Abstract
Purpose
Age-based causes and clinical characteristics of immediate-type food allergy (FA) have not been sufficiently studied. Therefore, we investigated age-dependent clinical profiles of FA in Korean children through an extensive multicenter investigation.
Methods
Using a case report form developed by the authors, a retrospective medical record review was performed of patients (0-18 years old) diagnosed with immediate-type FA between September 2014 and August 2015 in 14 tertiary hospitals in Korea.
Results
A total of 1,353 children and adolescents, 93% younger than 7 years, were enrolled in the present study, and 1,661 cases of immediate-type FA were recorded in these patients. The 7 major causative foods were cow's milk (28.1%), hen's eggs (27.6%), wheat (7.9%), walnuts (7.3%), peanuts (5.3%), buckwheat (1.9%), and shrimps (1.9%). Categorizing the patients into 4 age groups revealed that the most common causative food was different for each age group: cow's milk (<2 years), walnuts (2–6 years), walnuts (7–12 years), and buckwheat (13-18 years). The onset time of symptoms was less than 10 minutes in 49%, between 10 and 30 minutes in 17%, and between 30 minutes and 2 hours in 34% of cases. Food-induced anaphylaxis was reported in 506 (30.5%) out of 1,661 cases, and the 7 major causes of food-induced anaphylaxis was cow's milk (27.5%), hen's eggs (21.9%), wheat (11.3%), walnuts (10.5%), peanuts (5.9%), buckwheat (4.2%), and pine nuts (3.0%). The proportion of anaphylaxis was highest in the patients allergic to buckwheat (67.7%), followed by those allergic to pine nuts (57.7%), walnuts (43.8%), wheat (43.5%), and peanuts (34.1%).
Conclusions
The 5 major causative foods of immediate-type FA in Korean children were cow's milk, hen's eggs, wheat, walnuts, and peanuts. The distribution of causative foods was considerably distinctive according to different age groups. Anaphylaxis was reported in 30.5% of immediate-type FA cases.
Keywords: Food hypersensitivity, anaphylaxis, IgE-mediated, children, adolescents
INTRODUCTION
Food allergy (FA) most often begins early in life and can cause severe life-threatening symptoms, such as anaphylaxis.1,2 FA affects up to 10% of children and 6% of adults, and in recent decades the prevalence of FA has increased worldwide.3,4,5,6,7 The reactions of FA are often unpredictable and can have a significant impact on the health-related quality of life in children and their care givers.8
Causes of childhood FA show a distinct pattern according to different age groups. However, the changes in the age-dependent profiles of FA throughout childhood have not been adequately studied yet, despite the growing investigations on allergic diseases worldwide.9 Moreover, the previous reports on childhood FA in Korean children were restricted to small-scale studies or questionnaire-based self-reported surveys. In the present study, we aimed to identify the causes and clinical characteristics of immediate-type FA in different age groups of Korean children through an extensive multicenter investigation.
MATERIALS AND METHODS
Data were collected using a retrospective medical record review of patients younger than 19 years of age, diagnosed with immediate-type FA by experienced pediatric allergists in 14 tertiary hospitals in South Korea between September 2014 and August 2015. Cases with characteristic symptoms of immediate-type FA, such as cutaneous, respiratory, gastrointestinal, and/or cardiovascular system manifesting within 2 hours after exposure to single food items recognized by pediatric allergists by a thorough medical history, were included in the present study regardless of whether an allergy test was conducted or not.10 Unclear cases and cases with an onset time of symptoms longer than 2 hours were excluded from the present study even if sensitization was verified. This study was approved by the Institutional Review Boards of all the participating hospitals.
A 7-page case report form was used for data collection: the form requested the following information: 1) demographic data on the age of the first occurrence of FA symptoms and patient sex; 2) personal and family history of allergic diseases; 3) causes of immediate-type FA and route of exposure; 4) detailed information regarding allergic symptoms after exposure to causative foods, with separate questions for cutaneous, respiratory, gastrointestinal, cardiovascular and neurological/general symptoms; 5) elapsed time between contact with the triggering food and the onset of symptoms; and 6) the place where an allergic reaction occurred.
The clinical manifestations of immediate-type FA were categorized into 5 groups: cutaneous (itching, urticaria, erythema, swelling of lips/tongue/uvula, and angioedema), respiratory (cough, rhinorrhea, wheezing, dyspnea, stridor, and cyanosis), gastrointestinal (nausea, vomiting, diarrhea, and abdominal pain), cardiovascular (chest pain, pallor, collapse, diaphoresis, hypotension, and arrest), and neurological/general (dizziness, anxiety, paresthesia, weakness, confusion, and loss of consciousness). The diagnosis of anaphylaxis was made according to the criteria published in 2006 by the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network, and individual symptoms of anaphylaxis were not separately counted as cutaneous, respiratory, cardiovascular, gastrointestinal, nor neurologic & general.11
The primary aim of the present study was to investigate the distinctive patterns of causative food allergens and clinical profiles of immediate-type FA in different age groups; therefore, cases were categorized into the following 4 age groups based on developmental milestones: infants <2 years; preschoolers 2–6 years, school-aged children 7–12 years; and adolescents 13–18 years.
SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Using a cross-sectional approach, the results of this study are limited to raw and stratified descriptions of the variables.
RESULTS
Demographics
A total of 2,901 cases of FA were identified in 2,056 subjects from 14 participating hospitals during the study period of 1 year. Excluding 1,190 cases with symptom onset time not exactly specified and 50 cases with symptom onset time longer than 2 hours, a total of 1,661 cases of immediate-type FA were analyzed in this study (Supplementary Fig. 1). The number of subjects included was 1,353 since some patients had immediate-type allergic reactions to more than 2 food items; 350 subjects experienced allergic reactions to 2 food items, 117 subjects to 3, 48 subjects to 4, 15 subjects to 5, and 1 subject to 8 food items. The median age of the first occurrence of symptoms was 13 months, and 62.2% of patients were male. More than 90% of patients experienced the first symptoms of immediate-type FA at an age younger than 7 years. A history of concurrent allergic diseases was common. Of the 1,353 patients, 837 were of atopic dermatitis (61.9%), 262 were of allergic rhinitis (19.4%), 155 were of asthma (11.5%), 65 were of allergic conjunctivitis (4.8%), 22 were of chronic urticaria (1.6%), and 15 were of drug allergy (1.1%). More than half of the patients reported a family history of allergic diseases. Table 1 summarizes the demographic characteristics and allergy-related medical histories.
Table 1. Demographic and clinical characteristics of patients.
| N (%) | |
|---|---|
| Total number of subjects | 1,353 |
| Total number of immediate-type FA cases | 1,661 |
| Gender, Male | 842 (62.2) |
| Age at first symptom of FA (median age) | 13 months |
| <2 years | 1,073 (71.1*) |
| 2–6 years | 331 (21.9*) |
| 7–12 years | 76 (5.0*) |
| 13–18 years | 29 (1.9*) |
| Family history of allergic diseases | 692 (51.1) |
| History of concurrent allergic diseases | |
| Atopic dermatitis | 837 (61.9) |
| Allergic rhinitis | 262 (19.4) |
| Asthma | 155 (11.5) |
| Allergic conjunctivitis | 65 (4.8) |
| Chronic urticaria | 22 (1.6) |
| Drug allergy | 15 (1.1) |
*Percentages were calculated based on 1,509 cases of immediate-type FA, since only in 1,509 out of 1,661 cases information regarding the age at first symptom of FA was available.
Causes of FA and routes of exposure
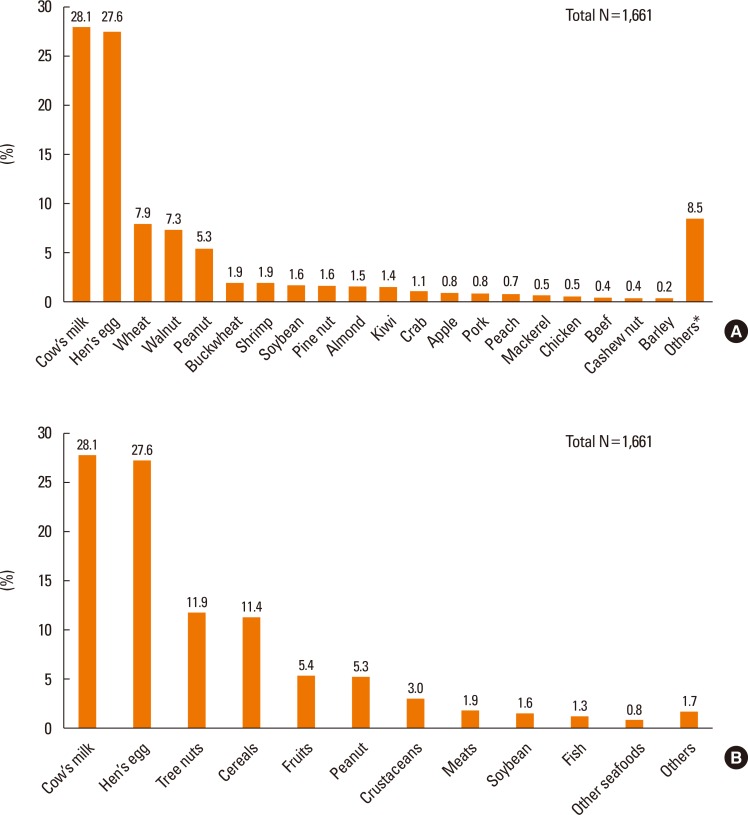
Cow's milk (28.1%) was the most common cause of immediate-type FA in the present study, followed by hen's eggs (27.6%), wheat (7.9%), walnuts (7.3%), peanuts (5.3%), buckwheat (1.9%), and shrimp (1.9%) in the decreasing order of frequencies. The rest of the top 20 causative foods are listed in Fig. 1A. When the triggering foods were analyzed by food groups instead of individual food items, tree nuts were ranked as the third most common triggering food (11.9%), followed by cereals and fruits (Fig. 2B).
