Abstract
Mutations in the Presenilin genes are the major genetic cause of Alzheimer’s disease. Presenilin and Nicastrin are essential components of γ-secretase, a multi-subunit protease that cleaves Type I transmembrane proteins. Genetic studies in mice previously demonstrated that conditional inactivation of Presenilin or Nicastrin in excitatory neurons of the postnatal forebrain results in memory deficits, synaptic impairment, and age-dependent neurodegeneration. The roles of Drosophila Presenilin (Psn) and Nicastrin (Nct) in the adult fly brain, however, are unknown. To knockdown (KD) Psn or Nct selectively in neurons of the adult brain, we generated multiple shRNA lines. Using a ubiquitous driver, these shRNA lines resulted in 80–90% reduction of mRNA and pupal lethality—a phenotype that is shared with Psn and Nct mutants carrying nonsense mutations. Furthermore, expression of these shRNAs in the wing disc caused notching wing phenotypes, which are also shared with Psn and Nct mutants. Similar to Nct, neuron-specific Psn KD using two independent shRNA lines led to early mortality and rough eye phenotypes, which were rescued by a fly Psn transgene. Interestingly, conditional KD (cKD) of Psn or Nct in adult neurons using the elav-Gal4 and tubulin-Gal80ts system caused shortened lifespan, climbing defects, increases in apoptosis, and age-dependent neurodegeneration. Together, these findings demonstrate that, similar to their mammalian counterparts, Drosophila Psn and Nct are required for neuronal survival during aging and normal lifespan, highlighting an evolutionarily conserved role of Presenilin in neuronal protection in the aging brain.
Keywords: γ-secretase, Alzheimer’s disease, conditional knockdown, shRNA, brain
PRESENILIN (PS) is the catalytic subunit of the γ-secretase complex, which also contains Nicastrin (Nct) and cleaves type I transmembrane proteins, such as the Notch receptors (De Strooper et al. 1999; Song et al. 1999; Struhl and Greenwald 1999; Li et al. 2000; Yu et al. 2000; Bai et al. 2015). The Presenilin-1 (PSEN1) and Presenilin-2 genes were identified as the major genes linked to familial Alzheimer’s disease (FAD) (Levy-Lahad et al. 1995; Rogaev et al. 1995; Schellenberg 1995). Genetic studies in mice have demonstrated that, in the developing brain, PS regulates neurogenesis and cell-fate decisions through the Notch signaling pathway (Shen et al. 1997; Handler et al. 2000; Kim and Shen 2008), and that deficiency of PS or Nct results in early embryonic lethality (Donoviel et al. 1999; Nguyen et al. 2006). Conditional gene targeting further demonstrated that PS and Nct are required for normal learning and memory, synaptic plasticity, and neuronal survival in the mouse cerebral cortex (Yu et al. 2001; Beglopoulos et al. 2004; Feng et al. 2004; Saura et al. 2004; Tabuchi et al. 2009; Zhang et al. 2009; Wines-Samuelson et al. 2010; Lee et al. 2014; Watanabe et al. 2014).
Drosophila Presenilin (Psn) and Nicastrin (Nct) share high sequence homology with their human and mouse counterparts (Hong and Koo 1997; Yu et al. 2000). Loss-of-function mutations in Drosophila Psn cause pupal lethality and Notch-like phenotypes, such as maternal neurogenic effects, loss of lateral inhibition within proneural cell clusters, and absence of wing margin formation (Struhl and Greenwald 1999; Ye et al. 1999). Loss-of-function mutations in Nct also cause pupal lethality, and abolish Psn accumulation and Psn-dependent intramembrane cleavage of Notch (Chung and Struhl 2001; Hu et al. 2002). Genetic modifier screens of Notch-like phenotypes in Psn loss-of-function mutants also confirmed the genetic interaction between Psn and Nct in the Drosophila eye and wing (Mahoney et al. 2006). However, the pupal lethality of Psn and Nct mutants precluded studies of their functions in mature neurons of the adult brain. As a result, the consequences of Psn or Nct inactivation in adult neurons of the fly remain unknown.
In this study, we developed multiple shRNA lines targeting Psn or Nct. Using the Act5c-Gal4 driver, we found that ubiquitous Psn or Nct knockdown (KD) in all cells leads to an ∼80–90% reduction of Psn or Nct mRNA and pupal lethality, similar to homozygous null mutant flies (Lukinova et al. 1999; Struhl and Greenwald 1999; Hu et al. 2002). Selective Psn or Nct KD in wing marginal discs caused notching phenotypes in the adult wing, further confirming that these Psn and Nct shRNA lines effectively suppress Psn and Nct function, respectively. Similar to Nct, neuron-specific Psn KD under the control of the elav-Gal4 driver led to developmental defects, with dramatic early mortality, severe climbing defects, and rough eye phenotypes. Expression of a fly Psn transgene rescued the early mortality and rough eye phenotypes of neuron-specific Psn KD. Strikingly, adult neuron-specific Psn or Nct conditional KD (cKD) flies using the elav-Gal4/tub-Gal80ts system, which permits inducible expression of shRNAs in adult neurons, displayed shortened lifespan, climbing defects, increases in apoptosis, and age-dependent neurodegeneration. Together, these findings demonstrate that similar to their mammalian orthologs, Drosophila Psn and Nct are required for adult neuronal survival during aging and normal lifespan, highlighting an evolutionally conserved role of Presenilin in neuronal protection in the aging brain.
Materials and Methods
Generation of Psn and Nct shRNA transgenic flies
We newly generated UAS-shRNA lines against Psn (shPsn1, 2, 3, 4) and Nct (shNct1, 2, 3) as described (Ni et al. 2011), which are different from those available at TRiP. Briefly, shRNA target regions were selected using a Perl program as described previously (Vert et al. 2006), and we avoided the 5′ and 3′ UTRs, and the regions that have >15 bp match to other Drosophila transcripts, to reduce the risk of off-target effects. The annealed top and bottom oligos containing the 21 bp shRNA target sequences against Psn and Nct were subcloned into pWALIUM20 (TRiP) vector following the UAS sequence. The sequences of the oligos used to generate UAS-shPsn and UAS-shNct transgenic flies are included in Supplemental Material, Table S1. As described previously (Groth et al. 2004; Venken et al. 2006; Markstein et al. 2008), each transgene was injected into embryos for targeted phiC31-mediated integration at genomic attP landing sites on the second (attP16 or attP40) or third (attP2 or VK0027) chromosomes.
Crosses and culture conditions
Flies were raised on standard cornmeal media, and maintained at either 22 or 25°, and 40–60% relative humidity. Control flies in all experiments were as closely related to the experimental transgenic flies as possible. Typically, UAS-shPsn or UAS-shNct, and Gal4 transgenic flies were crossed with w1118 (Bloomington Drosophila Stock Center: BL5905) or P(CarryP)attP2 (BL36303) to generate the heterozygous alleles of the UAS-shRNA or Gal4 transgene. We used ubiquitous (Act5C-Gal4), wing-specific (c96-Gal4) or neuron-specific (elav-Gal4c155) drivers to generate Psn or Nct KD; female Gal4 transgenic flies were crossed with male UAS-shRNA flies. For the rescue experiments, elav-Gal4/FM6;; UAS-shPsn2/TM6B female flies were crossed with male flies of human UAS-hPSEN1 or Drosophila UAS-Psn+14. The progeny with the desired genotype (elav-Gal4/+ (or y);; UAS-shPsn2/UAS-hPSEN1 or UAS-Psn+14) was collected based on the absence of genetic markers in the FM6 and TM6B balancers.
To generate adult neuron-specific Psn or Nct cKD flies, elav-Gal4;; tub-Gal80ts females were crossed with UAS-shPsn2 or UAS-shNct2 male flies at a permissive temperature of 22°, and maintained at 22° until adulthood, then male offspring (elav-Gal4/y;; tub-Gal80ts/UAS-shPsn2 or UAS-shNct2) were collected and shifted to a restrictive temperature of 27° until the end of each experiment. To generate adult neuron-specific Psn/Nct cKD flies, elav-Gal4;; tub-Gal80ts, UAS-shPsn2/TM6B females were crossed with UAS-shNct2 male flies at 22°, and maintained at 22° until adulthood, then male offspring (elav-Gal4/y;; tub-Gal80ts, UAS-shPsn2/UAS-shNct2) were collected and shifted to 27° until the end of each experiment. All stocks were obtained from the Bloomington Drosophila Stock Center unless otherwise indicated.
Quantitative RT-PCR (qRT-PCR)
Five third-instar larvae were washed twice in RNase free PBS and collected in lysis buffer. Fifteen third-instar larval brains were dissected in RNase free PBS, and homogenized in lysis buffer using a homogenizing blender (Next Advance). For adult fly RNA extraction, adult flies were collected and frozen immediately in liquid nitrogen. Five whole adult bodies were used for total RNA extraction. Twenty adult heads were removed from bodies by vortexing, and then collected. Total RNA was extracted according to the manufacturer’s instructions (Quick-RNA MicroPrep; Zymo Research). The eluted total RNA was reverse transcribed using iScript Select cDNA Synthesis Kit (Bio-Rad) with gene specific primers: 5′-TATAAACACCTGCTTGGCCG-3′ for Psn, 5′-CGTGCACTGAACTGTGACCA-3′ for Nct and 5′-GACAATCTCCTTGCGCTTCT-3′ for rp49. Real-time qPCR was performed with ViiA 7 Real-Time PCR System (ThermoFisher Scientific) using PowerUP SYBR Green Master Mix (ThermoFisher Scientific) with the following primers: for Psn, 5′-TCCCATCCTCGACAGAATCA-3′ and 5′-TATGCCACGTTCTTCTTGCC-3′; for Nct, 5′-GACTTCATGCTGGACATCGG-3′ and 5′-TTGCTGAGCCAAAGTCGTTC-3′; for rp49, 5′-AAGCGGCGACGCACTCTGTT-3′ and 5′-GCCCAGCATACAGGCCCAAG-3′. The level of rp49 mRNA was evaluated as an internal control for the total mRNA quantity in each sample. Data were analyzed with Microsoft Excel and Prism. More than four independent experiments were done for each analysis.
Adult viability and lifespan analysis
Fly viability was assessed by collecting and gently transferring 20 third-instar larvae to fresh vials at 25°. The total number of eclosed adult flies from the pupal cases was scored. Viability was calculated by dividing the total number of flies by the total number of pupae, and shown as the percentage of pupae surviving to adulthood. At least 100 flies per genotype were scored in >5 independent experiments.
