Abstract
Potassium channels are the targets of antiepileptic drugs (AEDs), which play important roles in the etiology of epilepsy. KCNA1 and KCNA2 encode mammalian Kv1.1 and Kv1.2 channels, which are essential roles in the initiation and shaping of action potentials. KCNV2 encodes Kv8.2, which is a regional overlap with Kv2 subunits as functional heterotetramers. In our study, we aim to investigate whether variants of KCNA1, KCNA2, and KCNV2 genes influence susceptibility to genetic generalized epilepsies (GGEs) and the efficacy of AEDs. Seven hundred sixty-seven subjects (284 healthy controls, 279 drug-responsive, and 204 drug-resistant GGE patients) were enrolled in our study. Eight variants of KCNA1, KCNA2, and KCNV2 were assessed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry method. Results showed that there were no statistically significant correlations between the 8 variants of KCNA1, KCNA2, and KCNV2 and the risk/drug resistance of GGEs. In conclusion, our study suggests that KCNA1, KCNA2, and KCNV2 variants may not be involved in the risk/drug resistance of GGEs. Further multicenter, multiethnic, and large sample size pharmacogenetic and case–control studies are warranted to confirm our negative results.
Keywords: drug resistant, genetic generalized epilepsies, KCNA1, KCNA2, KCNV1
1. Introduction
Epilepsy is a clinical syndrome characterized by abnormal electrical activity and is a common type of neurological diseases.[1] Genetic generalized epilepsies (GGEs) are characterized by unprovoked generalized seizures. And GGEs have no evidence for an acquired cause that results in absences, generalized myoclonic seizures, or primary generalized tonic-clonic seizures.[2,3]
As we know, there are a series of gene mutations that can change the channel function. Most of them encode ion channels, which play important roles in the pathogenesis of epilepsy. Among them, the dysfunction mutations of potassium channels were also identified to be associated with the pathology of GGEs.[1,4] Because of genes related to the dysfunction mutations of potassium channels related genes in epilepsies, they are called “K+ channelepsies.”[5] Among potassium channels related genes, KCNA1 and KCNA2 encode mammalian Kv1.1 and Kv1.2 channels, which are closely related to the initiation and shaping of action potentials. KCNV2 encodes Kv8.2, which is a regional overlap with Kv2 subunits as functional heterotetramers.[1,6,7]
Several KCNA1 knockout mouse models develop epileptic phenotypes that imply the importance of electrophysiological roles on brain neuron function.[8–10]KCNA1 gene loss-of-function mutations were reported in episodic ataxia type 1 patients, who have epileptic seizures.[11–15] The growing body of accepted evidence demonstrates that KCNA1 variants cause reduced current amplitude, thus contributing to the pathogenesis of epilepsy. Moreover, Kv1.2 knockout mouse model also shows the increased susceptibility of seizure.[16] Furthermore, recent studies found de novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy, ataxia, and myoclonic epilepsy.[17–20] Notably, KCNV2 encodes Kv8.2 potassium channel that are involved in mediating the suppression of Kv2.1 currents.[21] It has been reported that KCNV2 mutations are associated with febrile, afebrile partial seizures, and epileptic encephalopathy, because the 2 nonsynonymous variants R7K and M285R can alter the function of Kv2.1/Kv8.2 heterotetrameric potassium channels.[21]
Although there are sorts of antiepileptic drugs (AEDs) to control the onset of GGEs, still ports of seizures are not well controlled. Previously, studies found that variants of ion channel or drug transporters genes (SCN1A, SCN2A, ABCB1, ABCC2) contributed to the drug-resistant epilepsy and influenced the efficacy of AEDs.[22–24] However, the results are not consistent and not fully understood. Only a case of severe refractory epileptic patient was reported to suffer from KCNV2 p.Met285Arg mutation.[21] To our knowledge, no studies are reported about the relationship between the variants of KCNA1/KCNA2 and drug-resistant epilepsy. On the basis of the important electrophysiological roles of Kv1.1, Kv1.2, and Kv8.2 potassium channels in the brain neurons, we hypothesized that the variants of these potassium channel related genes would affect the efficacy of AEDs.
Herein, we performed a pharmacogenetic and case–control study to investigate the relationships between KCNA1/KCNA2/KCNV1 variants and AEDs efficacy and the risk of GGEs.