Fig. 1.
(A) Causes of immediate-type food allergy in Korean children (individual food items, 0–18 years). (B) Causes of immediate-type food allergy in Korean children (food groups, 0–18 years). *Others: rice, tomato, shellfish, cuttlefish, melon, duck, pistachio, lobster, tuna, salmon, codfish, and processed foods.
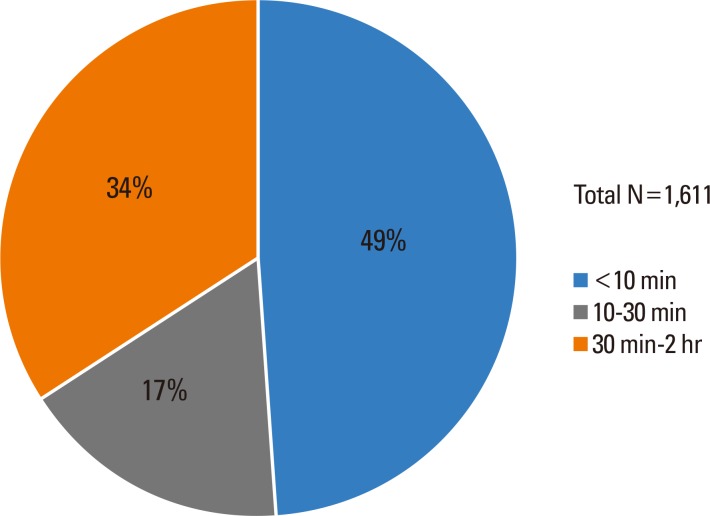
Fig. 2.
Onset time of immediate-type food allergy in Korean children.
The major causative foods were distinct in different age groups (Table 2). In infants, cow's milk and hen's eggs accounted for more than two-thirds of FA. In preschoolers, walnuts (16.6%) were the most common cause of FA, followed by hen's eggs (15.4%) and cow's milk (11.2%). In school-aged children, walnuts (13.2%) were the most common cause of FA, followed by buckwheat (9.2%). In adolescents, buckwheat (17.2%) was ranked the top cause of FA, followed by wheat.
Table 2. Causes of immediate-type food allergy in Korean children depending on the age groups.
| < 2 years | 2-6 years | 7-12 years | 13-18 years | |
|---|---|---|---|---|
| Sample size | 1,073 | 331 | 76 | 29 |
| 1st | Cow's milk (37.1%) | Walnut (16.6%) | Walnut (13.2%) | Buckwheat (17.2%) |
| 2nd | Hen's egg (34.3%) | Hen's egg (15.4%) | Buckwheat (9.2%) | Wheat (10.3%) |
| 3rd | Wheat (8.3%) | Cow's milk (11.2%) | Peanut (6.6%) | Shrimp (10.3%) |
| 4th | Walnut (4.0%) | Peanut (9.7%) | Wheat (6.6%) | |
| 5th | Peanut (3.7%) | Wheat (8.5%) | Crab (6.6%) | |
| 6th | Soybean (1.8%) | Almond (5.1%) | Apple (6.6%) | |
| 7th | Pine nut (0.9%) | Pine nut (3.6%) | Hen's egg (5.3%) | |
| 8th | Shrimp (0.9%) | Buckwheat, Kiwi (3.0% each) | Shrimp (5.3%) |
<2 years and 2–6 years: causative foods with ≥10 cases were included in this table; 7–12 years and 13–18 years: causative foods with ≥3 cases were included in this table.
There were slight variations in the distribution of causative foods among the participating hospitals, but the composition of top 5 food items in the major participating hospitals were not markedly different. Top 10 causative food items in the leading 8 hospitals are listed in Supplementary Table 1.
Of the 1,649 cases, for which the information regarding the route of exposure to causative food was available, 97.9% were exposed via ingestion and 2.1% via cutaneous contact.
