Abstract
Angiotensin-(1–7) (Ang-(1–7)) is expressed within the kidney and exhibits renoprotective actions that antagonize the inflammatory, fibrotic and pro-oxidant effects of the Ang II-AT1 receptor axis. We previously identified a peptidase activity from sheep brain, proximal tubules and human HK-2 proximal tubule cells that metabolized Ang-(1–7); thus, the present study isolated and identified the Ang-(1–7) peptidase. Utilizing ion exchange and hydrophobic interaction chromatography, a single 80 kDa protein band on SDS-PAGE was purified from HK-2 cells. The 80 kDa band was excised, the tryptic digest peptides analyzed by LC-MS and a protein was identified as the enzyme dipeptidyl peptidase 3 (DPP 3, EC: 3.4.14.4). A human DPP 3 antibody identified a single 80 kDa band in the purified enzyme preparation identical to recombinant human DPP 3. Both the purified Ang-(1–7) peptidase and DPP 3 exhibited an identical hydrolysis profile of Ang-(1–7) and both activities were abolished by the metallopeptidase inhibitor JMV-390. DPP 3 sequentially hydrolyzed Ang-(1–7) to Ang-(3–7) and rapidly converted Ang-(3–7) to Ang-(5–7). Kinetic analysis revealed that Ang-(3–7) was hydrolyzed at a greater rate than Ang-(1–7) [17.9 vs. 5.5 nmol/min/μg protein], and the Km for Ang-(3–7) was lower than Ang-(1–7) [3 vs. 12 μM]. Finally, chronic treatment of the HK-2 cells with 20 nM JMV-390 reduced intracellular DPP 3 activity and tended to augment the cellular levels of Ang-(1–7). We conclude that DPP 3 may influence the cellular expression of Ang-(1–7) and potentially reflect a therapeutic target to augment the actions of the peptide.
Keywords: Renin angiotensin system, Angiotensin-(1, 7), HK-2 cells, DPP 3
1. Introduction
We recently identified a peptidase activity in the brain medulla and cerebrospinal fluid (CSF) of sheep that metabolized angiotensin-(1–7) [Ang-(1–7)] at low picomolar concentrations [8,13-15]. Marshall et al. [15], using a partially purified fraction of the peptidase from brain medulla, showed the enzyme exhibited a very high affinity (IC50 < 1 nM) to the metallopeptidase agent JMV-390, but was insensitive to other metallopeptidase agents that inhibit neprilysin (NEP, EC. 3.4.24.11), neurolysin (EC 3.4.24.16) and thimet oligopeptidase (TOP, EC3.4.24.15) [15]. The enriched peptidase hydrolyzed Ang-(1–7) at a 12-fold higher rate than Ang II, but failed to metabolize the larger biologically active peptides apelin 13, bradykinin and neurotensin [15]. Interestingly, the Ang-(1–7) peptidase activity was 3-fold higher in the CSF of glucocorticoid-exposed sheep, an experimental model of in utero fetal programing that exhibits higher blood pressure, baroreceptor dysfunction and lower expression of the AT7/Mas receptor [14]. The peptidase activity negatively correlated with endogenous levels of Ang-(1–7) in the CSF. Therefore, central expression of an Ang-(1–7) peptidase may potentially regulate Ang-(1–7) tone within key cardiovascular centers of the brain to influence blood pressure and baroreflex function [8].
Although we originally identified Ang-(1–7) as an endogenous component of the brain renin-angiotensin system (RAS) over 25 years ago [7], the peptide and its receptor Mas were subsequently shown to be expressed within the kidney, as well as other peripheral tissues [5,20]. In contrast to the Ang II-AT1 receptor pathway, Ang-(1–7) increases renal blood flow and acts directly as a natriuretic to enhance sodium excretion likely through the stimulation of nitric oxide [5]. Ang-(1–7) also exhibits renoprotective actions and antagonizes the inflammatory, fibrotic and pro-oxidant actions of Ang II [4,6,21]. Indeed, Ang-(1–7) treatment was recently shown to be superior to AT1 receptor antagonists to attenuate the progression of diabetic renal damage and advanced glycation end product (AGE)-induced myofibroblast transition and cellular hypertrophy [3,28]. The proximal tubule of the kidney is considered a key site for an intrarenal RAS expressing the biosynthetic components angiotensinogen, renin, and ACE that participate in the local formation of Ang II [17]. The tubules also express the ACE homolog ACE2 that efficiently converts Ang II to Ang-(1–7), as well as the endopeptidases NEP and TOP that process Ang I directly to Ang-(1–7) [6,27]. Moreover, the Ang-(1–7) peptidase activity is expressed in the sheep renal cortex, isolated proximal tubules and in human HK-2 proximal tubule cells [25]. The Ang-(1–7) peptidase constituted the sole enzymatic activity in the HK-2 cells that metabolized Ang-(1–7) [25]. Therefore, the current study utilized the human HK-2 cells as a source of the Ang-(1–7) degrading activity to purify, identify and characterize the peptidase. Following extensive purification, a single protein band revealed on SDS-PAGE was analyzed by LC-MS and the enzyme dipeptidyl peptidase 3 (DPP 3, EC: 3.4.14.4) was identified in the HK-2 cell line.
2. Methods
2.1. Cell culture
Human HK-2 cells derived from human proximal tubular cells were obtained from ATCC (Manassas, VA, USA). The cells were incubated at 37°C under 5% CO2 humidified atmosphere, and were routinely maintained in DMEM/F12 supplemented with 10% FBS, insulin-transferrin-selenium-cortisol, 100μg/mL penicillin and 100 μg/mL streptomycin [26]. HK-2 cells were placed in serum-free DMEM/F12 media for 24 h prior to the metabolism and purification studies.