2. Methods
2.1. Subjects
The patients and controls were enrolled from Xiangya Hospital and the Second Xiangya Hospital of Central South University, from January 14, 2013, to February 2014. GGEs and the subtype definitions were created according to the guidelines of the International League Against Epilepsy and standardized protocols.[25,26] GGEs inclusion criteria are as follows: patients have normal intelligence, psychomotoric development, normal neurologic examination status; electroencephalography results present generalized spike-wave discharges (2.5–5 Hz) and normal background activity, and Han Chinese patients. GGEs exclusion criteria are as follows: patients have structural, metabolic, or degenerative brain disorders, exclusively stimulus-induced seizures, mental retardation, severe adverse drug reactions, unreliable or lacking records of seizure frequency, poor compliance with AEDs, a history of alcohol or drug abuse, the presence of progressive or degenerative neurological or systemic disorders, and hepatic or renal failure. The subgroups of GGEs were as follows: childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), juvenile myoclonic epilepsy (JME), and epilepsy with generalized tonic-clonic seizures (EGTCS), according to the definition of previous studies.[2,25] According to previous treatment guidelines of epilepsy,[26,27] patients were treated with AEDs such as carbamazepine, valproic acid, lamotrigine, oxcarbazepine, levetiracetam, topiramate, and so on. Drug-responsive patients were defined as the patients who had not experienced any type of seizures for a minimum of 1 year after receiving AEDs; drug-resistant patients were defined as patients who had at least 4 seizures during the previous year while trying at least 3 antiepileptic medications at maximal tolerated doses.[28,29] Healthy controls were recruited from Medical examination center of Xiangya hospital and were Han Chinese peoples who were found disease-free in a physical examination. Healthy controls were randomly selected from our Han Chinese Healthy controls DNA database. We designed a questionnaire to collect the clinical data and follow-up data. All patients or their parents wrote the consent agreement of participating in our research projects. The authors stated that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. Chinese Clinical Trail Register approved this study (the registration number: ChiCTR-RO-12002853). The Ethics Committee of Xiangya School of Medicine and Ethics Committee of Institute of Clinical Pharmacology of Central South University approved the study protocol.
2.2. Genotyping
After the whole blood was collected from the subjects, DNA was extracted by the phenol-chloroform method. The SNPs for investigation were selected with the tagged-single nucleotide polymorphisms (tagSNPs) method according to the linkage disequilibrium (LD) data from International HapMap Project. We used matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Sequenom, SanDiego, CA) method to assess the selected variants according to manufacturer's guideline. Five percent of genotyping results were validated by Sanger DNA sequencing.
2.3. Statistical analyses
We used the SPSS software package (Version 13.0 for Windows; SPSS, Chicago, IL) to input the data and to analyze the data. Student t test was used for measurement data. χ2 test and Fisher exact test were used for enumeration data. Hardy–Weinberg equilibrium was analyzed with χ2 test. After adjustment for age, sex, and seizure types, binary logistic regression was used to analyze the relationships between KCNA1/KCNA2/KCNV1 loci and AEDs efficacy. Haploview was used to analyze LDs and haplotypes. We use a 1000 bootstrap resampling technique to adjust the aforementioned variables. A Bonferroni correction was used to correct for multiple comparisons. P < .05 was accepted for statistical significance.
3. Results
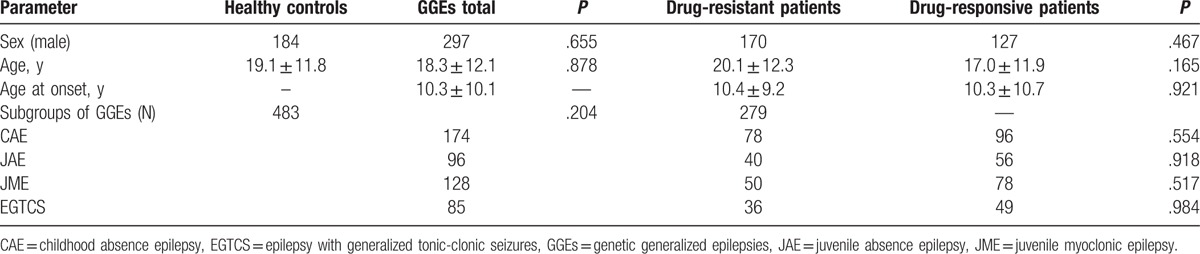
According to the inclusion and exclusion criteria, 767 subjects were enrolled in our study, consisting of 284 healthy controls and 483 Chinese GGE patients. Two hundred seventy-nine drug-responsive patients and 204 drug-resistant patients were identified according to the definition guideline. The characteristics of subjects are summarized in Table 1. There were no differences between drug-responsive and resistant patients in age, sex, age, at the onset, and in the distribution of subgroups. All relevant data were shown in the manuscript. For further information, please contact the corresponding author.
Table 1.
The characteristics of subjects.
