Scholar]
- 43.Wrangham R. 1977. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In Primate ecology (ed. Clutton-Brock T.), pp. 503–538. London, UK: Academic Press. [Google Scholar]
- 44.Wrangham R, Smuts B. 1980. Sex differences in the behavioral ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. 28, 13–31. [PubMed] [Google Scholar]
- 45.Hauser MD, Wrangham RW. 1987. Manipulation of food calls in captive chimpanzees. A preliminary report. Folia Primatol. 48, 207–210. ( 10.1159/000156298) [DOI] [PubMed] [Google Scholar]
- 46.Hauser MD, Teixidor P. 1993. Food-elicited calls in chimpanzees: effects of food quantity and divisibility. Anim. Behav. 45, 817–819. ( 10.1006/anbe.1993.1096) [DOI] [Google Scholar]
- 47.Chapman CA, Lefebvre L. 1990. Manipulating foraging group size: spider monkey food calls at fruiting trees. Anim. Behav. 39, 891–896. ( 10.1016/S0003-3472(05)80953-4) [DOI] [Google Scholar]
- 48.Clutton-Brock TH, Albon SD, Guinness FE. 1988. Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262. ( 10.1038/337260a0) [DOI] [PubMed] [Google Scholar]
- 49.Ellis L. 1995. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257–333. ( 10.1016/0162-3095(95)00050-U) [DOI] [Google Scholar]
- 50.Deag J. 1977. Aggression and submission in monkey societies. Anim. Behav. 25, 465–474. ( 10.1016/0003-3472(77)90021-5) [DOI] [Google Scholar]
- 51.Benkman CW. 1997. Feeding behavior, flock-size dynamics, and variation in sexual selection in crossbills. Auk 114, 163–178. ( 10.2307/4089158) [DOI] [Google Scholar]
- 52.Hino T. 2000. Intraspecific differences in benefits from feeding in mixed-species flocks. J. Avian Biol. 31, 441–446. ( 10.1034/j.1600-048X.2000.310402.x) [DOI] [Google Scholar]
- 53.Koenig A. 2002. Competition for resources and its behavioral consequences among female primates. Int. J. Primatol. 23, 759–783. ( 10.1023/A:1015524931226) [DOI] [Google Scholar]
- 54.Saito C. 1996. Dominance and feeding success in female Japanese macaques, Macaca fuscata: effects of food patch size and interpatch distance. Anim. Behav. 51, 967–980. ( 10.1006/anbe.1996.0100) [DOI] [Google Scholar]
- 55.Janson CH, van Schaik CP. 1988. Recognizing the many faces of primate food competition: methods. Behaviour 105, 165–186. ( 10.1163/156853988X00502) [DOI] [Google Scholar]
- 56.Janson CH. 1988. Food competition in brown capuchin monkeys (Cebus apella): quantitative effects of group size and tree productivity. Behaviour 105, 53–76. ( 10.1163/156853988X00449) [DOI] [Google Scholar]
- 57.Brown J. 1982. Optimal group size in territorial animals. J. Theor. Biol. 95, 793–810. ( 10.1016/0022-5193(82)90354-X) [DOI] [Google Scholar]
- 58.Cohen JE. 1971. Casual groups of monkeys and men: stochastic models of elemental social systems. London, UK: Oxford University Press. [Google Scholar]
- 59.Brown CR, Brown MB, Roche EA, O'Brien VA, Page CE. 2016. Fluctuating survival selection explains variation in avian group size. Proc. Natl Acad. Sci. USA 113, 5113–5118. ( 10.1073/pnas.1600218113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aureli F, et al. 2008. Fission–fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. [Google Scholar]
- 61.Sibly R. 1983. Optimal group size is unstable. Anim. Behav. 31, 947–948. ( 10.1016/S0003-3472(83)80250-4) [DOI] [Google Scholar]
- 62.Alberts SC, Altmann J. 2006. The evolutionary past and the research future: environmental variation and life history flexibility in a primate lineage. In Reproduction and fitness in baboons (eds Swedell L, Leigh SR), pp. 277–303. New York, NY: Springer. [Google Scholar]