For lifespan analysis, >100 flies per genotype were collected in individual vials containing no >30 flies and assayed for longevity as previously described (Wittmann et al. 2001). Flies were transferred to fresh vials every other day. Lifespans were measured by scoring dead flies remaining in the old vial and plotted using the Kaplan-Meir method. The median lifespan (MedLS) was calculated as the age when half of the flies have died, and the survival distribution of two genotypic groups were compared using the log-rank (Mantel-Cox) test.
Climbing assay
Climbing assays were performed as previously described (Rhodenizer et al. 2008). Briefly, ∼20 flies were gently tapped to the bottom of a plastic vial, and a picture was taken after 20 sec. This procedure was repeated four times with 1 min intervals between trials to allow the flies to recover from prior tapping. Climbing ability was evaluated by scoring the number of flies that failed to climb over 5 cm in each trial.
Analysis of adult wings and eyes
One wing per adult fly was separated from anesthetized flies, incubated in 100% ethanol for 3 min, dried, and mounted in 50% v/v Canada Balsam (Sigma-Aldrich) in methyl salicylate (Fisher Scientific). The images of eyes and dissected wings were obtained using an Olympus PD25 camera mounted on an Olympus BX40 microscope.
Western analysis
Adult flies were collected and frozen immediately in liquid nitrogen, and 30 adult heads were removed from bodies by vortexing and then collected. Heads were homogenized in an ice-cold stringent RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate), supplemented with protease and phosphatase inhibitor mixtures (Sigma-Aldrich), followed by sonication. Homogenates were centrifuged at 14,000 × g for 20 min at 4° to separate supernatants (RIPA buffer-soluble fractions). Equal amounts of total proteins from each preparation were loaded and separated in NuPAGE gels (Invitrogen), and transferred to nitrocellulose membranes. After blocking, membranes were incubated at 4° overnight with primary antibodies. Primary antibodies used were rabbit anti-APP-Y188 (1:2000; abcam) and rabbit anti-β actin (1:2000; abcam). Membranes were then incubated with dye-coupled secondary antibodies (goat anti-rabbit IRdye680 and goat anti-rabbit IRdye 800 from LI-COR). Signal was quantified using the Odyssey Infrared Imaging System (LI-COR bioscience).
Histology and TUNEL analysis
Heads from adult flies were fixed in 10% formalin, paraffinized, embedded in paraffin, and sectioned from a frontal orientation. Serial sections (4 μm) spanning the entire brain were collected and placed on glass slides, and subjected to further analysis. Brain morphology was evaluated by staining paraffin sections with hematoxylin and eosin (H&E) as previously described (Dias-Santagata et al. 2007). To quantify neurodegeneration, the number of vacuoles >5 μm in diameter was counted throughout the serial sections of the entire brain (usually 25–30 sections). At least 10 individual brains were analyzed per genotype for each time point.
Cells undergoing apoptosis were detected in the paraffin sections by TUNEL, according to the manufacturer’s instructions (TdT Enzyme DNA Fragmentation Detection Kit; EMD Millipore, Calbiochem, FragEL). Quantification of the TUNEL-positive (TUNEL+) cells was performed by counting cells labeled with visible markers in all the serial sections (4 μm) of the entire fly brain (usually 25–30 sections). At least 11 individual brains were analyzed per genotype.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are presented fully in the article. Drosophila strains used in this study are available upon request.
Results
Generation and validation of transgenic shRNA lines using a ubiquitous Gal4 driver
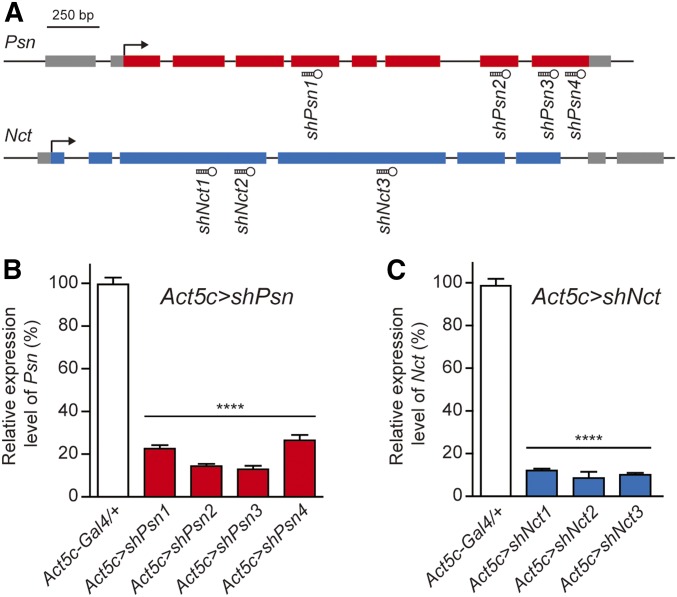
We designed 3–4 distinct shRNAs against Psn or Nct using the Perl program (Figure 1A), and generated UAS-Psn or UAS-Nct shRNA transgenic flies, which permit spatial and temporal restriction of Psn or Nct inactivation using the Gal4/UAS system (Brand and Perrimon 1993; Dietzl et al. 2007; Ni et al. 2011). Ubiquitous expression of Psn shRNA using the Actin5C-Gal4 driver in Act5c > shPsn (Actin5C-Gal4/+; UAS-shPsn/+) flies caused early- to mid-pupal lethality, similar to the previous observation in Psn-null homozygous mutants (Struhl and Greenwald 1999; Ye et al. 1999; Mahoney et al. 2006). Levels of Psn transcripts in Act5C > shPsn third-instar larvae were significantly reduced (∼80–90% depending on the specific shRNA line) compared to control flies (Figure 1B). Similar to Act5c > shPsn transgenic flies, Act5C > shNct (Actin5C-Gal4/+; UAS-shNct/+) transgenic flies also showed early pupal lethality, similar to Nct-null homozygous mutants (Chung and Struhl 2001; Zhang et al. 2005). Levels of Nct mRNA in Act5C > shNct third-instar larvae were also significantly reduced (∼90%) compared to control flies (Figure 1C). Furthermore, Psn and Nct KD using two additional ubiquitous drivers, tub-Gal4 and en-Gal4, also produced pupal lethality (data not shown).
Figure 1.
Generation and validation of UAS-shPsn and UAS-shNct transgenic lines using a ubiquitous Gal4 driver. (A) Schematic gene structures of Psn and Nct and the target regions of the Psn and Nct shRNAs. Gray boxes indicate the 5′- or 3′-UTR. Colored boxes indicate exons. Hairpins indicate the shRNAs and the corresponding target regions. (B, C) Ubiquitous expression of all of the Psn and Nct shRNAs using the Act5c-Gal4 driver result in pupal lethality and ∼80–90% reduction of mRNA levels in Act5c > shPsn and Act5c > shNct whole third-instar larvae, compared to control (Act5c-Gal4/+). qRT-PCR analysis of Psn (B) and Nct (C) mRNA levels was performed using total RNA extracted from five whole third-instar larvae per genotype. Psn and Nct mRNA levels were normalized to rp49 mRNA levels as internal control. n = 4 independent experiments. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons, ****P < 0.0001.
Notching wing phenotypes in Psn and Nct KD flies using a wing disc-specific Gal4 driver
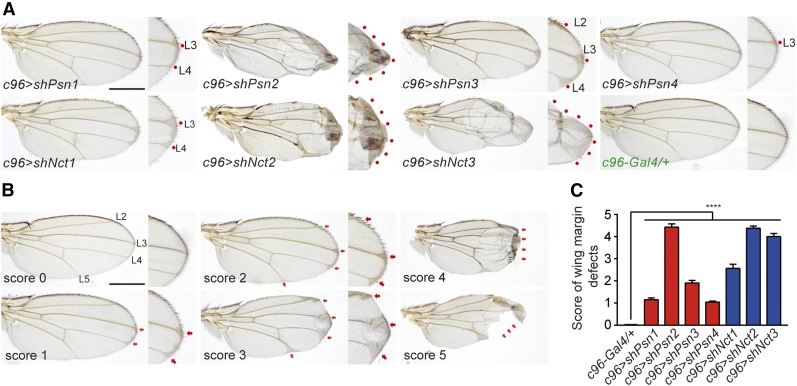
One of the most striking phenotypes of Psn and Nct loss-of-function mutant flies is the notching wing phenotype observed in the wing containing Psn−/− or Nct−/− mosaic clones (Struhl and Greenwald 1999; Klein et al. 2000; Chung and Struhl 2001; Lopez-Schier and St Johnston 2002). Consistent with these earlier findings, notching wing phenotypes were also observed in wing disc-specific Psn (c96 > shPsn) and Nct (c96 > shNct) KD flies (Figure 2A), using the wing imaginal disc driver c96-Gal4 (Gustafson and Boulianne 1996). We quantified the severity of the wing phenotypes to identify the most potent shRNA lines (Figure 2, B and C). The strongest shPsn line, shPsn2, produced the most severe notching phenotypes with loss of wing margins in ∼30% of the c96 > shPsn2 flies (Figure 2, A and C). The weaker shPsn lines, shPsn4 and shPsn1, only showed mildly thickened veins in the wing margins (L3 for c96 > shPsn4; L3 and L4 for c96 > shPsn1), whereas c96 > shPsn3 flies showed thickened L2 veins in addition to L3 and L4 (Figure 2A). Similar to c96 > shPsn flies, c96 > shNct flies also showed thickened veins in the L3 and L4 wing margins (c96 > shNct1), and severe notching with blistered phenotypes and loss of wing margins (c96 > shNct2 and c96 > shNct3) (Figure 2, A and C). These results indicate that these UAS-shPsn and UAS-shNct lines effectively KD Psn and Nct expression and function. Since the UAS-shPsn2 and UAS-shNct2 lines are most effective, we selected them for further extensive analysis. We also included the UAS-shPsn3 and UAS-shNct3 lines in further studies to rule out possible off-target effects of shPsn2 and shNct2.
Figure 2.
Notching wing phenotypes in wing-specific Psn and Nct KD flies. (A) Expression of shPsn or shNct under the control of the c96-Gal4 driver results in thickened veins and/or notching wing margins. Representative adult wing images of control (c96-Gal4/+) and wing-specific Psn (c96 > shPsn) or Nct (c96 > shNct) KD flies are shown. The wing margin is also shown in higher magnification views. Red dots mark thickened veins or blistered phenotypes in the wing margins. Bar, 0.5 mm. (B) Representative adult wing images used to establish the scoring system for quantifying the severity of the wing phenotypes. Score 0: wild-type wing morphology. Score 1: mildly thickened L3 and L4 veins near the margin. Score 2: thickened L2, L3, L4, and L5 veins near the margin. Score 3: more enhanced vein phenotypes. Score 4: severe vein and blistered phenotypes. Score 5: severe margin loss. (C) Quantification of the severity of wing phenotypes using the scoring system shown in (B). At least 20 wings were scored per genotype. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons, ****P < 0.0001.
