Abstract
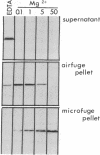
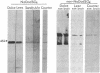
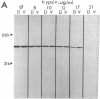
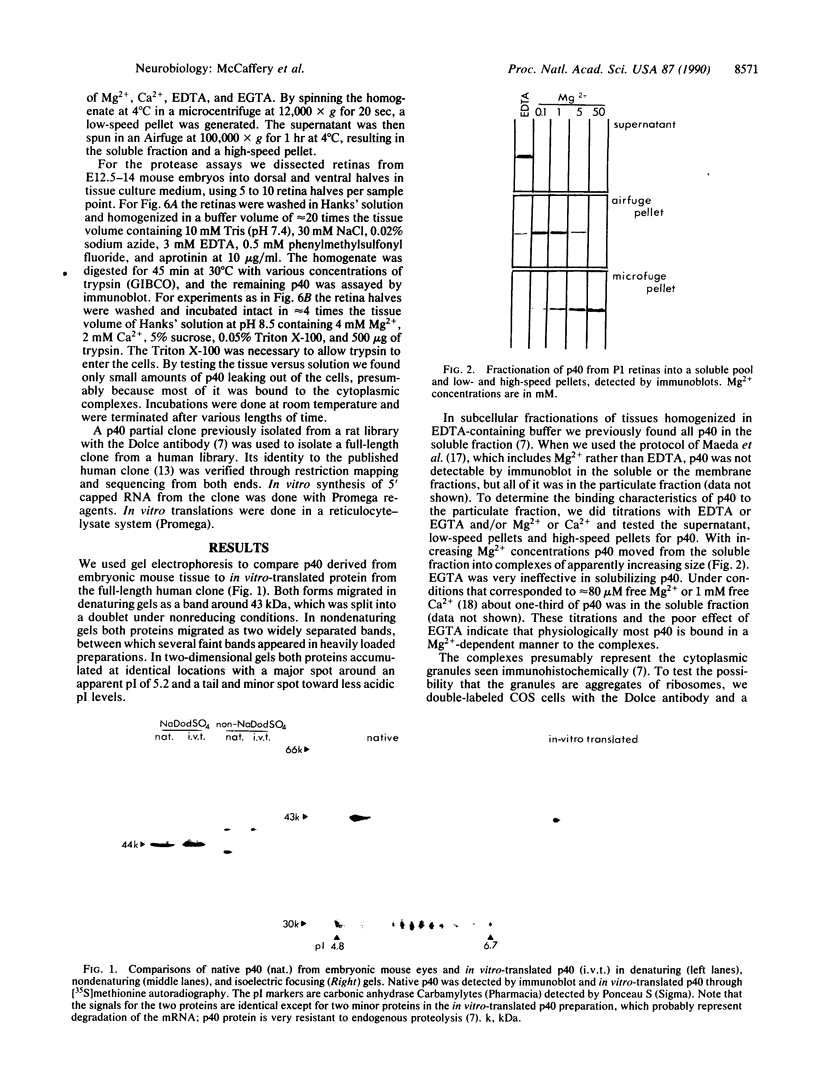
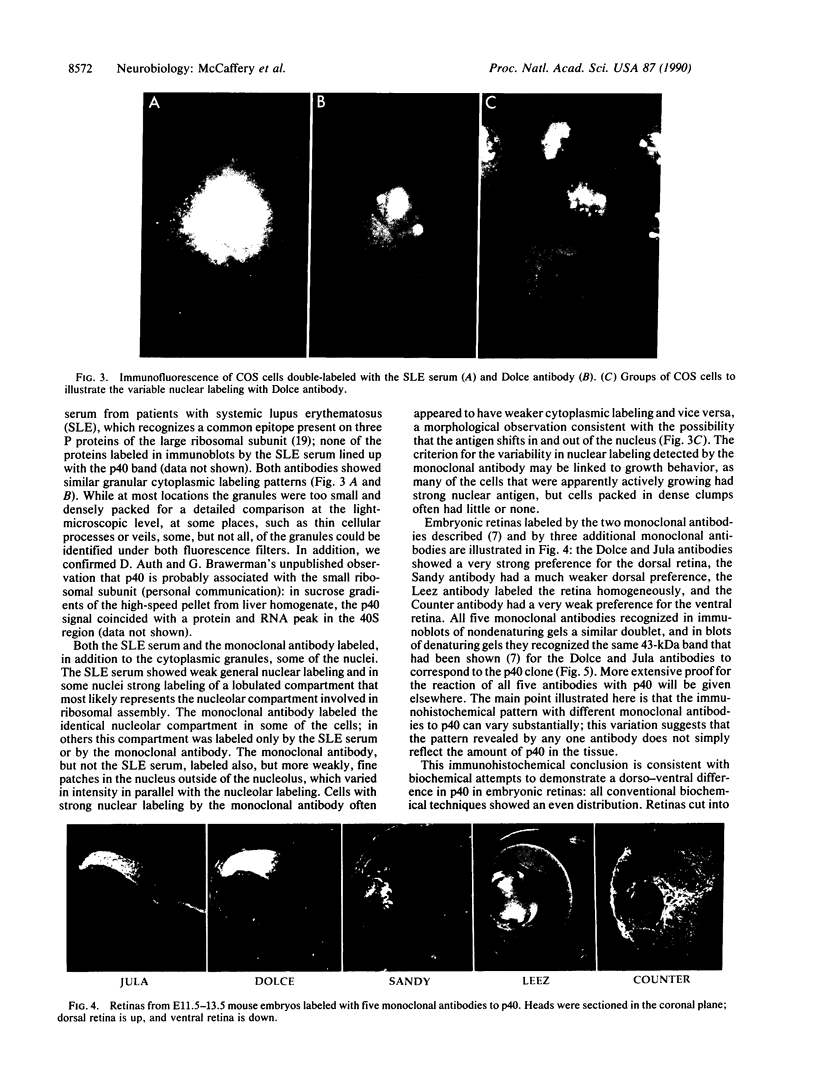
In a search for determinants of retinotopic specification we previously identified an antigen in the dorsal embryonic retina as a protein called the 68-kDa laminin receptor. A dorso-ventral asymmetry in a laminin receptor seemed consistent with the known responsiveness of embryonic optic axons to laminin, but there were three peculiar points. (i) The molecular mass of this presumed laminin receptor in immunoblots is not 68 kDa but 43 kDa, and the molecular mass of the protein deduced from the mRNA is only 33 kDa. (ii) The antigen does not have the localization expected of a receptor for the extracellular matrix: the antibodies label mainly a granular cytoplasmic antigen in dorsal retina; an additional sparse cell-surface antigen present on a few cells does not show a dorso-ventral asymmetry. (iii) Despite the pronounced dorso-ventral difference seen immunohistochemically, in immunoblots the 43-kDa protein (p40) is evenly distributed throughout the retina. Here we show that (i) native p40 and in vitro-translated gene product are indistinguishable and their anomalous migration in denaturing gels probably is due to low pI; (ii) p40 is bound in a Mg2(+)-dependent manner to large cytoplasmic complexes that appear to include ribosomes; and (iii) there is a labile conformational difference in p40 between dorsal and ventral retina: dorsally it is more accessible to proteolysis, suggesting a more open conformation. In conjunction with the recent hypothesis that p40 constitutes a translation initiation factor (D. Auth and G. Brawerman, personal communication), these observations point to a dorso-ventral asymmetry in some aspect of protein translation, which in turn may set up differences in recognition factors on retinal growth cones.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989 Feb 10;56(3):379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- Douville P. J., Harvey W. J., Carbonetto S. Isolation and partial characterization of high affinity laminin receptors in neural cells. J Biol Chem. 1988 Oct 15;263(29):14964–14969. [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hunt L. T., Barker W. C. Identification of a mouse homolog of the human laminin receptor. Nucleic Acids Res. 1988 Jun 10;16(11):5195–5195. doi: 10.1093/nar/16.11.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M. Adhesion molecules and the hierarchy of neural development. Neuron. 