- 63.Chapman CA, Pavelka MSM. 2005. Group size in folivorous primates: ecological constraints and the possible influence of social factors. Primates 46, 1–9. ( 10.1007/s10329-004-0093-9) [DOI] [PubMed] [Google Scholar]
- 64.Van Schaik CP, Noordwijk MA. 1986. The hidden costs of sociality: intragroup variation in feeding strategies in Sumatram long-tailed macaques (Macaca fascicularis). Behaviour 99, 296–315. ( 10.1163/156853986X00595) [DOI] [Google Scholar]
- 65.Van Schaik CP, Van Hooff J. 1983. On the ultimate causes of primate social systems. Behaviour 85, 91–117. ( 10.1163/156853983X00057) [DOI] [Google Scholar]
- 66.Lehmann J, Korstjens AH, Dunbar RIM. 2007. Fission–fusion social systems as a strategy for coping with ecological constraints: a primate case. Evol. Ecol. 21, 613–634. ( 10.1007/s10682-006-9141-9) [DOI] [Google Scholar]
- 67.Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. 2002. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Anim. Behav. 63, 347–360. ( 10.1006/anbe.2001.1916) [DOI] [Google Scholar]
- 68.Lehmann J, Boesch C. 2004. To fission or to fusion: effects of community size on wild chimpanzee (Pan troglodytes verus) social organisation. Behav. Ecol. Sociobiol. 56, 207–216. ( 10.1007/s00265-004-0781-x) [DOI] [Google Scholar]
- 69.Symington MM. 1988. Food competition and foraging party size in the black spider monkey (Ateles paniscus chamek). Behaviour 105, 117–134. ( 10.1163/156853988X00476) [DOI] [Google Scholar]
- 70.Chapman CA, Wrangham RW, Chapman LJ. 1995. Ecological constraints on group-size—an analysis of spider monkey and chimpanzee subgroups. Behav. Ecol. Sociobiol. 36, 59–70. ( 10.1007/BF00175729) [DOI] [Google Scholar]
- 71.White FJ, Wrangham RW. 1988. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105, 148–164. ( 10.1163/156853988X00494) [DOI] [Google Scholar]
- 72.Chapman CA. 1990. Ecological constraints on group size in three species of neotropical primates. Folia Primatol 55, 1–9. ( 10.1159/000156492) [DOI] [PubMed] [Google Scholar]
- 73.Williams JM, Lio H-Y, Pusey A. 2002. Costs and benefits of grouping for female chimpanzees at Gombe. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant LF), pp. 192–203. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 74.Henzi SP, Lycett JE, Piper SE. 1997. Fission and troop size in a mountain baboon population. Anim. Behav. 53, 525–535. ( 10.1006/anbe.1996.0302) [DOI] [Google Scholar]
- 75.Altmann J, Alberts SC. 2003. Intraspecific variability in fertility and offspring survival in a nonhuman primate: behavioral control of ecological and social sources. In Offspring: human fertility behavior in biodemographic perspective (eds Wachter KW, Bulatao RA), pp. 140–169. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 76.Van Horn RC, Buchan JC, Altmann J, Alberts SC. 2007. Divided destinies: group choice by female savannah baboons during social group fission. Behav. Ecol. Sociobiol. 61, 1823–1837. ( 10.1007/s00265-007-0415-1) [DOI] [Google Scholar]
- 77.Anderson DP, Nordheim EV, Boesch C, Moermond TC. 2002. Factors influencing fission–fusion grouping in chimpanzees in the Tai National Park, Cote d'Ivoire. In Behavioural diversity in chimpanzees and bonobos (eds Boesch C, Hohmann G, Marchant LF), pp. 90–101. Cambridge, UK: Cambirdge University Press. [Google Scholar]
- 78.Markham AC, Gesquiere LR, Alberts SC, Altmann J. 2015. Optimal group size in a highly social mammal. Proc. Natl Acad. Sci. USA 112, 14 882–14 887. ( 10.1073/pnas.1517794112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sueur C, et al. 2011. Collective decision-making and fission–fusion dynamics: a conceptual framework. Oikos 120, 1608–1617. ( 10.1111/j.1600-0706.2011.19685.x) [DOI] [Google Scholar]