Early mortality, climbing defects, and rough eyes in neuron-specific Psn KD flies
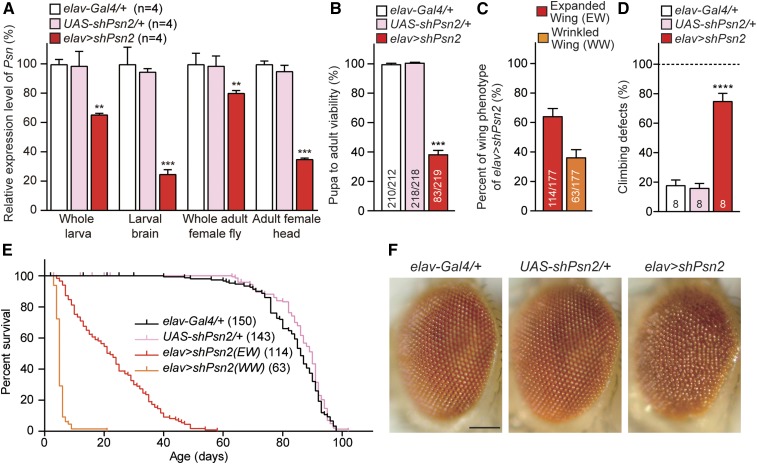
To examine the consequences of neuron-specific Psn and Nct KD, we used the elav-Gal4 driver to express shPsn and shNct in neurons beginning at embryonic stages (Yao and White 1994). We found that elav > shPsn2 flies show pupal lethality in males. At 25°, all elav > shPsn2 flies were females (83/219), and no males eclosed from 219 pupae (Figure 3B), likely due to the stronger Gal4 expression in males (Koushika et al. 1996). Among elav > shPsn2 female escapers, ∼65% (114/177) showed normal expanded wings, and ∼35% (63/177) showed wrinkled wings (Figure 3C). qRT-PCR analysis of third-instar larvae revealed that, relative to the control, levels of Psn mRNA are reduced by ∼34% in whole larvae, and ∼75% in dissected larval brains of elav > shPsn2 (Figure 3A). In adult elav > shPsn2 female escapers, qRT-PCR showed ∼20 and ∼65% reduction of Psn mRNA levels in the whole body and the head, respectively (Figure 3A). The remaining Psn mRNA detected in whole larvae or dissected larval brains and adult flies or heads is due largely to normal Psn expression in non-neuronal cells, where shPsn2 is not expressed.
Figure 3.
Severe early mortality, climbing defects and rough eyes in elav > shPsn flies. (A) Significant reduction of Psn mRNA level in elav > shPsn2. qRT-PCR analysis of Psn mRNA levels in third-instar larvae (whole larvae or dissected brains) or 3-day-old adults (whole flies or heads only). Psn mRNA levels were normalized to rp49 mRNA levels as internal control. Total RNA was extracted from whole larvae (five larvae per genotype), larval brains (15 brains per genotype), whole adult females (five adults per genotype) or adult female heads only (20 heads per genotype). n = 4 independent experiments. (B) Neuron-specific KD of Psn reduces pupa-to-adult viability. Viability was calculated by dividing the total number of flies by the total number of pupae, and shown as the percentage of pupae surviving to adulthood. No male flies eclosed from elav > shPsn2 pupae (0/219), and the number of adult females (83/219) were lower than anticipated. n = 11 independent experiments; ≥210 flies per genotype (∼20 flies per experiment) were used in the study. (C) Neuron-specific Psn KD results in defects on wing expansion. Percentage of elav > shPsn2 flies with expanded or wrinkled wing phenotypes. 63.9 ± 5.6% of elav > shPsn2 flies had normal expanded wings (EW; red) and 36.1 ± 5.6% of flies had wrinkled wings (WW; orange). n = 4 independent experiments. (D) Neuron-specific Psn KD causes defects in climbing ability. Only elav > shPsn2 females with normal expanded wings were used for the climbing assay. Bar indicates percentage of failed climbers. Age = 3 days, n = 8 independent experiments; ≥150 flies per genotype (∼20 flies per experiment) were used in the study. (E) Neuron-specific Psn KD causes severe mortality. Survival of Gal4 control (elav-Gal4/+, black), UAS control (UAS-shPsn2/+, pink), and neuron-specific Psn KD flies (elav-Gal4/+;; UAS-shPsn2/+) with expanded wings (EW, red) and wrinkled wings (WW, orange). Lifespans were plotted by the Kaplan-Meier method. (F) Neuron-specific Psn KD causes rough eye phenotypes. Representative images of the control and ealv > shPsn2 eyes are shown. Bar, 0.1 mm. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Next, we evaluated locomotor function using a well-established climbing assay (Feany and Bender 2000; Al-Ramahi et al. 2007; Rhodenizer et al. 2008). Adult elav > shPsn2 females with normal expanded wings at 3 days of age showed a severe defect in climbing ability (Figure 3D). Furthermore, the lifespan of elav > shPsn2 female escapers with either wrinkled or expanded wings was drastically reduced (Figure 3E). At 25° Culture conditions, most of elav > shPsn2 female escapers with wrinkled wings died before 10 days (Figure 3E), and the maximum (∼58 days) and median (∼22 days) lifespan of elav > shPsn2 female escapers with normal wings were significantly reduced compared to control flies (P < 0.0001, Mantel-Cox test; Figure 3E and Table 1). The elav > shPsn2 female escapers also exhibited rough eye phenotypes compared to control flies (Figure 3F).
Table 1. Analysis of lifespan using the Log-rank test.
| Experimental Group | Number of Flies Tested (n) | Median Lifespan (d) | % Change | χ2 | Log-Rank |
|---|---|---|---|---|---|
| elav > shPsn2 (Figure 3E) | |||||
| elav-Gal4/+ | 150 | 86 | |||
| +/+;; UAS-shPsn2/+ | 143 | 90 | 4.65 | 2.6 | P = 0.1079 |
| elav-Gal4/+;; UAS-shPsn2/+(EW) | 114 | 22 | −75.56 | 345.7 | P < 0.0001 |
| elav-Gal4/+;; UAS-shPsn2/+(WW) | 65 | 5 | −77.27 | 292.1 | P < 0.0001 |
| elav > shPsn3 male (Figure 4D) | |||||
| elav-Gal4/y | 118 | 62 | |||
| +/y; UAS-shPsn3/+ | 119 | 56 | −9.68 | 6.9 | P < 0.01 |
| elav-Gal4/y; UAS-shPsn3/+ | 99 | 23 | −58.93 | 214.1 | P < 0.0001 |
| elav > shPsn3 female (Figure 4E) | |||||
| elav-Gal4/+ | 147 | 87 | |||
| +/+; UAS-shPsn3/+ | 149 | 83 | −4.60 | 36.5 | P < 0.0001 |
| elav-Gal4/+; UAS-shPsn3/+ | 156 | 62 | −19.48 | 284.4 | P < 0.0001 |
| AN-Psn cKD (Figure 7D) | |||||
| elav-Gal4/y;; tub-Gal80ts/+ (control) | 198 | 53 | |||
| elav-Gal4/y;; tub-Gal80ts/UAS-shPsn2 | 222 | 46 | −13.21 | 146.4 | P < 0.0001 |
| AN-Nct cKD (Figure 7E) | |||||
| elav-Gal4/y;; tub-Gal80ts/+ (control) | 233 | 51 | |||
| elav-Gal4/y;; tub-Gal80ts/UAS-shNct2 | 197 | 46 | −9.80 | 76.6 | P < 0.0001 |
| AN-Psn/Nct cKD (Figure 7F) | |||||
| elav-Gal4/y;; tub-Gal80ts/+ (control) | 169 | 51 | |||
| elav-Gal4/y;; tub-Gal80ts, UAS-shPsn2/UAS-shNct2 | 123 | 32 | −37.26 | 388.0 | P < 0.0001 |
% change shows differences in median lifespan between control and experimental groups. P values were determined in each comparison between the control and the experimental group by the Mantel-Cox test.
Similar but less severe phenotypes associated with another Psn RNAi line
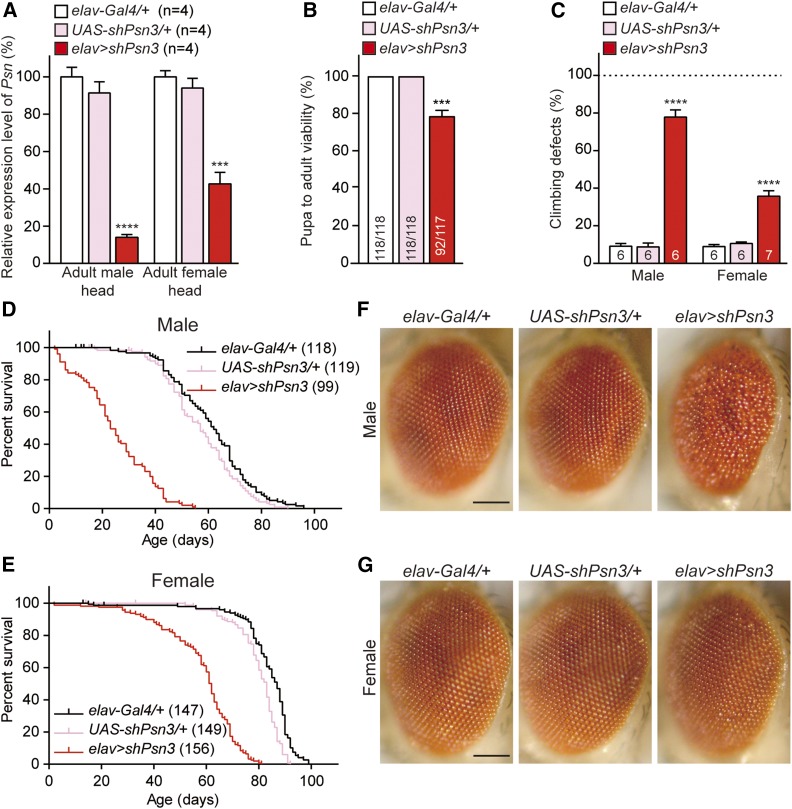
To confirm the specificity of the phenotypes of elav > shPsn2 flies (i.e., that they are not caused by off-target effects of the hairpin used), we tested an additional shRNA line against Psn (UAS-shPsn3), which targets the last exon of the Psn coding region. A lower score in wing margin defects of c96 > shPsn3 relative to c96 > shPsn2 (Figure 2C) suggested that shPsn3 is less effective. Indeed, we were able to obtain male elav > shPsn3 adult flies (33/117), albeit at a reduced number, in contrast to elav > shPsn2 (0/219), whereas female elav > shPsn3 flies were obtained at a normal ratio (59/117). Together, the pupa-to-adult viability of elav > shPsn3 flies was significantly decreased (∼21%) compared to controls (Figure 4B). Thus, two independent shPsn lines in elav > shPsn caused pupal lethality in a dose-dependent manner.
