Abstract
Background
Weak hand grip strength in later life is a risk factor for disability, morbidity and mortality and is central to definitions of sarcopenia and frailty. It is unclear whether rate of change in grip strength adds to level of grip strength as a risk factor for poor ageing outcomes.
Methods
Study participants were 292 community-dwelling men and women whose grip strength was measured during the 1994/5 (average age 67) and 2003/5 (average age 76) phases of the Hertfordshire Ageing Study, UK. Individual rate of change in grip strength was estimated using a residual change method. Mortality was followed-up to 2011 (42 men and 21 women died).
Results
Average grip strengths in 2003/5 were 38.4kg (standard deviation [SD] 8.1) and 23.7kg (SD 6.6) for men and women respectively. Average annualised rates of change in grip strength (2003/5 minus 1994/5) were modest owing to a healthy-participant effect (men: -0.12kg/year SD 0.71; women: 0.08kg/year SD 0.54) but varied widely. Mortality risk varied according to level and rate of change in grip strength (p=0.03); death rates per 100 person years of follow-up were 6.7 (95%CI 4.6,9.6) among participants who lost grip over time and had low grip in 2003/5, in contrast with 0.8 (95%CI 0.1,5.8) among participants whose grip changed little over time and remained high in 2003/5.
Conclusions
Levels of grip strength in later life should be considered in conjunction with estimates of change in grip strength identified by repeat measurement over time. Normative data for longitudinal change in grip strength are required.
Keywords: Sarcopenia, epidemiology, longevity, physical function, change
Introduction
Weak hand grip strength in later life is an established risk factor for subsequent disability [1], morbidity [2] and mortality [3], Grip strength is central to current definitions of sarcopenia [4,5] and physical frailty [6] and there is considerable interest in its role as a marker of healthy ageing [7], as an outcome in intervention studies[8], and as a potential tool for clinical assessment [9].
Cross-sectional and longitudinal studies have described the lifecourse trajectory of grip strength: a period of increase from childhood to a peak in early adult life is apparent, followed by maintenance through to midlife, and finally decline from midlife onwards [10,11]. It is unclear whether an individual’s rate of change in grip strength acts as a risk factor for poor ageing outcomes, over and above their level of grip strength in later life.
We are aware of only seven published studies which have taken longitudinal measurements of grip strength in later life and related rate of change in grip strength to subsequent risk of mortality [12–18]. These studies differed in many ways: three were based in the United States (the RUSH [12], Baltimore Longitudinal Study of Ageing [15], and Women’s Health and Ageing Study [18]), the others in Canada (Canadian Health Survey [13]), the Netherlands (Leiden 85+ Study [14]), Denmark (1905 Birth Cohort [16]) and Sweden (a study of twins [17]); sample sizes ranged from a few hundred to several thousand study participants; five of the studies considered men and women [12–14,16,17], one studied only men[15], and one only women[18]; the average baseline age of the study participants was typically late-seventies to early-eighties but ranged from mid-sixties to mid-nineties; longitudinal measurement of grip strength was conducted across periods of time ranging from two to twenty-five years, with a median number of four grip strength measurements per study (range: 2 to 12); and mortality follow-up ranged from two to forty years. The studies also differed in their conclusions about the relative importance of level and rate of change in grip strength in later life as risk factors for subsequent mortality: three studies concluded that level of grip strength is a more important risk factor for mortality than rate of change in grip strength [12,15,16]; two studies concluded that accelerated loss of grip strength is a stronger risk factor for mortality than level of grip strength [13,17]; and two studies concluded that level and rate of change in grip strength are both risk factors for mortality [14,18]. No UK studies to date have explored the association between level and rate of change in grip strength in later life as risk factors for mortality.
We have addressed this gap in knowledge by exploring the association between level, and rate of change, of grip strength in later life and risk of mortality among the 292 community-dwelling men and women who participated in the 1994/5 and 2003/5 phases of the Hertfordshire Ageing Study, UK, and for whom mortality was followed-up to 2011.
Methods
The Hertfordshire Ageing Study (HAS) has been described previously [19]. A full account of the methods pertinent to this paper may be found in Appendix 1 on the journal’s website. In brief, 292 men and women, born 1920-1930, attended research clinics in Hertfordshire in 1994/5 and 2003/5 and had grip strength measured at both waves of follow-up. All-cause mortality was ascertained for all participants till 13/01/2011.