- 80.Harestad AS, Bunnel FL. 1979. Home range and body weight—a reevaluation. Ecology 60, 389–402. ( 10.2307/1937667) [DOI] [Google Scholar]
- 81.Jetz W, Carbone C, Fulford J, Brown JH. 2004. The scaling of animal space use. Science 306, 266–268. ( 10.1126/science.1102138) [DOI] [PubMed] [Google Scholar]
- 82.Bokma F. 2004. Evidence against a universal metabolic allometry. Funct. Ecol. 18, 184–187. ( 10.1111/j.0269-8463.2004.00817.x) [DOI] [Google Scholar]
- 83.Westerterp KR. 1999. Body composition, water turnover and energy turnover assessment with labelled water. Proc. Nutr. Soc. 58, 945–951. ( 10.1017/S0029665199001251) [DOI] [PubMed] [Google Scholar]
- 84.Rosetta L, Lee PC, Garcia C. 2011. Energetics during reproduction: a doubly labeled water study of lactating baboons. Am. J. Phys. Anthropol. 144, 661–668. ( 10.1002/ajpa.21475) [DOI] [PubMed] [Google Scholar]
- 85.Nagy KA, Milton K. 1976. Energy metabolism and food consumption by wild howler monkeys (Alouatta palliata). Ecology 60, 475–480. ( 10.2307/1936066) [DOI] [Google Scholar]
- 86.Pontzer H, Raichlen DA, Shumaker RW, Ocobock C, Wich SA. 2013. Metabolic adaptation for low energy throughput in orangutans. Proc. Natl Acad. Sci. USA 107, 14 048–14 052. ( 10.1073/pnas.1001031107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. 2002. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J. Clin. Endocrinol. Metab. 88, 16–23. ( 10.1210/jc.2002-020405) [DOI] [PubMed] [Google Scholar]
- 88.Gittleman JL, Harvey PH. 1982. Carnivore home-range size, metabolic needs and ecology. Behav. Ecol. Sociobiol. 10, 57–63. ( 10.1007/BF00296396) [DOI] [Google Scholar]
- 89.Oates JF, Whitesides GH, Davies AG, Waterman PG. 1990. Determinants of variation in tropical forest primate biomass: new evidence from West Africa. Ecology 71, 328–343. ( 10.2307/1940272) [DOI] [Google Scholar]
- 90.Nunn CL, Barton RA. 2000. Allometric slopes and independent contrasts: a comparative test of Kleiber's Law in primate ranging patterns. Am. Nat. 156, 519–533. [DOI] [PubMed] [Google Scholar]
- 91.Milton K, May ML. 1976. Body weight, diet and home range area in primates. Nature 259, 459–462. ( 10.1038/259459a0) [DOI] [PubMed] [Google Scholar]
- 92.Carbone C, Cowlishaw G, Isaac NJB, Rowcliffe JM. 2005. How far do animals go? Determinants of day range in mammals. Am. Nat. 165, 290–297. ( 10.1086/426790) [DOI] [PubMed] [Google Scholar]
- 93.Altmann J, Alberts SC. 2005. Growth rates in a wild primate population: ecological influences and maternal effects. Behav. Ecol. Sociobiol. 57, 490–511. ( 10.1007/s00265-004-0870-x) [DOI] [Google Scholar]
- 94.Altmann SA. 1998. Foraging for survival: yearling baboons in Africa. Chicago, IL: University of Chicago Press. [Google Scholar]
- 95.Altmann J. 1980. Baboon mothers and infants. Chicago, IL: University of Chicago Press. [Google Scholar]
- 96.Kleiber M. 1947. Body size and metabolic rate. Physiol. Rev. 27, 511–541. [DOI] [PubMed] [Google Scholar]
- 97.Harvey P, Pagel M, Rees JA. 1991. Mammalian metabolism and life histories. Am. Nat. 137, 556–566. ( 10.1086/285183) [DOI] [Google Scholar]
- 98.Kleiber M. 1961. The fire of life: an introduction to animal energetics. New York, NY: Wiley. [Google Scholar]
- 99.Dodds PS, Rothman DH, Weitz JS. 2001. Re-examination of the ‘3/4-law’ of metabolism. J. Theor. Biol. 209, 9–27. ( 10.1006/jtbi.2000.2238) [DOI] [PubMed] [Google Scholar]