SNPs enrolled in our study are summarized in Table 2. The LD tests of tagSNPs with patients’ data are shown in Fig. 1. The results showed that KCNA1 rs2227910 and rs7974459 have significant LD relationship, and KCNV2 rs7029012 and rs10967705 have significant LD relationship (D’ = 74 and 70, respectively). The distributions of all tagSNPs in GGEs and healthy controls are summarized in Table 3. Notably, there was no mutation subject in GGEs and healthy controls about KCNA1 rs112561866, which is a missense (p.Met449Ile) variant. The investigated SNPs were all in Hardy–Weinberg equilibrium among the case–controls. Sanger sequencing validated the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry results were 100% correct. Comparisons with GGEs and healthy controls showed no difference in the distributions of all selected SNPs. In addition, we analyzed the distributions of SNPs by the subgroups of GGEs. The results showed that rs7029012 and rs10967705 of KCNV2 were associated with the susceptibility of JME. The frequency of KCNV2 rs7029012 CG+CC genotypes was higher in the JME patients than that in the healthy controls [51.6% vs 41.1%, odds ratio (OR) = 1.523 (1.001–2.32), P = .049]. KCNV2 rs10967705 CG+CC genotypes were higher in the JME patients than those in the healthy controls [48.4% vs 37.9%, OR = 1.57 (1.004–2.35), P = .047]. The results of 2 loci were also consistent with the LD result that these 2 loci were strong LD from each other. Moreover, we also found the frequency of KCNV2 rs10967728 GC + CC genotype was lower in EGTCS than that in the controls [83.1% vs 92.2%, OR = 0.90 (0.81–0.99), P = .015]. KCNV2 rs10967705 was also associated with the risk of CAE (Table 4). No significant KCNA1 and KCNV2 haplotypes that are related with the risk of GGEs were observed (Table 5).
Table 2.
Characteristics of selected tagSNPs of potassium channel related genes.
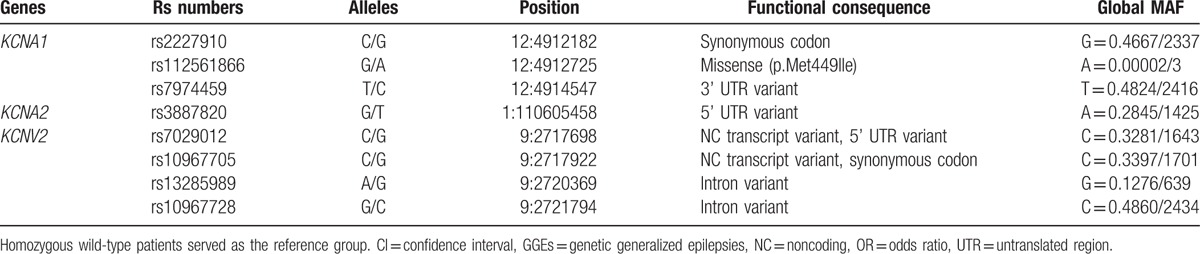
Figure 1.
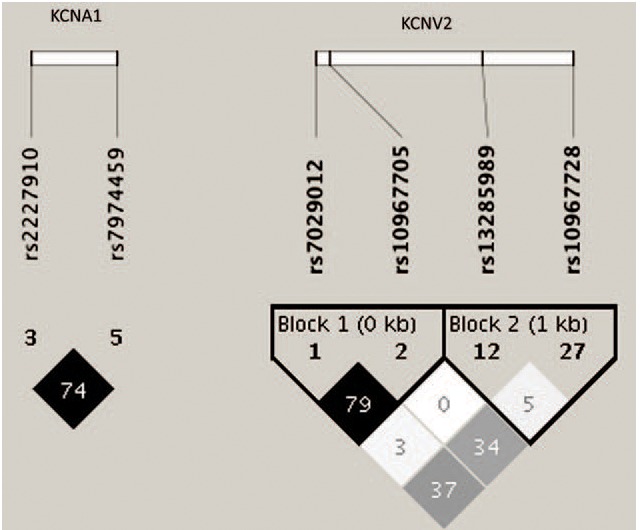
Linkage disequilibrium of tagSNPs with patients’ data. Linkage disequilibriums between pairs of polymorphisms are shown with diamonds (r2), with darker shading indicating greater r2.
Table 3.
The distributions of potassium channel related genes tagSNPs in the GGEs and the healthy controls.