A research nurse measured grip strength at each clinic using a standard protocol [20]; Harpenden and Jamar dynamometers were used in 1994/5 and 2003/5 respectively. Intra- and inter-observer studies were carried out during the fieldwork (see Appendix 1 for details). The HAS had ethical approval from the Hertfordshire and Bedfordshire Local Research Ethics Committee and all participants gave written informed consent. This work was supported by the Medical Research Council (MC_UP_A620_1015, MC_UU_12011/2).
Statistical methods
Maximum grip strength values were calculated from all available measurements at the 1994/5 clinic and similarly at the 2003/5 clinic.
Characterisation of change in a clinical phenotype is challenging when data are only available at two time points and the phenotype may be subject to the effects of regression to the mean; in this instance, simple calculation of absolute or percentage change may poorly reflect magnitude of change and will be artefactually negatively associated with initial value [21]. To overcome these problems, we characterised change in grip strength by estimating sex-specific linear regression models for grip strength in 2003/5 on grip strength in 1994/5 with adjustment for individual follow-up duration; the standardised residuals from these models function as Twisk’s recommended measure of “residual change” [21] in grip strength over time. For illustrative purposes only, thirds of the distribution of residual change were regarded as providing evidence of “loss”, “no change”, or “gain” of grip strength.
Cox’s proportional hazards models were used to analyse associations between “residual change” in grip strength between HAS clinics, and levels of grip strength in 1994/5 and 2003/5, and risk of mortality between the 2003/5 clinic and 13/01/2011. We considered independent, interaction, and quadratic (second order) effects of grip strength on mortality risk. To maximise sample size, analyses were principally conducted for men and women combined with adjustment for gender. Analyses were conducted using Stata, release 11.
Results
Participant characteristics are shown in table 1; 42 men and 21 women died prior to 13/01/2011. Consistent with a healthy survivor effect in HAS [19] a modest average decline in grip strength was observed between clinics among men (p=0.03) but not women (p=0.13). Nonetheless, substantial variation in annualised change in grip strength was evident for both men (median and [5th, 25th, 75th, 95th centiles]: -0.05kg/year [-1.34,-0.56, 0.46,0.93]) and women (0.08kg/year [-0.80,-0.35,0.43,0.91]) and older age at baseline clinic was associated with accelerated loss of grip strength among men and women. In addition, men who were shorter, had higher BMI and reported slower walking speed at baseline, experienced accelerated loss of grip strength (data not shown).
Table 1. Characteristics of Hertfordshire Ageing Study (HAS) participants.
| Mean (SD) | Men (n=172) | Women (n=120) |
|---|---|---|
| 1994/5 | ||
| Age (yrs) | 67.1 (2.3) | 67.2 (2.1) |
| Maximum grip strength (kg) | 39.5 (6.0) | 23.0 (5.4) |
| Social class: I-IIINM+ | 83 (49%) | 50 (42%) |
| 2003/05 | ||
| Age (yrs) | 76.5 (2.3) | 76.2 (2.1) |
| Follow-up time (yrs)* | 9.6 (9.0, 9.8) | 9.1 (8.7, 9.4) |
| Maximum grip strength (kg) | 38.4 (8.1) | 23.7 (6.6) |
| Annualised change in grip strength (kg/yr) | -0.12 (0.71) | 0.08 (0.54) |
| Height (cm) | 171.4 (6.3) | 158.2 (5.5) |
| Weight (kg) | 81.3 (13.9) | 69.9 (13.7) |
| Smoking status+: Never | 56 (33%) | 51 (43%) |
| Ex | 100 (58%) | 61 (51%) |
| Current | 16 (9%) | 8 (7%) |
| High weekly alcohol intake+ (≥22M; ≥15F units per week) | 16 (9%) | 7 (6%) |
| Walking speed (self-reported)+: Slow/Stroll | 75 (44%) | 59 (50%) |
| Normal speed | 71 (41%) | 40 (34%) |
| Brisk/Fast | 26 (15%) | 20 (17%) |
| Number of systems medicated* | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) |
| Fracture since 45 years age+ | 19 (11%) | 35 (29%) |
| Stroke/TIA+ | 18 (11%) | 7 (6%) |
| Hypertension+ | 116 (67%) | 71 (59%) |
| Deaths between 2003/05 follow-up and 13/01/2011+ | 42 (24%) | 21 (18%) |
n(%);
Median (Lower quartile, Upper quartile); SD: standard deviation; Yr: year; I-IIINM: classes I to III non-manual of registrar general’s social class coded to the SOC90 classification of occupations of most recent full-time occupation and with social class for ever married women assigned the social class of their husband; M: males; F: females; TIA: transient ischaemic attack
Older age, shorter stature and slower walking speed in 2003/5 were associated with lower concurrent grip strength among men and women, and women with many systems medicated had lower grip strength. Older age, slower walking speed and having more systems medicated at the 2003/5 clinic were associated with increased risk of mortality to 13/01/2011 among men and women. Lower social class was associated with increased mortality among men only. These characteristics were regarded as potential confounders of the relationships between level and loss of grip strength and mortality in subsequent analyses.