Onset time of FA and symptoms
Among 1,661 cases, 49% experienced symptoms within 10 minutes, 17% within 10 to 30 minutes, and 34% within 30 minutes to 2 hours (Fig. 2). Overall, two-thirds experienced symptoms within 30 minutes after exposure to the triggering food.
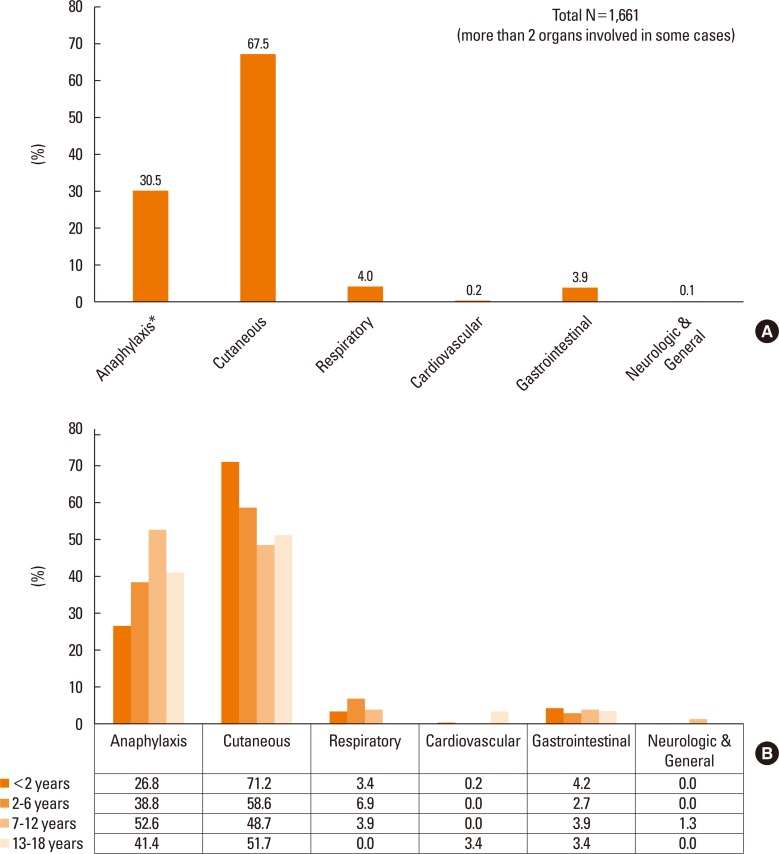
Among the 1,661 cases of immediate-type FA, anaphylaxis was observed in 506 cases (30.5%). Other reactions included predominantly cutaneous symptoms (67.5%), followed by respiratory (4.0%), gastrointestinal (3.9%), cardiovascular (0.2%), and neurological/general (0.1%) symptoms (Fig. 3A). Individual symptoms of anaphylaxis were not separately counted as cutaneous, respiratory, cardiovascular, gastrointestinal, nor neurologic & general. No deaths were reported in the present study. When the symptoms were evaluated according to different age groups, the percentage of anaphylaxis was highest (52.6%) in school-aged children and lowest in infants (26.8%). The cutaneous symptoms were more commonly reported in infants, whereas cardiovascular symptoms were predominantly noticed in adolescents (Fig. 3B).
Fig. 3.
(A) Symptoms of immediate-type food allergy in Korean children (0–18 years). (B) Symptoms of immediate-type FA in Korean children by age groups. *Individual symptoms of anaphylaxis were not separately counted as cutaneous, respiratory, cardiovascular, gastrointestinal, nor neurologic & general.
Place of FA occurrence
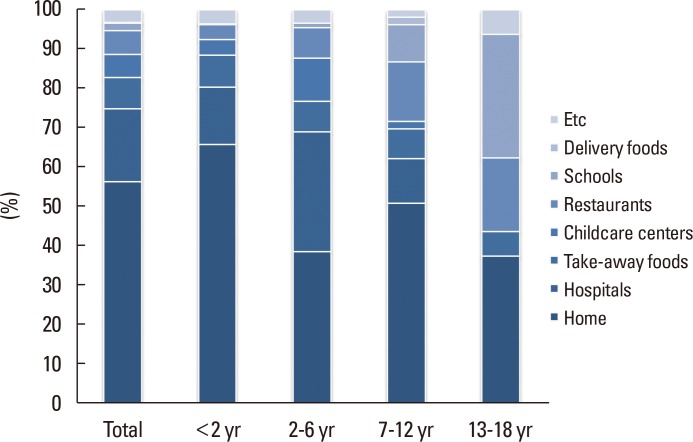
The information where the immediate-type FA occurred was collected in 961 out of 1,661 cases. Overall, the most common place of FA occurrence was at home (56.5%). Other places included hospitals (18.4%, mostly during oral food challenges), take-away food restaurants (8.0%), restaurants (6.1%), childcare centers (5.9%), and schools (1.8%). The percentage of children experiencing allergic reactions at home was highest in infants and lowest in adolescents. With increasing age of the children, the importance of schools and restaurants as places for FA occurrence increased. The detailed percentages for the different places of FA occurrence are given in Fig. 4. No significant differences were noticed between the demographic profiles of cases with and without the information for places of FA occurrence (Supplementary Table 2).