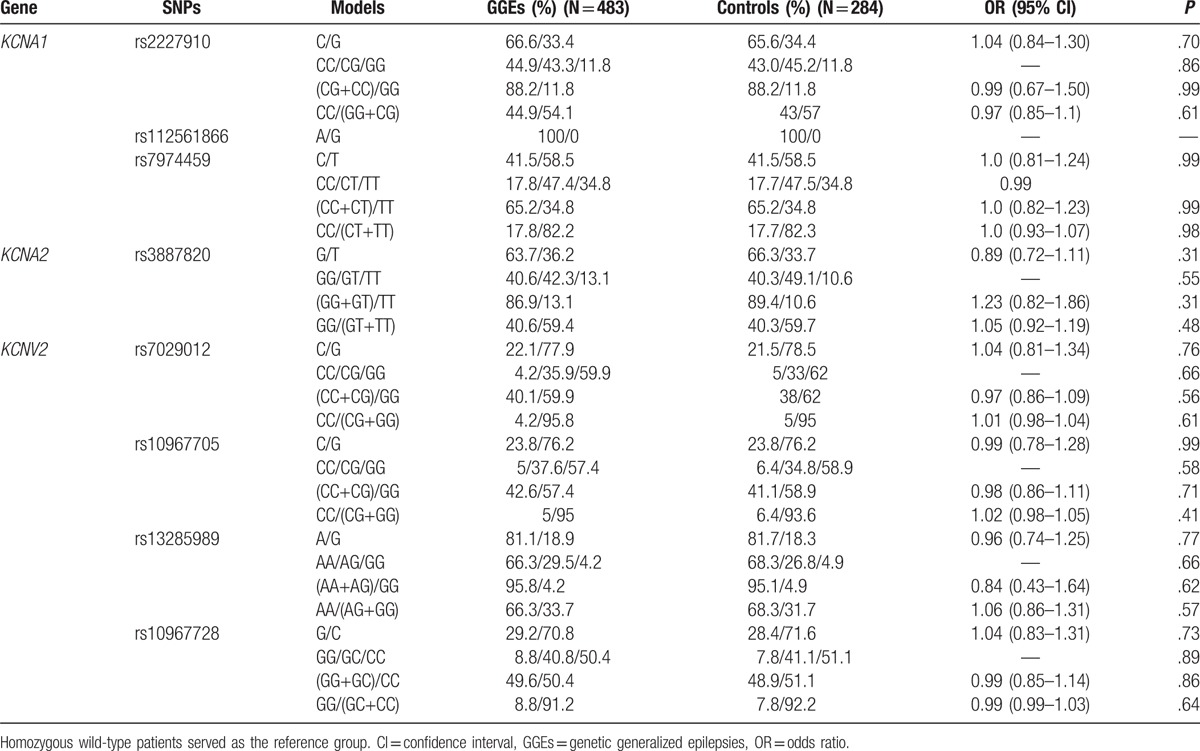
Table 4.
Significant differences on the distributions of potassium channel related genes tagSNPs in the GGE subtype patients and the healthy controls.
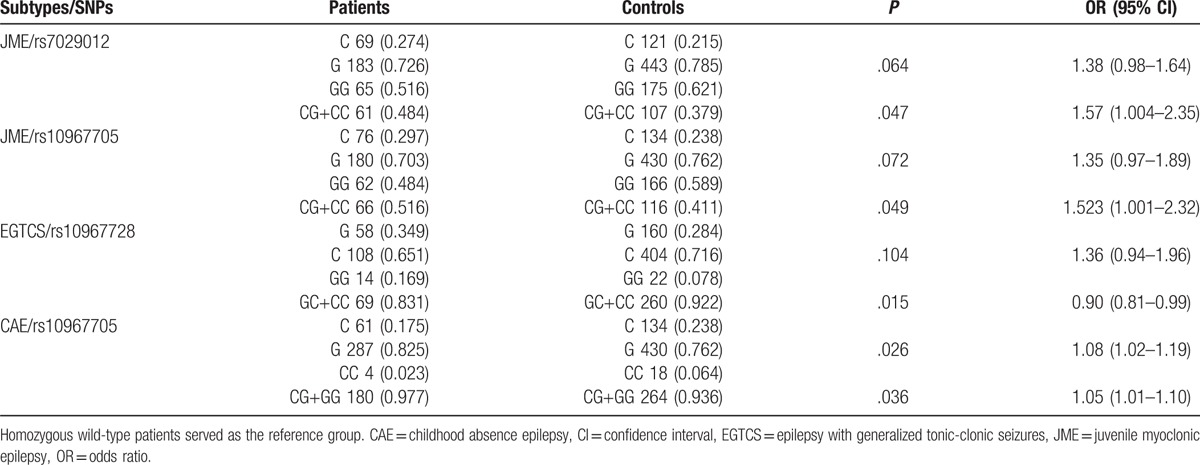
Table 5.
Frequencies of the haplotypes of KCNA1 and KCNV2 genes in the GGEs and the healthy controls.