Figure 4.
Similar but less severe phenotypes associated with a different Psn shRNA line. (A) Neuron-specific Psn KD using a different shRNA line results in significant reduction of Psn mRNA levels in 3-day-old adult heads of elav > shPsn3 males (83 ± 3.2%) and females (54 ± 2.8%), compared to controls. qRT-PCR analysis of Psn mRNA level in adult fly heads expressing shPsn3. Psn mRNA levels are normalized to rp49 mRNA levels as internal control. Total RNA was extracted from 20 adult heads per genotype. n = 4 independent experiments. (B) Neuron-specific Psn KD in elav > shPsn3 reduces pupa-to-adult viability (92/117). Viability was calculated by dividing the total number of flies by the total number of pupae, and shown as the percentage of pupae surviving to adulthood. n = 6 independent experiments, ≥110 flies per genotype (∼20 flies per experiment). (C) elav > shPsn3 causes defects in climbing ability in both males and females. Bar indicates percentage of failed climbers. Age = 3 days, n = 6 independent experiments, ≥110 flies per genotype (∼20 flies per experiment). (D, E) elav > shPsn3 causes severe mortality in male (D) and female (E) flies. Survival of Gal4 controls (elav-Gal4/+, black), UAS controls (UAS-shPsn3/+, pink), and elav > shPsn3 flies (elav-Gal4/+;; UAS-shPsn3/+, red). Lifespans were plotted using the Kaplan-Meir method. (F) elav > shPsn3 male flies exhibit severe rough eye phenotypes. (G) In contrast to females with a stronger shPsn2 line (elav > shPsn2), females of elav > shPsn3 show normal compound eyes. Representative images showing adult eye of controls and elav > shPsn3 flies. Bar, 0.1 mm. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons. ***P < 0.001, ****P < 0.0001.
qRT-PCR analysis showed that Psn mRNA levels are reduced in the adult head of elav > shPsn3 male escapers (∼82%) and female flies (∼54%) (Figure 4A). Climbing tests revealed that elav > shPsn3 flies also exhibit defects in locomotion in both males and females (Figure 4C). Furthermore, similar to elav > shPsn2, elav > shPsn3 also showed significantly earlier mortality in male escapers (MaxLS = 55 days, MedLS = 23 days; P < 0.0001; Figure 4D and Table 1) and female flies (MaxLS = 81 days, MedLS = 62 days; P < 0.0001; Figure 4E and Table 1) compared to control flies (male: MaxLS = 96 days, MedLS = 62 days; female: MaxLS = 99 days, MedLS = 87 days). Lastly, similar to elav > shPsn2, the male elav > shPsn3 escapers also exhibit severe rough eyes, a phenotype that is 100% penetrant (Figure 4F), whereas female elav > shPsn3 flies have normal compound eyes (Figure 4G).
Phenotypic rescues of neuron-specific Psn KD using a fly Psn transgene
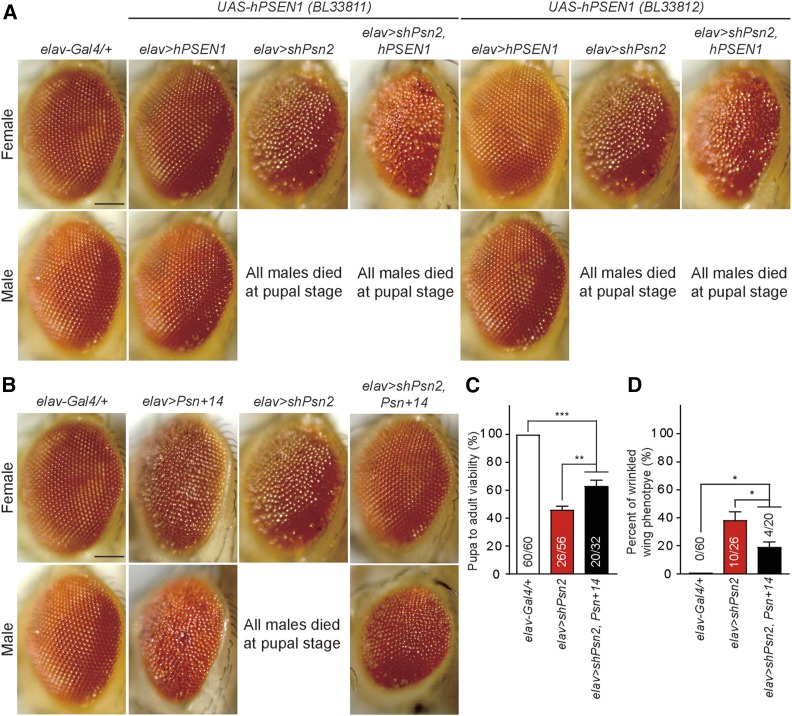
To determine whether the phenotypes observed in elav > shPsn flies can be rescued by human PSEN1 or fly Psn transgenes, we first used two independent lines of human UAS-hPSEN1 (BL33811 and BL33812). Neuron-specific expression of either wild-type human PSEN1 transgene in elav > hPSEN1 flies did not cause any detectable phenotypes in male lethality, wing expansion or eye roughness (Figure 5A). Furthermore, neither of the hPSEN1 transgenes in elav > shPsn2, hPSEN1 flies was able to rescue any of the phenotypes associated with elav > shPsn2 flies, such as male lethality, wrinkled wings, and rough eyes in the female escapers (Figure 5A). This lack of rescue may reflect that human PSEN1 is less efficient in participating in the Drosophila γ-secretase complex.
Figure 5.
Partial phenotypic rescue of neuron-specific Psn KD using a Drosophila Psn transgene. (A) The UAS-hPSEN1 transgenic lines (BL33811 and BL33812) are not able to rescue the male pupal lethality or rough eye phenotype of elav > shPsn2. Representative images show the adult eye of each genotype. Bar, 0.1 mm (B–D) Using a Drosophila Psn transgenic line, UAS-Psn+14 (BL63243), the male pupal lethality, rough eye phenotype, and defective wing expansion in elav > shPsn2 are rescued. (B) Both male and female of elav > Psn+14 show rough eye phenotypes. elav > shPsn2, Psn+14 female flies show full rescue of the rough eye phenotype exhibited in elav > shPsn2. Representative images of adult eye of each genotype. Bar, 0.1 mm (C) The pupa-to-adult viability is significantly rescued in elav > shPsn2, Psn+14 (20/32; P < 0.01) compared to elav > shPsn2 (26/56). However, elav > shPsn2, Psn+14 flies showed reduced viability compared to control (P < 0.001). Viability was calculated by dividing the total number of flies by the total number of pupae, and shown as the percentage of pupae surviving to adulthood. n = 3 independent experiments. (D) The wrinkled wing phenotype of elav > shPsn2 is significantly rescued in elav > shPsn2, Psn+14 (P < 0.05). Specifically, female elav > shPsn2, Psn+14 flies showed normal wing phenotypes, while male elav > shPsn2, Psn+14 flies showed wrinkled wing phenotypes. Percentage of wrinkled wing phenotypes was calculated by dividing the number of flies with wrinkled wings by the total number of flies. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Tukey’s post hoc comparisons. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we tested UAS-Psn+14 (BL63243)—a transgenic line expressing wild-type fly Psn—which exhibits rough eyes in males and females (Ye and Fortini 1999). The Psn+14 line was able to rescue significantly the pupa-to-adult viability of elav > shPsn2 male flies (Figure 5C). At 25°, we were able to obtain male elav > shPsn2, Psn+14 adult flies from pupae (4/32), in contrast to elav > shPsn2 (0/56), whereas female flies were obtained at normal ratios, elav > shPsn2, Psn+14 (16/32), and elav > shPsn2 (26/56). The pupa-to-adult viability of elav > shPsn2, Psn+14 flies was still significantly reduced (∼36%), compared to controls (Figure 5C). While the rough eye and the wrinkled wing phenotypes associated with elav > shPsn2 female flies were rescued in elav > shPsn2, Psn+14 female flies (Figure 5, B and D), elav > shPsn2, Psn+14 male flies still exhibited these phenotypes (Figure 5, B and D). Thus, the phenotypes observed in Psn KD flies, which could be rescued by expression of a fly Psn transgene, are due to loss of Psn function instead of off-target effects of shPsn2.