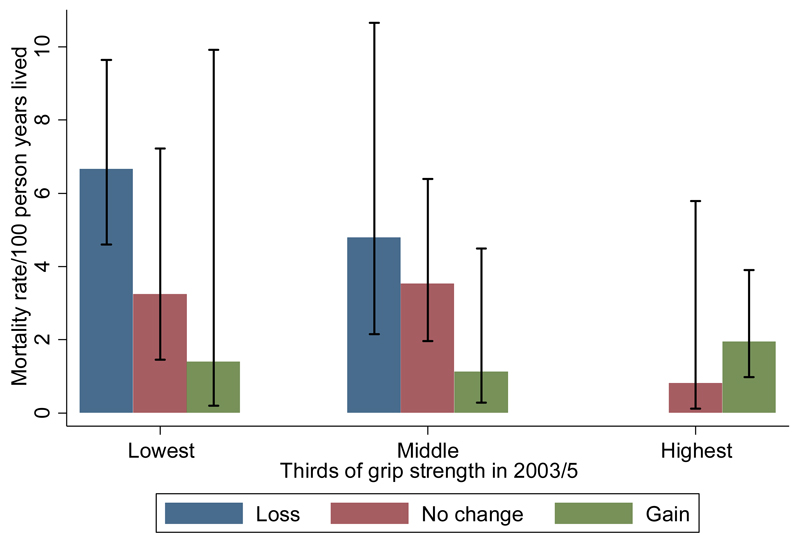
Figure 1 shows that mortality risk between 2003/5 follow-up and 13/01/2011 varied according to combinations of loss of grip strength between HAS follow-ups, and level of grip strength at 2003/5 clinic.. Loss of grip strength was associated with increased mortality but this was accentuated among participants with lower grip strengths in 2003/5 ; conversely, low grip strength in 2003/5 was associated with increased mortality risk but this was accentuated among individuals who experienced the greatest loss of grip strength between follow-ups. For example, the death rate per 100 person years of follow-up (pyrs) was 6.7 (95%CI 4.6,9.6) among individuals who lost grip strength between clinics and had a grip strength in the lowest third of the distribution in 2003/5; in contrast, the death rate was 0.8 (95%CI 0.1,5.8) per 100 pyrs among individuals who experienced no change in grip strength and maintained strength in the highest third of the distribution in 2003/5. Evidence for interaction between loss and level of grip strength as predictors of mortality was significant at the 5% level without and with adjustment for potential confounders (see footnote to figure 1) and the pattern of interaction was consistent for men and women (p=0.61 for a null hypothesis that supports a homogenous pattern of interaction between men and women).
Figure 1. Mortality rates in HAS men and women combined subsequent to 2003/5 clinic according to level of grip strength at the HAS 2003/5 clinic and change in grip strength between 1994/5 and 2003/5 clinics.
Footnotes:
P-value for interaction between level and residual change in grip strength, adjusted for gender: p=0.03. P=0.02 also adjusted for age, height and weight residual; p=0.01 also adjusted for social class, walking speed, smoking habit and alcohol intake; p=0.06 also adjusted for number of systems medicated
Error bars represent 95% confidence limits.
Thirds of the grip strength distribution at 2003/5 clinic were identified by: “lowest” third 15-34kg for men and 2-22kg for women; “middle” third 35-42kg for men and 23-27kg for women; “highest” third 43-58kg for men and 28-39kg for women.
Median crude annualised changes in grip strength (2003/5 minus 1994/5, divided by follow-up duration) within each third of the residual change in grip strength distribution were: “loss” -0.72 kg/year among men and -0.44 kg/year among women; “no change” -0.05kg/year for men and 0.08kg/year for women; “gain” 0.61kg/year among men and 0.59kg/year among women.
Only 2 men and 1 woman experienced loss of grip strength whilst maintaining a grip strength in the highest third of the sex-specific distribution at 2003/5 clinic; none of these men and women died. This death rate estimate has been regarded as missing and omitted from the figure.