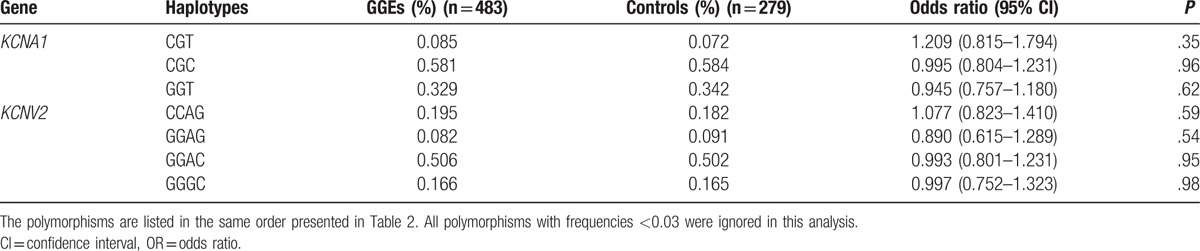
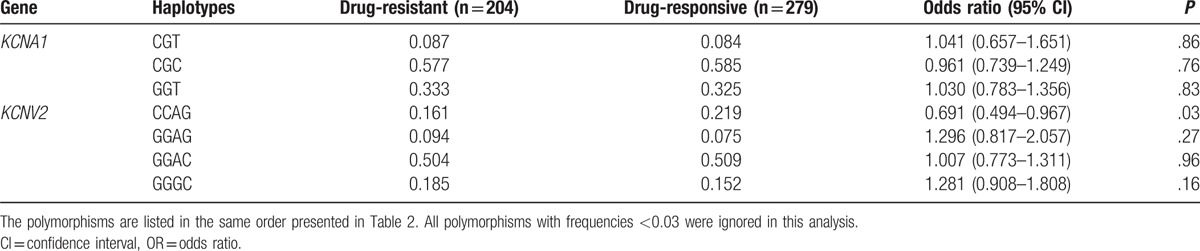
We analyzed the distributions of KCNA1, KCNA2 and KCNV2 loci in 204 drug-resistant patients and 279 drug-responsive patients. The frequency of KCNV2 rs10967728 CC genotype was higher in drug-resistant patients than that in drug-responsive patients [55.9% vs 46.4%, OR = 1.21 (1.01–1.44), P = .039] (Table 6). After analyzing the frequencies of potassium channel related genes haplotypes in the drug-resistant and drug-responsive patients, we found that a KCNV2 haplotype CCAG was associated with the risk of drug resistance. The frequency of KCNV2 haplotype CCAG were lower in the drug-resistant patients than that in the drug-responsive patients [16.1% vs 21.9%, OR = 0.691 (0.494–0.967), P = .03] (Table 7).
Table 6.
Allelic and genotypic tagSNPs frequencies of potassium channel related genes in the drug-responsive and drug-resistant GGE patients.
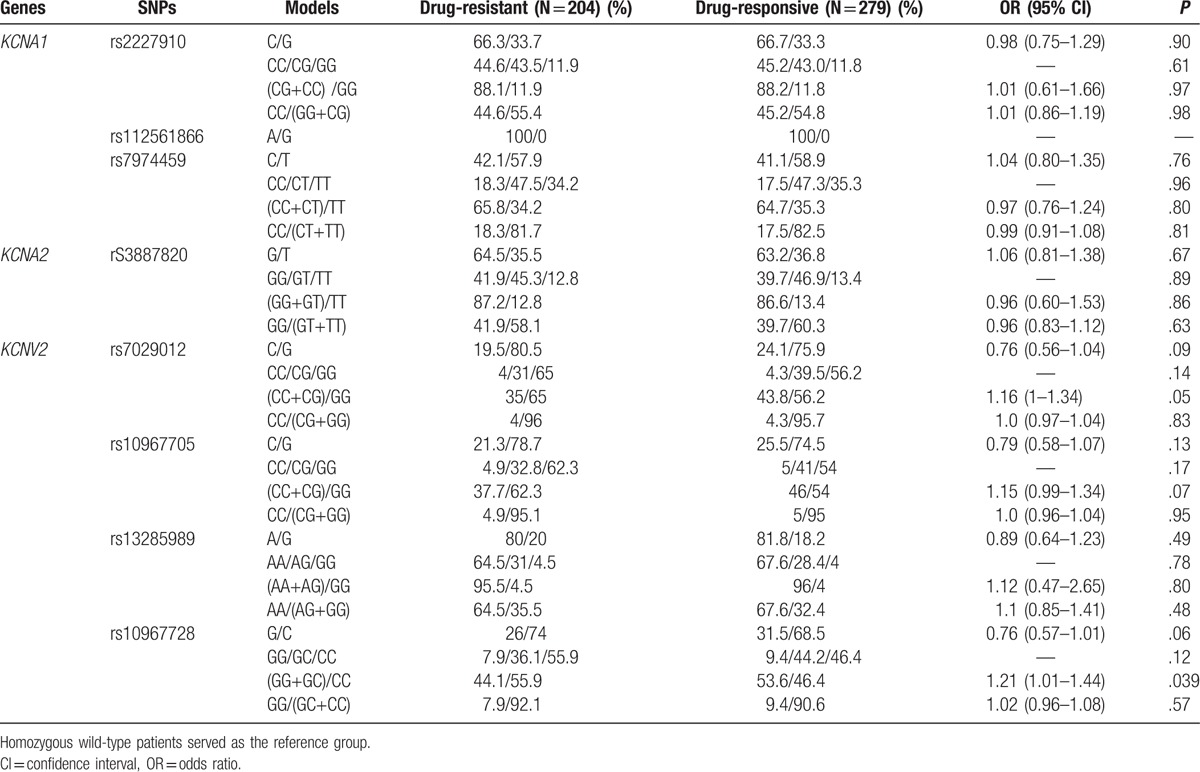
Table 7.