2.2. Cell preparation
Frozen cell pellets were resuspended in HEPES buffer (25 mM Na+ free HEPES, 10 μM ZnCl2, 125 mM NaCl, 0.01% Triton, pH 7.4) and sonicated briefly utilizing an ultrasonic processor (Model W-380 Heat Systems-Ultrasonics, Inc.) and centrifuged at 100,000 ×g for 30min at 4°C. Supernatants were stored on ice and utilized for peptide metabolism experiments or the enzyme purification (HK-2 supernatant).
2.3. HK-2 peptidase purification
The Ang-(1–7) peptidase was purified from HK-2 cells using a similar approach to enrich the brain peptidase but with an additional column step and SDS-PAGE fractionation (Fig. 1) [15]. The HK-2 supernatant was initially pooled and concentrated on a 30 kDa cut-off filter (Millipore, Bedford, MA, USA). The concentrated supernatant was resuspended in a final volume of 5 mL HEPES buffer (25 mM HEPES, 50 mM NaCl, 10 μM ZnCl2, 0.01% Triton, pH 8.0) and applied to a Cibacron Blue Sepharose Fast Flow (GE Healthcare Bio-Sciences, USA) column (1 × 5 cm) equilibrated in the same HEPES buffer. The flow-through fraction was applied directly to a diethylaminoethyl Sepharose column (DEAE, 1 × 10 cm, Sigma-Aldrich, St. Louis, MO, USA). The column was subsequently washed in HEPES buffer with an increasing step gradient of NaCl (75, 100, 125,250 mM). The eluted activity in the 125 and 250 mM NaCl fractions were diluted in HEPES buffer without NaCl then applied to a Q-Sepharose Fast Flow column (1 × 10 cm, Sigma-Aldrich) with a step gradient of increasing NaCl (155,185, 215, and 250 mM). Peptidase activity eluted at 185–215 mM NaCl and the two fractions were concentrated in the HEPES buffer containing 1 M NH4SO4 and applied to 2 × 10 cm Phenyl-Sepharose column (Sigma-Aldrich). This column was washed with 1 M NH4SO4 and a decreasing step gradient was applied (500, 250, 100, 0mM). The enzyme activity primarily eluted in 250 mM NH4SO4 and the fraction was concentrated on a 30 kDa filter in the HEPES buffer to remove the NH4SO4.
Fig. 1.
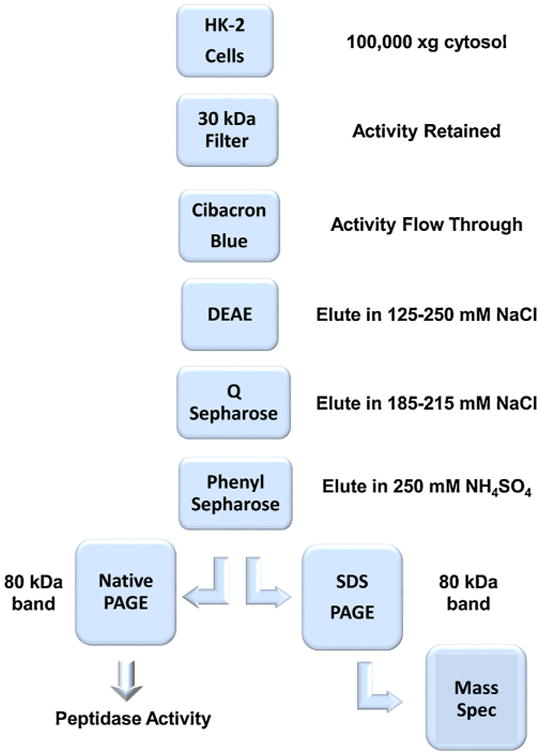
Purification scheme for Ang-(1–7) peptidase activity from human HK-2 cells.
2.4. Native and SDS polyacrylamide electrophoresis
The purified peptidase from the Phenyl Sepharose column was fractionated by both native and SDS 10% polyacrylamide gels for 1hat120Vin Tris-glycine. Gels were stained with the protein dye Imperial Blue and the sole band at 80 kDa excised for peptidase activity (native gel) or the SDS gel for MS analysis (MS Bioworks, Ann Arbor MI USA). For Western blot analysis of the purified enzyme and the human DPP3 standard (R&D Systems, Minneapolis MN USA), the SDS gels were transferred to a polyvinylidene difluoride (PVDF) membrane. Blots were blocked with 5% Bio-Rad Dry Milk (Bio-Rad, Hercules, CA, USA) and Tris buffered saline (TBS) with Tween (0.05%) and probed overnight at 4 °C with a human DPP 3 primary antibody (1:10,000; Abcam, Cambridge MA USA). Membranes were treated with horseradish peroxidase (HRP)-labeled polyclonal anti rabbit secondary antibodies (1:5000) for 1 h and detected with chemiluminescent substrates (Pierce Biotechnology, Rockford, Illinois, USA).
2.5. Peptidase assays
Metabolism reactions were conducted at 37°C in the HEPES assay buffer (25 mM HEPES, 125 mM NaCl, 10 μM ZnCl2, 0.01% Triton, pH 7.4) in the HK-2 cytosol, the column fractions, the supernatant from the excised band of the native gel and human DPP 3. The assay contained a final concentration 0.5 nM 125I-Ang-(1–7) and 100nM Ang-(1–7) in a volume of 250 μL. The reaction was terminated after 60min by addition of ice-cold 1.0% phosphoric acid. The supernatants were filtered (0.2 μm) prior to separation on a Shimadzu Prominence HPLC equipped with an Aeris Peptide XB-C18 (2.1 × 100 mm, Phenomenex, Torrance, CA, USA). The 125I-products were quantified using a Bioscan flow-through γ detector and activity were expressed as fmol of 125I-Ang-(3-4) formed per minute per mg protein (fmol/min/mg) [13].