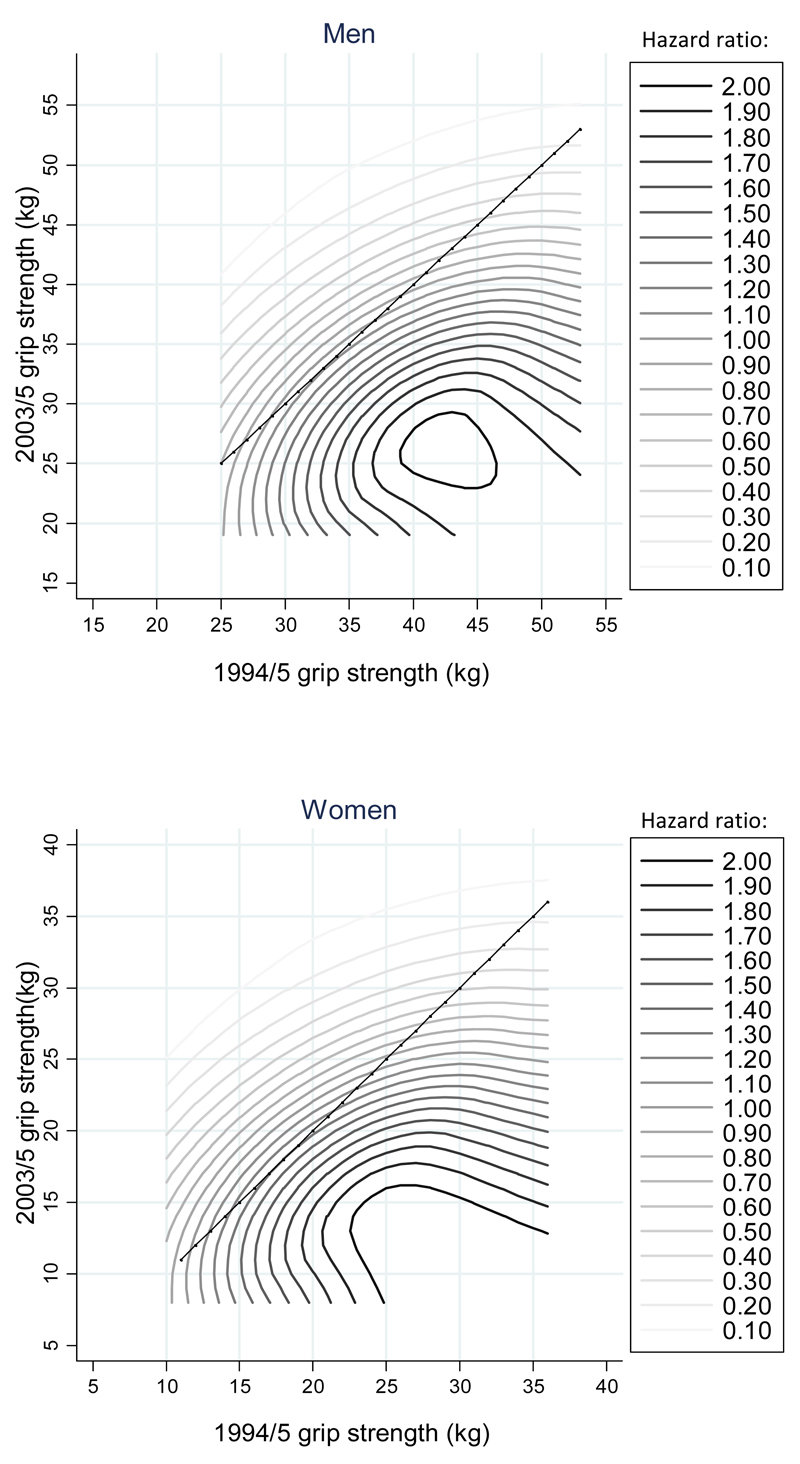
Figure 2 presents contour plots for combinations of measurements of grip strength in 1994/5 and 2003/5 which are estimated to have the same relative risk for mortality in comparison with a reference risk of 1 for individuals with average grip strength at both clinics. The contour patterns in Figure 2 confirm that changes in grip strength combine with levels of grip strength to influence mortality risk; overall, lower grip strength in 2003/5 was associated with increased mortality risk among men and women (note elevated risk contours in the bottom half of the plots) but risks were particularly accentuated if low strength in 2003/5 followed on from a higher level in 1994/5 (note particularly elevated risk contours in the bottom right quadrants of the plots).