Fig. 4.
Place of occurrence of immediate-type food allergy in Korean children.
Anaphylaxis as manifestation of immediate-type FA
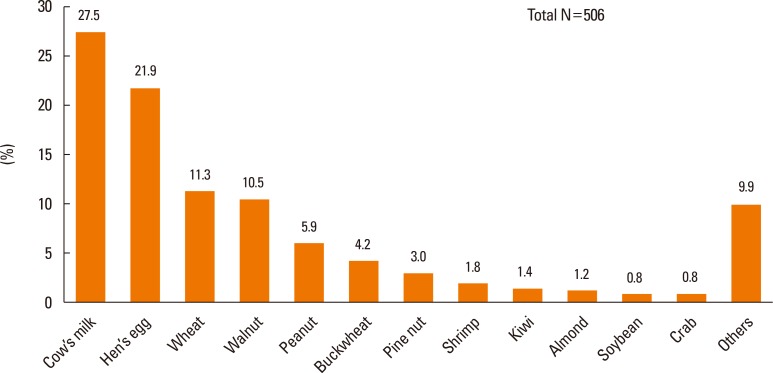
The major causes of food-induced anaphylaxis showed a similar pattern with the overall immediate-type FA (Fig. 5). Among the 506 cases of food-induced anaphylaxis in the present study, cow's milk (27.5%) was the most common cause, followed by hen's eggs (21.9%). The third to sixth common causes (wheat, walnuts, peanuts, and buckwheat) of food-induced anaphylaxis were the same as in the overall immediate-type FA cases, whereas the seventh common cause of food-induced anaphylaxis was pine nuts instead of shrimp.
Fig. 5.
Major causes of food-induced anaphylaxis in Korean children (0–18 years).
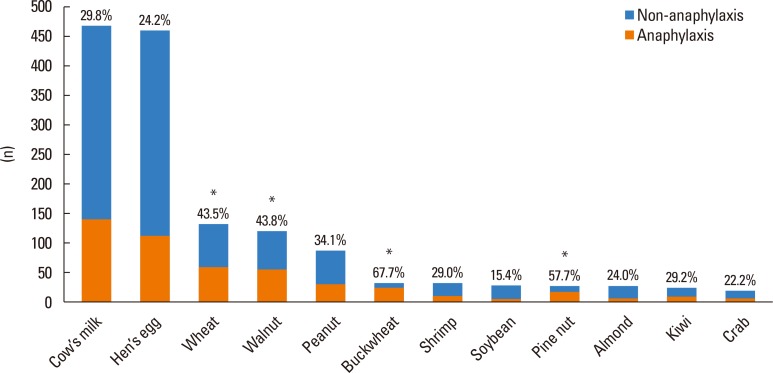
The overall proportion of cases of anaphylaxis was 30.5%; however, the percentages varied remarkably according to different causative foods (Fig. 6). The proportion of anaphylaxis was highest upon exposure to buckwheat (67.7%), followed by exposure to pine nuts (57.7%), walnuts (43.8%), wheat (43.5%), and peanuts (34.1%). Among the top 12 causative foods of FA, the proportion of anaphylaxis was lowest for soybean (15.4%).
Fig. 6.
Proportion of anaphylaxis according to different causative foods (0–18 years). *Proportion of anaphylaxis higher than 40%.
Allergic tests performed
Out of 1,661 cases, 1,444 (86.9%) underwent either one of skin prick test, serum specific immunoglobulin E (IgE) measurement, and oral food challenges. The specific numbers of cases for each test are represented in Supplementary Fig. 2, and the positive sensitization to the culprit food by at least one of the above tests was recognized in 1,311 (78.9%) out of 1,611 cases (detailed results of the tests not shown).
DISCUSSION
To our knowledge, this is the first large-scale, multicenter study to investigate the age-dependent causes and clinical features of immediate-type FA in Korean children and adolescents.