1988 Mar;1(1):3–13. doi: 10.1016/0896-6273(88)90204-8. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- Makrides S., Chitpatima S. T., Bandyopadhyay R., Brawerman G. Nucleotide sequence for a major messenger RNA for a 40 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988 Mar 25;16(5):2349–2349. doi: 10.1093/nar/16.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Rabacchi S. A., Neve R. L., Dräger U. C. A positional marker for the dorsal embryonic retina is homologous to the high-affinity laminin receptor. Development. 1990 Jul;109(3):521–531. doi: 10.1242/dev.109.3.521. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Castronovo V., Schmitt M. C., Wewer U. M., Claysmith A. P., Liotta L. A., Sobel M. E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry. 1989 Sep 5;28(18):7476–7486. doi: 10.1021/bi00444a047. [DOI] [PubMed] [Google Scholar]
- Roth S., Stein D., Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989 Dec 22;59(6):1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Han K., Manley J. L., Levine M. The graded distribution of the dorsal morphogen is initiated by selective nuclear transport in Drosophila. Cell. 1989 Dec 22;59(6):1165–1177. doi: 10.1016/0092-8674(89)90772-1. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Relocalization of the dorsal protein from the cytoplasm to the nucleus correlates with its function. Cell. 1989 Dec 22;59(6):1179–1188. doi: 10.1016/0092-8674(89)90773-3. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Liotta L. A., Jaye M., Ricca G. A., Drohan W. N., Claysmith A. P., Rao C. N., Wirth P., Coligan J. E., Albrechtsen R. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7137–7141. doi: 10.1073/pnas.83.19.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenofsky R., Cereghini S., Krowczynska A., Brawerman G. Regulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxide. Mol Cell Biol. 1983 Jul;3(7):1197–1203. doi: 10.1128/mcb.3.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yow H. K., Wong J. M., Chen H. S., Lee C. G., Davis S., Steele G. D., Jr, Chen L. B. Increased mRNA expression of a laminin-binding protein in human colon carcinoma: complete sequence of a full-length cDNA encoding the protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6394–6398. doi: 10.1073/pnas.85.17.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K., Risse G. Isolation and characterization of laminin receptors. Methods Enzymol. 1987;144:490–507. doi: 10.1016/0076-6879(87)44197-9. [DOI] [PubMed] [Google Scholar]