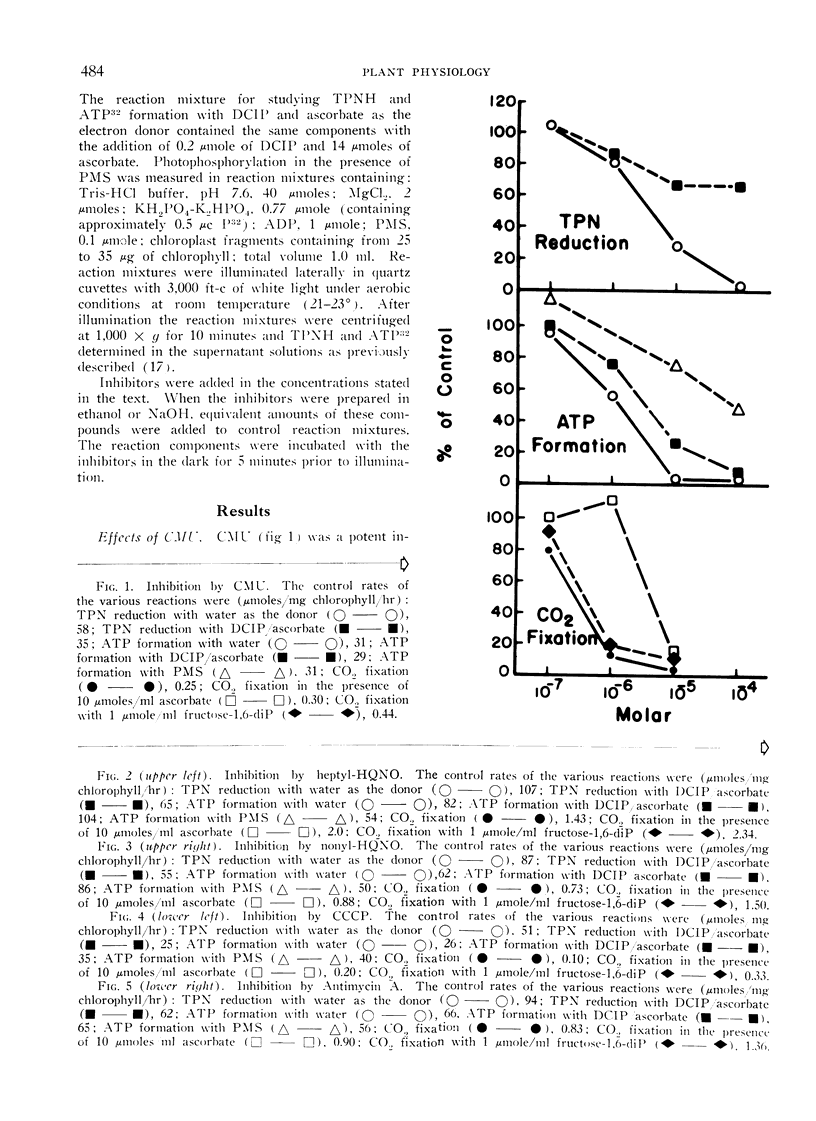
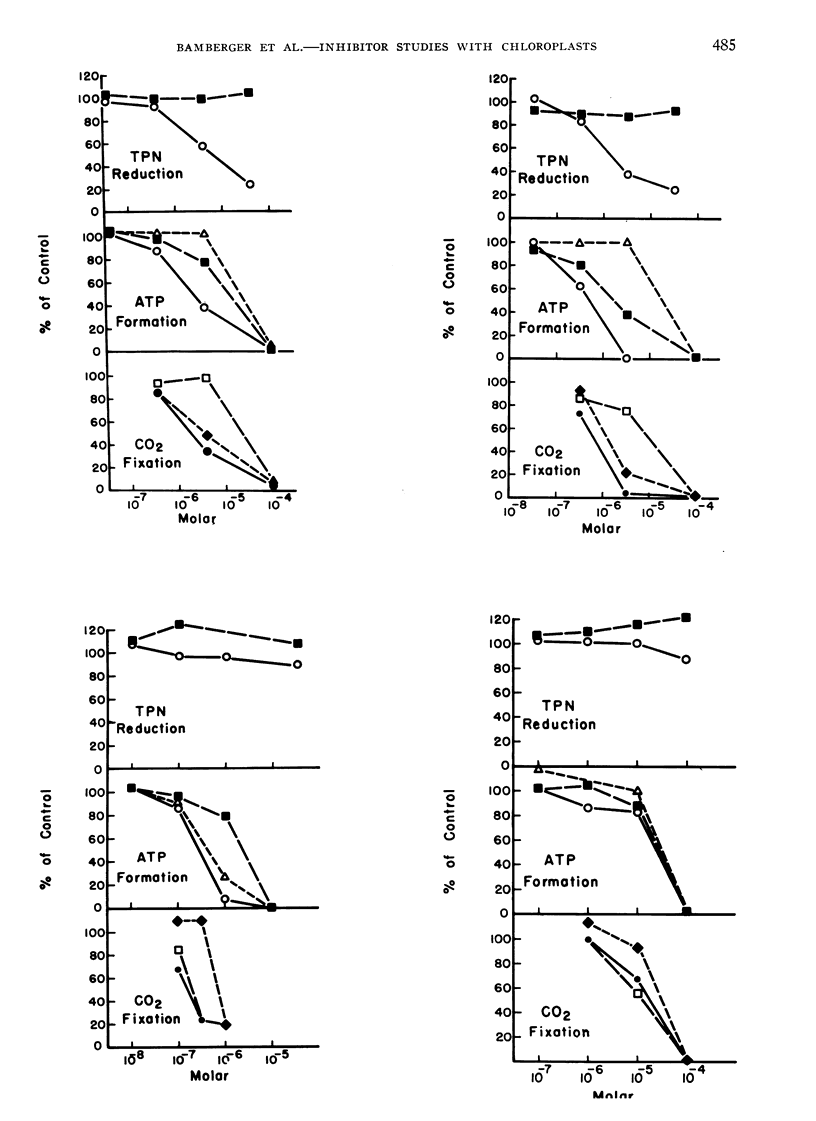
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., ALLEN M. B., WHATLEY F. R. Photosynthesis by isolated chloroplasts. IV. General concept and comparison of three photochemical reactions. Biochim Biophys Acta. 1956 Jun;20(3):449–461. doi: 10.1016/0006-3002(56)90339-0. [DOI] [PubMed] [Google Scholar]
- AVRON M. The inhibition of photoreactions of chloroplasts by 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J. 1961 Apr;78:735–739. doi: 10.1042/bj0780735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNFORTH J. W., JAMES A. T. Structure of a naturally occurring antagonist of dihydrostreptomycin. Biochem J. 1956 May;63(1):124–130. doi: 10.1042/bj0630124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARLEY B. G. Some results of computer simulation of neuron-like nets. Fed Proc. 1962 Jan-Feb;21:92–96. [PubMed] [Google Scholar]
- GELLER D. M. Oxidative phosphorylation in extracts of Rhodospirillum rubrum. J Biol Chem. 1962 Sep;237:2947–2954. [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- JAGENDORF A. T., MARGULIES M. Inhibition of spinach chloroplast photosynthetic reactions by p-chlorophenyll, 1-dimethylurea. Arch Biochem Biophys. 1960 Oct;90:184–195. doi: 10.1016/0003-9861(60)90566-x. [DOI] [PubMed] [Google Scholar]