In this study, the proportion of children experiencing the symptoms of immediate-type FA for the first time was highest (71.1%) in infants, and the proportions progressively decreased with increasing age. As reported in several previous studies, FAs are more prevalent among infants and young children and significantly decline with age.12,13,14 The median age of 13 months at the first onset of symptoms of immediate-type FA reported in this study was relatively lower than the median age of first diagnosis in other studies.6,15 As in most earlier pediatric studies, a male predominance was also observed in our study.13,15,16,17,18 Sex-related differences in immune response profiles regarding sensitization and hypersensitivity in early childhood have been discussed previously.19
The most common cause of immediate-type FA in the present study was cow's milk (28.1%), followed closely by hen's eggs (27.6%), reflecting a similar pattern as in several previous reports from other countries.12,15,20 Other studies from Korea also reported cow's milk and hen's eggs as the most common causes of food hypersensitivity among children.7,21 Similar to the results of Japanese studies, the third common food allergen was wheat in the present study, whereas it was peanuts in most of the Western countries.6,9,12,15,22 Walnuts, the fourth common food allergen closely following wheat in this study, were the most common causative tree nuts, whereas hazelnuts were regarded as more common food allergens than walnuts in other studies.6,15 Since different geographical regions have different eating customs, the causative food items as the trigger for immediate-type FA might vary. The progressive increase in the use of walnuts as an ingredient in daily Korean dishes compared to other countries might explain the higher ranking of walnuts in the present study. Similar to other studies, the 8 most prevalent food allergens overall accounted for nearly 80% of FAs.15
Our data revealed that the major food allergens, causing immediate-type FAs in children and adolescents changed with age. In infants, cow's milk was the most common cause of FA in the present study, as well as in numerous previous reports.15,23,24 Interestingly, in the age group of infants, walnut and peanut allergies were already common. In the age group of preschoolers, walnuts were surprisingly the most common trigger of FA, closely followed by hen's eggs and cow's milk. Other food allergens, such as almond, buckwheat, and kiwi, appeared for the first time in the top 8 list in this age group. In the age group of school-aged children, walnuts were the most common cause, while cow's milk and hen's eggs no more took up the higher rankings. The most common food allergens in adolescents were buckwheat, followed by wheat and shrimps. In contrast to studies from other countries, walnuts instead of peanuts accounted for a higher percentage of FA cases in all age groups except adolescents in the present study.3,6,22
Regarding the time between exposure to the triggering food and the onset of symptoms, 66% of the allergic reactions were reported to occur within 30 minutes, which was time frame reported by several other studies as well.17 Cutaneous symptoms like urticaria and angioedema were the most common clinical manifestations of immediate-type FA in children of each age group. Respiratory and gastrointestinal symptoms were the second and third most common in children, whereas cardiovascular symptoms were mainly noticed in adolescents. Previous studies with comparable results have been reported.6,7,15
The most common site of FA occurrence in the present study was the child's home, which was in accordance with other investigations regarding the place of occurrence of severe FA reactions in children.14,25,26 Results from the European anaphylaxis registry, including both adults and children, also reported home to be the most common site of FA occurrence.27 By further analyzing the places of FA occurrence based on the age groups, our data showed that with increasing age of the children the percentage of experiencing allergic reactions in an out-of-home setting also increased. The likelihood of allergic reactions occurring at school is considerably high, since many children spend up to half of their waking hours in school and in school consume foods containing common allergens. Among over 4,000 children registered in the US Peanut and Tree Nut Allergy Registry, 16% were reported to have had an allergic reaction at school.28
Anaphylaxis was observed in 30.5% of all immediate-type FA cases reported in the present study. The proportion of anaphylaxis was highest in the school-aged children (52.6%), followed by the adolescents (41.4%). Although a precise comparison with previous studies regarding the proportion of anaphylaxis among FA patients by specific age groups within children is not possible, owing to the lack of previous studies, a higher risk for severe food allergic reactions among adolescents and young adults has been reported in other studies.29,30 School-aged children and adolescents comprise an age group that frequently eats out and that has therefore little control of the ingredients in the food they are consuming, making them more susceptible to food-induced anaphylaxis compared to younger children who are under stricter supervision by their care givers.