Frequencies of the haplotypes of KCNA1 and KCNV2 genes in in the drug-responsive and drug-resistant GGE patients.
However, there were no significant associations between the 8 variants of KCNA1, KCNA2, and KCNV2 genes and the risk or drug resistance of GGEs after a Bonferroni correction for multiple comparisons.
4. Discussion
Ion channels are the electrophysiological basis of neuron activity. Notably, potassium channels play important roles in neuronal excitability and are associated with the inward-negative resting membrane potential.[1] Whether potassium channel related genes KCNA1, KCNA2, and KCNV1 affect the risk of GGEs and the efficacy of AEDs is still not clear. In our study, we collected 767 subjects, including 284 healthy controls and 483 Chinese GGEs patients (consisting of 279 drug-responsive patients and 204 drug-resistant patients) and assessed the 8 SNPs of KCNA1, KCNA2, and KCNV1. Before a Bonferroni correction for multiple comparisons, there were tendencies suggesting that rs7029012 and rs10967705 of KCNV2 might be associated with the susceptibility of JME; rs10967705 of KCNV2 might be associated with the risk of CAE; and KCNV2 rs10967728 might be associated with the risk of EGTCS. In addition, KCNV2 rs10967728 and a haplotype CCAG might be associated with the GGEs drug resistance. However, after a Bonferroni correction for multiple comparisons, no variants of KCNA1, KCNA2, and KCNV1 are found statistically significantly related to the risk and the drug resistance of GGEs.
KCNV2 encodes the K+ channel Kv8.2, which is electrophysiologically silent when assembled in homotetramer and KCNV2 modulates the properties of Kv2 and Kv3 channels to influence membrane translocation and channel properties.[1,21] Kv8.2 localizes with Kv2.1 as a contributor to the delayed rectifier potassium current in hippocampal pyramidal neurons, which is of particular importance for seizure generation.[30] Moreover, a study identified unique nonsynonymous variants R7K and M285R of KCNV2 in 2 unrelated children with epilepsy.[21] Clinical severity differs between these 2 variants of KCNV2, from relatively benign febrile and afebrile seizures in the R7K variant to severe drug-resistant epileptic encephalopathy in the M285R variant. Functional study found that the 2 variants could enhance Kv8.2-mediated suppression of Kv2.1 currents.[21]
Previously, no other variants of KCNV2 were found to be associated with the risk of epilepsy except the R7K and M285R loci. In our study, we found that KCNV2 rs7029012 was associated with JME before a Bonferroni correction. Rs7029012 is a 5’ UTR variant (c. -42C>G) within an exon, but not a translated 5’ end of the gene. As a promoter region variant, it may influence the binding of acting element in inducing the expression changes of KCNV2. Further studies on whether the variant affect KCNV2 are warranted. Rs10967705 was found to be associated with the risk of JME and CAE, as a synonymous variant (p.Gly61Gly), before a Bonferroni correction was made. Synonymous variants do not produce altered coding sequences. Hence, they are not expected to change the function of the protein if they mutate. However, studies found that the synonymous variants could change the function of their products protein.[31,32] The mechanisms were alteration in mRNA stability and induction of translational pausing to modify the protein abundance, structure, and activity. Synonymous variant rs10967705 does not change the amino acid of KCNV2. We hypothesized that it may influence the function of K+ channel Kv8.2 via these mechanisms. Moreover, Rs10967705 was a strong LD with rs7029012 and these 2 variants may jointly influence the function of Kv8.2. Rs10967728 was associated with the risk of EGTCS and drug resistance, as an intron variant. This variant may have the possibility of LD with functional SNPs.
KCNA1 and KCNA2 encode 2 Kv1 subfamilies called Kv1.1 and Kv1.2 subunits, which play essential roles in the initiation and shaping of action potentials in synaptic terminals, axons, soma, and proximal dendrites.[1] Several heterozygous point mutations of KCNA1 were reported with generalized or partial seizures in episodic ataxia type 1, which is a neurological disease characterized by generalized ataxia attacks and spontaneous muscle quivering.[12,14,15,33]KCNA2 was reported as having the risk of mild to severe epileptic encephalopathy and myoclonic epilepsy.[19,20] In our study, we have not observed rs2227910, rs112561866, and rs7974459 of KCNA1 and rs3887820 of KCNA2 loci associated with the risk of GGEs and their subgroups.