Similar phenotypes exhibited by two independent lines of neuron-specific Nct KD flies
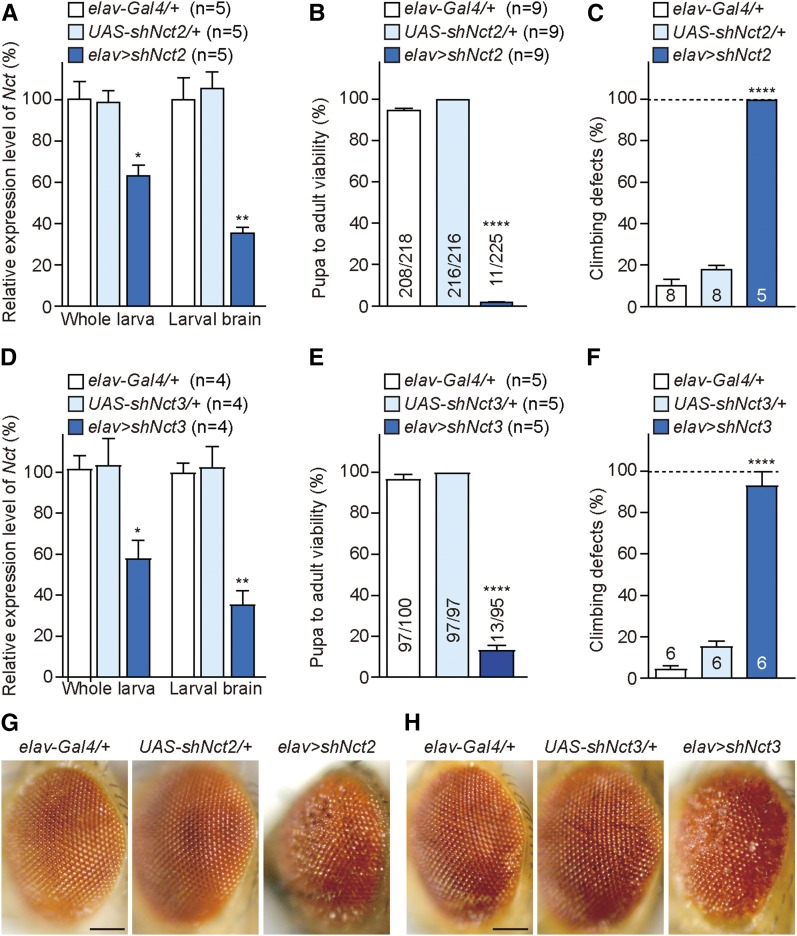
We further tested two independent UAS-shNct lines using the elav-Gal4 driver to determine whether elav > shNct flies exhibit similar phenotypes as those observed in elav > shPsn flies. Interestingly, elav > shNct2 flies showed even more severe phenotypes relative to elav > shPsn flies. At 25°, no males and only 11 females eclosed from 225 elav > shNct2 pupae (Figure 6B). qRT-PCR analysis of elav > shNct2 third-instar larvae revealed that levels of Nct mRNA are reduced by ∼36% in whole larvae, and ∼64% in dissected larval brains relative to the controls (Figure 6A). The remaining Nct mRNA detected in whole larvae or larval brains is largely due to normal Nct expression in non-neuronal cells, where shNct is not expressed. Climbing tests revealed that elav > shNct2 flies exhibit severe defects in locomotion in the female escapers (Figure 6C), and all female elav > shNct2 flies died within 7 days. In addition, all female escapers showed wrinkled wings and rough eye phenotypes (Figure 6G).
Figure 6.
Severe early mortality, climbing defects, and rough eyes in elav > shNct flies. (A, D) Neuron-specific Nct KD using two independent lines, UAS-shNct2 (A) and UAS-shNct3 (D), results in significant reduction of Nct mRNA levels in third-instar larvae compared to controls. qRT-PCR analysis of Nct mRNA level in third-instar larvae (whole larvae or dissected brains) of elav > shNct2 (A) and elav > shNct3 (D). Nct mRNA levels are normalized to rp49 mRNA levels as internal control. Total RNA was extracted from whole larvae (five larvae per genotype) or larval brains (15 brains per genotype). n = 4–5 independent experiments. (B, E) Neuron-specific Nct KD results in decreased pupa-to-adult viability. Viability was calculated by dividing the total number of flies by the total number of pupae, and shown as the percentage of pupae surviving to adulthood. (B) Only 11 females elav > shNct2 eclosed from 225 pupae. n = 9 independent experiments. Total numbers of flies tested: elav-Gal4/+ = 218, UAS-shNct2/+ = 216, and elav > shNct2 = 225 (20 flies per experiment). (E) Only 13 females elav > shNct3 eclosed from 95 pupae; n = 5 independent experiments. Total numbers of flies tested: elav-Gal4/+ = 100, UAS-shNct3/+ = 97, and elav > shNct3 = 95 (20 flies per experiment). (C, F) Neuron-specific Nct KD causes defects in climbing ability. Bar indicates percentage of failed climbers. Age = 3 days. Gal4 and UAS controls: n = 6–8 independent experiments, ≥110 flies per genotype (20 flies per experiment). elav > shNct2: n = 5 flies (C) and elav > shNct3: n = 14 flies (F). (G, H) Females of both elav > shNct2 (G) and elav > shNct3 (H) exhibit rough eye phenotypes. Representative images showing the adult eye of each genotype. Bar, 0.1 mm All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons. *P < 0.05, **P < 0.01, ****P < 0.0001.
Similar to elav > shNct2, elav > shNct3 flies also showed severe pupal lethality with no males, and only 13 females eclosing from 95 pupae (Figure 6E). qRT-PCR analysis of elav > shNct3 third-instar larvae showed reduced levels of Nct mRNA in whole larvae (∼42%), and in dissected larval brains (∼64%) relative to controls (Figure 6D). Furthermore, elav > shNct3 female escapers exhibited severe climbing defects (Figure 6F) and rough eyes (Figure 6H), and all died within 10 days.
These findings together demonstrate that neuron-specific Psn or Nct KD severely impairs development, resulting in pupal to early adult lethality. We therefore decided to impose temporal restriction of Psn or Nct KD to adult neurons to bypass the requirement of Psn and Nct in neural development.
Age-dependent climbing defects and shortened lifespan in adult neuron-specific Psn and Nct cKD flies
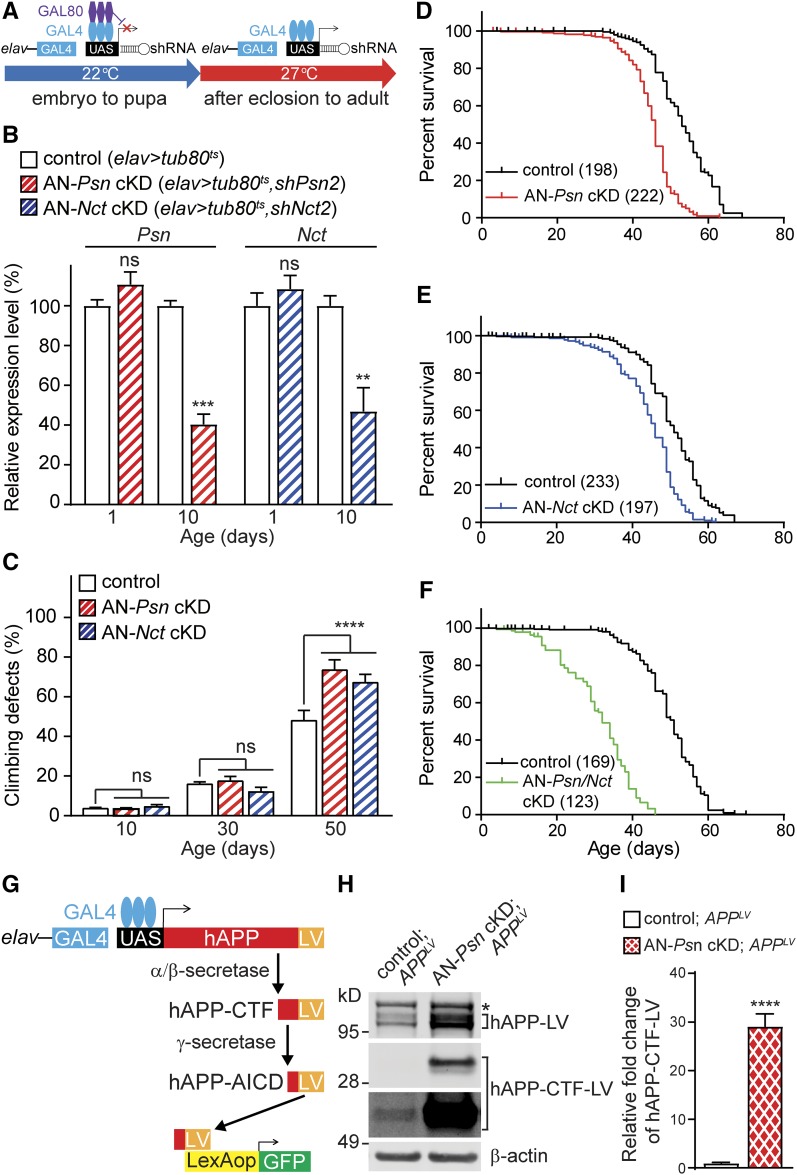
To restrict Psn or Nct KD to adult neurons, we used elav-Gal4 coupled with a ubiquitously expressed temperature-sensitive allele of the Gal80 repressor (tub-Gal80ts) (Ma and Ptashne 1987; McGuire et al. 2003). The temperature-sensitive Gal80 repressor under the control of the tubulin promoter can selectively control the binding affinity of Gal4 to the UAS sequence. To knockdown Psn expression selectively in adult neurons, we generated adult neuron specific Psn conditional KD flies (AN-Psn cKD; elav-Gal4/y;; tub-Gal80ts/UAS-shPsn2). AN-Psn cKD and control flies (elav-Gal4/y;; tub-Gal80ts/+) were cultured at a permissive temperature (22°) until eclosion, and then shifted to a restrictive temperature (27°) after metamorphosis into adults (Figure 7A). At 22°, AN-Psn cKD adult flies, examined 1 day after eclosion, were obtained at normal male/female ratio (0.99 ± 0.02), and showed no climbing defects or eye phenotypes (data not shown). Furthermore, Psn mRNA levels were normal in the adult heads of male AN-Psn cKD flies relative to controls (Figure 7B). Similarly, at 22°, AN-Nct cKD flies did not show male pupal lethality (male/female ratio = 1.00 ± 0.1), climbing defects, eye phenotypes, or reduction of Nct mRNA levels in the adult heads of male AN-Nct cKD flies relative to controls (Figure 7B). For further analysis, only male flies were used, as males of elav > shPsn3 showed greater reduction of Psn mRNA than females (Figure 4A).
Figure 7.