- KEISTER D. L., SAN PIETRO A., STOLZENBACH F. E. Photo-synthetic pyridine nucleotide reductase. III. Effect of phosphate acceptor system on triphosphopyridine nucleotide reduction. Arch Biochem Biophys. 1961 Aug;94:187–195. doi: 10.1016/0003-9861(61)90029-7. [DOI] [PubMed] [Google Scholar]
- KOGUT M., LIGHTBOWN J. W. Selective inhibition by 2-heptyl-4-hydroxyquinoline N-oxide of certain oxidation-reduction reactions. Biochem J. 1962 Aug;84:368–382. doi: 10.1042/bj0840368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOSADA M., WHATLEY F. R., ARNON D. I. Separation of two light reactions in noncyclic photo-phosphorylation of green plants. Nature. 1961 May 13;190:606–610. doi: 10.1038/190606a0. [DOI] [PubMed] [Google Scholar]
- REIF A. E., POTTER V. R. Oxidative pathways insensitive to antimycin A. Arch Biochem Biophys. 1954 Jan;48(1):1–6. doi: 10.1016/0003-9861(54)90298-2. [DOI] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]
- VERNON L. P., ZAUGG W. S. Photoreductions by fresh and aged chloropasts: requirement for ascorbate and 2, 6-dichlorophenolindophenol with aged chloroplasts. J Biol Chem. 1960 Sep;235:2728–2733. [PubMed] [Google Scholar]


