Abstract
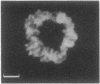
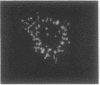
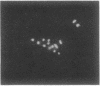
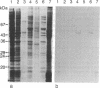
Several autoimmune sera from patients with Raynaud phenomenon decorated mammalian kinetochores and bound to a 47-kDa protein on immunoblots of nuclear lysates. Antibody affinity-purified from immunoblots of the 47-kDa band recognized kinetochores, but due to crossreaction with an 18-kDa protein, localization remains elusive. We used one of these sera to purify the antigen from HeLa cells synchronized in mitosis as a noncovalent complex with a 25-kDa protein. The antigen was released from DNA by intercalation with 25 mM chloroquine. Ion-exchange chromatography yielded the pure complex with an apparent molecular size of 68 kDa, which was separated into its components by gel filtration in 6 M guanidinium chloride. Upon two-dimensional gel electrophoresis the 47-kDa protein gave two main spots of pI 6.6 and 6.7, respectively. Posttranslational modification is indicated by additional antigenic spots, by lack of a free alpha-amino group, and by chromatographic behavior of peptides on reversed-phase chromatography. The amino acid sequence for 205 residues of the 47-kDa protein has been established. This sequence is highly homologous with the translated reading frame of RCC1, a gene reportedly involved in regulating onset of mammalian chromosome condensation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balczon R. D., Brinkley B. R. Tubulin interaction with kinetochore proteins: analysis by in vitro assembly and chemical cross-linking. J Cell Biol. 1987 Aug;105(2):855–862. doi: 10.1083/jcb.105.2.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram R. J., Kornberg R. D. Isolation of a Saccharomyces cerevisiae centromere DNA-binding protein, its human homolog, and its possible role as a transcription factor. Mol Cell Biol. 1987 Jan;7(1):403–409. doi: 10.1128/mcb.7.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M. W., Tan E., Brinkley B. R. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981 Oct;91(1):95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Stubblefield E. The fine structure of the kinetochore of a mammalian cell in vitro. Chromosoma. 1966;19(1):28–43. doi: 10.1007/BF00332792. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Schenk E. A., Olmsted J. B. Human anticentromere antibodies: distribution, characterization of antigens, and effect on microtubule organization. Cell. 1983 Nov;35(1):331–339. doi: 10.1016/0092-8674(83)90236-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Kinsella T. D. The CREST syndrome: a distinct serologic entity with anticentromere antibodies. Am J Med. 1980 Oct;69(4):520–526. doi: 10.1016/0002-9343(80)90462-3. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Sammak P. J., Borisy G. G. Chromosomes move poleward in anaphase along stationary microtubules that coordinately disassemble from their kinetochore ends. J Cell Biol. 1987 Jan;104(1):9–18. doi: 10.1083/jcb.104.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner H. H., Lakomek H. J., Bautz F. A. Human anti-centromere sera recognise a 19.5 kD non-histone chromosomal protein from HeLa cells. Clin Exp Immunol. 1984 Oct;58(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Hadlaczky G., Praznovszky T., Rasko I., Kereso J. Centromere proteins. I. Mitosis specific centromere antigen recognized by anti-centromere autoantibodies. Chromosoma. 1989 Jan;97(4):282–288. doi: 10.1007/BF00371967. [DOI] [PubMed] [Google Scholar]
- Huitorel P., Kirschner M. W. The polarity and stability of microtubule capture by the kinetochore. J Cell Biol. 1988 Jan;106(1):151–159. doi: 10.1083/jcb.106.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell B., Rattner J. B. Mammalian kinetochore/centromere composition: a 50 kDa antigen is present in the mammalian kinetochore/centromere. Chromosoma. 1987;95(6):403–407. doi: 10.1007/BF00333991. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Mitchison T. J., Kirschner M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988 Feb 11;331(6156):499–504. doi: 10.1038/331499a0. [DOI] [PubMed] [Google Scholar]
- Maschek U., Pülm W., Hämmerling G. J. Altered regulation of MHC class I genes in different tumor cell lines is reflected by distinct sets of DNase I hypersensitive sites. EMBO J. 1989 Aug;8(8):2297–2304. doi: 10.1002/j.1460-2075.1989.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilage L. J., Whittingham S., McHugh N., Barnett A. J. A highly conserved 72,000 dalton centromeric antigen reactive with autoantibodies from patients with progressive systemic sclerosis. J Immunol. 1986 Oct 15;137(8):2541–2547. [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M., Kai R., Furuno N., Sekiguchi T., Sekiguchi M., Hayashida H., Kuma K., Miyata T., Fukushige S., Murotsu T. Isolation and characterization of the active cDNA of the human cell cycle gene (RCC1) involved in the regulation of onset of chromosome condensation. Genes Dev. 1987 Aug;1(6):585–593. doi: 10.1101/gad.1.6.585. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Okazaki H., Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989 Oct;109(4 Pt 1):1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B. The organization of the mammalian kinetochore: a scanning electron microscope study. Chromosoma. 1987;95(3):175–181. doi: 10.1007/BF00330348. [DOI] [PubMed] [Google Scholar]
- Roos U. P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma. 1973;41(2):195–220. doi: 10.1007/BF00319696. [DOI] [PubMed] [Google Scholar]
- Schröter H., Maier G., Ponstingl H., Nordheim A. DNA intercalators induce specific release of HMG 14, HMG 17 and other DNA-binding proteins from chicken erythrocyte chromatin. EMBO J. 1985 Dec 30;4(13B):3867–3872. doi: 10.1002/j.1460-2075.1985.tb04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]