Age-dependent climbing defects and shortened lifespan in adult neuron-specific Psn and Nct KD flies. (A) Strategy for imposing temporal control of shRNA expression using Gal80ts. (B) Adult neuron-specific conditional KD of Psn (AN-Psn cKD; red) and Nct (AN-Nct cKD; blue) results in significant reduction of Psn (red) or Nct (blue) mRNA level in 10-day-old male fly heads, but no significant difference in 1-day-old male fly heads compared to control. qRT-PCR analysis of Psn and Nct mRNA level in the male adult heads of 1 and 10 day-old flies. Total RNA was extracted from 20 male adult heads. Psn and Nct mRNA levels were normalized to rp49 mRNA levels as internal control. n = 4 independent experiments. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons. **P < 0.01, ***P < 0.001. (C) Adult neuron-specific Psn or Nct KD causes defects in climbing ability at the age of 50 days. Bar indicates percentage of failed climbers. n ≥ 5 independent experiments, ≥100 flies per genotype (20 flies per experiment). All data are expressed as mean ± SEM. Statistical analysis was performed using two-way ANOVA with Dunnett’s post hoc comparisons. Two-way ANOVA showed main effects of genotype [F(2, 45) = 6.471; P = 0.0034] and age [F(2, 45) = 556.1; P < 0.0001] with an interaction between these factors [F(4, 45) = 9.905; P < 0.0001]. ns, nonsignificant; ****P < 0.0001. (D–F) Adult neuron-specific Psn and Nct cKD reduce lifespan. AN-Psn/Nct cKD flies showed a greater reduced lifespan relative to AN-Psn cKD and AN-Nct cKD flies. Survival of control (black), AN-Psn cKD (red, D), AN-Nct cKD (blue, E), and AN-Psn/Nct cKD (green, F). Lifespans were plotted by the Kaplan-Meier method. (G–I) Impairment of γ-secretase activity in AN-Psn cDK flies. (G) Strategy for assessing γ-secretase dependent cleavage of the APP-LV reporter system. (H) Western analysis shows dramatic increases of γ-secretase substrate hAPP-CTF-LV in the adult head of male AN-Psn cKD; APPLV flies, indicating reduction of γ-secretase activity. β-actin was used as loading control. Asterisk marks nonspecific band. (I) Quantification of hAPP-CTF-LV levels in AN-Psn cKD; APPLV and control fly heads. hAPP-CTF-LV levels were normalized to β-actin and full length APP protein; n = 3 independent experiments. All data are expressed as mean ± SEM. Statistical analysis was performed using unpaired Student’s t-test. ****P < 0.0001.
At the restrictive temperature (27°), AN-Psn cKD and AN-Nct cKD flies showed significant reduction (∼60%) in Psn or Nct mRNA levels in the heads of 10-day-old males (Figure 7B). Although ∼40% of Psn or Nct mRNA was still detected in the head of AN-Psn cKD or AN-Nct cKD flies relative to controls; this is likely due to normal Psn or Nct expression in non-neuronal cells in the adult fly head, and the reduced efficiency of Gal4 in the presence of Gal80. We then evaluated locomotor function at various ages to determine whether there is age-dependent decline in climbing ability. Climbing tests revealed that control flies showed an age-dependent reduction in climbing [one-way ANOVA F(2, 15) = 108.6, P < 0.0001, Figure 7C]. While AN-Psn cKD and AN-Nct cKD flies at 10 and 30 days of age performed comparably to control flies in the climbing test, AN-Psn cKD and AN-Nct cKD flies at 50 days of age performed significantly worse relative to control flies (P < 0.0001, Figure 7C). A two-way ANOVA showed main effects of genotype [F(2, 45) = 6.471; P= 0.0034] and ages [F(2, 45) = 556.1; P < 0.0001] with an interaction between these factors [F(4, 45) = 9.905; P < 0.0001].
The lifespan of AN-Psn cKD flies was significantly decreased (MaxLS = 63 days, MedLS = 46 days; P < 0.0001) compared to controls (MaxLS = 69 days, MedLS = 53 days; Figure 7D). Similarly, the lifespan of AN-Nct cKD flies was also significantly decreased (MaxLS = 62 days, MedLS = 46 days; P < 0.0001) compared to controls (MaxLS = 67 days, MedLS = 51 days; Figure 7E). Importantly, AN-Psn/Nct cKD (elav-Gal4/y;; tub-Gal80ts, UAS-shPsn2/UAS-shNct2) flies, which knocked down both Psn and Nct in adult neurons, showed an even greater reduction in lifespan (MaxLS = 46 days, MedLS = 32 days; P < 0.0001) compared to AN-Psn cKD and AN-Nct cKD (Figure 7F).
Reduced γ-secretase activity in adult neuron-specific Psn cKD flies
To evaluate γ-secretase activity in AN-Psn cKD flies, we used the Gal4/UAS and LexA/LexAop system to express selectively in adult neurons the APP-LV chimeric protein, in which the full length human APP is fused to the LexA DNA-binding domain and the VP16 activator domain (LV) (Loewer et al. 2004). APP-LV is cleaved to generate the APP intracellular domain (AICD) and LV (AICD-LV) fusion fragment following sequential cleavages by α- or β- and γ-secretases. The AICD-LV fragment translocates to the nucleus, and binds and activates the LexA operator, which drives the expression of a green fluorescent protein (GFP) reporter, permitting visualization of the γ-secretase-mediated cleavage event in vivo (Figure 7G). Western analysis of protein lysates from adult heads (3 days post eclosion) at the restrictive temperature of 27° revealed ∼30-fold accumulation of hAPP-CTF-LV, which is the γ-secretase substrate, in AN-Psn cKD; APPLV flies (elav-Gal4/y; LexAop-hrGFP/+; tub-Gal80, UAS-shPsn2/UAS-APPLV) relative to controls (elav-Gal4/y; LexAop-hrGFP/+; tub-Gal80/UAS-APPLV), indicating severe impairment of γ-secretase activity (Figure 7, H and I). Consistent with the biochemical findings, GFP signals were also diminished in AN-Psn cKD; APPLV brains relative to controls.
Age-dependent neurodegeneration in adult neuron-specific Psn and Nct cKD flies
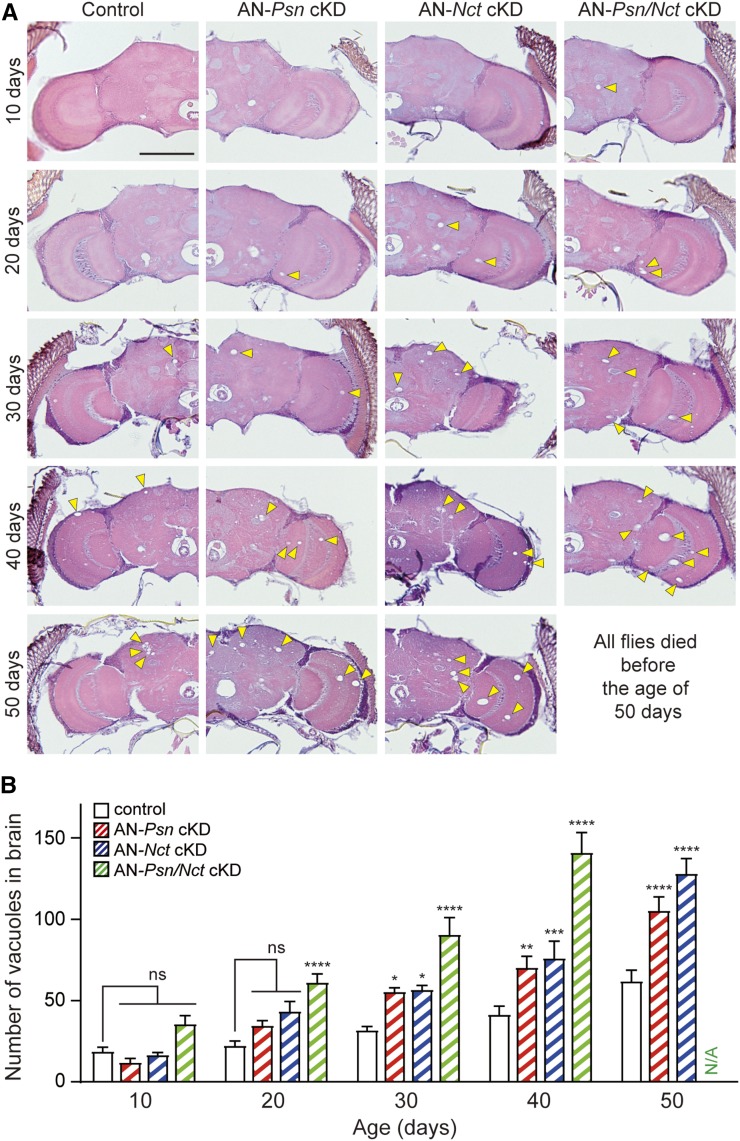
To determine the impact of Psn or Nct KD in neurons of the adult brain during aging, we performed histological analysis of AN-Psn, -Nct and -Psn/Nct cKD flies at multiple ages. Examination by H&E staining revealed age-dependent increases of neuropil vacuolization in the adult brains of AN-Psn, -Nct and -Psn/Nct cKD flies compared to controls (Figure 8A). Quantification of the total number of large vacuoles (≥5 μm) present in the cortex and neuropil of AN-Psn, -Nct and -Psn/Nct cKD flies at the age of 10, 20, 30, 40, and 50 days revealed age-dependent increases of large vacuoles compared to the brains of control flies (Figure 8B). While the number of large vacuoles is not significantly increased in the AN-Psn and AN-Nct cKD brains at 20 days of age compared to control brains (two-way ANOVA with Dunnett’s post hoc), AN-Psn/Nct cKD brains exhibited significant increases in large vacuoles (Figure 8). This is likely due to further reduction of active γ-secretase complexes in AN-Psn/Nct cKD brains, relative to AN-Psn and AN-Nct cKD brains, because both Psn and Nct mRNA are 90% reduced in neurons of AN-Psn/Nct cKD brains. Beginning at 30 days, both AN-Psn and AN-Nct cKD brains showed significant increases in large vacuoles relative to control brains, and the number of vacuoles was even higher in AN-Psn/Nct cKD brains (Figure 8). By 50 days of age, the neurodegeneration phenotype was more severe in AN-Psn and AN-Nct cKD brains, and all AN-Psn/Nct cKD flies died before the age of 50 days. Two-way ANOVA showed main effects of genotype [F(3, 197) = 22.77; P < 0.0001] and age [F(4, 197) = 57.92; P < 0.0001] with an interaction between these factors [F(12, 197) = 19.94; P < 0.0001]. These neuropathology results show that neuronal reduction of Psn and/or Nct in adult flies results in age-dependent and dose-related neurodegenerative changes in the adult brain.
Figure 8.