- 100.Feldman HA. 1995. On the allometric mass exponent, when it exists. J. Theor. Biol. 172, 187–197. ( 10.1006/jtbi.1995.0015) [DOI] [PubMed] [Google Scholar]
- 101.Strandburg-Peshkin A, Farine DF, Couzin ID, Crofoot MC. 2015. Shared decision-making drives collective movement in wild baboons. Science 348, 1358–1361. ( 10.1126/science.aaa5099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lazaro-Perea C. 2001. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21. ( 10.1006/anbe.2000.1726) [DOI] [Google Scholar]
- 103.Koch F, Signer J, Kappeler PM, Fichtel C. 2016. Intergroup encounters in Verreaux's sifakas (Propithecus verreauxi): who fights and why? Behav. Ecol. Sociobiol. 70, 797–808. ( 10.1007/s00265-016-2105-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crofoot MC. 2007. Mating and feeding competition in white-faced capuchins (Cebus capucinus): the importance of short- and long-term strategies. Behaviour 144, 1473–1495. ( 10.1163/156853907782512119) [DOI] [Google Scholar]
- 105.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. ( 10.1016/S0018-506X(02)00024-7) [DOI] [PubMed] [Google Scholar]
- 106.Wingfield JC, Moore MC, Farner DS. 1983. Endocrine responses to inclement weather in naturally breeding popluations of white-crowned sparrows (Zonotrichia leucophrys pugetensis). Auk 100, 56–62. [Google Scholar]
- 107.Valeggia C, Ellison PT. 2009. Interactions between metabolic and reproductive functions in the resumption of postpartum fecundity. Am. J. Hum. Biol. 21, 559–566. ( 10.1002/ajhb.20907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McEwen BS. 1998. Protective and damaging effects of stress mediators. N. Engl. J. Med. 338, 171–179. ( 10.1056/NEJM199801153380307) [DOI] [PubMed] [Google Scholar]
- 109.Romero LM, Dickens MJ, Cyr NE. 2009. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389. ( 10.1016/j.yhbeh.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 110.Thompson ME, Muller MN, Wrangham RW. 2012. The energetics of lactation and the return to fecundity in wild chimpanzees. Behav. Ecol. 23, 1234–1241. ( 10.1093/beheco/ars107) [DOI] [Google Scholar]
- 111.Goymann W, Wingfield JC. 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. ( 10.1016/j.anbehav.2003.08.007) [DOI] [Google Scholar]
- 112.Clutton-Brock TH. 1988. Reproductive success. In Reproductive success: studies if individual variation in contrasting breeding systems (ed. Clutton-Brock TH.), pp. 472–476. Chicago, IL: University of Chicago Press. [Google Scholar]
- 113.Crossin GT, Trathan PN, Phillips RA, Gorman KA, Dawson A, Sakamoto KQ, Williams TD. 2012. Corticosterone predicts foraging behavior and parental care in macaroni penguins. Am. Nat. 180, E31–E41. ( 10.1086/666001) [DOI] [PubMed] [Google Scholar]
- 114.Ouyang JQ, Muturi M, Quetting M, Hau M. 2013. Small increases in corticosterone before the breeding season increase parental investment but not fitness in a wild passerine bird. Horm. Behav. 63, 776–781. ( 10.1016/j.yhbeh.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 115.Astheimer LB, Buttemer WA, Wingfield JC. 1992. Interactions of corticosterone with feeding, activity and metabolism in passerine birds. Ornis Scand. 23, 355–365. ( 10.2307/3676661) [DOI] [Google Scholar]
- 116.Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. 2001. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B: Biochem. Syst. Environ. Physiol. 171, 701–709. ( 10.1007/s003600100230) [DOI] [PubMed] [Google Scholar]
- 117.Wahab F, Aziz F, Irfan S, Zaman WU, Shahab M. 2008. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta). Life Sci. 83, 633–637. ( 10.1016/j.lfs.2008.09.001) [DOI] [PubMed] [Google Scholar]