Antiepileptic drug resistance is an important and complex problem. Although multiple drugs were used for the treatment, the conditions of some of the patients were not under good control. AEDs target multiple but not specific ion channels to play the pharmacological action, which creates complex electrophysiological situations in brain neurons. Previous research found that SCN1A gene polymorphism IVS5–91 rs3812718 G>A and SCN2A IVS7-32A>G rs2304016 might be associated with the drug-resistant epilepsy,[23,24,34] which implies that the variants of the target genes of AEDs may influence the drug efficacy, as the potential drug target and the important electrophysiological roles in brain, KCNA1, KCNA2, and KCNV2 may influence the pharmacoresistance of AEDs. In our study, we also carried out the pharmacogenetic study to investigate the association between variants of KCNA1, KCNA2, and KCNV2 and GGEs drug resistance. We found that rs10967728 and a haplotype CCAG of KCNV2 might be related with GGEs drug resistance before a Bonferroni correction for multiple comparisons. But after a Bonferroni correction, the significant association has disappeared. Herein, further large-scale studies of whether and how the variant and haplotype affect the function of Kv8.2 are needed.
Some limitations exist in our study. First, the SNPs and genes selected for analysis in our study were limited and the sample size was relatively small. Second, the evaluation of AEDs’ efficacy and the definition of subgroups of GGEs were not as accurate as we had expected. All these limitations need further improvements in future research.
In conclusion, we have conducted a pharmacogenetic and case–control study to evaluate the role of the variants of KCNA1, KCNA2, and KCNV2 in the susceptibility and drug resistance of GGEs. Our results have revealed no significant association between 8 variants of KCNA1, KCNA2, and KCNV2 genes and risk or drug resistance of GGEs after a Bonferroni correction for multiple comparisons. Further larger sample clinical studies would be warranted to confirm our negative results.
Acknowledgments
The authors thank all the funds that supported the study including the National Scientific foundation of China (No. 81503166, No. 81603208) and the Youth Foundation of Xiangtan Hospital in Central South University (2014Q08) and all patients who participated in the study.
Footnotes
Abbreviations: AEDs = antiepileptic drugs, CAE = childhood absence epilepsy, EGTCS = epilepsy with generalized tonic-clonic seizures, GGEs = genetic generalized epilepsies, JAE = juvenile absence epilepsy, JME = juvenile myoclonic epilepsy, LD = linkage disequilibrium, OR= odds ratio, tagSNPs = tagged-single nucleotide polymorphisms.
JQ and SHL contributed equally.
Authorship: Conceptualization: JQ, SHL, PX, DXX, QQ. Data collection: JQ, SHL. Formal analysis: JQ, QQ. Funding acquisition: JQ, QQ. Investigation: PX, DXX, SHL. Methodology: JQ, SHL. Project administration: JQ, SHL, QQ. Resources: PX, DXX. Software: JQ, SHL. Writing original draft: JQ, SHL, QQ. Manuscript revising: JQ, ZLL, QQ.
The authors report no conflicts of interest.
References
- [1].Villa C, Combi R. Potassium channels and human epileptic phenotypes: an updated overview. Front Cell Neurosci 2016;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nordli DR., Jr Idiopathic generalized epilepsies recognized by the International League Against Epilepsy. Epilepsia 2005;46(Suppl 9):48–56. [DOI] [PubMed] [Google Scholar]
- [3].Elshahabi A, Klamer S, Sahib AK, et al. Magnetoencephalography reveals a widespread increase in network connectivity in idiopathic/genetic generalized epilepsy. PLoS One 2015;10:e0138119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kohling R, Wolfart J. Potassium channels in epilepsy. Cold Spring Harb Perspect Med 2016;6:pii: a022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].D’Adamo MC, Catacuzzeno L, Di Giovanni G, et al. K(+) channelepsy: progress in the neurobiology of potassium channels and epilepsy. Front Cell Neurosci 2013;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Czirjak G, Toth ZE, Enyedi P. Characterization of the heteromeric potassium channel formed by kv2.1 and the retinal subunit kv8.2 in Xenopus oocytes. J Neurophysiol 2007;98:1213–22. [DOI] [PubMed] [Google Scholar]
- [7].Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci 1995;15(5 Pt 2):3840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Smart SL, Lopantsev V, Zhang CL, et al. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron 1998;20:809–19. [DOI] [PubMed] [Google Scholar]
- [9].Rho JM, Szot P, Tempel BL, et al. Developmental seizure susceptibility of kv1.1 potassium channel knockout mice. Dev Neurosci 1999;21:320–7. [DOI] [PubMed] [Google Scholar]