Age-dependent neurodegeneration in adult neuron-specific Psn and Nct cKD flies. (A) H&E staining in frontal brain sections revealed age-dependent neurodegeneration. Increased number of vacuoles (marked by arrowheads) indicates neurodegeneration in the brain. Bar, 100 μm. (B) Quantification of vacuoles in the brain of control (elav-Gal4/y;; tub-Gal80ts/+, black), AN-Psn cKD (elav-Gal4/y;; tub-Gal80ts/UAS-shPsn2, red), AN-Nct cKD (elav-Gal4/y;; tub-Gal80ts/UAS-shNct2, blue), and AN-Psn/Nct cKD flies (elav-Gal4/y;; tub-Gal80ts, UAS-shPsn2/UAS-shNct2, green). Neurodegeneration is minimal in the brain of control flies. The brains of AN-Psn cKD and -Nct cKD flies show significant age-dependent neurodegeneration compared to brains of control flies at the age of 30, 40, and 50 days. The brains of AN-Psn/Nct cKD flies show significant age-dependent neurodegeneration compared to the brains of control flies at the age of 20, 30, and 40 days. All AN-Psn/Nct cKD flies died before the age of 50 days. Total number of vacuoles >5 μm in diameter was counted throughout all sections of entire brains. n = 10–17 brains per genotype. All data are expressed as mean ± SEM. Statistical analysis was performed using two-way ANOVA with Dunnett’s post hoc comparisons. Two-way ANOVA showed main effects of genotype [F(3, 197) = 22.77; P < 0.0001] and age [F(4, 197) = 57.92; P < 0.0001], with an interaction between these factors [F(12, 197) = 19.94; P < 0.0001]; ns, nonsignificant; *P < 0.05, **P < 0.01, ****P < 0.001, ****P < 0.0001.
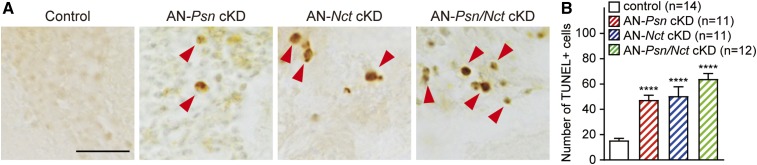
To evaluate whether neurons undergo apoptosis in AN-Psn, -Nct and -Psn/Nct cKD adult brains, we performed TUNEL staining on 30-day-old flies. The number of TUNEL-positive cells in AN-Psn, -Nct and -Psn/Nct cKD brains is significantly increased compared to control brains (number of TUNEL+ cells per brain: control, 15.5 ± 1.6; AN-Psn cKD, 47.6 ± 3.6; AN-Nct cKD, 50.6 ± 7.3; AN-Psn/Nct cKD, 64.17 ± 4.2; P < 0.0001; n ≥ 11 per each genotype; Figure 9). These data demonstrate that adult neuron-specific knockdown of Psn or Nct indeed causes increased apoptotic death in the brain.
Figure 9.
Increased apoptosis in the brain of adult neuron-specific Psn and Nct cKD flies. (A) Apoptotic cells (marked by arrowheads) were identified by TUNEL staining. Bar, 10 μm. (B) Quantification of TUNEL+ cells. The number of TUNEL+ cells is increased in AN-Psn cKD, AN-Nct cKD, and AN-Psn/Nct cKD brains compared to control brains; n = 11–14 brains per genotype at 30 days of age. All data are expressed as mean ± SEM. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc comparisons. ****P < 0.0001.
Discussion
Mutations in the PSEN genes account for ∼90% of the identified FAD mutations, and it has been proposed that PSEN mutations cause FAD via a loss-of-function mechanism (Shen and Kelleher 2007). FAD PSEN mutations are mostly missense mutations (>260) scattered throughout the coding sequence. Genetic studies in mice have demonstrated that Presenilins are essential for synaptic function, learning and memory, and age-dependent neuronal survival (Yu et al. 2001; Beglopoulos et al. 2004; Feng et al. 2004; Saura et al. 2004; Zhang et al. 2009; Wines-Samuelson et al. 2010; Watanabe et al. 2014). Furthermore, studies using cultured cells and knockin mice have shown that FAD PSEN mutations are loss-of-function mutations, causing reduced γ-secretase activity and recapitulating phenotypes of knockout mice (Heilig et al. 2010, 2013; Xia et al. 2015, 2016).
In the current study, we investigated whether Drosophila Psn is also required for neuronal survival in the aging fly brain. We circumvented the pupal lethality associated with germline Psn loss-of-function mutants by generating conditional KD flies expressing Psn shRNA selectively in neurons of the adult brain. Using a ubiquitous driver, we first confirmed that our Psn shRNA lines result in 80–90% reduction of mRNA levels (Figure 1) and recapitulate developmental phenotypes of Psn germ-line mutant flies (Struhl and Greenwald 1999; Ye et al. 1999; Chung and Struhl 2001; Mahoney et al. 2006). Consistent with notching wings in Notch heterozygous mutant flies, and in wing-specific Notch or Psn KD flies (Boyles et al. 2010; Casso et al. 2011), wing disc-specific expression of Psn shRNA leads to similar wing phenotypes at varying severity, allowing selection of the most effective Psn shRNA line (Figure 2). Selective neuronal expression of two independent Psn shRNA lines beginning at embryonic stages caused earlier lethality, rough eye phenotypes, and severe climbing defects (Figure 3 and Figure 4), and these phenotypes were partially rescued by expressing a Drosophila Psn transgene (Figure 5). Further restriction of Psn shRNA expression to adult neurons using the elav-Gal4/tub-Gal80ts system bypassed developmental phenotypes associated with elav > shPsn flies, and resulted in age-dependent climbing defects, shortened lifespan and impairment of γ-secretase activity (Figure 7), age-dependent neurodegeneration (Figure 8), and elevated cell death in the adult brain (Figure 9). Therefore, Drosophila Psn is also required for neuronal survival in the aging brain as well as normal lifespan and behavior, demonstrating that the essential role of PS in neuronal protection during aging is evolutionarily conserved from fruit flies to mammals.
Drosophila Psn models have previously provided significant insight into the normal physiological function of Psn. Impairment of synaptic plasticity and associated learning and memory has been reported in Psn-null mutant larvae (Knight et al. 2007). Heterozygous Psn loss-of-function mutant flies displayed age-dependent learning and memory deficits at old ages without any loss of neurons from TUNEL analysis or gross defects in the mushroom body, suggesting that heterozygosity of Psn in flies is insufficient to cause neurodegeneration (McBride et al. 2010). These findings are consistent with our previous report showing that Psen1 cKO mice exhibit impairment in spatial learning and memory in the absence of neurodegeneration (Yu et al. 2001). Transgenic flies expressing Psn FAD mutations under the endogenous Psn promoter in a Psn-null background showed severe developmental phenotypes including pupal lethality and notching wings, similar to those of Psn loss-of-function mutant flies, indicating that these FAD mutations cause loss of Psn function (Seidner et al. 2006). In addition, large-scale genetic screens in flies with Psn hypomorphic alleles using Notch-like developmental phenotypes in the eye and wing identified genetic modifiers of the Notch pathway (Mahoney et al. 2006). Another screen in flies with Notch-related phenotypes using Psn overexpression in notum and wing recovered modifiers involved in the regulation of Psn activity, calcium signaling and Notch signaling (van de Hoef et al. 2009). While Presenilin regulates neurogenesis via Notch signaling in the developing mouse brain (Song et al. 1999; Handler et al. 2000; Kim and Shen 2008), PS-dependent neuronal survival in the aging brain is Notch-independent, as inactivation of both Notch1 and Notch2 in excitatory neurons of the postnatal forebrain does not cause neurodegeneration (Zheng et al. 2012).
In addition to PS, genetic studies of another γ-secretase component Nct, in mice also demonstrated the essential role of Nct in learning and memory, synaptic function, and neuronal survival during aging, highlighting the importance of γ-secretase in these processes relevant to AD pathogenesis (Tabuchi et al. 2009; Lee et al. 2014). We therefore developed multiple Drosophila shNct lines, and tested them in parallel with shPsn lines using ubiquitous or cell type-specific drivers. Similar to Psn KD flies, selective Nct KD in wing discs resulted in notching wing phenotypes, whereas earlier lethality, climbing defects, and rough eyes were observed in neuron-specific Nct KD flies (Figure 2 and Figure 6). Furthermore, selective Nct KD in adult neurons caused age-dependent climbing defects and shortened lifespan (Figure 7), age-dependent neurodegeneration (Figure 8), and elevated cell death in the adult brain (Figure 9). These phenotypes were more severe in adult neuron-specific Psn/Nct double cKD flies (Figure 7, Figure 8, and Figure 9), likely due to further reduction of functional γ-secretase complexes, and, thereby, its activity. Consistent with genetic findings from analysis of PS cDKO and Nct cKO mice (Beglopoulos et al. 2004; Saura et al. 2004; Tabuchi et al. 2009; Wines-Samuelson et al. 2010), the findings from the current Drosophila study provide further evidence for the importance of PS and Nct in the promotion of neuronal survival during aging, highlighting the evolutionary conservation of this pathway in the protection of the aging brain.
A recent report in C. elegans described mitochondrial morphology defects and elevated cytosolic calcium levels in sel-12 loss-of-function mutants (Sarasija and Norman 2015). Indeed, PS cDKO mice exhibit age-dependent reduction of mitochondrial density and selective increases of larger mitochondria in cortical neurons at 6 months of age (Wines-Samuelson et al. 2010). Presenilin plays a key role in the regulation of calcium homeostasis but the regulatory site by PS has been hotly debated because of varying experimental systems employed in the studies (Ho and Shen 2011). Using physiologically relevant experimental systems, such as the hippocampus of PS cDKO mice and primary dissociated hippocampal neurons lacking PS, we previously reported that PS modulates presynaptic short-term plasticity through its regulation of ryanodine receptor-mediated calcium release from the ER (Zhang et al. 2009; Wu et al. 2013). Future studies in adult neuron-specific Psn and Nct fly models using calcium and cell death reporters could provide insight into how calcium dysregulation may be involved in neuronal cell death in vivo.
Despite the importance of Presenilin in promoting neuronal survival in the aging brain, the underlying molecular pathway remains unknown. This is partly due to the fact that only 0.1% of excitatory neurons lacking PS in the cerebral cortex undergo apoptosis at a given time (Wines-Samuelson et al. 2010), making it challenging to study the molecular mechanisms underlying neuronal death in these cells. These unique adult neuron-specific Psn and Nct cKD fly models will permit a functional, genetic dissection to identify downstream targets of PS and physiological substrates of γ-secretase that mediate neuronal survival during aging, which may be further explored as novel therapeutic targets for Alzheimer’s disease.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.196881/-/DC1.