For the kinetic studies, we varied the concentration of unlabeled Ang-(1–7) or Ang-(3–7) from 0 to 50 μM in the presence of 0.5 nM 125I-Ang-(1–7)or125I-Ang-(3–7).The reaction conditions were 1 ng DPP 3 for 15 min for Ang-(3–7) and 5 ng for 60 min for Ang-(1–7) at 37 °C. The generation of 125I-Ang-(3–4) was determined following HPLC and the Bioscan flow-through γ detector.
For the metabolism of unlabeled Ang-(1–7)by DPP 3, 100 μM of Ang-(1–7) was incubated with 100 ng of human DPP 3 for 60 min at 37 °C with or without the JMV-390 inhibitor (1 μM, Tocris Bioscience, Bristol, UK) under the assay conditions described above. In this case, the HPLC profile of peptide products was monitored at 220 nM using an inline spectrophotometer.
2.6. Cell inhibitor studies
To establish the effect of peptidase inhibition on the cellular expression of Ang-(1–7), the HK-2 cells were placed in serum-free DMEM/F12 media for 24 h and then treated with 20 or 200 nM JMV-390 every 24 h for 3 days. Cell plates were placed on ice, rinsed in cold PBS, scraped and stored at -80 °C. The cell pellet was brought up to 5 mL of Milli-Q water and immediately placed in a boiling water bath for 15min. The solution was acidified with 0.1% formic acid, sonicated and then centrifuged at 25,000 × g (30min at 4°C). The supernatant was applied to a Strata-X (Phenomenex, CA, USA) SPE reversed phase extraction column. The column was washed with Milli-Q water and the peptide fraction eluted in 90% methanol with 0.1% formic acid. The eluent was evaporated in a Savant vacuum centrifuge, reconstituted in the Ang-(1–7) RIA buffer and peptide content determined. As previously described, the Ang-(1–7) RIA recognizes Ang-(2–7) and Ang-(3–7), but does not recognize (<0.01%) other angiotensin peptides including Ang II and AngI [27]. The Ang-(1–7) RIA exhibits a sensitivity of 2.5 fmol per tube. The peptide content of Ang-(1–7) in the HK-2 cells was expressed as fmol per mg protein of the cell extract.
2.7. Statistics
Data are expressed as mean±SEM. A Student's t-test and one-way ANOVA with Bonferroni posttest were applied for the statistical analysis of the data (GraphPad Prism 6, San Diego, CA USA). The criterion for statistical significance was set at p<0.05. The inhibitory constants (IC50) for the peptidase activity were determined by non-linear regression one-site competition with no constraints (GraphPad Prism 6). The Michealis-Menten constants (Km, Vmax) for DPP 3 hydrolysis of Ang-(1–7) and Ang-(3–7) were derived in Prism 6.
3. Results
As shown in the purification outline (Fig. 1), the 100,000 × g cytosolic fraction from the HK-2 cells was initially concentrated on a 30 kDa cut-off filter and applied to a Cibacron Blue 3AG column. The peptidase activity was not retained and the flow through was immediately applied to DEAE Sepharose; Ang-(1–7) degrading activity primarily eluted in 125/250 mM NaCl fractions. These fractions were diluted in HEPES buffer and applied to a Mono-Q ion exchange column, washed and the majority of peptidase activity eluted in the 185/215 mM NaCl fractions. The Q fractions were concentrated, the buffer adjusted to 1 M NH4SO4 and applied to a Phenyl-Sepharose column. The peptidase activity was retained on the column under the 1 M NH4SO4 loading conditions and a reverse step gradient was applied; the activity eluted in the 250 mM NH4SO4 fraction. This fraction was concentrated on a 30 kDa filter and analyzed by SDS-PAGE stained with Imperial Blue. As shown in Fig. 2A, the full-length SDS gel reveals a single protein band at ~80 kDa for the Phenyl-Sepharose fraction. We excised the 80 kDa band from the SDS gel and submitted it for MALDI-MS analysis. The MS analysis revealed 8 human proteins that included keratin, heat shock protein 70, and DPP 3, as well as bovine serum albumin (BSA) that likely reflects internalization from fetal bovine serum by the tubule cells (Fig. 2B). Of the proteins identified, only DPP 3 exhibited the expected molecular size and proteolytic activity to hydrolyze Ang-(1–7). We then obtained an antibody to human DPP 3 and the recombinant human enzyme to evaluate immunostaining of the purified Ang-(1–7) peptidase and human DPP 3. As shown in Fig. 2C, the full-length immunoblot reveals an identical band at 80 kDa for human DPP 3 (lanes 1-2) and the purified Ang-(1–7) peptidase (lanes 3-4) from the HK-2 cells. Although not shown, the Phenyl Sepharose fraction was also analyzed by native PAGE and the stained gel revealed a single band at 80 kDa. This band contained peptidase activity that metabolized 125I-Ang-(1–7) to 125I-Ang-(3-4) (Fig. 2D).
Fig. 2.
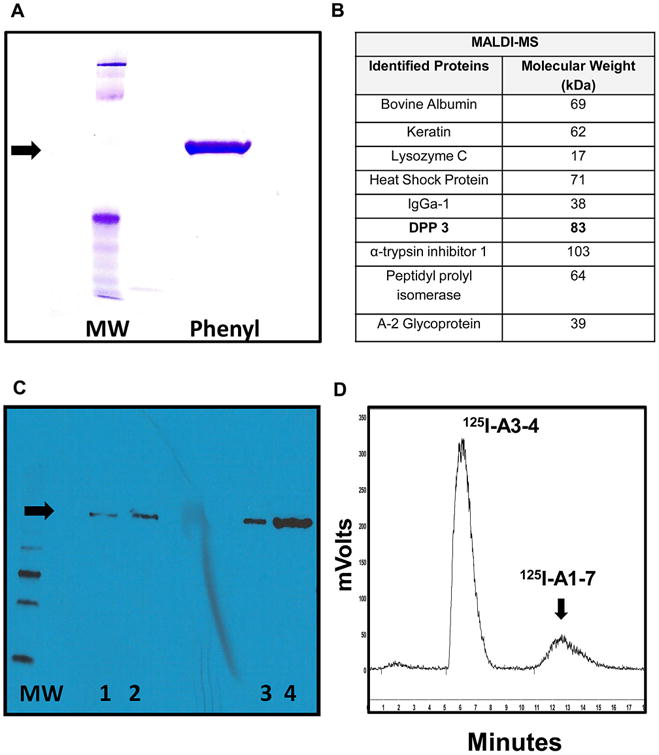
Characterization of purified Ang-(1–7) peptidase from HK-2 cells. A: Phenyl column fraction reveals a single 80kDa band (arrow) on SDS-PAGE gel stained with Imperial Blue. B: Proteins identified by MALDI-MS in the 80kDa band excised from SDS-PAGE gel. C: Western blot of human DPP 3 (lanes 1-2) and purified Ang-(1–7) peptidase with human DPP 3 antibody reveals a single 80 kDa band (arrow). D: Excised 80 kDa band from native PAGE gel of purified Ang-(1–7) peptidase from Phenyl column reveals activity that hydrolyzed 125I-Ang-(1–7) to 125I-Ang-(3-4) (panel D). Molecular weight marker, MW.