- [10].Simeone KA, Matthews SA, Rho JM, et al. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia 2016;57:e178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Imbrici P, D’Adamo MC, Kullmann DM, et al. Episodic ataxia type 1 mutations in the KCNA1 gene impair the fast inactivation properties of the human potassium channels Kv1.4-1.1/Kvbeta1. 1 and Kv1. 4-1. 1/Kvbeta1. 2. Eur J Neurosci 2006;24:3073–83. [DOI] [PubMed] [Google Scholar]
- [12].D’Adamo MC, Imbrici P, Sponcichetti F, et al. Mutations in the KCNA1 gene associated with episodic ataxia type-1 syndrome impair heteromeric voltage-gated K(+) channel function. FASEB J 1999;13:1335–45. [DOI] [PubMed] [Google Scholar]
- [13].Tristan-Clavijo E, Scholl FG, Macaya A, et al. Dominant-negative mutation p.Arg324Thr in KCNA1 impairs Kv1.1 channel function in episodic ataxia. Mov Disord 2016;31:1743–8. [DOI] [PubMed] [Google Scholar]
- [14].Spauschus A, Eunson L, Hanna MG, et al. Functional characterization of a novel mutation in KCNA1 in episodic ataxia type 1 associated with epilepsy. Ann N Y Acad Sci 1999;868:442–6. [DOI] [PubMed] [Google Scholar]
- [15].Zuberi SM, Eunson LH, Spauschus A, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain 1999;122:817–25. [DOI] [PubMed] [Google Scholar]
- [16].Brew HM, Gittelman JX, Silverstein RS, et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 2007;98:1501–25. [DOI] [PubMed] [Google Scholar]
- [17].Allen NM, Conroy J, Shahwan A, et al. Unexplained early onset epileptic encephalopathy: exome screening and phenotype expansion. Epilepsia 2016;57:e12–7. [DOI] [PubMed] [Google Scholar]
- [18].Drogemoller BI. Maintaining the balance: both gain- and loss-of-function KCNA2 mutants cause epileptic encephalopathy. Clin Genet 2015;88:137–9. [DOI] [PubMed] [Google Scholar]
- [19].Syrbe S, Hedrich UB, Riesch E, et al. De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet 2015;47:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pena SD, Coimbra RL. Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet 2015;87:e1–3. [DOI] [PubMed] [Google Scholar]
- [21].Jorge BS, Campbell CM, Miller AR, et al. Voltage-gated potassium channel KCNV2 (Kv8.2) contributes to epilepsy susceptibility. Proc Natl Acad Sci U S A 2011;108:5443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qu J, Zhou BT, Yin JY, et al. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci Ther 2012;18:647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou BT, Zhou QH, Yin JY, et al. Effects of SCN1A and GABA receptor genetic polymorphisms on carbamazepine tolerability and efficacy in Chinese patients with partial seizures: 2-year longitudinal clinical follow-up. CNS Neurosci Ther 2012;18:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ma CL, Wu XY, Zheng J, et al. Association of SCN1A, SCN2A and ABCC2 gene polymorphisms with the response to antiepileptic drugs in Chinese Han patients with epilepsy. Pharmacogenomics 2014;15:1323–36. [DOI] [PubMed] [Google Scholar]
- [25].Consortium E, Consortium EM, Steffens M, et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum Mol Genet 2012;21:5359–72. [DOI] [PubMed] [Google Scholar]
- [26].Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989;30:389–99. [DOI] [PubMed] [Google Scholar]
- [27].Nunes VD, Sawyer L, Neilson J, et al. Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ 2012;344:e281. [DOI] [PubMed] [Google Scholar]
- [28].Qu J, Zhang Y, Yang ZQ, et al. Gene-wide tagging study of the association between KCNT1 polymorphisms and the susceptibility and efficacy of genetic generalized epilepsy in Chinese population. CNS Neurosci Ther 2014;20:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Qu J, Yang ZQ, Zhang Y, et al. Common variants of ATP1A3 but not ATP1A2 are associated with Chinese genetic generalized epilepsies. J Neurol Sci 2015;354:56–62. [DOI] [PubMed] [Google Scholar]
- [30].Murakoshi H, Trimmer JS. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J Neurosci 1999;19:1728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525–8. [DOI] [PubMed] [Google Scholar]
- [32].Fung KL, Pan J, Ohnuma S, et al. MDR1 synonymous polymorphisms alter transporter specificity and protein stability in a stable epithelial monolayer. Cancer Res 2014;74:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Eunson LH, Rea R, Zuberi SM, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol 2000;48:647–56. [PubMed] [Google Scholar]
- [34].Kwan P, Poon WS, Ng HK, et al. Multidrug resistance in epilepsy and polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, and SCN3A: correlation among phenotype, genotype, and mRNA expression. Pharmacogenet Genomics 2008;18:989–98. [DOI] [PubMed] [Google Scholar]