Figure 2. Contour plots for combinations of grip strength measurements at HAS 1994/5 and 2003/5 clinics which were estimated to have the same hazard ratio for mortality in comparison with a reference group of individuals with average grip strength at each phase of follow-up.
Footnotes:
These contour plots were derived by fitting a second order model for mortality on linear terms for grip strength at 1994/5 and 2003/5 clinics, in addition to an interaction term, and quadratic terms for each of these grip strength values. A second order model provided a better description of mortality risk than a model that only included linear terms for grip strength at each follow-up (p<0.05 with or without adjustment for potential confounders).
Average grip strengths among men were 39.5kg and 38.4kg at HAS 1994/5 and 2003/5 clinics respectively; corresponding figures for women were 23.0kg and 23.7kg.
The diagonal lines reflect identical values of grip strength at 1994/5 and 2003/5 follow-ups; combinations of grip strength measurements located to the bottom right of the lines reflect loss of grip strength (lower in 2003/5 than 1994/5) and combinations to the top left of the lines reflect gain in grip strength.
Discussion
Using data from the HAS we have identified elevated mortality risks among older people who experience accelerated loss of grip strength in later life, especially when in combination with a low level of grip strength. These results have implications for understanding of the utility of grip strength measurements made in research and clinical settings. Our results suggest that levels of grip strength at any given point in later life should be considered in conjunction with estimates of change in grip strength from repeat measurement over time.
Our conclusion that change combines with level of grip strength as a predictor of mortality in later life is consistent with findings by Ling [14] and Xue [18] who studied older people participating in the Leiden 85-plus and the Women’s Health and Aging Study II respectively. In contrast, Buchman [12], Hirsch [13], Metter [15], Oksuzyan [16], and Proctor [17] found that level of grip strength was more predictive of mortality risk than change in grip strength, and Hirsch [13] and Proctor [17] identified change in grip strength as a stronger predictor of mortality than level. Further studies are required to elucidate the relative importance of level and change in grip strength as risk factors for poor ageing outcomes. Future studies would be well advised: to include many repeated measurements of grip strength within the same individuals over time; to study different phases of later life; and to examine a wide range of ageing outcomes such as disability and morbidity in addition to mortality.
If level and rate of change in grip strength in later life are to have utility as outcomes in intervention studies, and are to have prognostic value in clinical assessment, normative data are required. Dodds et al have recently established lifecourse normative data for grip strength by pooling cross-sectional data from twelve British studies [10]; however, these normative data are unlikely to be applicable in low to middle-income countries and in different ethnic settings [22]. Studies from Sweden [23], Japan [24], the United States [11,25], Denmark [26] and Finland [27] have described within-person longitudinal change in grip strength in later life but no normative data for longitudinal change in grip strength are available for the UK; the development of such norms is an important area for future research.
Our study has some limitations. First, a healthy participant effect is, unsurprisingly, evident in HAS [19]; this selection effect has the potential to bias our results. However, our analyses were internal to the HAS sample; bias would only be introduced if the associations between mortality and level and loss of grip strength were systematically different among those who participated in our study, and those who did not; this seems unlikely. Second, grip strength measurements were only available at two time points; our change measure may not fully reflect underlying individual rate of change in grip strength. However, we were careful to implement Twisk’s [21] recommended “residual change” approach and also conducted complementary analyses based on second order models for baseline and follow-up grip strength measurements in relation to mortality which avoided direct calculation of change; results were consistent which suggests that the results from either one of the approaches are not simply due to statistical artefact. Nonetheless, our results require replication and extension in datasets including many longitudinal measurements of grip strength. Finally, although measured according to identical protocol [20], grip strength was measured using different dynamometers in 1994/5 and 2003/5; we acknowledge this as a limitation of our study. However, Dodds et al have shown that different dynamometers have minimal impact on normative values for grip strength across the lifecourse [10]. Moreover, use of different dynamometers is unlikely to have affected the ranking of grip strength loss for HAS participants.
Our study also has many strengths. First, few studies to date have explored the association between mortality and longitudinal change in grip strength in later life; we have added to this limited literature with the first study of its kind from the UK. Second, we conducted a careful analysis of the HAS longitudinal grip strength data which acknowledged the potential impact of regression to the mean and the limitation of only two repeat measurements. Finally, all measurements in HAS were made according to strict protocol by trained research nurses working as part of an experienced multi-disciplinary research team.