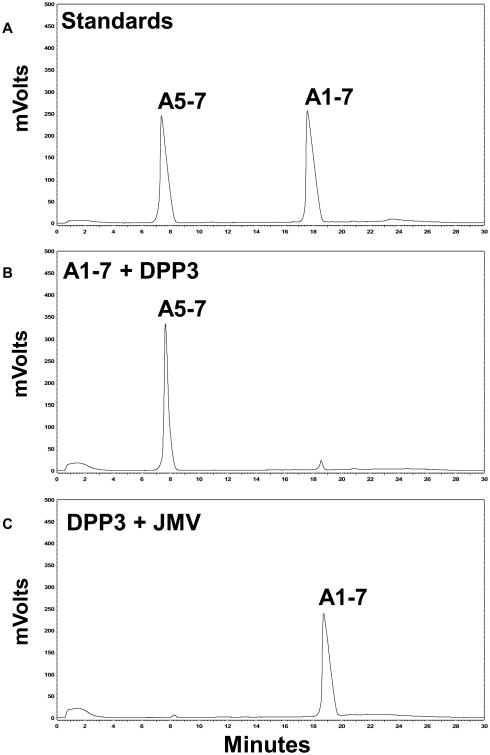
The metabolism of 125I-Ang-(1–7) by the purified Ang-(1–7) peptidase and human DPP 3 was then compared (Fig. 3). As shown in the chromatographs, both the Ang-(1–7) peptidase and DPP 3 cleaved 125I-Ang-(1–7) to predominantly 125I-Ang-(3-4) (Fig. 3A and B). Addition of increasing concentrations of JMV-390 attenuated the metabolism of 125I-Ang-(1–7) by the Ang-(1–7) peptidase (Fig. 3C and 3E) and by DPP 3 (Fig. 3D and 3F). The inhibition studies of JMV-390 for 125I-Ang-(1–7) hydrolysis revealed an IC50 of 0.20 ± 0.01 nM for the Ang-(1–7) peptidase and 1.40 ± 0.01 nM for DPP 3 (Fig. 3G). We confirmed that DPP 3 also hydrolyzed unlabeled Ang-(1–7). As shown in the chromatograph (Fig. 4B), incubation of Ang-(1–7) with DPP 3 for 60min at 37°C revealed a major peak corresponding to the metabolite Ang-(5–7). Treatment of DPP 3 with the JMV-390 inhibitor essentially abolished the metabolism of Ang-(1–7) to Ang-(5–7) (Fig. 4C). Note that unlabeled Ang-(1–7) and Ang-(3–7) co-elute under the current HPLC-UV conditions while their 125I-forms are distinguished (Fig. 3). Addition of the 125I moiety results in greater retention of Ang-(3–7) than Ang-(1–7) such that 125I-Ang-(3–7) elutes approximately 3 min after 125I-Ang-(1–7) (Figs. 2 and 3). Moreover, the HPLC-γ detection of 125I-peptides identifies only their 125I-Tyr residue and as the Ang-(5–7) metabolite lacks the Tyr residue, this peak is not detected in the chromatographs from Fig. 3.
Fig. 3.
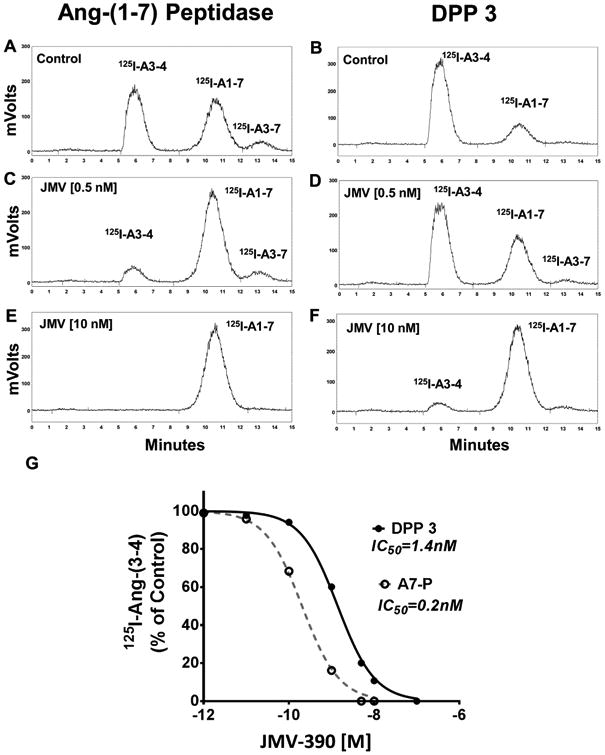
Hydrolysis of 125I-Ang-(1–7) by the purified Ang-(1–7) peptidase and human DPP3. Chromatographs show Ang-(1–7) peptidase and DPP 3 hydrolysis of 125I-Ang-(1–7) alone (A and B) or with 0.5 nM JMV-390 (C and D) or with 10nM JMV-390 (E and F). G: Mean values forJMV-390 inhibition of Ang-(1–7) peptidase (A7-P, -◌) and DPP 3 (-●-). A monophasic curve fit and IC50 values were derived by Prism 6 (n=3).