- 118.Romero LM. 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocr. 128, 1–24. ( 10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- 119.Abbott DH, et al. 2003. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 43, 67–82. ( 10.1016/S0018-506X(02)00037-5) [DOI] [PubMed] [Google Scholar]
- 120.Creel S. 2001. Social dominance and stress hormones. Trends Ecol. Evol. 16, 491–497. ( 10.1016/S0169-5347(01)02227-3) [DOI] [Google Scholar]
- 121.Dhabhar FS. 2014. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 58, 193–210. ( 10.1007/s12026-014-8517-0) [DOI] [PubMed] [Google Scholar]
- 122.Romero LM, Wikelski M. 2001. Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370. ( 10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648–652. ( 10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 124.Higham JP, Vitale AB, Rivera AM, Ayala JE, Maestripieri D. 2010. Measuring salivary analytes from free-ranging monkeys. Physiol. Behav. 101, 601–607. ( 10.1016/j.physbeh.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meyer JS, Novak MA. 2012. Minireview: Hair cortisol: a novel biomarker of hypothalamic–pituitary–adrenocortical activity. Endocrinology 153, 4120–4127. ( 10.1210/en.2012-1226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muller MN, Wrangham RW. 2004. Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 55, 332–340. ( 10.1007/s00265-003-0713-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. 2000. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen. Comp. Endocr. 120, 260–275. ( 10.1006/gcen.2000.7557) [DOI] [PubMed] [Google Scholar]
- 128.Whitten PL, Brockman DK, Stavisky RC. 1998. Recent advances in noninvasive techniques to monitor hormone–behavior interactions. Yearb. Phys. Anthropol. 41, 1–23. ( 10.1002/(SICI)1096-8644(1998)107:27%2B%3C1::AID-AJPA2%3E3.0.CO;2-H) [DOI] [PubMed] [Google Scholar]
- 129.Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS. 2013. Stress, the HPA axis, and nonhuman primate well-being: a review. Appl. Anim. Behav. Sci. 143, 135–149. ( 10.1016/j.applanim.2012.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. 2011. Life at the top: rank and stress in wild male baboons. Science 333, 357–360. ( 10.1126/science.1207120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boonstra R, Boag PT. 1992. Spring declines in Microtus pennsylvanicus and the role of steroid hormones. J. Anim. Ecol. 61, 339–352. ( 10.2307/5326) [DOI] [Google Scholar]
- 132.Foley CAH, Papageorge S, Wasser SK. 2001. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 15, 1134–1142. ( 10.1046/j.1523-1739.2001.0150041134.x) [DOI] [Google Scholar]
- 133.Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR. 2006. Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim. Behav. 71, 39–48. ( 10.1016/j.anbehav.2005.03.027) [DOI] [Google Scholar]
- 134.Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217. ( 10.1126/science.1235765) [DOI] [PubMed] [Google Scholar]
- 135.Dettmer AM, Novak MA, Meyer JS, Suomi SJ. 2014. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42, 59–67. ( 10.1016/j.psyneuen.2014.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Viblanc VA, Gineste B, Stier A, Robin JP, Groscolas R. 2014. Stress hormones in relation to breeding status and territory location in colonial king penguin: a role for social density? Oecologia 175, 763–772. ( 10.1007/s00442-014-2942-6) [DOI] [PubMed] [Google Scholar]
- 137.Creel S, Dantzer B, Goymann W, Rubenstein DR. 2013. The ecology of stress: effects of the social environment. Funct. Ecol. 27, 66–80. ( 10.1111/j.1365-2435.2012.02029.x) [DOI] [Google Scholar]