In conclusion, our results have implications for understanding of the utility of grip strength measurements made in research and clinical settings and suggest that levels of grip strength at any given point in later life should be considered in conjunction with estimates of change in grip strength identified by repeat measurement over time. Normative data for longitudinal changes in grip strength in later life are now required.
Supplementary Material
Key points.
It is unclear whether an individual’s rate of change in hand grip strength acts as a risk factor for poor ageing outcomes, over and above their level of grip strength in later life.
Using data from the HAS we have identified elevated mortality risks among older people who experience accelerated loss of grip strength in later life, especially when in combination with a low level of grip strength.
Our results have implications for understanding of the utility of grip strength measurements made in research and clinical settings and suggest that levels of grip strength in later life should be considered in conjunction with estimates of change in grip strength identified by repeat measurement over time.
Footnotes
Conflicts of interest
None declared.
References
- 1.Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC geriatrics. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayer AA, Robinson SM, Patel HP, et al. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42(2):145–50. doi: 10.1093/ageing/afs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. JGerontolA BiolSciMedSci. 2001;56(3):M146–M56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Sayer AA, Kirkwood TB. Grip strength and mortality: a biomarker of ageing? Lancet. 2015;386(9990):226–7. doi: 10.1016/S0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Fielding R, Visser M, et al. Tools in the assessment of sarcopenia. Calcified tissue international. 2013;93(3):201–10. doi: 10.1007/s00223-013-9757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallinen J, Stenholm S, Rantanen T, et al. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58(9):1721–6. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahhas RW, Choh AC, Lee M, et al. Bayesian longitudinal plateau model of adult grip strength. American journal of human biology : the official journal of the Human Biology Council. 2010;22(5):648–56. doi: 10.1002/ajhb.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. Journal of the American Geriatrics Society. 2007;55(1):11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch CH, Buzkova P, Robbins JA, Patel KV, Newman AB. Predicting late-life disability and death by the rate of decline in physical performance measures. Age and Ageing. 2012;41(2):155–61. doi: 10.1093/ageing/afr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling CHY, Taekema D, De Craen AJM, et al. Handgrip strength and mortality in the oldest old population: The Leiden 85-plus study. Cmaj. 2010;182(5):429–35. doi: 10.1503/cmaj.091278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2002;57(10):B359–B65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 16.Oksuzyan A, Maier H, McGue M, Vaupel JW, Christensen K. Sex differences in the level and rate of change of physical function and grip strength in the Danish 1905-cohort study. Journal of aging and health. 2010;22(5):589–610. doi: 10.1177/0898264310366752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proctor DN, Fauth EB, Hoffman L, et al. Longitudinal changes in physical functional performance among the oldest old: Insight from a study of Swedish twins. Aging Clinical and Experimental Research. 2006;18(6):517–30. doi: 10.1007/BF03324853. [DOI] [PubMed] [Google Scholar]
- 18.Xue QL, Beamer BA, Chaves PHM, Guralnik JM, Fried LP. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: The women's health and aging study II. Journal of the American Geriatrics Society. 2010;58(11):2076–84. doi: 10.1111/j.1532-5415.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syddall HE, Simmonds SJ, Martin HJ, et al. Cohort profile: The Hertfordshire Ageing Study (HAS) IntJEpidemiol. 2010;39(1):36–43. doi: 10.1093/ije/dyn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–29. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 21.Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press; 2003. [Google Scholar]
- 22.Dodds R, Syddall HE, Cooper R, et al. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age and Ageing. 2016;45(2):209–16. doi: 10.1093/ageing/afv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly RM, Rosengren BE, Alwis G, et al. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC geriatrics. 2013;13:71. doi: 10.1186/1471-2318-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizaki T, Furuna T, Yoshida Y, et al. Declines in physical performance by sex and age among nondisabled community-dwelling older Japanese during a 6-year period. Journal of epidemiology / Japan Epidemiological Association. 2011;21(3):176–83. doi: 10.2188/jea.JE20100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dam T, Lee C, Guo M, Mantell E. Gender differences in lean mass and grip strength trajectory among community dwelling adults. Journal of the American Geriatrics Society. 2014;62:S91. [Google Scholar]
- 26.Frederiksen H, Hjelmborg J, Mortensen J, et al. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Annals of epidemiology. 2006;16(7):554–62. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Stenholm S, Harkanen T, Sainio P, Heliovaara M, Koskinen S. Long-term changes in handgrip strength in men and women--accounting the effect of right censoring due to death. The journals of gerontology 2012;Series A, Biological sciences and medical sciences. 67(10):1068–74. doi: 10.1093/gerona/gls064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.