Fig. 4.
Hydrolysis of Ang-(1–7) by human DPP 3. A: HPLC-UVchromatograph of Ang-(5–7) and Ang-(1–7) standards. B: DPP 3 cleaves Ang-(1–7) to the tripeptide Ang-(5–7). C: Pretreatment of DPP 3 withJMV-390 (1 (1 μM) attenuated the metabolism of Ang-(1–7).
DPP 3 is a metallothiolpeptidase that cleaves two amino acids from the N-terminus of peptide substrates 4-8 residues in length [1].Thus, the predicted pattern of Ang-(1–7) metabolism by DPP 3 should be the immediate formation of the pentapeptide Ang-(3–7) from the initial hydrolysis of the Arg2-Val3 bond and the subsequent formation of the tripeptide Ang-(5–7) by hydrolysis of the Tyr4-Ile4 bond; there should be no further metabolism as DPP 3 does not cleave peptides of 3 or less residues [1]. However, based on the expected metabolism, it was unclear why we failed to observe a predominant peak of 125I-Ang-(3–7). Therefore, we performed a kinetic analysis of 125I-Ang-(1–7) and 125I-Ang-(3–7) metabolism by human DPP 3. For these studies, we used a fixed concentration of the 125I-substrate and increasing concentrations of the unlabeled substrate assuming the peptidase equally recognizes both labeled and unlabeled peptides. Shown in Fig. 5 are the saturation curves for the hydrolysis of Ang-(1–7) and Ang-(3–7) by DPP 3. Derivation of the Michealis-Menten constants revealed a higher Km value of 12 μM for Ang-(1–7) as compared to a Km of 3 μM for Ang-(3–7) (Table 1). Moreover, the Vmax value for Ang-(3–7) was 3-fold higher than that for Ang-(1–7) [17.9 vs 5.5 nmol/min/μg] (Table 1). Calculation of the specificity constant (kcat/Km) also revealed a higher value for Ang-(3–7) than Ang-(1–7) [7.9 vs 0.6] (Table 1). These data likely explain the absence of a peak for Ang-(3–7) as the pentapeptide is rapidly degraded to Ang-(5–7) following the initial hydrolysis of Ang-(1–7) (Fig. 5, inset).
Fig. 5.
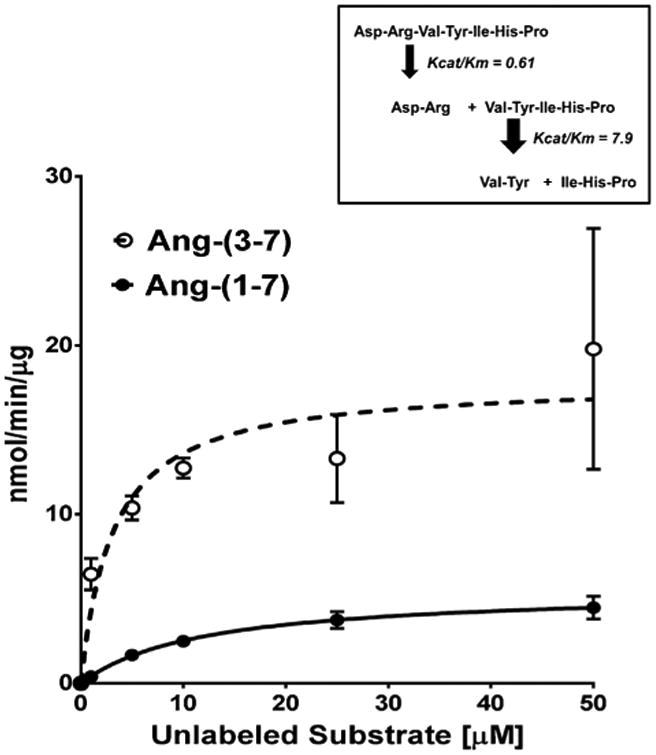
Saturation curves forthe hydrolysis of 125I-Ang-(1–7) and 125I-Ang-(3–7) by human DPP 3. Conversion of either 125I-Ang-(1–7) (-⋏-) or 125I-Ang-(3–7) (-⋌) to 125I-Ang-(3-4) in the presence of increasing concentrations of unlabeled peptide substrates. Curve fit and kinetic constants were derived by Prism 6 (n=3). Inset: Scheme forthe cleavage of Ang-(1–7) and Ang-(3–7) by DPP 3 based on the kinetic data.
Table 1.
Kinetic constants for Ang-(1–7) and Ang-(3–7) metabolism by DPP 3.
| Ang-(1–7) | Ang-(3–7) | |
|---|---|---|
| Vmax | 5.5 ± 0.4 nmol/min/μg | 17.9 ± 1.8 nmol/min/μg |
| Km | 12±2 μM | 3±1 μM |
| kcat | 7.3 s−1 | 23 8 s−1 |
| kcat/Km | 0.61 | 7.9 |
Data are mean±SEM, n=3
Finally, we sought to address whether DPP 3 influences the intracellular expression of Ang-(1–7) in the HK-2 cells by chronically treating the cells with JMV-390. Cells were exposed to 20 and 200 nM JMV-390 for 72 h; the chosen doses of JMV-390 were 100-1000-fold higher than that of the IC50 (0.2 nM) for the purified Ang-(1–7) peptidase. As shown in Fig. 6A, basal or control peptidase activity was 136±6 fmol/min/mg (n = 3). Treatment with 20nM and 200 nM JMV reduced activity by 35% (76 ±13 fmol/min/mg, n = 2) and 85% (17±3 fmol/min/mg; p<0.05, n = 3), respectively. In Fig. 6B, basal content of Ang-(1–7) in the HK-2 cells was 22 ±2 fmol/mg protein (n = 3). The 20 nM JMV-390 dose increased Ang-(1–7) by 68% (37 ±5 fmol/mg protein, n = 3); however, the increase did not achieve statistical significance (Fig. 6B). In contrast, treatment with 200 nM JMV-390 significantly lowered Ang-(1–7) levels (15 ±3 fmols/mg protein; p<0.05, n = 3) as compared to 20 nM JMV-390 (Fig. 6B).