The major causes of anaphylaxis observed in the present study were similar to those of Japanese studies, which documented cow's milk, hen's eggs, and wheat as the top 3 food allergens that induce anaphylaxis in children.14,31 On the other hand, the most common trigger of food-induced anaphylaxis in Europe and North America was peanuts.3,26,32 This variation in the major triggers of food-induced anaphylaxis is possibly due to regional differences in the consumption of food, different assignment of patients to age groups, and different timing of data collection. A recent multicenter case study conducted in Korea reported cow's milk, hen's eggs, walnuts, wheat, and buckwheat as the 5 major triggers of food-induced anaphylaxis in children and adolescents.33
The overall percentage of cases with anaphylaxis of the total immediate-type FA cases was 30.5%; however, each of the major causative food allergens showed a different proportion of anaphylaxis. The proportion of anaphylaxis was highest for buckwheat (67.7%) and lowest for soybean (15.4%). Buckwheat, a member of the Polygonaceae group of herbaceous species, has already been reported to cause severe allergic symptoms, and a recent study in Korea reported the high proportion of anaphylaxis among children allergic to buckwheat.34,35,36 Tree nuts, such as pine nuts and walnuts, showed the second (57.7%) and third (43.8%) highest proportion of anaphylaxis in the present study, followed by other plant food allergens, such as wheat and peanuts. Similar to our study, other studies also report tree nuts and peanuts are generally associated with more severe reactions.37,38,39
Although the present study was very comprehensive, it is not without limitations. One limitation was that some details for FAs might have been missed due to the retrospective study design. Another limitation was that our results might not be directly transferable to other populations, due to environmental, cultural, and genetic differences.
However, the strength of the present study is that it is the first large-scale multicenter case study, which collected and revealed the most recent data of immediate-type FA reactions in a stratified report according to different age in Korean children and adolescents.
ACKNOWLEDGMENTS
This research was supported by a grant from Korea Ministry of Food and Drug Safety (MFDS) in 2015.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
Supplementary Materials
Flow-chart for case selection according to symptom onset time.
Top 10 causative foods of 8 leading hospitals
Demographic profiles of cases with and without information for places of FA occurrence
The number of cases of serum specific IgE, skin prick test, and oral food challenges performed.
References
- 1.Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet. 2013;382:1656–1664. doi: 10.1016/S0140-6736(13)60309-8. [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-García S, Cipriani F, Ricci G. Food allergy in childhood: phenotypes, prevention and treatment. Pediatr Allergy Immunol. 2015;26:711–720. doi: 10.1111/pai.12514. [DOI] [PubMed] [Google Scholar]
- 3.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–e17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 4.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Soller L, Ben-Shoshan M, Harrington DW, Fragapane J, Joseph L, St Pierre Y, et al. Overall prevalence of self-reported food allergy in Canada. J Allergy Clin Immunol. 2012;130:986–988. doi: 10.1016/j.jaci.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J, Malinovschi A, Alving K, Lidholm J, Borres MP, Nordvall L. Ten-year review reveals changing trends and severity of allergic reactions to nuts and other foods. Acta Paediatr. 2014;103:862–867. doi: 10.1111/apa.12687. [DOI] [PubMed] [Google Scholar]
- 7.Park M, Kim D, Ahn K, Kim J, Han Y. Prevalence of immediate-type food allergy in early childhood in Seoul. Allergy Asthma Immunol Res. 2014;6:131–136. doi: 10.4168/aair.2014.6.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flokstra-de Blok BM, Dubois AE, Vlieg-Boerstra BJ, Oude Elberink JN, Raat H, DunnGalvin A, et al. Health-related quality of life of food allergic patients: comparison with the general population and other diseases. Allergy. 2010;65:238–244. doi: 10.1111/j.1398-9995.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. doi: 10.1186/s12887-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowak-Wegrzyn A, Sampson HA. Adverse reactions to foods. Med Clin North Am. 2006;90:97–127. doi: 10.1016/j.mcna.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama H, Imai T, Ebisawa M. Japan food allergen labeling regulation--history and evaluation. Adv Food Nutr Res. 2011;62:139–171. doi: 10.1016/B978-0-12-385989-1.00004-1. [DOI] [PubMed] [Google Scholar]
- 13.Crespo JF, Pascual C, Burks AW, Helm RM, Esteban MM. Frequency of food allergy in a pediatric population from Spain. Pediatr Allergy Immunol. 1995;6:39–43. doi: 10.1111/j.1399-3038.1995.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 14.Imamura T, Kanagawa Y, Ebisawa M. A survey of patients with self-reported severe food allergies in Japan. Pediatr Allergy Immunol. 2008;19:270–274. doi: 10.1111/j.1399-3038.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari GG, Eng PA. IgE-mediated food allergies in Swiss infants and children. Swiss Med Wkly. 2011;141:w13269. doi: 10.4414/smw.2011.13269. [DOI] [PubMed] [Google Scholar]