- 138.Michelena P, Pillot MH, Henrion C, Toulet S, Boissy A, Bon R. 2012. Group size elicits specific physiological response in herbivores. Biol. Lett. 8, 537–539. ( 10.1098/rsbl.2012.0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blondel DV, Wallace GN, Calderone S, Gorinshteyn M, St Mary CM, Phelps SM. 2016. Effects of population density on corticosterone levels of prairie voles in the field. Gen. Comp. Endocr. 225, 13–22. ( 10.1016/j.ygcen.2015.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pride RE. 2005. Optimal group size and seasonal stress in ring-tailed lemurs (Lemur catta). Behav. Ecol. 16, 550–560. ( 10.1093/beheco/ari025) [DOI] [Google Scholar]
- 141.Snaith TV, Chapman CA, Rothman JM, Wasserman MD. 2008. Bigger groups have fewer parasites and similar cortisol levels: a multi-group analysis in red colobus monkeys. Am. J. Primatol. 70, 1072–1080. ( 10.1002/ajp.20601) [DOI] [PubMed] [Google Scholar]
- 142.Ebensperger LA, et al. 2011. Sociality, glucocorticoids and direct fitness in the communally rearing rodent, Octodon degus. Horm. Behav. 60, 346–352. ( 10.1016/j.yhbeh.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 143.Porte D Jr, Baskin DG, Schwartz MW. 2005. Insulin signaling in the central nervous system: a critical role in metabolic homestasis and disease from C. elegans to humans. Diabetes 54, 1264–1276. ( 10.2337/diabetes.54.5.1264) [DOI] [PubMed] [Google Scholar]
- 144.Benedict C, et al. 2011. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60, 114–118. ( 10.2337/db10-0329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sliwowska JH, Fergani C, Gawalek M, Skowronska B, Fichna P, Lehman MN. 2014. Insulin: its role in the central control of reproduction. Physiol. Behav. 133, 197–206. ( 10.1016/j.physbeh.2014.05.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thompson ME, Knott CD. 2008. Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Horm. Behav. 53, 526–535. ( 10.1016/j.yhbeh.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 147.Deschner T, Kratzsch J, Hohmann G. 2008. Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Horm. Behav. 54, 620–626. ( 10.1016/j.yhbeh.2008.06.005) [DOI] [PubMed] [Google Scholar]
- 148.Higham JP, Heistermann M, Maestripieri D. 2011. The energetics of male–male endurance rivalry in free-ranging rhesus macaques, Macaca mulatta. Anim. Behav. 81, 1001–1007. ( 10.1016/j.anbehav.2011.02.001) [DOI] [Google Scholar]
- 149.Thompson ME, Muller MN, Wrangham RW, Lwanga JS, Potts KB. 2009. Urinary C-peptide tracks seasonal and individual variation in energy balance in wild chimpanzees. Horm. Behav. 55, 299–305. ( 10.1016/j.yhbeh.2008.11.005) [DOI] [PubMed] [Google Scholar]
- 150.Georgiev AV. 2012. Energetic costs of reproductive effort in male chimpanzees. Cambridge, MA: Harvard University. [Google Scholar]
- 151.Surbeck M, Deschner T, Behringer V, Hohmann G. 2015. Urinary C-peptide levels in male bonobos (Pan paniscus) are related to party size and rank but not to mate competition. Horm. Behav. 71, 22–30. ( 10.1016/j.yhbeh.2015.03.007) [DOI] [PubMed] [Google Scholar]
- 152.Flier JS, Harris M, Hollenberg AN. 2000. Leptin, nurtition, and the thyroid: the why, the wherefore, and the wiring. J. Clin. Invest. 105, 859–861. ( 10.1172/JCI9725) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hornick JL, Van Eenaeme C, Gerard O, Defrasne I, Istasse L. 2000. Mechanisms of reduced and compensatory growth. Domest. Anim. Endocrinol. 19, 121–132. ( 10.1016/S0739-7240(00)00072-2) [DOI] [PubMed] [Google Scholar]