Acknowledgments
We thank M. Feany and G. Struhl for their advice during this project and Bloomington Drosophila Stock Center for providing fly stocks. We are grateful for the technical support provided by the staff at the Drosophila RNAi Screening Center. We thank R. Binari, C. Hu, and members of the Shen and Perrimon labs for discussion. This work was supported by grants from the National Institutes of Health (R01NS041783, R01NS042818, R01NS101745 to J.S.) and an award from the MetLife Foundation. N.P. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Communicating editor: H. J. Bellen
Literature Cited
- Al-Ramahi I., Perez A. M., Lim J., Zhang M., Sorensen R., et al. , 2007. dAtaxin-2 mediates expanded Ataxin-1-induced neurodegeneration in a Drosophila model of SCA1. PLoS Genet. 3: e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X. C., Yan C., Yang G., Lu P., Ma D., et al. , 2015. An atomic structure of human gamma-secretase. Nature 525: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglopoulos V., Sun X., Saura C. A., Lemere C. A., Kim R. D., et al. , 2004. Reduced β-amyloid production and increased inflammatory responses in presenilin conditional knock-out mice. J. Biol. Chem. 279: 46907–46914. [DOI] [PubMed] [Google Scholar]
- Boyles R. S., Lantz K. M., Poertner S., Georges S. J., Andres A. J., 2010. Presenilin controls CBP levels in the adult Drosophila central nervous system. PLoS One 5: e14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Casso D. J., Biehs B., Kornberg T. B., 2011. A novel interaction between hedgehog and Notch promotes proliferation at the anterior-posterior organizer of the Drosophila wing. Genetics 187: 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H. M., Struhl G., 2001. Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila. Nat. Cell Biol. 3: 1129–1132. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., et al. , 1999. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522. [DOI] [PubMed] [Google Scholar]
- Dias-Santagata D., Fulga T. A., Duttaroy A., Feany M. B., 2007. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 117: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Donoviel D. B., Hadjantonakis A. K., Ikeda M., Zheng H., Hyslop P. S., et al. , 1999. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13: 2801–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Bender W. W., 2000. A Drosophila model of Parkinson’s disease. Nature 404: 394–398. [DOI] [PubMed] [Google Scholar]
- Feng R., Wang H., Wang J., Shrom D., Zeng X., et al. , 2004. Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1 and presenilin-2. Proc. Natl. Acad. Sci. USA 101: 8162–8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A. C., Fish M., Nusse R., Calos M. P., 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage φC31. Genetics 166: 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson K., Boulianne G. L., 1996. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39: 174–182. [DOI] [PubMed] [Google Scholar]
- Handler M., Yang X., Shen J., 2000. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development 127: 2593–2606. [DOI] [PubMed] [Google Scholar]
- Heilig E. A., Xia W., Shen J., Kelleher R. J., III, 2010. A presenilin-1 mutation identified in familial Alzheimer disease with cotton wool plaques causes a nearly complete loss of γ-secretase activity. J. Biol. Chem. 285: 22350–22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig E. A., Gutti U., Tai T., Shen J., Kelleher R. J., III, 2013. Trans-dominant negative effects of pathogenic PSEN1 mutations on γ-secretase activity and Aβ production. J. Neurosci. 33: 11606–11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A., Shen J., 2011. Presenilins in synaptic function and disease. Trends Mol. Med. 17: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C. S., Koo E. H., 1997. Isolation and characterization of Drosophila presenilin homolog. Neuroreport 8: 665–668. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ye Y., Fortini M. E., 2002. Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev. Cell 2: 69–78. [DOI] [PubMed] [Google Scholar]
- Kim W. Y., Shen J., 2008. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol. Neurodegener. 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T., Seugnet L., Haenlin M., Martinez Arias A., 2000. Two different activities of Suppressor of Hairless during wing development in Drosophila. Development 127: 3553–3566. [DOI] [PubMed] [Google Scholar]
- Knight D., Iliadi K., Charlton M. P., Atwood H. L., Boulianne G. L., 2007. Presynaptic plasticity and associative learning are impaired in a Drosophila presenilin null mutant. Dev. Neurobiol. 67: 1598–1613. [DOI] [PubMed] [Google Scholar]
- Koushika S. P., Lisbin M. J., White K., 1996. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr. Biol. 6: 1634–1641. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Sharma M., Sudhof T. C., Shen J., 2014. Synaptic function of nicastrin in hippocampal neurons. Proc. Natl. Acad. Sci. USA 111: 8973–8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., et al. , 1995. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269: 973–977. [DOI] [PubMed] [Google Scholar]
- Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., et al. , 2000. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature 405: 689–694. [DOI] [PubMed] [Google Scholar]
- Loewer A., Soba P., Beyreuther K., Paro R., Merdes G., 2004. Cell-type-specific processing of the amyloid precursor protein by Presenilin during Drosophila development. EMBO Rep. 5: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H., St Johnston D., 2002. Drosophila nicastrin is essential for the intramembranous cleavage of notch. Dev. Cell 2: 79–89. [DOI] [PubMed] [Google Scholar]
- Lukinova N. I., Roussakova V. V., Fortini M. E., 1999. Genetic characterization of cytological region 77A-D harboring the Presenilin gene of Drosophila melanogaster. Genetics 153: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M., 1987. The carboxy-terminal 30 amino acids of GAL4 are recognized by GAL80. Cell 50: 137–142. [DOI] [PubMed] [Google Scholar]
- Mahoney M. B., Parks A. L., Ruddy D. A., Tiong S. Y., Esengil H., et al. , 2006. Presenilin-based genetic screens in Drosophila melanogaster identify novel notch pathway modifiers. Genetics 172: 2309–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E., Perrimon N., 2008. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M., Choi C. H., Schoenfeld B. P., Bell A. J., Liebelt D. A., et al. , 2010. Pharmacological and genetic reversal of age-dependent cognitive deficits attributable to decreased presenilin function. J. Neurosci. 30: 9510–9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L., 2003. Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302: 1765–1768. [DOI] [PubMed] [Google Scholar]
- Nguyen V., Hawkins C., Bergeron C., Supala A., Huang J., et al. , 2006. Loss of nicastrin elicits an apoptotic phenotype in mouse embryos. Brain Res. 1086: 76–84. [DOI] [PubMed] [Google Scholar]
- Ni J. Q., Zhou R., Czech B., Liu L. P., Holderbaum L., et al. , 2011. A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat. Methods 8: 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D., Martin I., Bhandari P., Pletcher S. D., Grotewiel M., 2008. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp. Gerontol. 43: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., et al. , 1995. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 376: 775–778. [DOI] [PubMed] [Google Scholar]
- Sarasija S., Norman K. R., 2015. A γ-secretase independent role for presenilin in calcium homeostasis impacts mitochondrial function and morphology in Caenorhabditis elegans. Genetics 201: 1453–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura C. A., Choi S. Y., Beglopoulos V., Malkani S., Zhang D., et al. , 2004. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42: 23–36. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., 1995. Genetic dissection of Alzheimer disease, a heterogeneous disorder. Proc. Natl. Acad. Sci. USA 92: 8552–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidner G. A., Ye Y., Faraday M. M., Alvord W. G., Fortini M. E., 2006. Modeling clinically heterogeneous presenilin mutations with transgenic Drosophila. Curr. Biol. 16: 1026–1033. [DOI] [PubMed] [Google Scholar]
- Shen J., Kelleher R. J., III, 2007. The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc. Natl. Acad. Sci. USA 104: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Bronson R. T., Chen D. F., Xia W., Selkoe D. J., et al. , 1997. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell 89: 629–639. [DOI] [PubMed] [Google Scholar]
- Song W., Nadeau P., Yuan M., Yang X., Shen J., et al. , 1999. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc. Natl. Acad. Sci. USA 96: 6959–6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Greenwald I., 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398: 522–525. [DOI] [PubMed] [Google Scholar]
- Tabuchi K., Chen G., Sudhof T. C., Shen J., 2009. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J. Neurosci. 29: 7290–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Hoef D. L., Hughes J., Livne-Bar I., Garza D., Konsolaki M., et al. , 2009. Identifying genes that interact with Drosophila presenilin and amyloid precursor protein. Genesis 47: 246–260. [DOI] [PubMed] [Google Scholar]
- Venken K. J., He Y., Hoskins R. A., Bellen H. J., 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. [DOI] [PubMed] [Google Scholar]
- Vert J. P., Foveau N., Lajaunie C., Vandenbrouck Y., 2006. An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics 7: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Iqbal M., Zheng J., Wines-Samuelson M., Shen J., 2014. Partial loss of presenilin impairs age-dependent neuronal survival in the cerebral cortex. J. Neurosci. 34: 15912–15922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines-Samuelson M., Schulte E. C., Smith M. J., Aoki C., Liu X., et al. , 2010. Characterization of age-dependent and progressive cortical neuronal degeneration in presenilin conditional mutant mice. PLoS One 5: e10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C. W., Wszolek M. F., Shulman J. M., Salvaterra P. M., Lewis J., et al. , 2001. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293: 711–714. [DOI] [PubMed] [Google Scholar]
- Wu B., Yamaguchi H., Lai F. A., Shen J., 2013. Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc. Natl. Acad. Sci. USA 110: 15091–15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D., Watanabe H., Wu B., Lee S. H., Li Y., et al. , 2015. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron 85: 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D., Kelleher R. J., III, Shen J., 2016. Loss of Abβ43 production caused by Presenilin-1 mutations in the knockin mouse brain. Neuron 90: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K. M., White K., 1994. Neural specificity of elav expression: defining a Drosophila promoter for directing expression to the nervous system. J. Neurochem. 63: 41–51. [DOI] [PubMed] [Google Scholar]
- Ye Y., Fortini M. E., 1999. Apoptotic activities of wild-type and Alzheimer’s disease-related mutant presenilins in Drosophila melanogaster. J. Cell Biol. 146: 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y., Lukinova N., Fortini M. E., 1999. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature 398: 525–529. [DOI] [PubMed] [Google Scholar]
- Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., et al. , 2000. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407: 48–54. [DOI] [PubMed] [Google Scholar]
- Yu H., Saura C. A., Choi S. Y., Sun L. D., Yang X., et al. , 2001. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron 31: 713–726. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wu B., Beglopoulos V., Wines-Samuelson M., Zhang D., et al. , 2009. Presenilins are essential for regulating neurotransmitter release. Nature 460: 632–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Tran S., Clouser C., Pignoni F., 2005. Nicastrin controls aspects of photoreceptor neuron specification and differentiation in the Drosophila eye. Dev. Dyn. 234: 590–601. [DOI] [PubMed] [Google Scholar]
- Zheng J., Watanabe H., Wines-Samuelson M., Zhao H., Gridley T., et al. , 2012. Conditional deletion of Notch1 and Notch2 genes in excitatory neurons of postnatal forebrain does not cause neurodegeneration or reduction of Notch mRNAs and proteins. J. Biol. Chem. 287: 20356–20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are presented fully in the article. Drosophila strains used in this study are available upon request.