Fig. 6.
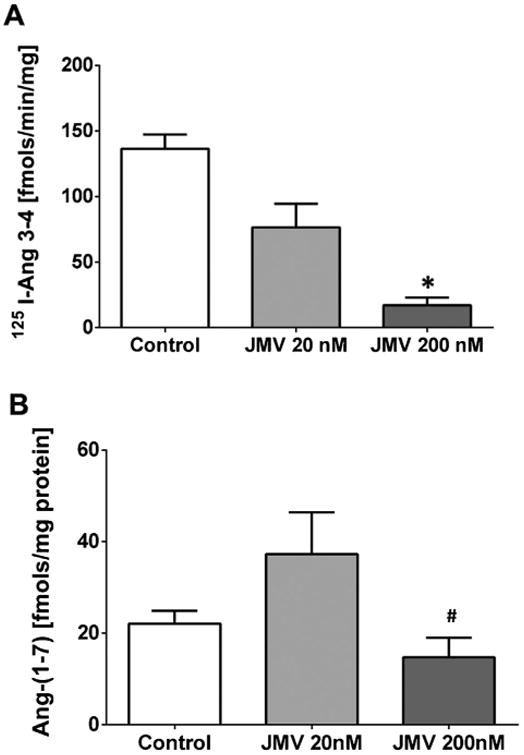
Influence of JMV-390 on 125I- Ang-(1–7) metabolism and intracellular Ang-(1–7) content in HK-2 cells. Cells were treated with 20 or 200 nM of JMV-390 for 72h. A: JMV-390 significantly reduced the metabolism of 125I-Ang-(1–7) to 125I-Ang-(3-4) in the 100,000 × g cytosolic fraction of HK-2 cells. Metabolism data are mean±SEM; *p< 0.05 versus Control, n = 3, except for 20 nM JMV dose, n = 2. Statistical analysis by Student's t-test (Prism 6). B: The 20 nM dose of JMV-390 increased the intracellular content of Ang-(1–7) in the HK-2 cell extracts while 200 nM JMV-390 significantly decreased the content of Ang-(1–7). Ang-(1–7) content data are mean±SEM;, #p<0.05 versus 20nM JMV, n = 3 all groups. Statistical analysis by one-way ANOVA with Bonferroni post-test (Prism 6).
4. Discussion
Current evidence strongly supports an alternative Ang-(1–7) axis of the RAS in the circulation, kidney and other tissues that may buffer or antagonize the actions of the Ang II-AT1 receptor axis [4,5,8,20]. Enzymes that form Ang-(1–7) directly from Ang I in vivo include the endopeptidases neprilysin and thimet oligopep-tidase while ACE2 and prolyl carboxypeptidase hydrolyze Ang II to Ang-(1–7) [6,26]. In regards to the metabolism of Ang-(1–7), we identified ACE as a primary pathway in the circulation that degrades Ang-(1–7) [6]. Circulating levels of Ang-(1–7) markedly increase following administration of ACE inhibitors that reflect the reduced metabolism of Ang-(1–7), as well as the shunting of Ang I away from Ang II and towards Ang-(1–7)-forming pathways [6]. Indeed, Marshall et al. [8,15] demonstrated ACE activity in the sheep CSF that hydrolyzed Ang-(1–7); however, these studies also revealed another peptidase activity that metabolized Ang-(1–7) to a greater extent than ACE. Moreover, this Ang-(1–7)-degrading activity was 3-fold higher in the sheep exposed to antenatal betamethasone, an experimental model of fetal programming that exhibits hypertension and reduced baroreflex sensitivity [8]. Wilson et al. [25] identified a similar if not identical peptidase activity that degraded Ang-(1–7) in the sheep proximal tubules and the human HK-2 cell line. As this activity was the sole pathway for the metabolism of Ang-(1–7) in the HK-2 cells, we isolated the Ang-(1–7) peptidase activity from these cells by multiple chromatographic steps and SDS-PAGE and identified the enzyme as DPP 3. DPP 3 is a soluble metallopeptidase that is widely distributed in both central and peripheral tissues [18]. The enzyme cleaves 2 residues at a time from the N-terminus of various peptides with an optimal substrate length of 4-8 amino acids [1]. The fact that DPP 3 is a thiol metallopeptidase explains the unusual sensitivity of the enzyme to the thiol inhibitors PCMB and APMA, as well as the chelating agents ophenanthroline and EDTA [2,15]. We reported that the Ang-(1–7) peptidase in brain and renal tubules exhibited a low IC50 (∼1 nM) to the metallopeptidase inhibitor JMV-390, and the recombinant form of DPP 3 was also potently inhibited by the JMV compound (IC50 of ∼1 nM) [15,25].