- 154.Gobush KS, Booth RK, Wasser SK. 2014. Validation and application of noninvasive glucocortocoid and thyroid hormone measures in free-ranging Hawaiian monk seals. Gen. Comp. Endocr. 195, 174–182. ( 10.1016/j.ygcen.2013.10.020) [DOI] [PubMed] [Google Scholar]
- 155.Behringer V, Deschner T, Murtagh R, Stevens JMG, Hohmann G. 2014. Age-related changes in thyroid hormone levels of bonobos and chimpanzees indicate heterochrony in development. J. Hum. Evol. 66, 83–88. ( 10.1016/j.jhevol.2013.09.008) [DOI] [PubMed] [Google Scholar]
- 156.Wagner MS, Wajner SM, Maia AL. 2008. The role of thyroid hormone in testicular development and function. J. Endocrinol. 199, 351–365. ( 10.1677/JOE-08-0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cristobal-Azkarate J, Marechal L, Semple S, Majolo B, Ann M. 2016. Metabolic strategies in wild Barbary macaques: evidence from faecal measurement of thyroid hormone. Biol. Lett. 12, 20160168 ( 10.1098/rsbl.2016.0168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ayres KL, et al. 2012. Distinguishing the impacts of inadequate prey and vessel traffic on an endangered killer whale (Orcinus orca) population. PLoS ONE 7, e36842 ( 10.1371/journal.pone.0036842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wasser SK, et al. 2010. Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species. Gen. Comp. Endocr. 168, 1–7. ( 10.1016/j.ygcen.2010.04.004) [DOI] [PubMed] [Google Scholar]
- 160.Joly K, Wasser SK, Booth R. 2015. Non-invasive assessment of the interrelationships of diet, pregnancy rate, group composition, and physiological and nutritional stress of barren-ground caribou in late winter. PLoS ONE 10, e0127586 ( 10.1371/journal.pone.0127586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schaebs FS, Wolf TE, Behringer V, Deschner T. 2015. Monitoring energy balance in yellow breasted capuchins (Sapajus xanthosternos) by measuring total T3 in non-invasively collected faecal samples. Folia Primatol 86, 353–354. [Google Scholar]
- 162.Genin F, Perret M. 2000. Photoperiod-induced changes in energy balance in gray mouse lemurs. Physiol. Behav. 71, 315–321. ( 10.1016/S0031-9384(00)00335-8) [DOI] [PubMed] [Google Scholar]
- 163.Oritz RM, Long B, Casper D, Ortiz CL, Williams TM. 2010. Biochemical and hormonal changes during acute fasting and re-feeding in bottlenose dolphins (Tursiops truncatus). Mar. Mammal Sci. 26, 409–419. ( 10.1111/j.1748-7692.2009.00309.x) [DOI] [Google Scholar]
- 164.Wingfield JC, Hahn TP, Maney DL, Schoech SJ, Wada M, Mortion ML. 2003. Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows Zonotrichia leucophrys oriantha. Gen. Comp. Endocr. 131, 143–158. ( 10.1016/S0016-6480(02)00648-2) [DOI] [PubMed] [Google Scholar]
- 165.Hayward LS, Bowles AE, Ha JC, Wasser SK. 2011. Impacts of acute and long-term vehicle exposure on physiology and reproductive success of the northern spotted owl. Ecosphere 2, 1–20. ( 10.1890/ES10-00199.1) [DOI] [Google Scholar]
- 166.Dunbar RIM. 1988. Primate social systems. Ithaca, NY: Cornell University Press. [Google Scholar]
- 167.Markham AC, Gesquiere LR. 2017. Data from: Costs and benefits of group living in primates: an energetic perspective. Dryad Digital Repository. ( 10.5061/dryad.r00n0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Markham AC, Gesquiere LR. 2017. Data from: Costs and benefits of group living in primates: an energetic perspective. Dryad Digital Repository. ( 10.5061/dryad.r00n0) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data underlying these analyses have been deposited in the Dryad repository (http://dx.doi.org/10.5061/dryad.r00n0) [167].