- 16.Namork E, Fæste CK, Stensby BA, Egaas E, Løvik M. Severe allergic reactions to food in Norway: a ten year survey of cases reported to the food allergy register. Int J Environ Res Public Health. 2011;8:3144–3155. doi: 10.3390/ijerph8083144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mäkinen-Kiljunen S, Haahtela T. Eight years of severe allergic reactions in Finland: a register-based report. World Allergy Organ J. 2008;1:184–189. doi: 10.1097/WOX.0b013e3181898224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63:1418–1427. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 19.Uekert SJ, Akan G, Evans MD, Li Z, Roberg K, Tisler C, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006;118:1375–1381. doi: 10.1016/j.jaci.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Pyrhönen K, Näyhä S, Kaila M, Hiltunen L, Läärä E. Occurrence of parent-reported food hypersensitivities and food allergies among children aged 1–4 yr. Pediatr Allergy Immunol. 2009;20:328–338. doi: 10.1111/j.1399-3038.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim HO, Cho SI, Kim JH, Chung BY, Cho HJ, Park CW, et al. Food hypersensitivity in patients with childhood atopic dermatitis in Korea. Ann Dermatol. 2013;25:196–202. doi: 10.5021/ad.2013.25.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Mousan G, Kamat D. Cow’s milk protein allergy. Clin Pediatr (Phila) 2016;55:1054–1063. doi: 10.1177/0009922816664512. [DOI] [PubMed] [Google Scholar]
- 24.Doğruel D, Bingöl G, Altıntaş DU, Yılmaz M, Güneşer Kendirli S. Clinical features of food allergy during the 1st year of life: the ADAPAR Birth Cohort Study. Int Arch Allergy Immunol. 2016;169:171–180. doi: 10.1159/000444639. [DOI] [PubMed] [Google Scholar]
- 25.Mehl A, Wahn U, Niggemann B. Anaphylactic reactions in children--a questionnaire-based survey in Germany. Allergy. 2005;60:1440–1445. doi: 10.1111/j.1398-9995.2005.00909.x. [DOI] [PubMed] [Google Scholar]
- 26.de Silva IL, Mehr SS, Tey D, Tang ML. Paediatric anaphylaxis: a 5 year retrospective review. Allergy. 2008;63:1071–1076. doi: 10.1111/j.1398-9995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 27.Worm M, Moneret-Vautrin A, Scherer K, Lang R, Fernandez-Rivas M, Cardona V, et al. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69:1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 28.Sicherer SH, Furlong TJ, DeSimone J, Sampson HA. The US Peanut and Tree Nut Allergy Registry: characteristics of reactions in schools and day care. J Pediatr. 2001;138:560–565. doi: 10.1067/mpd.2001.111821. [DOI] [PubMed] [Google Scholar]
- 29.Muñoz-Furlong A, Weiss CC. Characteristics of food-allergic patients placing them at risk for a fatal anaphylactic episode. Curr Allergy Asthma Rep. 2009;9:57–63. doi: 10.1007/s11882-009-0009-2. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher M, Worth A, Cunningham-Burley S, Sheikh A. Strategies for living with the risk of anaphylaxis in adolescence: qualitative study of young people and their parents. Prim Care Respir J. 2012;21:392–397. doi: 10.4104/pcrj.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai T, Sugizaki C, Ebisawa M. A report on 2011 Nationwide Survey of Immediate Type Food Allergies in Japan (supported by a grant from “Consumer Affairs Agency, Government of Japan”) Arerugi. 2016;65:942–946. doi: 10.15036/arerugi.65.942. [DOI] [PubMed] [Google Scholar]
- 32.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee SY, Ahn K, Kim J, Jang GC, Min TK, Yang HJ, et al. A multicenter retrospective case study of anaphylaxis triggers by age in Korean children. Allergy Asthma Immunol Res. 2016;8:535–540. doi: 10.4168/aair.2016.8.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SY. IgE mediated food allergy in Korean children: focused on plant food allergy. Asia Pac Allergy. 2013;3:15–22. doi: 10.5415/apallergy.2013.3.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieslander G, Norbäck D. Buckwheat allergy. Allergy. 2001;56:703–704. doi: 10.1034/j.1398-9995.2001.056008703.x. [DOI] [PubMed] [Google Scholar]
- 36.Park K, Jeong K, Lee S. Clinical and laboratory findings of childhood buckwheat allergy in a single tertiary hospital. Korean J Pediatr. 2016;59:402–407. doi: 10.3345/kjp.2016.59.10.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelman FD. Peanut allergy and anaphylaxis. Curr Opin Immunol. 2010;22:783–788. doi: 10.1016/j.coi.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skripak JM, Wood RA. Peanut and tree nut allergy in childhood. Pediatr Allergy Immunol. 2008;19:368–373. doi: 10.1111/j.1399-3038.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- 39.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow-chart for case selection according to symptom onset time.
Top 10 causative foods of 8 leading hospitals
Demographic profiles of cases with and without information for places of FA occurrence
The number of cases of serum specific IgE, skin prick test, and oral food challenges performed.