Although we originally considered the Ang-(1–7) degrading activity to be an endopeptidase that forms Ang-(1-4), the present studies confirm that the DPP 3 hydrolyzes Ang-(1–7) to the pentapeptide Ang-(3–7) which undergoes rapid conversion to Ang-(5–7) [15,25]. Indeed, using 125I-Ang-(1–7) as the peptide substrate, accumulation of 125I-Ang-(3–7) was not evident. Moreover, kinetic studies on the hydrolysis of Ang-(1–7) and Ang-(3–7) by DPP 3 revealed that Ang-(3–7) was a superior substrate. The kcat for the hydrolysis of Ang-(3–7) by DPP 3 was 3-fold higher than Ang-(1–7) while the Km for Ang-(3–7) was 6-fold lower than that for Ang-(1–7); the overall kcat/Km was 12-fold higher for Ang-(3–7) and this likely explains the apparent absence of an Ang-(3–7) peak following the initial hydrolysis of Ang-(1–7). These data are also consistent with our previous study in which the fluorescently quenched Ang-(1–7) analog [Abz-Asp1, Tyr (NO2)7]-Ang-(1–7)] was resistant to the HK-2 peptidase [25]. Peptide substrates that contain a N-terminally blocked group are likely resistant to hydrolysis by DPP 3 [18].
DPP 3 is primarily an intracellular peptidase, although we detected activity in the cell media of the HK-2 cells suggesting the possible secretion or release of the enzyme [25]. The HK-2 cells are reported to express a complete RAS including renin, angiotensinogen, ACE and ACE2; therefore, we assessed whether chronic treatment of the HK-2 cells with the JMV-390 inhibitor would influence the intracellular levels of Ang-(1–7) [21]. We find that the lower dose of JMV-390 reduced the activity of DPP 3 by 35% in the cytosol of the HK-2 cells and increased the cellular levels of Ang-(1–7) approximately 2-fold, although this increase was not statistically significant. The higher dose of JMV-390 markedly lowered DPP 3 activity (>80%), but also reduced Ang-(1–7) content. We previously reported that a high dose of JMV-390 inhibited the direct conversion of 125I-Ang I to 125I-Ang-(1–7) by TOP expressed in the HK-2 cells [25]. It is possible that the higher dose of JMV-390in the current study may have elicited a greater effect to block the generation of Ang-(1–7) rather than attenuate the metabolism of the peptide, thus leading to the lower cellular levels of Ang-(1–7). Additional studies are required to determine whether more selective approaches to knockdown DPP 3 would yield a more robust increase in the cellular content of Ang-(1–7), as well as better distinguish the intracellular pathways for the generation and metabolism of the peptide.
Prajapati et al. [19] recently reported that overexpression of DPP 3 and the AT1 receptor in HEK 293 cells attenuated the calcium response to extracellular Ang II. These investigators postulate that DPP 3 degrades intracellular peptide substrates that may influence the signaling of the Ang II-AT1 receptor complex [19]. It is possible that enhanced degradation of intracellular Ang-(1–7) contributes to the inhibitory actions of DPP 3 on Ang II signaling; however, evidence favors that Ang-(1–7) antagonizes or opposes the actions of the Ang II-AT1 receptor axis [4,8,20]. Indeed, we would predict that metabolism of Ang-(1–7) by DPP 3 would augment the Ang II response provided the HEK 293 cells have the capacity to generate Ang-(1–7). Others have reported that DPP 3 activity is enhanced in ovarian cancers and that DPP 3 is associated with the aggressiveness of the tumor which may potentially reflect a lower intracellular content of Ang-(1–7) [21-23]. In this regard, Ang-(1–7) exhibits both anti-proliferative and anti-angiogenic actionsto reduce growth in various tumor models [9-12,16,24].
In conclusion, the present studies reveal that the Ang-(1–7) degrading activity in the HK-2 proximal tubule cells is DPP 3 and that the peptidase activity present in the sheep CSF, brain medulla, and proximal tubules is likely DPP 3. The extent that DPP 3 participates in the intracellular expression of Ang-(1–7) in central and peripheral compartments may reflect the co-localization of the peptidase and Ang-(1–7), as well as the presence of other competing peptide substrates for DPP 3 in these tissues. The intracellular pathways involved in the expression of angiotensin peptides are not clearly established; however, elucidation of the role of DPP 3 to regulate Ang-(1–7) may be important to establish the cellular actions of the alternative axis of the RAS.
Acknowledgments
Dr. Cruz-Diaz is supported by a fellowship from the PRIME Institutional Research and Academic Career Development AwardK12-GM102773. Mr. Wilson is supported by an American Heart Association (AHA) predoctoral fellowship grant (15PRE25120007). Additional support for this study was provided by NIH grant HD-084227; AHA grant 14GRNT20480131; the Groskert Heart Fund, the Wake Forest Venture Fund the Farley-Hudson Foundation (Jacksonville, NC). These studies constitute the partial fulfillment of the requirements for the degree of Doctorate of Philosophy in the Department of Molecular Medicine and Translational Sciences at Wake Forest University School of Medicine for Mr. Wilson. Portions of this work were originally presented at the 2016 Experimental Biology meeting in San Diego CA.
Glossary
- Ang
Angiotensin
- Ang I
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10]
- Ang II
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8]
- Ang
(1–7) – [Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7]
- Ang
(1-4) – [Asp1-Arg2-Val3-Tyr4]
- Ang
(3–7) – [Val3-Tyr4-Ile5-His6-Pro7]
- Ang
(5–7) – [Ile5-His6-Pro7]
- Ang
(3-4) – [Val3-Tyr4]
- ACE
Angiotensin converting enzyme
- ACE2
Angiotensin converting enzyme 2
- CSF
Cerebrospinal fluid
- DPP 3
Dipeptidyl peptidase III
- RAS
Renin-angiotensin system
Footnotes
Conflict of interest: The authors declare that there are no competing financial interests in the work described.
References
- 1.Abramic M, Schleuder D, Dolovcak L, Schroder W, Strupat K, Sagi D, Peter-Katalini J, Vitale L. Human and rat dipeptidyl peptidase III: biochemical and mass spectrometric arguments for similarities and differences. Biol Chem. 2000;381:1233–1243. doi: 10.1515/BC.2000.151. [DOI] [PubMed] [Google Scholar]
- 2.Abramic M, Simaga S, Osmak M, Cicin-Sain L, Vukelic B, Vlahovicek K, Dolovcak L. Highly reactive cysteine residues are part of the substrate binding site of mammalian dipeptidyl peptidases III. Int J Biochem Cell Biol. 2004;36:434–446. doi: 10.1016/s1357-2725(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 3.Alzayadneh EM, Chappell MC. Angiotensin-(1–7) abolishes AGE-induced cellular hypertrophy and myofibroblast transformation via inhibition of ERK 1/2. Cell Signalling. 2014;26:3027–3036. doi: 10.1016/j.cellsig.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7) mas receptor axis; more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 5.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol. 2012;2:2733–2752. doi: 10.1002/cphy.c120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute. Am J Physiol Heart Circ Physiol. 2016;310:H137–H152. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1–7) in rat brain: evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523. [PubMed] [Google Scholar]
- 8.Chappell MC, Marshall AC, Alzayadneh EM, Shaltout HA, Diz DI. Update on the angiotensin converting enzyme 2-angiotensin (1–7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 2014;4:201–215. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher PE, Arter AL, Deng G, Tallant EA. Angiotensin-(1–7): a peptide hormone with anti-cancer activity. Curr Med Chem. 2014;21:2417–2423. doi: 10.2174/0929867321666140205133357. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by Angiotensin-(1–7) Carcinogenesis. 2004;25:2045–2052. doi: 10.1093/carcin/bgh236. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan B, Smith TL, Dubey P, Zapadka ME, Torti FM, Willingham MC, Tallant EA, Gallagher PE. Angiotensin-(1–7) attenuates metastatic prostate cancer and reduces osteoclastogenesis. Prostate. 2013;73:71–82. doi: 10.1002/pros.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan B, Torti FM, Gallagher PE, Tallant EA. Angiotensin-(1–7) reduces proliferation and angiogenesis of human prostate cancer xenografts with a decrease in angiogenic factors and an increase in sFlt-1. Prostate. 2013;73:60–70. doi: 10.1002/pros.22540. [DOI] [PubMed] [Google Scholar]
- 13.Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC. Antenatal betamethasone exposure is associated with lower ANG-(1–7) and increased ACE in the CSF of adult sheep. Am J Physiol Regul Integr Comp Physiol. 2013;305:R679–R688. doi: 10.1152/ajpregu.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall AC, Shaltout HA, Pirro NT, Rose JC, Diz DI, Chappell MC. Enhanced activity of an angiotensin-(1–7) neuropeptidase in glucocorticoid-induced fetal programming. Peptides. 2014;52:74–81. doi: 10.1016/j.peptides.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC. Evidence for an angiotensin-(1–7) neuropeptidase in the brain medulla and CSF of sheep. J Neurochem. 2014;130:313–323. doi: 10.1111/jnc.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA, Gallagher PE. Angiotensin-(1–7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res. 2007;67:2809–2815. doi: 10.1158/0008-5472.CAN-06-3614. [DOI] [PubMed] [Google Scholar]
- 17.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prajapati SC, Chauhan SS. Dipeptidyl peptidase III: a multifaceted oligopeptide N-end cutter. FEBSJ. 2011;278:3256–3276. doi: 10.1111/j.1742-4658.2011.08275.x. [DOI] [PubMed] [Google Scholar]
- 19.Prajapati SC, Singh R, Chauhan SS. Human dipeptidyl peptidase III regulates G-protein coupled receptor-dependent Ca2+ concentration in human embryonic kidney 293T cells. Biol Chem. 2016;397:457–462. doi: 10.1515/hsz-2016-0117. [DOI] [PubMed] [Google Scholar]
- 20.Santos RA. Angiotensin-(1–7) Hypertension. 2014:631138–631147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 21.Shalamanova L, Wilkinson MC, Mc Ardle F, Jackson MJ, Rustom R. Characterization of the expression of the Renin-Angiotensin system in primary and immortalized human renal proximal tubular cells. Nephron Exp Nephrol. 2010;116:e53–e61. doi: 10.1159/000318176. [DOI] [PubMed] [Google Scholar]
- 22.Simaga S, Babic D, Osmak M, Ilic-Forko J, Vitale L, Milicic D, Abramic M. Dipeptidyl peptidase III in malignant and non-malignant gynecological tissue. Eur J Cancer. 1998;34:399–405. doi: 10.1016/s0959-8049(97)00401-2. [DOI] [PubMed] [Google Scholar]
- 23.Simaga S, Babic D, Osmak M, Sprem M, Abramic M. Tumor cytosol dipeptidyl peptidase III activity is increased with histological aggressiveness of ovarian primary carcinomas. Gynecol Oncol. 2003;91:194–200. doi: 10.1016/s0090-8258(03)00462-1. [DOI] [PubMed] [Google Scholar]
- 24.Soto-Pantoja DR, Menon J, Gallagher PE, Tallant EA. Angiotensin-(1–7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol Cancer Ther. 2009;8:1676–1683. doi: 10.1158/1535-7163.MCT-09-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson BA, Cruz-Diaz N, Marshall AC, Pirro NT, Su Y, Gwathmey TM, Rose JC, Chappell MC. An angiotensin-(1–7) peptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme. Am J Physiol Renal Physiol. 2015;308:F594–F601. doi: 10.1152/ajprenal.00609.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson BA, Marshall AC, Alzayadneh EM, Chappell MC. The ins and outs of angiotensin processing within the kidney. Am J Physiol Regul Integr Comp Physiol. 2014;307:R487–R489. doi: 10.1152/ajpregu.00177.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson BA, Nautiyal M, Gwathmey TM, Rose JC, Chappell MC. Evidence for a mitochondrial angiotensin-(1–7) system in the kidney. Am J Physiol Renal Physiol. 2016;310:F637–F645. doi: 10.1152/ajprenal.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Meng X, Li D, Yang J, Kong J, Hao P, Guo T, Zhang M, Zhang Y, Zhang C. Angiotensin-(1–7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin i receptor blockade. Kidney Int. 2015;87:359–369. doi: 10.1038/ki.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]